SUMMARY
mRNAs enriched in membraneless condensates provide functional compartmentalization within cells. The mechanisms that recruit transcripts to condensates are under intense study, however how mRNAs organize once they reach a granule remains poorly understood. Here, we report on a self-sorting mechanism by which multiple mRNAs derived from the same gene assemble into discrete homotypic clusters. We demonstrate that in vivo mRNA localization to granules and self-assembly within granules are governed by different mRNA features; localization is encoded by specific RNA regions while self-assembly involves the entire mRNA, does not involve sequence-specific, ordered intermolecular RNA:RNA interactions and is thus RNA sequence-independent. We propose that the ability of mRNAs to self-sort into homotypic assemblies is an inherent property of an mRNP that is augmented under conditions that increase RNA concentration, such as upon enrichment in RNA-protein granules, a process that appears conserved in diverse cellular contexts and organisms.
Graphical Abstract

eTOC Blurb
mRNAs enriched in Drosophila germ granules form homotypic assemblies. In vivo, this assembly is not specified by germ granule proteins but instead relies on the mRNA itself. mRNA self-assembly involves the entire mRNP, is RNA sequence-independent and sequence-specific. Intermolecular, ordered RNA:RNA interactions among clustered transcripts were not observed.
INTRODUCTION
RNAs, like other biopolymers tend to assemble both in vivo and in vitro (Eno et al., 2019, Khong et al., 2017, Little et al., 2015, Pitchiaya et al., 2019, Roovers et al., 2018, Trcek et al., 2015) through the process of phase separation (Eisenberg and Felsenfeld, 1967, Jain and Vale, 2017, Langdon et al., 2018, Van Treeck et al., 2018). In most cases, the molecular principles that allow RNA assembly have not been experimentally determined however RNA binding proteins (RBPs), non-Watson-Crick interactions as well as promiscuous Watson-Crick and sequence-specific trans RNA:RNA interactions have been implicated in driving RNA self-assembly (Eisenberg and Felsenfeld, 1967, Jain and Vale, 2017, Langdon et al., 2018, Van Treeck et al., 2018). Experimentally, three RNAs have been shown to assemble via direct base-pairing in vivo: the RNA genome of the human immunodeficiency virus (HIV) and the Drosophila oskar (osk) and bicoid (bcd) mRNP transport messenger ribonucleoprotein (mRNP) particles. These RNAs use distinct palindromic sequences presented by conserved stem-loop structures to homodimerize (Ferrandon et al., 1997, Jambor et al., 2015, Moore and Hu, 2009, Marquet et al., 1991). Depending on the cellular environment, RNA structures can hide or expose interacting sequences and thus spatially control RNA assembly in cells (Langdon et al., 2018). Understanding the principles of RNA assembly in vivo within the endogenous cellular context is critical in determining how these assemblies shape the biology of a cell.
We have previously shown that upon enrichment into germ granules, the Drosophila CycB, nanos (nos), polar granule component (pgc) and germ-cell-less (gcl) mRNAs form homotypic clusters that contain multiple transcripts derived from the same gene (Little et al., 2015, Trcek et al., 2015). These clusters are de-mixed from each other and located at distinct positions within the homogeneously-distributed protein environment of the granule (Trcek et al., 2015). Germ granules, composed of homogeneously mixed core granule proteins Oskar (Osk), Vasa, Aubergine (Aub) and Tudor (Tud), are up to 500 nm big (Arkov et al., 2006) and contain territories occupied by homotypic mRNA clusters (Trcek et al., 2015). The centers harbor core granule proteins and the CycB clusters, while the gcl clusters position at the granule boundary (Trcek et al., 2015). Using in vivo quantitative super-resolution microscopy and single-molecule Fluorescent In Situ Hybridization (smFISH) combined with genetic manipulations we now show that homotypic assemblies are common among germ granule-enriched mRNAs and that granule proteins are critically important during this process, as clustering does not occur outside of granules. However, once in granules, mRNAs self-assemble into clusters. In contrast to the sequence-driven recruitment of mRNAs by germ granule proteins (Rangan et al., 2009, Gavis et al., 1996, Eagle et al., 2018), the specificity for homotypic mRNA self-assembly is independent of specific RNA sequences, their associated proteins or conserved RNA structures, as well as of core granule proteins. Stochastic optical reconstruction microscopy (STORM) (Rust et al., 2006) measurements support a model in which contacts among clustered mRNAs in granules are independent of any one spatially constrained interaction in trans. We propose that granule proteins increase mRNA concentration by recruiting them to granules, thereby increasing the probability of mRNAs to self-recognize and self-sort. Thus, the propensity of mRNAs to homotypically assemble is an inherent property of an mRNA, independent of its sequence and occurs when mRNAs become sufficiently crowded, such as upon their enrichment in RNA condensates. Our in vivo work uncovers a transcript-specific yet sequence-independent organizing principle of cellular mRNAs, which could be applicable to diverse RNA condensates across the organismal spectrum.
