Abstract
Introduction
Both high or low plasma amyloid levels have been associated with risk of dementia in nondemented subjects.
Methods
We examined baseline plasma β-amyloid (Aβ) levels in relationship to incident dementia during a period of 8.5 years in 2840 subjects age >75 years; 2381 were cognitively normal (CN) and 450 mild cognitive impairment.
Results
Increased plasma Aβ1–40 and Aβ1–42 levels were associated with gender (women), age, low education, creatinine levels, history of stroke, and hypertension. CN participants who developed dementia had lower levels of Aβ1–42 and Aβ1–42/Aβ1–40 ratio compared with those who did not. Aβ levels did not predict dementia in mild cognitive impairment participants.
Discussion
There was an inverse association between Aβ1–42 and Aβ1–42/Aβ1–40 ratio to risk of dementia in CN participants. Cerebral and cardiovascular disease and renal function are important determinants of increased Aβ levels and must be considered in evaluations of relationship of plasma Aβ and subsequent risk of dementia.
Keywords: Dementia, Epidemiology, Aβ1–42, Apolipoprotein-E, Blood levels
1. Introduction
Aggregation and accumulation of β-amyloid (Aβ) proteins as well as the deposition of hyperphosphorylated tau proteins in the brain are the defining pathology associated with neurodegeneration in patients with Alzheimer’s disease (AD) [1]. There is growing interest, but limited evidence, that blood biomarkers can predict subsequent risk of AD [2– 12]. Evidence suggests that in monogenic early-onset dementia, increased production of the 42 amino acid peptide Aβ (Aβ1–42) in the brain account for increased brain amyloid and high levels of cerebrospinal fluid (CSF) and blood Aβ1–42, whereas in late-onset AD, decreased clearance of Aβ1–42 from the brain possibly due to damage to the blood-brain barrier or decreased transportation of Aβ across the blood-brain barrier may account for higher brain amyloid deposition and decreased levels of CSF and plasma Aβ1–42 [13–15].
Ideally, a blood test based on levels of Aβ1–42 in blood, as a reflection of a central nervous system pathological process, could be used to predict the risk of dementia much like levels of serum cholesterol are used to predict risk of coronary heart disease and candidates for drug therapy to lower low-density lipoprotein cholesterol. CSF levels of Aβ are much higher than blood levels of either Aβ1–42 or Aβ140. Blood levels of Aβ1–40 and 1–42 are highly correlated with each other but blood levels of Aβ1–42 and CSF Aβ142 are weakly but significantly correlated [16].
There is great variability among existing studies on whether plasma amyloid levels can predict risk of dementia in nondemented individuals, finding that both high or low Aβ1–40 or Aβ1–42 was associated with incident dementia. Two meta-analyses in 2011 and 2012 of plasma amyloid and risk of dementia came to different conclusions. The first concluded that Aβ1–42 in plasma was not a significant predictor of risk of dementia. The Aβ1–42/Aβ1–40 ratio was a significant predictor of dementia but with substantial variability across studies [17]. The second study found that the ratio of Aβ1–42/Aβ1–40 in plasma was not significantly related to the risk of dementia. Furthermore, they concluded that individuals who converted from cognitively normal (CN) to AD had higher rather than lower levels of Aβ1–42 [4].
The long-term relationship between plasma amyloid levels and incident dementia has been examined in large epidemiological studies. The Framingham Study found that higher Aβ1–42 was associated with lower risk of dementia over 7.6 ± 3.0 years, HR 0.80 (0.71–0.90) and a lower ratio of Aβ1–42/Aβ1–40 with higher risk of dementia [18]. The recent update from the Rotterdam Study over 20 years of follow-up also found that higher levels of Aβ1–42 in plasma was associated with decreased to risk of dementia, HR, 0.64 (0.53–0.78) [19]. The Australian Biomarkers and Lifestyles Study reported a lower Aβ1–42/Aβ1–40 ratio in prevalent AD cases as compared with CN and in participants who converted from CN to mild cognitiveimpairment (MCI) over 18 months [20].
In the Health, Aging and Body Composition Study lower Aβ1–42/Aβ1–40 ratio and Aβ1–42 were inversely related to decline in global cognition as measured with the Modified Mini-Mental State Examination (3MSE). The Health, Aging and Body Composition Study also reported that low Aβ1–42/ Aβ1–40 ratio was directly related to lower education, history of diabetes, higher HDL cholesterol, and higher serum creatinine [21,22]. Lower levels of Aβ1–42 were significantly related to the presence of the apolipoprotein E4 (APOE-4) allele [22].
The purpose of this study was to examine the association between baseline plasma Aβ1–40 and Aβ1–42 levels and incident dementia in 2840 of the 3070 participants from the Ginkgo Evaluation of Memory Study (GEMS) over an 8.5 year follow-up [23].
