Abstract
Targeting virulence factors represents a promising alternative approach to antimicrobial therapy, through the inhibition of pathogenic pathways that result in host tissue damage. Yet, virulence inhibition remains an understudied area in parasitology. Several medically important protozoan parasites such as Plasmodium, Entamoeba, Toxoplasma, and Leishmania secrete an inflammatory macrophage migration inhibitory factor (MIF) cytokine homolog, a virulence factor linked to severe disease. The aim of this study was to investigate the effectiveness of targeting parasite-produced MIF as combination therapy with standard antibiotics to reduce disease severity. Here, we used Entamoeba histolytica as the model MIF-secreting protozoan, and a mouse model that mirrors severe human infection. We found that intestinal inflammation and tissue damage were significantly reduced in mice treated with metronidazole when combined with anti–E. histolytica MIF antibodies, compared to metronidazole alone. Thus, this preclinical study provides proof-of-concept that combining antiparasite MIF-blocking antibodies with current standard-of-care antibiotics might improve outcomes in severe protozoan infections.
Keywords: virulence, MIF, protozoan, antibody, antibiotic, parasite–host interactions, tissue damage, immunopathology, immunotherapeutic, antivirulence
Protozoan parasites represent a major threat to global public health, causing >1 million deaths yearly [1]. No effective vaccines have been developed to prevent disease from any of the protozoan parasites to date. Therefore, the management of infected patients continues to rely on treatment with antibiotics and supportive care. New strategies to fight such infections are urgently needed due to the emergence of multidrug-resistant pathogens that limit available treatment options. In addition, poor clinical outcomes are associated with severe infections, even when appropriate therapy is administered [2]. For example, Entamoeba histolytica is a protozoan parasite that causes inflammatory diarrhea, termed amebic colitis, which is characterized by colonic inflammation and tissue damage. Entamoeba histolytica infects millions of people annually, making amebic colitis a leading cause of severe diarrhea worldwide, estimated to kill approximately 50 000–100 000 people each year [3, 4]. Severe forms of amebic colitis carry high fatality exceeding 50%, even despite treatment with the nitroimidazole antibiotics, such as metronidazole, which are the treatment choice. New therapeutic strategies are needed as metronidazole alone is sometimes not enough, and even drastic measures such as the surgical resection of the inflamed portion of colon may not prevent death [4–7].
Virulence factors are molecules or proteins produced by pathogens that promote disease by damaging host tissue. Targeting virulence factors by inhibiting specific mechanisms that promote tissue damage and disease symptoms is a promising alternative strategy to new antimicrobial development. Also, removing pathogens of their virulence properties without harming their survival hopefully will reduce the potential of antimicrobial selection pressure and development of drug-resistant mutations [2, 8, 9]. While substantial progress in antivirulence approaches have been made in the field of bacteriology, virulence factor inhibition in parasitology remains significantly understudied.
Macrophage migration inhibitory factor (MIF) is an inflammatory cytokine that is a critical upstream mediator of inflammation. Secreted MIF binds to its receptor, CD74, on immune and epithelial cells and stimulates expression of various cytokines, for example, interleukin 8 (IL-8) and tumor necrosis factor alpha (TNF-α) [10, 11]. Pathogenic protozoan parasites, such as Plasmodium, Entamoeba, Toxoplasma, and Leishmania, secrete homologs of the cytokine MIF. Avoiding immune clearance allows these protozoa to persist in their host, which exacerbates the damage caused by the lingering inflammatory response to invading parasites [12], compounded by the fact that these parasites secrete MIF cytokine that can directly drive inflammation [13]. The inflammatory properties of protozoan-produced MIF contribute to immunopathology, damaging the host, and are linked to more severe disease [14–22]. However, a critical unanswered question is whether antibodies to protozoan MIF can reduce disease severity. The aim of this preclinical study was to investigate the benefit of neutralizing antiprotozoan MIF antibodies as an add-on therapy to antibiotics in severe disease using E. histolytica as the model organism. We found that blocking the virulence factor E. histolytica MIF (Eh-MIF) with neutralizing antibodies combined with antibiotics resulted in improved inflammatory outcomes and less host damage in severe infection.
MATERIALS AND METHODS
Coculture of Human Cells With E. histolytica Parasites
Human intestinal epithelial cells (HCT-116) and human macrophages (differentiated THP-1 cells) were cultured with E. histolytica strain HM1:IMSS trophozoites at a ratio of 10:1 human cells to parasite in M199 medium [17, 23]. IL-8 and TNF-α in cell culture supernatant were measured by enzyme-linked immunosorbent assay (ELISA; eBioscience).