RESULTS
mRNAs self-sort into homotypic clusters in germ granules
To visualize germ granule mRNAs, we coupled smFISH with structured illumination microscopy (SIM) (York et al., 2013), a super-resolution approach, to quantify mRNA cluster abundance (number of mRNAs/homotypic cluster) both within granules demarcated by the Vasa:GFP signal and outside (Figure 1A–D; S1A–E) (Little et al., 2015, Trcek et al., 2015). We found that clustering was common among granule-enriched mRNAs and that mRNA cluster abundance varied among different genes without a defined stoichiometry (Table S1, Figure S1F). Clustering only occurred within granules regardless of the somatic mRNA concentration (Table S1) and thus depended on the ability on an mRNA to enrich in granules (Little et al., 2015, Trcek et al., 2015). Furthermore, cluster abundance only moderately correlated with protein abundance of core granule proteins (Figure 1E,F; S1G), suggesting that mRNA cluster abundance depends on factors other than the core germ granule components (Niepielko et al., 2018).
FIGURE 1: mRNAs self-sort into homotypic clusters.
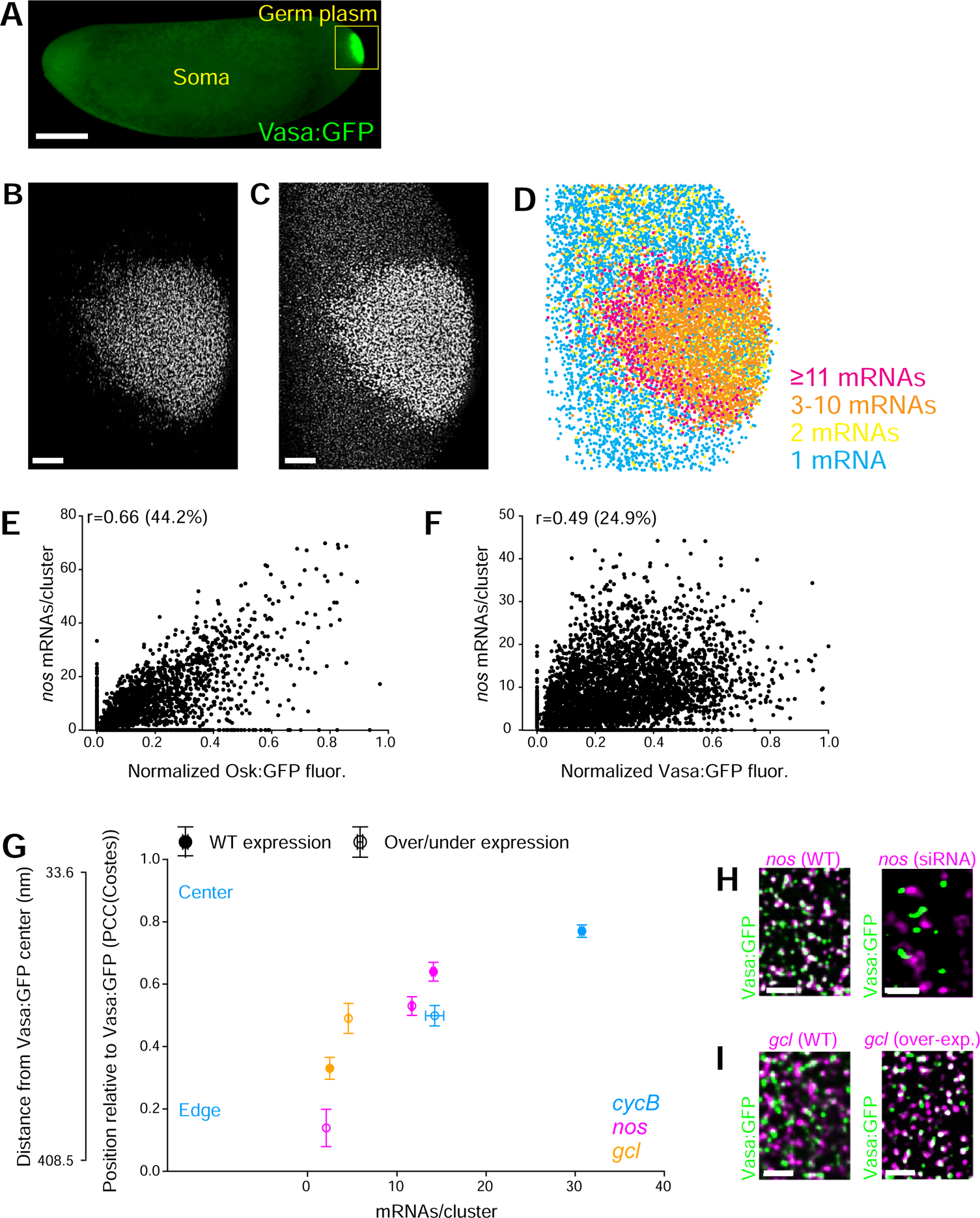
See also Figure S1,2 and Table S1. A Embryo with maternally expressed Vasa:GFP transgene. B,C smFISH reveals the spatial distribution of nos (C) in germ granules (B). D Spatial distribution of nos clusters at the posterior pole. E,F Correlation of nos cluster abundance with the Osk:GFP protein (D) and Vasa:GFP protein (D) abundance quantified by the Pearson correlation coefficient (r). Each dot represents one granule. % = coefficient of determination. ≥3483 granules / pair were analyzed. G Position and abundance of CycB (empty blue circles), nos (empty magenta circles) and gcl (empty yellow circles) clusters in over- and under-expression experiments and in WT condition (full blue, magenta and yellow circles, respectively) in Vasa:GFP granules. 33.6 nanometer (nm) on the Y axis indicates co-localization of a doubly-labeled pgc with itself and 408.5 nm shows co-localization between Vasa:GFP and non-granule-enriched ccr4 (Trcek et al., 2015). PCC(Costes) in over- and under-expression assays: mean±SEM of 3 embryos/gene. Cluster abundance: 337 to 3178 clusters /gene/condition. H Co-localization of nos (magenta) with Vasa:GFP granules (green) in WT embryos and embryos treated with RNAi against nos. I Co-localization of gcl (magenta) with Vasa:GFP granules (green) in WT embryos and embryos over-expressing gcl. Scale bar: C,D = 2 μm, B = 10 μm, A = 50 μm.