2. Methods
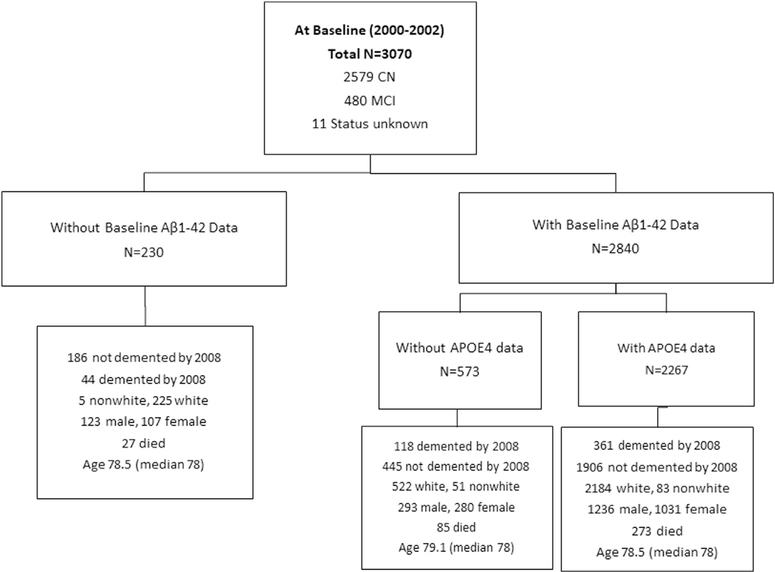
A full description of recruitment and screening procedures of GEMS has been reported. Briefly, volunteers were recruited from September 2000 to May 2002 (n = 3070) using voter registration and other purchased mailing lists in 4 US communities (Fig. 1). Volunteers were aged 75 years or older. Participants were required to identify a proxy willing to be interviewed at 6-month study visits. Signed informed consent was obtained from participants and their proxies. A list of exclusion criteria from the study has been previous published [23] and included taking warfarin and antidepressants, history of bleeding disorders, hospitalization for depression, history of Parkinson’s disease, abnormal fiver function, low B12, low platelet count, estimated life expectancy <5 years, abnormal thyroid function, and high serum creatinine >2 mg [23].
Fig. 1.
GEMS participants with and without plasma Aβ measures stratified by the presence of the APOE-4 allele.
Individuals with prevalent dementia were excluded from participation. MCI at the baseline was defined as having impairments at or below the 10th percentile of the Cardiovascular Health Study normative data stratified by age and education on at least two of ten selected neuropsychological test scores from five cognitive domains and a Clinical Dementia Rating global score of 0.5 [23,24].
2.1. Neuropsychological testing
A 3MSE was administered at every six-month visit as was the Alzheimer’s Disease Assessment Scale-Cognitive Subscale. The Telephone Interview for Cognitive Status was administered when in-person visits were missed. A comprehensive neuropsychological test battery was administered to all participants annually. The neuropsychological test battery was designed to assess multiple cognitive domains and to be maximally sensitive to detecting cognitive decline associated with preclinical or incident dementia [23,24].
Details of the therapeutic intervention (240 mg of Ginkgo biloba extract [Egb761; Schwabe Pharmaceuticals, Karlsruhe, Germany] or an identical placebo tablet). The study protocol was approved by the Institutional Review Boards of all universities involved in the study as well as the National Institutes of Health. Randomization in the trial was performed separately for each clinical site [23].
The primary outcome of the trial was incident dementia based on the Diagnostic and Statistical Manual of Mental Disorders version IV criteria as determined by an expert consensus panel of clinicians knowledgeable in dementia evaluations [23]. Secondary objectives of GEMS were overall cognitive decline, functional disability, and incidence of cardiovascular disease defined as the combined incidence of confirmed coronary heart disease, angina, stroke, transient ischemic attack, coronary artery bypass surgery, or angioplasty. Incidence was confirmed by review of the participants’ medical records after self-report at 6 months and annual visits.
There were 523 (17%) participants with incident dementia over 8.4 years of follow-up; 477 (91%) had AD and 46 (9%) had another type of dementia.
Incident dementia was diagnosed as follows: (1) incident abnormal scores were made on 5 or more tests and at least one of the abnormal scores was on a memory test; (2) incident abnormal scores were made on 4 tests and at least one of the abnormal scores was on a memory test and the participant failed to complete 1 or more of the neuropsychological tests; and (3) incident abnormalities on 2 or more cognitive domains scored below the cutoff for age and education, adjusted norms in both tests of that domain and one of those domains was memory. Participants classified as reaching at this point were then referred for full neurological evaluation, magnetic resonance imaging scan, at the clinic site to confirm that the participant met clinical criteria for dementia and assessed for atypical causes of dementia. The GEMS dementia adjudication panel blinded to treatment assignment reviewed all confirmed cases again to assign a specific dementia classification. This final review incorporated study-related assessments and clinical information. Specific diagnostic criteria were used for designation of dementia classification [23].
There were 3070 randomized in the trial (11 did not have cognitive status in 2000–2002 and 5 did not have a study start date), 1524 to the placebo, 1545 to active therapy, and 1 undetermined. There were 180 in the Ginkgo biloba arm and 198 in the placebo arm who did not contribute longitudinal neuropsychological evaluation. Median follow-up time was 6.1 years with a maximum of 7.3 years. The percentage of participants who were nonadherent at each visit ranged from 3 to about 8.5% throughout the study. Mean age at randomization was 79.1 years, a range of 72 to 96 years; 54% of participants were men, 95.5% were white, 2.9% African American, 0.9% Asian/Pacific Islanders, and 0.7% other. Education of <12 years was reported by 36% of the participants and ≥16 years by 38%.
There were 385 deaths before the diagnosis of dementia in which 323 (12.5%) were among 2579 CN participants and 59 (12.3%) among the 480 with MCI and 3 among 11 unknown cognitive status at the baseline in 2000–2002. Families were contracted as well as health care providers and a review of medical records was performed to determine whether dementia had occurred before death for those who died between the 6-month visits. The administration of Ginkgo biloba did not modify the incidence of dementia [23].