Mice and Amebic Colitis
Entamoeba histolytica strains capable of evading immune clearance were generated by passing trophozoites through mice intestine. Entamoeba histolytica trophozoites that persisted in an inflamed intestine for at least 5 days were used for severe colitis experiments. Wild-type CBA/J mice were obtained from the Jackson Laboratory. Male mice were used at 10 weeks of age. Mice were treated with granulocyte colony-stimulating factor (G-CSF) 125 μg/kg subcutaneously twice per day for 3 days [24]. On day 4, animals were anesthetized, laparotomized, and intracecally infected with 106E. histolytica trophozoites [25]. Treatment began 24 hours after infection [6] and continued for a total of 3 days. One group received metronidazole (10 mg/kg per day) [26] plus 1 mg mouse anti–Eh-MIF blocking antibodies given by parenteral (intraperitoneal) injection. The control group received equivalent amounts of metronidazole plus control antibody. At the end of the treatment course mice were killed, and the cecal tissue and luminal contents were obtained for further analysis.
ELISA
Intestinal tissue was prepared for ELISA as described previously [27]. Intestinal tissue lysates and luminal contents were evaluated by ELISA for CXCL1 (R&D Systems), TNF-α (eBioscience), myeloperoxidase (MPO; R&D Systems) [28], and albumin (Bethyl Laboratories) according to the manufacturers’ instructions. Total protein concentration was measured using the Pierce BCA Protein Assays Kit (Thermo Scientific).
Histopathological Examination
Mouse tissue was fixed in Bouin solution (Sigma) and stored in 70% ethanol. Tissue staining with hematoxylin and eosin was performed by the University of Virginia Research Histology Core [17]. Histological scoring was performed by 2 independent blinded scorers as previously described [29].
Structure Analysis and Bioinformatics
The coordinates of the Eh-MIF protein structure have been deposited in the Protein Data Bank under the accession code 6CUQ. Structural comparison between the Eh-MIF and human MIF proteins were done using the University of California, San Francisco Chimera software version 1.10.2. Amino acid sequences of MIF proteins from human and E. histolytica were aligned by Multiple Sequence Comparison by Log Expectation (MUSCLE) software [30].
Protein Expression, Purification, and Biotinylation
The CD74 ectodomain cDNA was subcloned from pGEX-6P-1-CD74 plasmid (previously described in [16]) into pET28-MBP-TEV vector (Addgene plasmid number 69929) within 5′BamH1 and 3′XhoI sites followed by transformation into Escherichia coli BL21 (DE3) cells for expression and purification of the recombinant MBP-CD74 protein. Both MBP and MBP-CD74 proteins were expressed by induction with 1 mM isopropyl β-d–thiogalactoside for 18 hours at 15°C. Purification of these proteins was done as previously described [31]. In brief, proteins were affinity purified with amylose resin (New England Biotechnologies) and eluted with 10 mM maltose. The expression and purification of Eh-MIF and human MIF recombinant proteins were done as previously described [16]. Purified proteins were concentrated and buffer exchanged into 1× phosphate-buffered saline using Amicon Ultra-15 centrifugal filter units (Millipore Sigma). Biotinylation of MBP and MBP-CD74 proteins were done by the EZ-Link Sulfo-NHS-LC-Biotinylation kit (Thermo Scientific).
Binding Kinetics Using Biolayer Interferometry Assay
The binding affinity of MBP-CD74 for Eh-MIF and human MIF was determined using the Blitz System (Octet Red 96 system, ForteBio). In brief, Streptavidin Dip and Read Biosensors (ForteBio) were hydrated for 10 minutes followed by baseline stabilization for 5 minutes in the sample dilution buffer (1× Dulbecco’s PBS, 0.02% Tween 20), and 25 µg biotinylated MBP-CD74 or MBP was loaded onto the biosensors for 5 minutes. After washing the loaded biosensors in sample dilution buffer for 5 minutes, they were exposed for 10 minutes to five 2-fold dilution series of Eh-MIF or human MIF protein starting at 800 nM concentration. Postbinding dissociation was done for 10 minutes in the sample dilution buffer. Binding affinities (KD) were calculated using the Blitz system software (ForteBio).
Immunoblotting
Recombinant Eh-MIF and human MIF were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by transfer onto polyvinylidene difluoride membranes (Millipore). The membranes were incubated overnight with affinity purified mouse anti–Eh-MIF antibody at 4°C followed by antimouse immunoglobulin G (IgG) horseradish peroxidase conjugate (Sigma) secondary antibody. Enhanced chemiluminescence (Thermo Scientific)–based substrates were used to detect antibody conjugated peroxidase activity.