To determine which mRNA property best predicted the position of clusters within granules we evaluated different parameters that may influence RNA regulation and behavior, while using the PCC(Costes) approach to determine the position of clusters within Vasa:GFP granules (STAR Methods and Figure S2A) (Trcek et al., 2015). We determined that the strongest positive indicator for the positioning of RNA clusters within germ granules was mRNA cluster abundance (r=0.88, Figure S2A,B, STAR Methods). For example, CycB clusters, with a mean abundance (MA) of 30.8±0.2 CycB mRNAs, were located in the center of granules (PCC(Costes) of 0.77±0.02) (Figure 1G full blue circle, Table S1) (Trcek et al., 2015), nos clusters (MA 14.1±0.2) were located in the middle (PCC(Costes) of 0.64±0.02) (Figure 1G, full magenta circle, Table S1) (Trcek et al., 2015), while gcl clusters (MA 2.9±0.1) resided at the edge of granules (PCC(Costes) of 0.33±0.04) (Figure 1G, full yellow circle; Table S1) (Trcek et al., 2015). The different cluster positions within granules could not be explained by the differences in their physical size (nm2) since mRNAs that position differently within granules nevertheless form clusters of similar physical size (Trcek et al., 2015). These results, which extended to all germ granule-enriched mRNAs tested (Figure S2B), suggested that the abundance of homotypic clusters and hence the concentration of mRNAs within granules could determine the position of mRNA clusters within germ granules.
To test this hypothesis, we manipulated the expression levels of CycB, nos and gcl to drive the formation of more or less abundant clusters and determined their position within granules. By using RNAi to reduce total embryonic nos levels, we decreased the average nos cluster abundance from 14.1±0.2 to 2.1±0.2 mRNAs per cluster, and recorded that nos clusters moved towards the edge of granules in a dosage-dependent manner: from PCC(Costes)nos-WT of 0.64±0.03 to PCC(Costes)nos-RNAi of 0.14±0.06 (Figure 1G, full and empty magenta circles, Figure 1H, S2E,F). This change was specific for nos RNAi induced under-expression, as RNAi against mCherry transcripts caused no change in the abundance of nos mRNA or in the position of nos clusters in Vasa:GFP-labeled granules (Figure 2E,G). A less drastic under-expression of nos using embryos derived from nosBN/+ mothers expressing nos from only one WT gene copy, decreased the average nos cluster abundance to 11.7±0.1 mRNAs and moved nos clusters towards the edge of granules ((PCC(Costes)nosBN/+: 0.53±0.03 (Figure 1G, full and empty magenta circles, Figure S2E,F)). Similarly, when we decreased the mean cluster abundance of CycB from 30.8±0.2 to 14.2±1.0 mRNAs using RNAi, we recorded that CycB clusters moved towards the periphery of granules in a dosage-dependent manner: from PCC(Costes)CycB-WT 0.77±0.02 to PCC(Costes)CycB-RNAi: 0.49±0.03 (Figure 1G, full and empty blue circles, Figure S2H–J). Conversely, when we increased the mean gcl cluster abundance from 2.9±0.1 to 4.5±0.1 by over-expressing gcl, gcl clusters moved towards the center of the granule: from PCC(Costes)gcl-WT of 0.33±0.04 to PCC(Costes)gcl-over-exp of 0.49.±0.05 (Figure 1G; open and empty yellow circles; Figure 1I, S2K,L). All these changes were statistically significant (Figure S2F,J,L). Apart from the changes in the overall concentration and cluster abundance of tested mRNAs, other germ granule parameters such as the average amount of Vasa:GFP in granules appeared unaffected (Figure S2M).
FIGURE 2: Germ granule proteins are more mobile than germ granule mRNAs.

See also Figure S3 and Table S2. A,B Fluorescence recovery of Vasa:GFP (A) and nos MS2-MCP:GFP (B) after photobleaching an ROI (magenta square) within germ plasm. C Labeling of nos with MS2-MCP:GFP. D Recovery of Vasa:GFP (mean±STDEV, gray bars) of 5 normalized recovery curves. Magenta line: fit to the data. E Kinetics of fluorescence recovery of core granule proteins and nos. The half-time to recovery (t1/2 (s)) and the percent mobile fraction of the population that exchanges between granule and the intergranular space is shown. Mean±STDEV of 5 Osk:GFP, 5 Vasa:GFP, 12 Tud:GFP, 9 Aub:GFP and 5 nos MS2-MCP:GFP recovery curves. Scale bar = 10 μm.