2.2. Laboratory tests
All participants had blood tests at the baseline, including complete blood count, thyroid stimulating hormone, and vitamin B12 levels, liver enzymes, creatinine, as well as APOE genotyping [25]. The Aβ proteins were measured using a sandwich ELISA initially developed by Eli Lilly and further implemented at the University of Vermont Laboratory for Clinical Biochemistry. The detailed methodology has been published. Interassay coefficients of variation ranged from 3.1 to 7.9% for Aβ1–40 and 12.0–20.0% for Aβ1–42 [26].
2.3. Statistical analysis
Continuous variables were evaluated for normality before the analyses, and log-transformations were applied to Aβ1–42, Aβ1–40, its ratio, and creatinine because of skewed distributions. Plasma amyloid data were also analyzed using quartile groups. Between-group comparisons for categorical variables and for continuous variables were examined by chi-square tests and analysis of variance, respectively. Linear regression models were used to determine the baseline factors that were associated with Aβ1–42 and Aβ1–40. To study the effect of amyloid marker on the development of dementia, a Cox model with death before dementia as a competing risk was carried out. Time to the event was defined as the duration from the study starting date to the date of dementia diagnosis, date of death, or the end of study. Covariates of the Cox model included study site, intervention group, age, gender, education, history of stroke, creatinine, presence of the APOE-4 allele, hypertension, and diabetes. Each amyloid marker was examined individually in the Cox model. All the analyses were conducted using SAS 9.4.
3. Results
3.1. Participant characteristics
The GEMS included 3070 participants in 2000–2002 and 523 developed incident dementia over 8 years of follow-up to 2008, including 477 with AD and 24 with other dementia. Mean age at the baseline was 78.5 years for those without dementia and 79.9 for those with incident dementia (P = .0001). Risk of dementia was much greater for those with MCI than CN participants and increased with age for both MCI and CN participants (Supplementary Table 1). Among 2579 CN participants, 323 (12.5%) and for the 480 MCI, 199 (41.5%) developed incident dementia between 2000–2002 and 2008.
3.2. Measures of blood Aβ1–42 and Aβ1–40
Of the 3070 participants, 2840 had determinations of plasma Aβ1 in 2000–2002 (Fig. 1). The participants without determinations of plasma Aβ1–42 had higher creatinine levels (0.95 vs. 1.00 ng) and had more hypertension (36% vs. 44%), and diabetes mellitus (4.5% vs. 9.5%) than those without. In addition, 45 participants had Aβ1–42 measurements at the baseline but had <3 years of follow-up and were alive with no history of dementia at the time of drop out. Among those alive and no history of dementia, about 1/3 (14 of 45) with <3 years of follow-up were diagnosed with MCI at the baseline as compared with 196 (10%) of 1952 with follow-up > 3 years. Baseline Aβ1–42 was higher for participants with follow-up > 3 years, mean Aβ1–42 15.9 ± 24.2 versus 12.4 ± 13.7 (n = 45) with <3 years of follow-up, P =.078 for comparing the differences. Exclusion of these 45 participants from the analysis had no effect on the overall results of the study. There were 133 participants who dropped out after 3 years and were alive and not demented at the time of withdrawal from the study, also in the entire sample, 2452 with and 618 without APOE genotyping. There were no differences in blood levels of Aβ1–42 between those with and without APOE genotype measurements, 15.8 ± 23.7 versus 15.1 ± 19.4 pg/mL (Fig. 1).
Measurement of Aβ1–40 and Aβ1–42 was carried out at the baseline in 2000 and repeated in 2008, at the end of the study. The Pearson correlation between the two measurements of Aβ1–42 (n = 1434) was 0.63 (P = .0001) and for Aβ1–40 (n = 1659) was 0.53 (P = .0001). The levels of Aβ1–40 and 1–42 were also moderately correlated at the baseline 0.47 (P = .0001), and in 2009, n = 1498, 0.52 (P = .0001). There was only a very small change in Aβ142 and 1–40 between baseline and 2008. For Aβ1–42, the average change in concentration was only 0.84 ± 14.9 pg/ mL and a median of 0.70 pg/mL, 25th percentile of −4.39 pg/mL, and for Aβ1–40, concentration was 7.6 ± 71 pg/ mL, 25th percentile –0.38 pg/mL.
Mean time to dementia for participants who were CN at the baseline was 4.7 years (n = 323), and for MCI at the baseline, 3.7 years (n = 196, 3 without study starting date). Mean time to death for CN participants from the year 2000 to 2002 was 3.96 years as compared with 3.20 years for MCI at the baseline. There were no significant associations between time to death and Aβ1–40 or Aβ1–42 levels or the ratio in the blood.
3.3. Aβ1–42 and risk of dementia
Participants who developed dementia during the study were significantly older at the baseline and had lower 3MSE scores and higher Centers for Epidemiologic Studies Depression Scale [3] scores. Levels of Aβ1–42 were significantly lower for incident dementia, 13.8 ± 16.1 versus 16.1 ± 24.0 pg/mL (P = .001 for those with no dementia). There was no association of Aβ1–40 levels and risk of dementia, 190.6 versus 189.2 (P = .725) pg/mL. The ratio of Aβ1–42/Aβ1–40 was lower for incident dementia, 0.07 versus 0.08 (0 = 0.0001), all age-adjusted (not shown).
In analysis restricted to participants who were CN at the baseline (Table 1), levels of Aβ1–42 were higher for those who remained CN, 16.0 pg/mL, as compared with those who converted to incident dementia, 13.6 pg/mL (0 = 0.0001). At the baseline, there were no differences for Aβ1–40 levels and significantly lower ratio of Aβ1–42/Aβ1–40. In addition, age history of stroke, APOE-4 allele, and low 3MSE and high Centers for Epidemiologic Studies Depression Scale scores were significantly related to risk of dementia by 2008. There was no significant association of either race or gender with dementia. However, there were only 11 dementia cases among the 84 nonwhite participants.