MIF Homologs and IL-8 Secretion Assay
IL-8 secretion assays were carried out as previously described [17, 18]. In brief, 106 cells/mL human Caco2 colonic epithelial cells were treated with 0.5 µg/mL human or E. histolytica MIF in the presence of 5, 20, and 50 µg/mL anti–E. histolytica antibodies for 8 hours. IL-8 in cell culture supernatant was measured by ELISA (eBioscience).
Antibody Purification
Antibodies used in cytokine secretion assays and passive immunization were purified using the Melon Gel IgG Purification Kit (Thermo Scientific) for purification of IgG from Eh-MIF immunized or control mice as previously described [32]. This allows both groups to have the same antibody profile except for neutralizing antibodies against the Eh-MIF protein.
Statistical Analysis
Statistical differences between 2 groups were determined using Student t test and Mann–Whitney U test. Pearson correlation was used for correlation analysis. A P value <.05 was considered statistically significant.
Study Approval
All animal procedures were approved by the University of Virginia Institutional Animal Care and Use Committee (IACUC). All animal studies were performed in compliance with the federal regulations set forth in the Animal Welfare Act, the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the guidelines of the University of Virginia IACUC.
RESULTS
E. histolytica MIF Protein is a Bona Fide Homolog of Human MIF
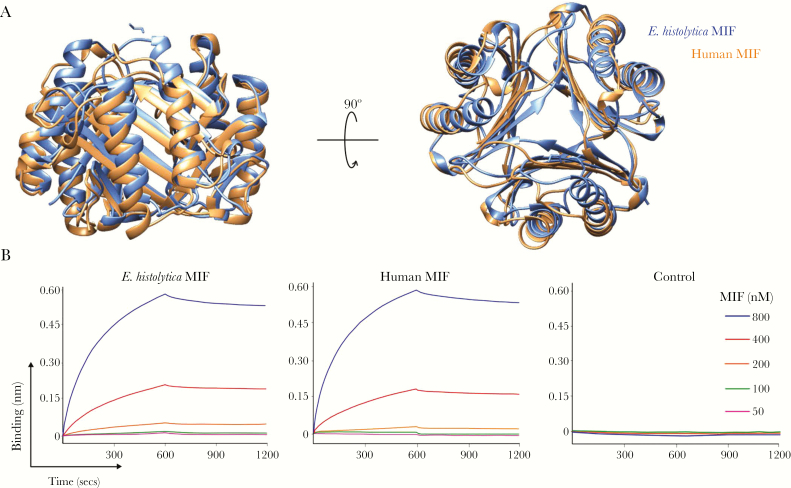
Given that structural similarity between proteins is strong predictor of functional similarity [33], we determined the crystal structure of the Eh-MIF protein and investigated whether it is an authentic homolog of human MIF. The X-ray crystal structure of Eh-MIF was solved at a resolution of 2.45 Å (Protein Data Bank identifier: 6CUQ). Similar to the human protein, Eh-MIF formed a stable trimer (Figure 1A). Structural homology between Eh-MIF and human MIF was measured by the root mean square deviation (RMSD) [34, 35]. Superimposition of all the 113 α-carbon atoms of the backbone resulted in a RMSD value of 1.678 Å, which falls well within the range of RMSD for homologous proteins, which is <3 Å [36]. Since RMSD calculation is inherently biased by further apart atoms [35], we also found that the RMSD value of 100 residues best aligned with the human protein was 1.076 Å. Therefore, our findings indicate that Eh-MIF protein is structurally homologous to human MIF.
Figure 1.
Characterization of Entamoeba histolytica macrophage migration inhibitory factor (MIF) as a bona fide homolog of human MIF. A, Structural homology between E. histolytica MIF and human MIF. The trimeric form of the human MIF (orange) and E. histolytica MIF (blue) was superimposed as shown in the overlapping backbone ribbon diagram. B, Biolayer interferometry analysis of E. histolytica MIF and human MIF binding to the MIF receptor CD74. Analysis of the representative binding curves (association analysis: 0–600 seconds) and dissociation curves (dissociation analysis: 600–1200 seconds) revealed a dissociation constant (KD) of 1.12 × 10−8 M and 1.82 × 10−8 M for E. histolytica MIF and human MIF, respectively. Biotinylated MBP-CD74 was loaded onto streptavidin biosensors and placed into solutions with MIF (concentration range, 50–800 nM). Biosensors loaded with biotinylated maltose binding protein only were used as a control.