To probe the effect of mRNA localization on cluster position within granules further, we analyzed the spatial distribution of chimeric nos that localize to the posterior less efficiently than endogenous nos (Gavis et al., 1996). In these chimeras, the nos 5′UTR and nos open reading frame (ORF) were fused with the tubulin 3′UTR containing different segments of the nos 3′UTR (here termed nos-tub B,C or D chimeras (Figure S2N)), which had previously been identified as partially redundant determinants of nos localization to germ granules (Gavis et al., 1996). These chimeras expressed lower overall levels of RNA and enriched less efficiently at the posterior pole than the endogenous nos (Gavis et al., 1996). Consistently, the three nos-tub chimeras formed less abundant clusters ranging from 2.2±0.3 (nos-tub B) to 3.6±0.6 (nos-tub C) mRNAs (Figure S2O) located at the granule’s periphery (Figure S2O,P).
Collectively, our analysis revealed that in vivo a given germ granule-enriched mRNA can occupy any position within the granule and that the position is dependent on the concentration of the mRNA in germ granules as well as the effectiveness of its localization to germ granules. Surprisingly, the position is not driven by germ granule proteins but relies directly on the mRNA itself. We conclude that germ granule mRNAs self-instruct sorting into homotypic clusters.
Germ granule proteins are more mobile than germ granule mRNAs
The ability of granule mRNAs to self-organize suggests that these mRNAs could display properties distinct from those of core granule proteins, such as their mobility. To this end, we used fluorescence recovery after photo-bleaching (FRAP) assays to record how GFP-tagged Osk, Vasa, Aub and Tud, as well as MS2-MCP:GFP-tagged nos mRNA exchanged with the granule environment in live embryos (STAR Methods, Figure 2A,B).
Our FRAP analysis showed that 35.8±0.4%, 36.2±0.3%, 60.0±0.2% and 57.4±0.6 % of Osk, Vasa, Tud and Aub exchanged with the intergranular space, respectively, with slow kinetic half-lives (t1/2) of 49.5±3.7s (Osk), 33.0±0.9s (Vasa), 14.2±0.6s (Tud) and 16.0±0.4s (Aub) (Figure 2E, Table S2). We found that only between 1.4% (Osk:GFP) and 16.0% (Tud:GFP) of the protein was located in the intergranular space while the majority was located in granules (Figure 2E, S3C, Table S2) (Kistler et al., 2018). The mobilities recorded for these four proteins in the intergranular space were similar to the mobility of free cytoplasmic GFP protein recorded in follicle cells of stage 11 Drosophila oocytes, which do not form germ plasm (Figure S3D, Table S2). Therefore, in the intergranular space, the mobility of Osk, Vasa, Tud and Aub seemed unrestricted. Importantly, Vasa displayed a similar mobility to Osk, while Aub was similar to Tud (Figure 3E, Table S2), an anticipated result given that Osk recruits and physically interacts with Vasa in granules (Breitwieser et al., 1996) while Tud recruits and physically interacts with Aub (Liu et al., 2010, Kirino et al., 2010).
FIGURE 3: Self-assembly into homotypic clusters is RNA sequence independent.

See also Table S3,4. Ai-iii Endogenous nos hybridized with spectrally-distinct smFISH probes (i), displaying highly co-localization (ii) with a PCC(Costes): 0.84±0.01 (mean±SEM of 5 embryos) (iii). Bi-iii Endogenous nos and the nos 3′UTR chimera hybridized with distinct probes (i); poorly co-localized (ii) with a PCC(Costes): 0.55±0.03 (mean±SEM of 6 embryos) (iii). Ci-iii Two full-length nos chimeras hybridized with distinct probes (i) poorly co-localized (ii) with a PCC(Costes): 0.47±0.02 (mean±SEM of 6 embryos) (iii). Scale bar: 1 μm.
Strikingly, nos-MS2-MCP:GFP mRNA was less mobile than any of the proteins assayed (mobile fraction: 28.6±0.2% and t1/2 (s) of 165.0±48.1) (Figure 3E, Table S2). This slower mobility is not caused by the propensity of MCP:GFP to retain in granules, since in the absence of nos-MS2 mRNA, this protein does not accumulate in granules (Figure S3E). Additionally, this kinetic of exchange may be over-estimated given that we were detecting the exchange of MCP-GFP protein bound to the nos-MS2 mRNA rather than the mobility of nos mRNA itself. We conclude that in vivo granule-enriched nos mRNA is stably associated within granules and far less mobile than any of the four core granule proteins. Thus, mRNAs such as nos display slower mobilities from those of core granule proteins and constitute the stable component of germ granules.
Self-assembly into homotypic clusters is RNA sequence independent
To understand how mRNAs self-assemble, we sought to identify the part of the RNA that generates specificity for homotypic clustering. We speculated that if embryos expressed two mRNAs that shared features needed to co-assemble but were otherwise distinct, the two transcripts would highly co-localize with each other within the same cluster. Alternatively, if the two mRNAs did not share such features, they may localize to the same granule but organize two distinct homotypic clusters that would poorly co-localize with each other.