Table 1.
Baseline data by dementia status at 2008 among GEMS participants who were cognitively normal at 2000–2002 (n = 2579)
| Baseline 2000–2002 | No dementia (n = 2256) |
Dementia (n = 323) |
P value | Age adjusted P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean or N (%) | SD | Median | Mean or N (%) | SD | Median | |||
| Age, years | 78.2 | 3.1 | 77.0 | 79.6 | 3.5 | 79.0 | <.0001 | - |
| Race—White | 2183 (97.3) | 312 (97.2) | .89 | .81 | ||||
| Gender—Women | 1021 (45.3) | 155 (48.0) | .35 | .35 | ||||
| History of stroke | 45 (2.0) | 20 (6.3) | <.0001 | <.0002 | ||||
| Heart disease | 202 (9.1) | 39 (12.3) | .06 | .16 | ||||
| Hypertension | 952 (43.2) | 143 (45.7) | .40 | .44 | ||||
| Diabetes mellitus | 196 (8.8) | 28 (8.9) | .97 | .78 | ||||
| APOE-4 carriers | 345 (18.9) | 89 (35.3) | <.0001 | <.0001 | ||||
| SBP, mm | 132.5 | 17.9 | 132.0 | 134.4 | 17.9 | 133.0 | .07 | .25 |
| Creatinine, mg% | 1.0 | 0.2 | 1.0 | 1.0 | 0.3 | 1.0 | .76 | .29 |
| GGT, mg% | 29.6 | 16.3 | 25.0 | 29.7 | 16.5 | 24.0 | .95 | .68 |
| ALT, mg% | 25.6 | 8.8 | 24.0 | 25.7 | 8.5 | 25.0 | .68 | .28 |
| AST, mg% | 22.2 | 6.8 | 21.0 | 22.1 | 6.2 | 21.0 | .98 | .99 |
| BMI, kg/m2 | 27.3 | 4.3 | 26.9 | 26.8 | 4.0 | 26.4 | .03 | .17 |
| 3MSE score | 94.3 | 4.1 | 95.0 | 92.1 | 4.6 | 92.0 | <.0001 | <.0001 |
| CES-D | 3.3 | 3.3 | 2.2 | 4.4 | 3.9 | 3.0 | <.0001 | <.0001 |
| Aβ1–42, pg/mL | 16.0 | 23.7 | 11.8 | 13.6 | 17.7 | 11.2 | .008 | .001 |
| Aβ 40, pg/mL | 189.0 | 91.3 | 180.1 | 190.1 | 74.0 | 183.6 | .49 | .98 |
| Aβ1–42/Aβ 40 | 0.08 | 0.07 | 0.07 | 0.07 | 0.06 | 0.1 | .0003 | .0001 |
| Aβ1–42*, pg/mL | 13.6 | 9.8 | 11.7 | 12.7 | 9.6 | 11.1 | .02 | .007 |
Abbreviations: GEMS, Ginkgo Evaluation of Memory Study; N, number of participants; 3MSE, Modified Mini-Mental State Examination; CES-D, Center for Epidemiological Studies Depression Scale; BMI, body mass index; SBP, systolic blood pressure; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; AST, aspartate aminotransferase.
Removed outliers (value ≥ 84.31 [i.e. 3SD above the mean; SD and mean were based on the full sample]).
Outliers were defined as >3 SD from the mean (Table 1) and were excluded from the analysis. The association of Aβ1–42 and dementia remained significant (age-adjusted P value = .007) after this exclusion (Table 1). There was no association of Aβ1–42, 1–40, or ratio of Aβ1–42/1–40 and risk of dementia for those who were MCI at the baseline.
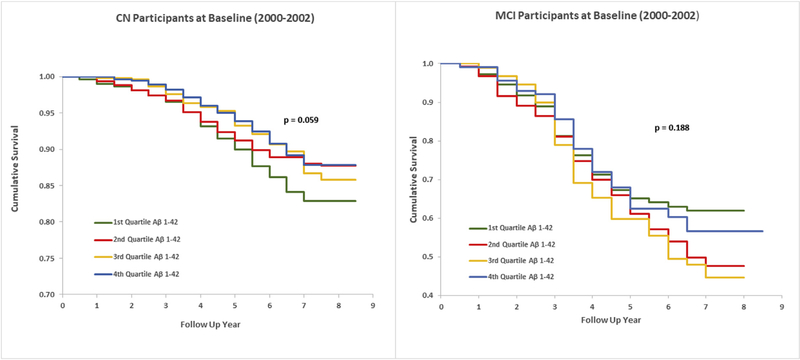
Aβ1–42 levels were divided into quartiles for the entire sample and for the CN and MCI groups separately. Age-adjusted incidence rates per 1000 person-years (PYs) were then evaluated for total and CN and MCI participants. The HR for the first versus the fourth quartiles of Aβ1–42 and the risk of dementia was 1.4 (1.1–1.8) (Table 2) for the total sample, and for CN at the baseline, age-adjusted HR was 1.7 (1.2–2.3) (Table 2, Fig. 2). The association of the Aβ1–42/ Aβ1–40 ratio to risk of dementia was similar to the risk associated with Aβ1–42 (Table 2).
Table 2.