Next, we compared the binding affinity of E. histolytica and human MIF proteins to the MIF receptor CD74. Biolayer interferometry (BLI) is a useful technique for measuring interaction affinity between proteins in real time [37]. Biotinylated MBP-CD74 fusion protein was loaded onto the surface of streptavidin BLI sensors, followed by binding measurements in different concentrations of E. histolytica or human MIF proteins. Analysis revealed a dissociation constant (KD) of 1.12 × 10−8 M for Eh-MIF (Figure 1B), similar to that for the human MIF, which was 1.82 × 10−8 M (Figure 1B). Biotinylated MBP alone coupled to the streptavidin BLI sensors was used as control. BLI measurements demonstrated that E. histolytica and human MIF proteins did not bind to the MBP-only control, KD not applicable (Figure 1B). Therefore, the MIF receptor CD74 has similar high binding affinity for both E. histolytica and human MIF proteins.
Collectively, these structural and receptor binding data along with previously described proinflammatory properties of Eh-MIF [16–18], supports Eh-MIF as a bona fide homolog of the human proinflammatory cytokine MIF.
Antibodies Against E. histolytica MIF Do Not Cross-React With Human MIF
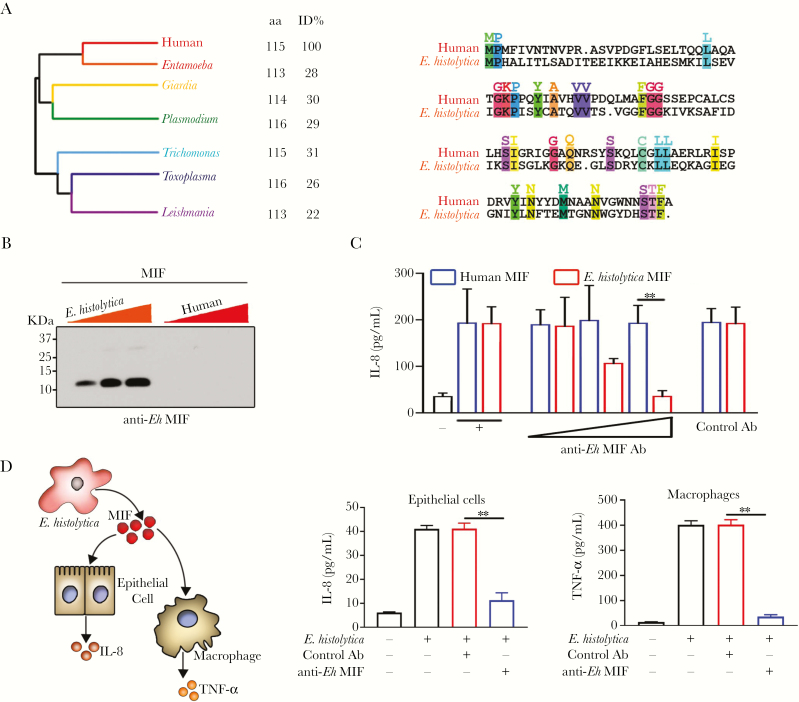
While human and Eh-MIF homologs share structural and functional similarities, their sequence homology is low; they share only 28% sequence identity (Figure 2A). Antibody cross-reactivity depends on the extent of protein sequence similarity. Therefore, the low sequence homology facilitates generating antibodies against Eh-MIF that do not cross-react with human MIF. In support of this, we found that anti–Eh-MIF antibodies were highly specific to the parasite protein and did not cross-react with the host protein by immunoblot analysis (Figure 2B). This is consistent with our earlier data that human and animal sera containing anti–Eh-MIF antibodies did not cross react with the recombinant host MIF [16, 17]. In addition to immunoblot assay, we evaluated cross-reactivity with functional analysis of neutralizing antibodies against Eh-MIF. Both human and Eh-MIF stimulate IL-8 production from Caco-2 intestinal epithelial cells [17]. Using this assay, we found that these neutralizing antibodies inhibited Eh-MIF activity but had no effect on human MIF–induced IL-8 production (Figure 2C). These results, which include sequence identity, immunoblot, and functional analysis, support the lack of cross-reactivity of anti–Eh-MIF antibodies with human MIF.
Figure 2.
Anti–Entamoeba histolytica macrophage migration inhibitory factor (MIF) antibody does not cross-react with human MIF. A, Medically important protozoan parasite MIF homologs. Pairwise amino acid sequence alignment of human and E. histolytica MIF proteins. Identical residues are color coded and highlighted. B, Immunoblot analysis of E. histolytica MIF and human MIF using anti–E. histolytica MIF antibody. Proteins were loaded in increasing amounts: 100 ng, 200 ng, and 300 ng as indicated by the colored triangles. C, Anti–E. histolytica MIF antibodies blocked E. histolytica MIF-induced interleukin 8 production but had no effect on human MIF activity. D, Entamoeba histolytica parasites cocultured with human intestinal epithelial cells (HCT116 cells) or macrophages (differentiated THP-1 cells) in the presence of anti–E. histolytica MIF antibodies. Data represent mean and standard deviation of triplicates from 1 experiment and are representative of 3 independent experiments. **P < .01. Abbreviations: aa, amino acid; Ab, antibody; Eh, Entamoeba histolytica; ID, identity; IL-8, interleukin-8; MIF, macrophage migration inhibitory factor; TNF-α, tumor necrosis factor alpha.