To test this hypothesis, we focused on nos mRNA whose localization signals, like those of several other germ granule mRNAs, reside in its 3’UTRs (Rangan et al., 2009, Gavis et al., 1996, Eagle et al., 2018). We reasoned that the determinants required for homotypic nos assembly could also reside in its 3’UTR. To this end, we constructed chimeras that only shared the nos 3’UTR with the endogenous gene and fused it to an open reading frame (ORF) of a farred fluorescent protein (IRFP670), which allowed for the simultaneous detection of the chimeric mRNA and the endogenous nos within the same granule using distinct smFISH probes (Figure 3Bi). To define co-localization of multiple nos molecules within the same cluster, we stained the endogenous nos with two sets of spectrally-distinct smFISH probes (Figure 3Ai) and detected high co-localization (Figure 3Aii) with a PCC(Costes) of 0.84±0.01 (Figure 3Aiii). This value establishes the expected co-localization coefficient should two distinct nos transcripts sort into the same cluster.
Our measurements revealed that while the chimeric nos formed abundant clusters (Table S3), it displayed a low co-localization coefficient with endogenous nos (PCC(Costes) of 0.55±0.03) and thus organized into distinct clusters within the same granule (Figure 3Bii,iii). We measured a similar low PCC(Costes) between chimeras fused with the gcl or pgc 3′UTRs and their respective endogenous counterparts (Table S3,4). The low co-localization coefficient of the nos 3’UTR reporter with the endogenous nos could not be attributed to the IRF670 ORF since clusters of the nos chimera where the nos 3’UTR was fused with the ORF of EGFP and the actin-interacting domain of the moesin protein (moe-EGFP) (Edwards et al., 1997) also co-localized with endogenous nos with a low PCC(Costes) (Table S3,4). Therefore, the 3’UTRs of germ granule mRNAs, while required for their recruitment to granules, were insufficient to generate the specificity for mRNA sorting into homotypic clusters within granules.
To determine whether other mRNA features regulate self-assembly, we tested chimeras fused with the 5’UTR of nos, pgc and gcl as well as the promoter of nos and found that these too sorted independently from their respective endogenous counterparts (Suppl. Table 3,4). Finally, we analyzed two chimeras that had all the features of the endogenous nos (5’ and 3’UTRs, ORF, introns and whose expression was driven by the nos promoter), but differed in heterologous sequences inserted into these complete transcripts, which allowed binding of spectrally-distinct smFISH probes and differentiation of the two chimeras within the same granule. The first chimera carried 18 MS2 RNA loops inserted at the end of nos 3’UTR (Brechbiel and Gavis, 2008), while the ORF of the second was fused with EGFP (Figure 3Ci) (Forrest et al., 2004). The fluorescence originating from the EGFP reporter was minimal and did not interfere with the fluorescence originating from Alexa488 smFISH probes (Figure S3F,G)). Importantly, these MS2 and EGFP chimeras fully rescue the nanos null phenotype (Sinsimer et al., 2013, Forrest et al., 2004) indicating that the relevant regulatory elements of endogenous nos including its structured translational control element were preserved in both chimeras. Our experiments revealed that while the two chimeras formed abundant mRNA clusters within the same granules (Table S3), they sorted into distinct clusters with a low PCC(Costes) of 0.47±0.02 (Figure 3Cii,iii; Table S4).
Lastly, we speculated that the length of a transcript could generate the specificity for self-sorting. However, CycB, nos and gcl mRNAs, which have similar lengths (2556 nucleotides (nt), 2349 nt and 2464 nt, respectively (Table S3)) nevertheless organized into distinct clusters (Figure 1G, S2A) (Trcek et al., 2015), while nos chimeras fused with moe-EGFP or with IRF670, which also had a similar length as endogenous nos similarly organized into distinct clusters (Table S3,4).
In the context of a full-length nos sequence, the insertion of heterologous sequences is sufficient to cause de-mixing. This strongly suggests that nos lacks essential and mappable regions required to generate specificity for homotypic assembly. This finding is therefore inconsistent with a model where the homotypic mRNA assembly would be driven by direct, sequence-specific trans mRNA-mRNA base-pairing or recognition via proteins deposited on specific nos mRNA features. Instead, our data argue that the features of the entire mRNA including completely unrelated, heterologous sequences contribute to the specificity of sorting.
mRNA organization within homotypic clusters is disordered
Our genetic data demonstrate that the specificity for mRNA self-assembly does not require distinct RNA sequences. Therefore, mRNAs in clusters are likely unrestricted by any one sequence-encoded, trans RNA:RNA interaction and contacts between clustered transcripts may be less stereotypical than those between mRNAs bound by specific sequence complementarity. To address this possibility in vivo, we characterized the spatial organization of mRNAs within homotypic clusters and transport mRNPs using STORM (Figure S4A–E). We reasoned that if we hybridized a single probe conjugated to a photoswitchable dye within the region of the mRNA that engages in sequence-specific trans RNA:RNA interaction, for instance with sequences located within their 3′UTRs, then these intermolecular interactions will spatially constrain that region to a small cluster radius (Figure 4A). Conversely, if a probe is hybridized to the region of the mRNA that does not engage in sequence-specific trans RNA:RNA interactions, then that region would be expected to be less spatially constrained and would create areas with a larger cluster radius (Figure 4A). However, for mRNAs within homotypic assemblies that do not rely on specific sequences for their association, we would expect that no specific mRNA region would be spatially constrained and that all probes would report cluster radii of similar sizes regardless of their position on the mRNA (Figure 4B).