Dementia status at 2008 by baseline 2000–2002 Aβ1–42 and Aβ1–42/Aβ1–40 ratio quartile* groups for CN and MCI participants in GEMS
| Baseline 2000–2002 | N | N dem | Age-adjusted HR | Age-adjusted Rate/1000 PYs | P value for trend |
|---|---|---|---|---|---|
| Aβ1–42 | |||||
| All | |||||
| 1Q | 710 | 131 | 1.4 (1.1–1.8) | 30.9 (23.2–41.3) | .07 |
| 2Q | 710 | 122 | 1.3 (1.0–1.6) | 28.5 (21.2–38.5) | |
| 3Q | 708 | 117 | 1.2 (0.9–1.5) | 27.1 (20.0–36.8) | |
| 4Q | 708 | 106 | 1.0 | 23.0 (16.7–31.8) | |
| CN at 2000–2002 | |||||
| 1Q | 599 | 92 | 1.6 (1.2–2.3) | 25.3 (18.0–35.8) | .02 |
| 2Q | 588 | 66 | 1.1 (0.8–1.6) | 18.0 (12.0–27.0) | |
| 3Q | 609 | 73 | 1.2 (0.8–1.7) | 19.0 (12.9–28.1) | |
| 4Q | 584 | 62 | 1.0 | 16.0 (10.6–24.4) | |
| MCI at 2000–2002 | |||||
| 1Q | 111 | 39 | 1.0 (0.6–1.5) | 66.8 (38.9–115.4) | .89 |
| 2Q | 122 | 56 | 1.5 (1.0–2.2) | 91.4 (58.7–143.2) | |
| 3Q | 95 | 43 | 1.4 (0.9–2.1) | 87.2 (52.1–152.4) | |
| 4Q | 119 | 44 | 1.0 | 66.0 (39.3–114.3) | |
| Aβ1–42/Aβ1–40 ratio | |||||
| All | |||||
| 1Q | 700 | 136 | 1.5 (1.2–2.0) | 32.1 (24.2–42.7) | .0004 |
| 2Q | 707 | 136 | 1.5 (1.2–2.0) | 32.0 (24.2–42.5) | |
| 3Q | 720 | 110 | 1.1 (0.9–1.5) | 24.5 (18.0–33.7) | |
| 4Q | 704 | 94 | 1.0 | 21.0 (15.0–29.6) | |
| CN at 2000–2002 | |||||
| 1Q | 579 | 89 | 1.8 (1.3–2.5) | 24.9 (17.6–35.4) | .0003 |
| 2Q | 595 | 85 | 1.6 (1.1–2.2) | 23.2 (16.3–33.1) | |
| 3Q | 612 | 64 | 1.1 (0.8–1.6) | 16.3 (10.8–24.7) | |
| 4Q | 589 | 55 | 1.0 | 14.3 (9.2–22.4) | |
| MCI at 2000–2002 | |||||
| 1Q | 121 | 47 | 1.1 (0.7–1.7) | 72.6 (44.4–120.0) | .53 |
| 2Q | 111 | 51 | 1.6 (1.0–2.4) | 93.4 (58.4–150.7) | |
| 3Q | 107 | 46 | 1.3 (0.9–2.0) | 80.2 (49.3–134.8) | |
| 4Q | 108 | 38 | - | 62.4 (36.1–110.7) |
Abbreviations: Aβ, amyloid-β; CN, cognitively normal; MCI, mild cognitive impairment; GEMS, Ginkgo Evaluation of Memory Study; HR, hazards ratio; PY, person-years; Q, quartile; N, number of participants; N with dem, number of participants with incident dementia.
Aβ1–42 quartile: 1Q: ≤7.8; 3Q: 11.69–16.75; 4Q: >16.75.
Fig. 2.
Kaplan–Meier survival curves for incident dementia by Aβ1–42 quartiles in CN and MCI participants. Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment.
Incidence of dementia among CN participants at the baseline in relationship to quartiles of Aβ1–42 concentration was further analyzed separately for men and women. The HR for the first versus the fourth quartiles of Aβ1–42 concentration for women was 1.69 (1.08–2.63) and for men, 1.65 (1.03– 2.64) (not shown). There was no association of Aβ1–40 and risk of dementia for either men or women.
3.4. Association of blood Ab1–42 levels and other variables
The independent associations of Aβ1–42 blood levels in a linear regression model included higher levels of Aβ1–42 in older age, among women, hypertension, and higher creatinine levels. Education levels were inversely related to Aβ1–42. The association of these variables to Aβ1–40 was very similar to 1–42 (Supplementary Tables 2 and 3). There was no association of liver enzymes and Aβ1–42 levels.
3.5. APOE-4 allele, Ab1–42, and risk of dementia
The APOE-4 was a strong determinant of the risk of dementia. APOE-4 carriers had higher incidence of dementia within each of the quartiles of Aβ1–42 as compared with noncarriers (Table 3). Age-adjusted incidence rates of dementia went from 42/1000 PYs for those who were in the first quartile of Aβ1–42 and were APOE-4 carriers to 13.4/ 1000 PYs for those who were noncarriers and in the highest quartile of Aβ1–42. The highest quartile of Aβ1–42 was also associated with a lower risk of dementia among APOE-4 carriers (Table 3).
Table 3.