We further tested if the antibody could neutralize the effects of the parasite-secreted MIF on human epithelial and immune cells using coculturing assays [17, 23]. We found that neutralizing antibodies blocked E. histolytica–stimulated IL-8 and TNF-α production by human intestinal epithelial cells (HCT116) and macrophages (differentiated THP-1), respectively (Figure 2D). Together, these data further support the concept that Eh-MIF can be specifically targeted in E. histolytica–induced inflammation.
Combination Therapy With Neutralizing Anti–E. histolytica MIF Antibodies Is Superior to Metronidazole Alone
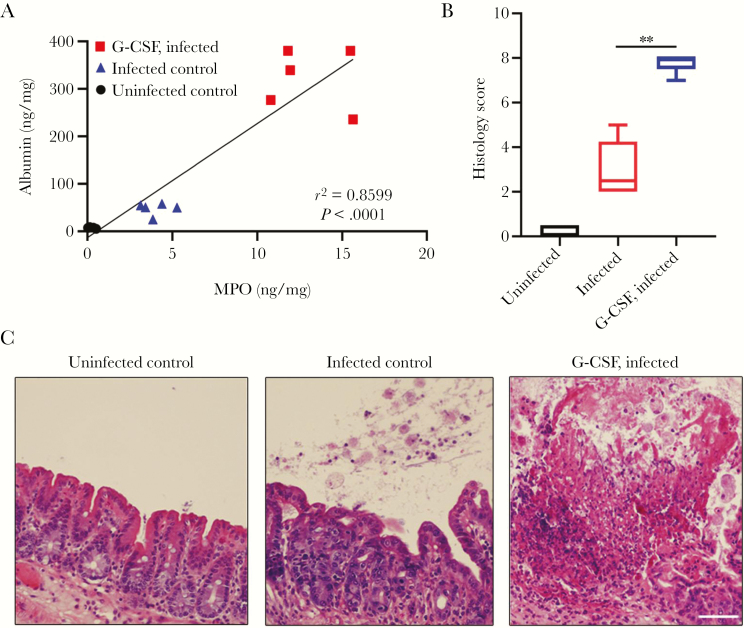
Since elevated parasite MIF levels positively correlate with inflammation and disease severity [13, 14, 17, 38], we hypothesize that blocking Eh-MIF activity would be most beneficial in the setting of severe infections. To test this hypothesis, we used a mouse model that simulates severe human amebic colitis. That is, in patients with amebic colitis, excess intestinal tract neutrophil infiltration is associated with more tissue destruction and severe disease [39–42]. First we serially infected mice, then obtained E. histolytica strains that were capable of evading the immune clearance by persisting in the inflamed intestine for at least 5 days. These strains were used for future studies. G-CSF is a potent stimulator of white blood cell production, and in particular, neutrophil production [24]. MPO, a major component of neutrophils, is a widely used marker of neutrophil influx and intestinal inflammation [43]. A healthy intestine with an intact epithelial and endothelial barrier prevents the spilling of albumin into the gut lumen [44]. Intestinal tissue damage caused by E. histolytica infection results in loss of the intestinal permeability barrier and can be quantified by measuring the flux of albumin from the serum into the intestinal lumen [45]. Infected mice pretreated with G-CSF had increased neutrophil infiltration as measured by tissue MPO levels. As expected, increased neutrophil infiltration correlated with intestinal damage as evidenced by more severe histopathology and elevated luminal albumin, modeling severe human amebic colitis (Figure 3).
Figure 3.
Tissue destruction caused by Entamoeba histolytica parasite. A, Increased tissue myeloperoxidase (MPO) and luminal albumin levels in infected mice pretreated with granulocyte colony-stimulating factor (G-CSF) compared with infected mice without G-CSF pretreatment and uninfected controls. B and C, Representative hematoxylin and eosin–stained images and histology scores. Scale bars, 50 μm. Data represent mean and standard deviation (n = 5 mice per group). **P < .01.