FIGURE 4: mRNA organization within homotypic clusters is disordered.

See also Figure S4 and Table S5,6. A,B Detection scheme of sequence-specific, ordered trans RNA:RNA interactions in using smFISH (magenta circle) and STORM where mRNAs base-pair with their 3′UTRs and become spatially constrained (right panel) compared to the 5′UTR that do not base-pair (left panel). Magenta dot: smFISH probes. Magenta circles: cluster radius recorded by STORM. B In the absence of sequence-specific, ordered trans RNA:RNA interaction, the same radius of the mRNA clusters is recorded regardless of the position of the smFISH probe hybridization. For simplicity, mRNAs are drawn as linear polymers in A, B. C Radii of osk clusters. Statistical significance P: two tailed t test. Magenta and green circles: Alexa647, Alexa568 probes, respectively. Per probe, mean±STDEV of 11 to 30 ROIs is plotted, with localization uncertainty of 15.5±6.2 to 26.6±2.6 nm. D Radii of nos clusters. Magenta and green circles: Alexa647 or Alexa568 probes, respectively. Per probe, mean±STDEV of 17 to 40 ROIs is plotted with localization uncertainty of 12.4±4.3 to 20.7±2.2 nm. E osk mRNA transport particle coupled to a dynein motor (i) (Sanghavi et al., 2013) and homodimerized dimerized via a palindromic sequence (ii) (Jambor et al., 2011). F Germ granule mRNAs distinguish among distinct mRNAs near near cognate mRNAs.
To determine if we could detect sequence-specific trans RNA:RNA interactions, we examined osk mRNA. osk localizes to the posterior pole via kinesin motors but does not enrich in germ granules (Trcek et al., 2015, Little et al., 2015, Lehmann, 2016). Its 3’UTR harbors a stem-loop, which contains a palindrome that promotes osk dimerization (Figure 4Ei,ii) (Jambor et al., 2011). As a result, localized osk forms clusters at the posterior pole that in the early embryo on average contained 21.8±0.5 mRNAs (Figure S4G).
When single smFISH probes hybridized to the osk 5’UTR, ORF or the beginning of its 3’UTR, we detected cluster radii ranging from 50.1±5.4 to 55.9±12.4 nm (Figure 4C). The measurement of the clusters’ physical size was invariant of the type of the photoswitchable dye, since probes coupled to Alexa647 or Alexa568 produced cluster radii of similar sizes (Figure 4C, radii marked with green and magenta circles). However, when probes hybridized directly adjacent to osk’s palindrome, we recorded a much smaller cluster radius of 31.4±11.1 nm, a statistically significant 40% decrease (Figure 4C). Using the mean cluster radius determined by STORM and cluster abundance measured by smFISH and SIM, we calculated the mean compactness of osk clusters (mean volume derived from the radius of a cluster occupied by one osk mRNA) at various positions. We found that outside of the palindrome, osk transcripts within the cluster were far less compact than within the palindromic region (mean compactness of 2.8±.4 and 0.6±0.1, respectively (Figure S4H)). These data also imply that potential non-specific RNA:RNA interactions among osk molecules outside of the palindrome cannot spatially constrict osk mRNPs as effectively as a single RNA region engaged in direct sequence-specific base-pairing. Notably, we were able to record this constriction in situ despite the fact that osk is translated at the posterior pole (Kimha et al., 1995, Gunkel et al., 1998). Thus the ribosome-crowded environment of osk clusters did not preclude smFISH probes from hybridizing to osk nor did it prevent photoswitching of fluorescent dyes, firmly validating STORM as a tool to characterize trans RNA:RNA interactions in vivo. Our direct observation at the posterior pole of the embryo revealed that osk mRNPs are spatially constrained within their 3’UTR, where osk engages in sequence-specific and ordered trans RNA:RNA interactions, and showed a more relaxed configuration along regions of the RNA not engaged in sequence-specific trans RNA:RNA interactions.
We next employed the same strategy to the analysis of nos clusters. In contrast to osk, we did not observe any specific constriction of the cluster radius regardless of where on the nos mRNA we hybridized smFISH probes and despite the fact that the average concentration of nos within clusters was 4761 times higher than outside of granules (Table S5). Instead, we recorded an average cluster radius of 42.9±7.4 nm (Figure 4D) with an average compactness of 2.4±1.2 (Figure S4H). This compactness was similar to that recorded for osk mRNA outside of its palindromic region (Figure S4H). Additionally, we also analyzed gcl mRNA and recorded a mean cluster radius of 35.4±2.3 nm and a mean compactness of 6.5±1.2 across the length of the gcl transcript (Figure 4H,I). As with osk, these measurements were not influenced by the choice of the photoswitchable dye that was conjugated to the probes (Figure 4D; S4I, radii marked with green and magenta circles). We conclude that unlike osk, in vivo clustered nos and gcl are not engaged in sequence-specific base-pairing and that contacts between these mRNAs are not stereotypical, consistent with our genetic data (Figure 3). We conclude that spatial organization among clustered transcripts in Drosophila germ granules is disordered.