Baseline Aβ1–42 quartile* groups and APOE-4 allele among CN participants at 2000–2002 and risk of dementia to 2008 (n = 1921) in GEMS
| Aβ1–42 | APOE-4 | N | N Dem (%) | Age adjusted Rate/1000 PYs | Age adjusted HR (95% CI) |
|---|---|---|---|---|---|
| 1Q | no | 391 | 44 (11.3) | 18.7 (11.5–30.6) | 1.4 (0.9–2.3) |
| yes | 106 | 26 (24.5) | 41.6 (21.4–81.5) | 3.4 (2.0–5.7) | |
| 2Q | no | 356 | 26 (7.3) | 11.7 (6.5–22.2) | 0.9 (0.5–1.4) |
| yes | 106 | 25 (24) | 38.0 (19.4–76.6) | 3.5 (2.1–5.8) | |
| 3Q | no | 401 | 43 (11) | 16.7 (10.1–27.9) | 1.3 (0.8–2.0) |
| yes | 88 | 18 (20.5) | 36.1 (16.4–79.3) | 2.8 (1.6–5.0) | |
| 4Q | no | 378 | 34 (9) | 13.4 (7.7–23.9) | 1.0 |
| yes | 95 | 12 (12.6) | 19.0 (7.2–52.7) | 1.6 (0.8–3.0) |
Abbreviations: Aβ, amyloid-β; APOE-4, apolipoprotein E-4; CN, cognitively normal; GEMS, Ginkgo Evaluation of Memory Study; N, number of participants; N dem, number of participants with incident dementia; PY, person-years; HR, hazards ratio; Q, quartile.
Aβ1–42 quartile: 1Q: ≤7.8; 3Q: 11.69–16.75; 4Q: >16.75.
3.6. Effect of field center and randomization
Blood samples were obtained from the participants before randomization to the placebo or the Ginkgo biloba arm. There was no difference in levels of Aβ1–42 at the baseline between the placebo (16.1 pg/mL) and Ginkgo biloba groups (15.4 pg/mL), P = .9. There was also no significant difference in the incidence of dementia by level of Aβ1–42 at the baseline between the placebo and Ginkgo biloba arm. The inverse association of Aβ1–42 and risk of dementia was significant in the both arms of the study (data not shown).
The four field centers in the GEMS independently randomized their participants. There was a significant difference in the distribution of Aβ1–40 or Aβ1–42 across the four field centers. The incidence of dementia was significantly higher in one of the four field centers as compared with the other three. There was an inverse relationship between risk of dementia and quartiles of Aβ1–42, similar to the total trial, in three of the four sites, and the relationship was significant for two sites. However, in the center with the highest incidence of dementia, average Aβ1–42 was higher than the other three centers and there was no relationship of Aβ1–42 and risk of dementia (not shown). Exclusion of this center from the analysis had no effect on the relationship of low Aβ1–42 in blood and risk of dementia.
3.7. Multivariate models
In Cox proportional hazards analysis, both Aβ1–42 and Aβ1–42/Aβ1–40 ratio were significant predictors of dementia in CN participants at study entry. Other significant predictors of dementia were older age, history of stroke, and APOE-4 (Table 4). The Cox models were run separately for participants who were APOE-4 carriers and noncarriers (Supplementary Table 4). Lower levels of Aβ1–42 were predictors of risk of dementia, as were age, history of stroke, and women who were APOE-4 carriers and noncarriers. The lack of significance of the lower quartile of Aβ1–42 in APOE-4 carriers shown in Supplementary Table 4 in the Appendix is likely a function of smaller sample size. Results for the above Cox models were similar when models included death as a competing risk (not shown).
Table 4.
Predictors of incident dementia (n = 216) in CN participants (n = 1841) in GEMS by Aβ1–42 and Aβ1–42/Aβ1–40 quartiles: Cox model
| Variables in the model | Baseline Aβ1–42* |
Baseline Aβ1–42/Aβ1–40 ratio† |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Field center | ||||
| WFU | 1.08 (0.73–1.61) | 1.02 (0.69–1.51) | ||
| UCD | 1.16 (0.81–1.64) | 1.11 (0.78–1.57) | ||
| JHU | 1.38 (0.93–2.04) | .44 | 1.35 (0.91–2.00) | .44 |
| Intervention | ||||
| Ginkgo biloba | 1.03 (0.79–1.35) | .83 | 1.02 (0.78–1.34) | .87 |
| Age | 1.13 (1.09–1.17) | <.0001 | 1.13 (1.09–1.17) | <.0001 |
| Gender, Women | 1.28 (0.95–1.74) | .10 | 1.23 (0.91–1.67) | .17 |
| Years of education | 1.01 (0.96–1.06) | .84 | 1.01 (0.96–1.06) | .77 |
| History of stroke | 2.69 (1.49–4.86) | .001 | 2.71 (1.50–4.89) | .001 |
| 1Q | 1.91 (1.28–2.85) | 1.83 (1.23–2.71) | ||
| 2Q | 1.29 (0.85–1.97) | 1.57 (1.05–2.34) | ||
| 3Q | 1.47 (0.99–2.19) | .01 | 1.19 (0.79–1.80) | .01 |
| log Creatinine | 1.13 (0.58–2.20) | .72 | 1.00 (0.52–1.91) | .99 |
| APOE-4 allele | 2.47 (1.86–3.28) | <.0001 | 2.41 (1.82–3.21) | <.0001 |
| Hypertension | 1.13 (0.86–1.49) | .39 | 1.13 (0.86–1.49) | .38 |
| Diabetes mellitus | 0.97 (0.61–1.55) | .90 | 0.97 (0.61–1.54) | .88 |
Abbreviations: CN, cognitively normal; GEMS, Ginkgo Evaluation of Memory Study; Aβ, amyloid-β; HR, hazards ratio; WFU, Wake Forest University; UCD, University California, Davis; JHU, Johns Hopkins University; Q, quartile; APOE-4, apolipoprotein E-4.
Aβ1–42 quartile: 1Q: ≤7.8; 2Q: 7.9–11.68; 3Q: 11.69–16.75.