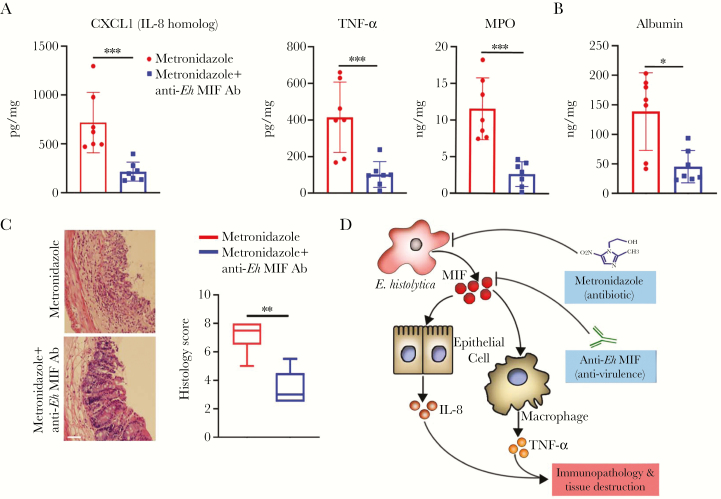
Next, we use this model to evaluate the benefit of add-on therapy with neutralizing anti–Eh-MIF antibodies in severe infection. Both treatment groups received metronidazole and were able to clear the parasites within 72 hours. Adding anti–Eh-MIF antibodies to the treatment regimen as opposed to metronidazole only, significantly reduced parasite-induced gut inflammation, as measured by CXCL1 (IL-8 homolog), TNF-α, and MPO levels, and the E. histolytica–induced tissue damage, as analyzed by histological score and by mucosal barrier integrity (Figure 4). These data provide evidence that antibodies to the amebic virulence factor MIF can provide additional benefits over antibiotics alone in severe amebic colitis.
Figure 4.
Anti–Entamoeba histolytica macrophage migration inhibitory factor (MIF) antibody adjunctive therapy reduces tissue damage. A–C, Reduced tissue CXCL1, tumor necrosis factor–α, and myeloperoxidase, luminal albumin levels, and histology scores in infected mice treated with anti–E. histolytica MIF antibodies combined with metronidazole compared to metronidazole alone (control). Scale bars, 50 μm. D, Schematic diagram of the possible mechanism by which combination therapy reduces tissue damage. Data represent mean and standard deviation (n = 7 mice per group). *P < .05; **P < .01; ***P < .001. Abbreviations: Ab, antibody; Eh, Entamoeba histolytica; IL-8, interleukin-8; MIF, macrophage migration inhibitory factor; MPO, myeloperoxidase; TNF-α, tumor necrosis factor alpha.
DISCUSSION
During an infection, host tissue destruction occurs by direct damage by the pathogen and inflammatory-mediated damage (immunopathology) [46]. Extensive tissue destruction correlates with poor clinical outcomes, even when appropriate antibiotics are administered promptly for treatment [5, 39, 46]. This implies that antibiotics alone are not always sufficient to disrupt the effects arising from severe parasitic infection. Disarming the parasite of important virulence factors offers an attractive targeted approach that can be used as an adjunct to protozoacidal antibiotics. Neutralizing secreted virulence factors, as well as virulence factors released during parasite death and cell lysis, may help to limit ongoing damage and inflammation. We have used E. histolytica as a prototype to show a novel therapeutic strategy for reducing tissue damage by directing therapy against the parasitic MIF virulence factor.
The targeting of microbial virulence factors offers several putative therapeutic advantages. As the pathogen itself is typically not destroyed, antivirulence treatments should not cause selection pressure of drug-resistant mutants, which has been a major challenge with traditional antibiotics [2]. Another benefit of antivirulence strategies may be lack of direct effects on beneficial host commensals, which are unlikely to harbor virulence factors [2]. Metronidazole, for example, in addition to having antiprotozoal activity, can cause undesired disturbance to a wide range of enteric anaerobes. Additionally, antivirulence may be able to more quickly inactivate targets than antibiotics, which act by inhibition of growth and replication [2]. Hence anti–Eh-MIF holds potential as a rapid-onset strategy for decreasing disease severity in amebic colitis without anticipated selection of resistance or disturbance of host microbiomes.
The concept of using antibodies to neutralize virulence factors is well described for several bacterial infections, and has been used for more than a century in the treatment of children with life-threatening diphtheria who are given both antibiotics and horse antiserum derived against diphtheria toxin, for example [2]. To our knowledge, this strategy for parasitic infections has been understudied [2], and we believe that anti–Eh-MIF offers a suitable and rationale prime candidate. Anti–Eh-MIF is a naturally occurring antibody in humans, produced as part of the adaptive immune response to E. histolytica infection. Eh-MIF is structurally identical to human MIF, but exhibits relatively low identity sequence homology. Hence, Eh-MIF antibodies can be generated with high affinity and specificity, but without significant cross-reactivity with human MIF, as we have shown here and in prior human and animal studies [16, 17]. Taken together, these data further support the safety of anti–Eh-MIF as an antivirulence candidate that can effectively neutralize parasite MIF, without anticipated adverse off-target effects on human MIF.