DISCUSSION
In this study, we addressed the molecular parameters of homotypic mRNA assembly within RNA granules in vivo. Our data suggest that models relying on specific trans RNA:RNA interactions generated by RBPs or by base-paired RNA sequences observed for osk and bcd mRNPs and the HIV genome cannot explain the specificity for homotypic mRNA assembly in Drosophila germ granules. Instead we show that RNA:RNA interactions among clustered nos and gcl are sequence-independent and disordered.
The exact molecular mechanism of homotypic mRNA self-assembly is not clear however it is distinct from the localization principles that generally rely on the 3′UTR-encoded features for enrichment of mRNAs in germ granules (Figure 3). Since RNA modifications are typically sequence-encoded (Roundtree et al., 2017), these similarly are likely not providing the specificity for homotypic assembly. Furthermore, accumulation of nos, CycB, pgc and gcl occurs concurrently (Little et al., 2015), indicating that nos chimeras expressed from the same promoter arrive at granules simultaneously and that therefore different time of arrival of transcripts cannot give rise to demixed clusters within granules. Similarly, full-length nos transgenes tagged with EGFP or MS2 loops were inserted at multiple genomic loci and their mRNAs each demixed into a single distinct cluster excluding the possibility that epigenetic mRNA imprinting could drive homotypic assembly. Additionally, given that the position of clusters changes simply when cluster mRNA abundance is altered independently of granule protein composition (Figure 1G) it is unlikely that granule proteins other than core proteins could drive homotypic assembly. Most compelling, a protein-driven model would have predicted that full length nos chimeras, which only differed in their EGFP or MS2 tag, would be recognized by the same trans-factors and thus organize into the same cluster, a model not supported by our in vivo measurements (Figure 3C). Lastly, the specificity for self-assembly could be generated by distinct RNA structures that could control self-assembly (Langdon et al., 2018). Such RNA structures are expected to be encoded by conserved RNA sequences (Clever et al., 1996, Ferrandon et al., 1997, Jambor et al., 2011).
However, nos chimeras that included all nos sequences but differed in their tags demixed from each other (Figure 3Ci). This suggests that neither sequence-specific RNA:RNA interactions nor specific structural RNA elements are generating the specificity for homotypic clustering. A recent study revealed that RNAs self-recruit to stress granules and revealed that in vitro transcribed nos, pgc and gcl RNAs interact with each other suggesting that sequence-specific base-pairing could give rise to homotypic mRNA assembly (Tauber et al., 2020). While this in vitro study cannot account for the sequence-independent mRNA sorting into homotypic assemblies we observed in vivo, it might explain how RNA:RNA interactions initially recruit these transcripts to germ granules. Indeed, nos, pgc and gcl have been shown to self-recruit to granules (Niepielko et al., 2018) possibly via specific trans RNA:RNA interactions.
Given that RNAs phase separate in vitro (Eisenberg and Felsenfeld, 1967, Van Treeck et al., 2018, Jain and Vale, 2017, Langdon et al., 2018) and in vivo (Jain and Vale, 2017), it is possible that homotypic RNA assemblies could form by phase separation according to their miscible properties. Instead of a single determinant such as an RNA sequence or fold, the miscibility of an mRNA could be specified by its many features including bound proteins, modifications, structures and length. Thus, granule mRNAs could “read” the sum of all of its characteristics to discriminate between transcripts rather than rely on one single determinant. In this “global” approach, if an mRNA becomes altered, so would the sum of its features, causing even near cognate transcripts to sort into distinct clusters. mRNPs that share their primary sequences but are distinctly modified, structured or protein-bound, could also sort into distinct clusters. Thus, chimeras that contained all nos sequences but were modified by EGFP or MS2 repeats have a different sum of their features, therefore become differently miscible and sort distinctly.
Finally, rather than sorting towards each other, mRNAs could simply sort away from transcripts that are different. Future experiments are required to decipher the mechanistic principles that govern the specificity of this mRNA sorting in vivo.
STAR METHODS
LEAD CONTACT
Further information about this work should be directed to the lead contact, Ruth Lehmann (ruth.lehmann@med.nyu.edu) and co-corresponding author Tatjana Trcek (ttrcekp1@jhu.edu).
MATERIALS AVAILABILITY
Requests for resources and reagents should be directed and will be fulfilled by the lead contact, Ruth Lehmann (ruth.lehmann@med.nyu.edu) and co-corresponding author Tatjana Trcek (ttrcekp1@jhu.edu). All unique reagents generated in this study are available from the Lead Contact and co-corresponding author without restriction.
DATA AND CODE AVAILABILITY
Requests for data and codes generated in this study should be directed and will be fulfilled by the lead contact, Ruth Lehmann (ruth.lehmann@med.nyu.edu) and co-corresponding author Tatjana Trcek (ttrcekp1@jhu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly lines
Flies were maintained on cornmeal molasses/yeast medium at RT or 25°C using standard procedures. To create the nos chimeras described in this study, the 5′UTR of nos was fused with the Drosophila codon optimized ORF sequence of the near-infrared fluorescent protein IRF670 (Addgene, #45457) and nos 3′UTR. The expression of this chimera was driven by the nos promoter (Rangan et al., 2009). Alternatively, the IRF670 ORF and nos 3′UTR were fused with the 5′UTR of P element transposase gene and driven by the Gal4-responsive UASp promoter (Rorth, 1998). These two constructs were inserted into the attP40 site (2nd chromosome) via the PhiC31 integrase-mediated site-specific integration by Best Gene.