Aβ1–42/Aβ1–40 ratio quartile: 1Q: ≤0.045; 2Q: 0.065–0.087; 3Q: 0.046–0.064.
4. Discussion
This study reported an inverse association between low Aβ1–42 levels and Aβ1–42/Aβ1–40 ratio but not Aβ1–40 levels to dementia risk, 91% AD, over an approximate 8.5 years of follow-up in CN individuals, but not in those with MCI. This is one of the longest studied and largest samples analyzed, especially with detailed annual evaluations of cognitive status and separation of CN and MCI at the baseline. The results are consistent with those recently reported from both the Rotterdam and Framingham Studies [18,19].
Aβ1–40 and 1–42 are cleared from the peripheral circulation primarily by renal excretion, accounting for the higher levels associated with creatinine levels and with factors related to kidney function, such as hypertension, diabetes, age, and markers of brain vascular disease [27,28]. In the Rotterdam Study, Aβ1–42 levels increased with age, hypertension, and with lacunar infarcts, microbleeds, and decreased white matter volume in the brain [29]. The liver is the primary organ for peripheral metabolism of Aβ1–42. Levels of liver enzymes were not related to Aβ−142 levels.
Several new methods of measuring Aβ1–42 in the periphery have been reported, including one recent study that used gas chromatography mass spectroscopy ratios of Aβ1–42 and different molecular species of Aβ. The authors reported a very high correlation between peripheral plasma Aβ1–42 and amyloid precursor protein 669–711 ratios and the extent of amyloid in the brain [30–32]. In the present study, there was a very high correlation of two samples of Aβ1–42 concentration measured approximately 8 years apart, and relatively little change over time in measurements carried out in the same laboratory. Measures of Aβ1–42 were therefore repeatable in the laboratory and little variability over the long term within individuals. Accuracy of blood measurements of Aβ1–42 or 1–40 cannot be determined without a “gold standard”.
4.1. Limitations
The GEMS sample was a randomized clinical trial and not a population sample. However, inclusion of randomization group and specific field centers did not affect the results. In addition, the GEMS did not have measurements of magnetic resonance imaging at the baseline so that we could not evaluate the relationship of Aβ1–42 concentration and brain vascular disease.
Because there was no follow-up after the diagnosis of dementia, the total mortality in relationship to Aβ1–42 concentration in the cohort cannot be evaluated. The short period between evaluations and the retrospective review and evaluation of the deaths probably precludes a potential limitation due to selected survival of the cohort.
No measures in the blood of other important biomarkers, such as phosphorylated tau, which has recently been directly related to risk of dementia, were available [33,34]. No follow-up of the total cohort was carried out after 2008 so that measures of change in Aβ1–42 concentration between baseline and 8.5 years later cannot be used to evaluate subsequent risk of dementia.
Finally, one major limitation is that we do not know the source of blood Aβ1–42. However, we know that Aβ1–42 is metabolized in the liver and individuals with chronic liver disease have high levels of Aβ1–42 [35]. Only liver enzymes were measured in GEMS and were not related to levels of Aβ1–42. Aβ1–42 and 1–40 are transported primarily via binding to albumin in plasma. No measures of albumin were available. Studies of plasma exchange of albumin to lower Aβ1–42 are ongoing and show a short-term reduction in Aβ1–42 [36]. Kidney dialysis is also associated with clearance of Aβ1–42 in plasma and decrease in Aβ1–42 levels [37,38].
5. Conclusions
Low levels of peripheral Aβ1–42 are related to the risk of incident dementia as now reported in three large longitudinal studies. Better measurements of not only Aβ1–42 but other species of Aβ in the blood may improve the use of blood amyloid measurements to predict risk of AD, especially when combined with other measures in the blood, such as tau. A biomarker blood test to predict risk of AD remains an important goal.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the published literature (e.g., PubMed) on the relationship between plasma amyloid levels in nondemented individuals and incident dementia.
Interpretation: Blood levels of Aβ1–42 and Aβ1–42/ Aβ1–40 ratio were independent predictors of incident dementia, primarily Alzheimer’s disease (AD). The lower levels may be a measure of decreased clearance of Aβ1–42 from the brain to periphery. By contrast, age, sex (women), low education level, and multiple age-related pathological processes, especially cerebral and cardiovascular disease, and renal function that are important determinants of increased Aβ1–42 and Aβ1–40 levels.
Future directions: Studies of the prediction of incident AD by plasma Ab levels should be conducted in the context of multiple factors that increase or decrease these levels. The understanding of the contribution of different mechanisms to remove Aβ1–42 from the interstitial fluid to the cerebrospinal fluid and peripheral circulation to reduce brain load of Aβ1–42 may be an important approach to reducing the extent of AD pathology and the incidence of AD dementia.
Acknowledgments
This work was supported by grants U01 AT000162 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements, and P01-AG025204 and P50-AG05133 from the National Institute on Aging.
Footnotes
Conflict of interest: The authors have no conflict of interest with the results of this study.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2019.04.008.