Limitations of this approach include the following: Anti–Eh-MIF does not impair amebic growth or have amebicidal activity, meaning that as antibody levels wane with time, if the parasite has not been killed, there could be a risk of recurrence of disease. To overcome this, we suggest that at this time anti–Eh-MIF be developed as a strategy to minimize inflammation-induced tissue damage in combination with antibiotic therapy, rather than as monotherapy. Second, an early microbiologic diagnosis is required to deploy antivirulence therapy in an effective manner, as these strategies are too specific for broad-range empiric use. The increasing availability of rapid molecular diagnostics such as multiplex enteric pathogen panels, however, will facilitate the ability to carry this out in a timely manner in the future [4].
We can further improve upon this work by pursuing pathways for polyclonal antibody development, such as standardization and supply of anti–Eh-MIF, in order to study clinical applications. Polyclonal antibodies offer the advantage of binding to multiple epitopes and hence offer higher affinity with less vulnerability to minor antigenic changes, but large-scale production can be challenging. One solution may be to engineer a monoclonal antibody candidate, through the use of recombinant DNA or other technology, which can be made more readily in larger quantities. Previously such technologies required large investments in time and technical skills, but the increasing demand for monoclonal antibodies in the treatment of cancer, autoimmune conditions, and other infections is paving the way for faster production capacity and improved manufacturing processes, making this a viable and perhaps even affordable option with time [47, 48].
In summary, we have demonstrated that anti–E. histolytica MIF offers a promising candidate for adjunctive treatment of severe amebic colitis, which otherwise carries a high fatality, even when treated with appropriate antibiotics. We also more broadly demonstrate the concept that antiparasite virulence factors can be blocked to treat disease, providing a preclinical basis for the development of innovative strategies to save and protect lives from these devastating neglected tropical diseases.
Notes
Acknowledgments. We thank Richard Locksley, William Petri, Carol Gilchrist, and Mayuresh Abhyankar for helpful advice and assistance; and the University of Virginia’s Research Histology, and Biorepository and Tissue Research facilities.
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers R01AI026649-27S1 and K08AI119181); a University of Virginia seed grant; and the Robert Wood Johnson Foundation–Harold Amos Medical Faculty Development Program Award (to S. M.). The Seattle Structural Genomics Center for Infectious Disease is funded by the National Institute of Allergy and Infectious Diseases of the NIH (contract number HHSN272201700059C).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2015. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Dickey SW, Cheung GYC, Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 2017; 16:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shirley DT, Farr L, Watanabe K, Moonah S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect Dis 2018; 5:ofy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirley DA, Moonah S. Fulminant amebic colitis after corticosteroid therapy: a systematic review. PLoS Negl Trop Dis 2016; 10:e0004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debnath A, Parsonage D, Andrade RM, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 2012; 18:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehrenkaufer GM, Suresh S, Solow-Cordero D, Singh U. High-throughput screening of Entamoeba identifies compounds which target both life cycle stages and which are effective against metronidazole resistant parasites. Front Cell Infect Microbiol 2018; 8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 2008; 6:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 2010; 9:117–28. [DOI] [PubMed] [Google Scholar]
- 10. Harris J, VanPatten S, Deen NS, Al-Abed Y, Morand EF. Rediscovering MIF: new tricks for an old cytokine. Trends Immunol 2019; 40:447–62. [DOI] [PubMed] [Google Scholar]
- 11. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 2003; 3:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moonah SN, Jiang NM, Petri WA Jr. Host immune response to intestinal amebiasis. PLoS Pathog 2013; 9:e1003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Jiang N, Farr L, Ngobeni R, Moonah S. Parasite-Produced MIF Cytokine: Role in Immune Evasion, Invasion, and Pathogenesis. Frontiers in Immunology 2019; 10. doi: 10.3389/fimmu.2019.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun T, Holowka T, Song Y, et al. A Plasmodium-encoded cytokine suppresses T-cell immunity during malaria. Proc Natl Acad Sci U S A 2012; 109:E2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baeza Garcia A, Siu E, Sun T, et al. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat Commun 2018; 9:2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moonah SN, Abhyankar MM, Haque R, Petri WA Jr. The macrophage migration inhibitory factor homolog of Entamoeba histolytica binds to and immunomodulates host macrophages. Infect Immun 2014; 82:3523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ngobeni R, Abhyankar MM, Jiang NM, et al. Entamoeba histolytica-encoded homolog of macrophage migration inhibitory factor contributes to mucosal inflammation during amebic colitis. J Infect Dis 2017; 215:1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghosh S, Leaton LA, Farr L, Barfield A, Moonah S. Interaction between parasite-encoded JAB1/CSN5 and macrophage migration inhibitory factor proteins attenuates its proinflammatory function. Sci Rep 2018; 8:10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Twu O, Dessí D, Vu A, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A 2014; 111:8179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommerville C, Richardson JM, Williams RA, et al. Biochemical and immunological characterization of Toxoplasma gondii macrophage migration inhibitory factor. J Biol Chem 2013; 288:12733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holowka T, Castilho TM, Garcia AB, Sun T, McMahon-Pratt D, Bucala R. Leishmania-encoded orthologs of macrophage migration inhibitory factor regulate host immunity to promote parasite persistence. FASEB J 2016; 30:2249–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchko GW, Abendroth J, Robinson H, et al. Crystal structure of a macrophage migration inhibitory factor from Giardia lamblia. J Struct Funct Genomics 2013; 14:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marie C, Verkerke HP, Theodorescu D, Petri WA. A whole-genome RNAi screen uncovers a novel role for human potassium channels in cell killing by the parasite Entamoeba histolytica. Sci Rep 2015; 5:13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lord BI, Woolford LB, Molineux G. Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (PEG-r-metHu G-CSF). Clin Cancer Res 2001; 7:2085–90. [PubMed] [Google Scholar]
- 25. Deloer S, Nakamura R, Mi-Ichi F, Adachi K, Kobayashi S, Hamano S. Mouse models of amoebiasis and culture methods of amoeba. Parasitol Int 2016; 65:520–5. [DOI] [PubMed] [Google Scholar]
- 26. Becker S, Hoffman P, Houpt ER. Efficacy of antiamebic drugs in a mouse model. Am J Trop Med Hyg 2011; 84:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe K, Gilchrist CA, Uddin MJ, et al. Microbiome-mediated neutrophil recruitment via CXCR2 and protection from amebic colitis. PLoS Pathog 2017; 13:e1006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Couturier-Maillard A, Froux N, Piotet-Morin J, et al. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol 2018; 11:1181–90. [DOI] [PubMed] [Google Scholar]
- 29. Spalinger MR, Kasper S, Gottier C, et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J Clin Invest 2016; 126:1783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 2004; 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Currinn H, Guscott B, Balklava Z, Rothnie A, Wassmer T. APP controls the formation of PI(3,5)P(2) vesicles through its binding of the PIKfyve complex. Cell Mol Life Sci 2016; 73:393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akahata W, Yang ZY, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med 2010; 16:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian W, Skolnick J. How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol 2003; 333:863–82. [DOI] [PubMed] [Google Scholar]
- 34. Kamir D, Zierow S, Leng L, et al. A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J Immunol 2008; 180:8250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kufareva I, Abagyan R.. Methods of protein structure comparison. In: Homology modeling. New York Dordrecht Heidelberg London: Springer, 2011:231–57. [Google Scholar]
- 36. Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. EMBO J 1986; 5:823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tao L, Zhang J, Meraner P, et al. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature 2016; 538:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han C, Lin Y, Shan G, et al. Plasma concentration of malaria parasite-derived macrophage migration inhibitory factor in uncomplicated malaria patients correlates with parasitemia and disease severity. Clin Vaccine Immunol 2010; 17:1524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghosh S, Padalia J, Moonah S. Tissue destruction caused by Entamoeba histolytica parasite: cell death, inflammation, invasion, and the gut microbiome. Curr Clin Microbiol Rep 2019; 6:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickson-Gonzalez SM, de Uribe ML, Rodriguez-Morales AJ. Polymorphonuclear neutrophil infiltration intensity as consequence of Entamoeba histolytica density in amebic colitis. Surg Infect (Larchmt) 2009; 10:91–7. [DOI] [PubMed] [Google Scholar]
- 41. Sierra‐Puente R, Campos‐Rodríguez R, Jarillo‐Luna RA, et al. Expression of immune modulator cytokines in human fulminant amoebic colitis. Parasite Immunol 2009; 31:384–91. [DOI] [PubMed] [Google Scholar]
- 42. Ventura‐Juárez J, Barba‐Gallardo L, Muñoz‐Fernández L, et al. Immunohistochemical characterization of human fulminant amoebic colitis. Parasite Immunol 2007;29:201–9. [DOI] [PubMed] [Google Scholar]
- 43. Hao XP, Lucero CM, Turkbey B, et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun 2015; 6:8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015; 421:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(-/-) mice. Am J Pathol 2013; 182:852–65. [DOI] [PubMed] [Google Scholar]
- 46. Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012; 335:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sparrow E, Friede M, Sheikh M, Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull World Health Organ 2017; 95:235–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tse BN, Adalja AA, Houchens C, Larsen J, Inglesby TV, Hatchett R. Challenges and opportunities of nontraditional approaches to treating bacterial infections. Clin Infect Dis 2017; 65:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]