References
- [1].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012; 123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol 2015;77:301–11. [DOI] [PubMed] [Google Scholar]
- [3].Johansson P, Äberg D, Johansson JO, Mattsson N, Hansson O, Ahrén O, et al. Serum but not cerebrospinal fluid levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) are increased in Alzheimer’s disease. Psychoneuroendocrinology 2013;38:1729–37. [DOI] [PubMed] [Google Scholar]
- [4].Song F, Poljak A, Smythe GA, Sachdev P. Plasma biomarkers for mild cognitive impairment and Alzheimer’s disease. Brain Res Rev 2009; 61:69–80. [DOI] [PubMed] [Google Scholar]
- [5].O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: Current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement 2017; 13:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging 2010; 31:357–67. [DOI] [PubMed] [Google Scholar]
- [7].Hanon O, Vidal JS, Lehmann S, Bombois S, Allinquant B, Tréluyer JM, et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement 2018;14:858–68. [DOI] [PubMed] [Google Scholar]
- [8].Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016; 15:673–84. [DOI] [PubMed] [Google Scholar]
- [9].Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018;14:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma apolipoprotein E levels and risk of dementia: A Mendelian randomization study of 106,562 individuals. Alzheimers Dement 2018;14:71–80. [DOI] [PubMed] [Google Scholar]
- [11].Frank RA, Galasko D, Hampel H, Hardy J, de Leon MJ, Mehta PD, et al. Biological markers for therapeutic trials in Alzheimer’s disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging 2003; 24:521–36. [DOI] [PubMed] [Google Scholar]
- [12].van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement 2018;14:707–22. [DOI] [PubMed] [Google Scholar]
- [13].Rissman RA, Trojanowski JQ, Shaw LM, Aisen PS. Longitudinal plasma amyloid beta as a biomarker of Alzheimer’s disease. J Neural Transm (Vienna) 2012;119:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010;330:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci 2004; 23:247–54. [DOI] [PubMed] [Google Scholar]
- [16].Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW, et al. Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 2011;122:401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 2012; 69:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chouraki V, Beiser A, Younkin L, Preis SR, Weinstein G, Hansson O, et al. Plasma amyloid-beta and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimers Dement 2015;11:249–257 e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolters FJ. Amyloid in cardiovascular disease In: Wolters FJ, ed. On the Origin of Dementia. A Population Perspective on Risk and Aetiology; 2018;. p. 191–205. Rotterdam, the Netherlands. [Google Scholar]
- [20].Rembach A, Faux NG, Watt AD, Pertile KK, Rumble RL, Trounson BO, et al. Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer’s disease. Alzheimers Dement 2014; 10:53–61. [DOI] [PubMed] [Google Scholar]
- [21].Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 2011; 305:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Metti AL, Cauley JA, Newman AB, Ayonayon HN, Barry LC, Kuller LM, et al. Plasma beta amyloid level and depression in older adults. J Gerontol A Biol Sci Med Sci 2013;68:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA 2008;300:2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Snitz BE, O’Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA 2009;302:2663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kamboh MI, Sanghera DK, Ferrell RE, DeKosky ST. APOE 4associated Alzheimer’s disease risk is modified by alpha1antichymotrypsin polymorphism. Nat Genet 1995;10:486–8. [DOI] [PubMed] [Google Scholar]
- [26].Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension 2012; 59:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gronewold J, Klafki HW, Baldelli E, Kaltwasser B, Seidel UK, Todica O, et al. Factors responsible for plasma beta-amyloid accumulation in chronic kidney disease. Mol Neurobiol 2016;53:3136–45. [DOI] [PubMed] [Google Scholar]
- [28].Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR. Serum creatinine levels correlate with plasma amyloid beta protein. Alzheimer Dis Associated Disord 2002;16:187–90. [DOI] [PubMed] [Google Scholar]
- [29].Hilal S, Wolters FJ, Verbeek MM, Vanderstichele H, Ikram MK, Stoops E, et al. Plasma amyloid-beta levels, cerebral atrophy and risk of dementia: a population-based study. Alzheimers Res Ther 2018;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018;554:249–54. [DOI] [PubMed] [Google Scholar]
- [31].Figurski MJ, Waligorska T, Toledo J, Vanderstichele H, Korecka M, Lee VM, et al. Improved protocol for measurement of plasma beta-amyloid in longitudinal evaluation of Alzheimer’s Disease Neuroimaging Initiative study patients. Alzheimers Dement 2012;8:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pérez-Grijalba V, Fandos N, Canudas J, Insua D, Casabona D, Lacosta AM, et al. Validation of immunoassay-based tools for the comprehensive quantification of Abeta40 and Abeta42 peptides in plasma. J Alzheimers Dis 2016;54:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of plasma total tau level with cognitive Decline and risk of mild cognitive impairment or dementia in the Mayo Clinic study on aging. JAMA Neurol 2017;74:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dage JL, Wennberg AMV, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement 2016;12:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang YR, Wang QH, Zhang T, Liu YH, Yao XQ, Zeng F, et al. Associations between hepatic functions and plasma amyloid-beta levels-implications for the capacity of liver in peripheral amyloid-beta clearance. Mol Neurobiol 2017;54:2338–44. [DOI] [PubMed] [Google Scholar]
- [36].Boada M, Anaya F, Ortiz P, Olazaran J, Shua-Haim JR, Obisesán TO, et al. Efficacy and safety of plasma exchange with 5% albumin to modify cerebrospinal fluid and plasma amyloid-beta concentrations and cognition outcomes in Alzheimer’s disease patients: A multicenter, randomized, controlled clinical trial. J Alzheimers Dis 2017; 56:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kitaguchi N, Hasegawa M, Ito S, Kawaguchi K, Hiki Y, Nakai S, et al. A prospective study on blood Abeta levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm (Vienna) 2015;122:1593–607. [DOI] [PubMed] [Google Scholar]
- [38].Kitaguchi N, Kato T, Matsunaga S, Hirano K, Iwata K, Kawaguchi K, et al. Removal of blood amyloid-beta with hemodialysis reduced brain amyloid-beta, confirmed by brain imaging: a case report. Neuropsychiatr Dis Treat 2018;14:2931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.