METHOD DETAILS
Microscopes
Unless otherwise specified, images were acquired with an instant Structural Illumination Microscope (iSIM) (Curd et al., 2015, York et al., 2013) in 3D with a 150nm Z step as previously described (Trcek et al., 2017). Using Huygens (Scientific Volume Imaging), images were deconvolved and corrected in 3D for chromatic aberrations using multicolored beads (ThermoFisher Scientific; T7279) (Trcek et al., 2015, Trcek et al., 2017).
To image co-localization of nosp-5′gcl-EGFP-3′gcl and nosp-5′pgc-EGFP-3′pgc chimeras with endogenous gcl or pgc a Nikon Structured Illumination Microscope using a 100× 1.5 NA oil immersion objective, with pixels of 33 × 33 nm was used(Eagle et al., 2018). For each embryo, a z series of 21 slices was taken, with a step size of 150 nm. To minimize fluorescent signal distortion, all images were taken within 5 μm of the embryo cortex.
For FRAP assays, a Zeiss LSM780, AxioOberver inverted, laser scanning confocal microscope, equipped with an argon, an HeNe 633 laser and a DPSS 561–10 laser, a Plan-Apo40X/1.4 Oil DIC and EC Plan-Neofluar 10X/0.30 objectives was used.
To quantify nos and pgc mRNA levels in the soma and in the germ plasm through early embryogenesis (Suppl. Fig. 3F,G), an API DeltaVision personal DV widefield epifluorescence microscope equipped with the Photometrics CoolSNAP HQ2 CCD camera and Olympus PlanApo N 60X/1.42 oil and UPlanSApo 20X/0.75 objectives was used (Trcek et al., 2015).
For auto-correlation experiments, a custom Stochastic Optical Reconstruction Microscope (STORM) was used. The system was built on an optical imaging platform equipped with the Leica DMI 3000 inverted microscope, 488-nm (OBIS, Coherent) and 639-nm (MRL-FN-639–800, CNI) laser lines, as previously described (Nemoz et al., 2018). Laser lines were reflected into an HCX PL APO 63X NA = 1.47 OIL CORR TIRF Objective (Zeiss) by a penta-edged dichroic beam splitter (FF408/504/581/667/762-Di01–22×29). Afterwards, the emitted fluorescence was extended by a 2X lens tube (Diagnostic Instruments), filtered by single-band filters (Semrock FF01–531/40, FF01–607/36, and FF01–676/37 for GFP, Alexa Fluor 568, and Alexa Fluor 647, respectively), and collected onto a scientific (sCMOS) camera (Prime95B, Photometrics). To reactivate Alexa Fluor647 fluorophores, the microscope was also equipped with a 405-nm laser line (MDL-III-405–150, CNI). To achieve super-resolution imaging, the 561 and 639 laser lines were adjusted to ~ 1.0 and 1.5 kW cm−2, and a highly inclined and laminated optical sheet illumination mode was used for sample excitation. Alexa568 and Alexa647 dyes were sequentially exited and their emitted fluorescence sequentially collected by switching the single-band filters in a filter wheel. A minimum of 2,000 frames at 33 Hz were recorded for each image stack (Nemoz et al., 2018).
For cross-correlation experiments a second Stochastic Optical Reconstruction Microscope (STORM) was used. This system was built on a Leica DMI 3000 inverted microscope base (Whelan et al., 2018). In brief, the 473 nm (Opto Engine LLC, MBL-473–300 mW), 532 nm (OEM Laser Systems, MLL-III 200 mW), and 640 nm (OEM Laser Systems, MLL-III 150 mW) laser beams were collimated and reflected into an HCX PL APO 100x NA = 1.47 TIRF objective (Leica) via a multi-band dichroic (Chroma, zt405/488/532/640/730rpc, UF1C165837). The illumination was adjusted into a Highly Inclined and Laminated Optical (HILO) illumination mode to achieve an image depth of ~ 400 – 600 nm. Multi-color imaging was achieved by sequentially imaging different fluorophores. Emitted fluorescence was collected with the same objective and further filtered by single-band filters (Semrock FF01–531/40, FF01–607/36, and FF01–676/37 for GFP, Alexa Fluor 568, and Alexa Fluor 647, respectively) switched in a filter wheel (Thorlabs, FW102C) accordingly. The photons from each color were then recorded on an EMCCD (Andor iXon +897) at 33Hz for 2000 frames with an EM gain of 300. Note that the microscope was also equipped with a 405 nm laser line (MDL-III-405–150, CNI) to reactivate Alexa Fluor 647. A minimum of 2,000 frames at 33 Hz were recorded for each image stack.
Embryo collection
Unless otherwise noted, caged flies were allowed to lay eggs at room temperature (RT) on a fresh apple juice plate containing a dollop of fresh yeast paste for 1.5 hours, after which the embryos were dechorionated and the vitelline membrane removed by methanol cracking. Embryos were then stored in 100% methanol at 4°C until further use (Trcek et al., 2015, Trcek et al., 2017).
Single-molecule fluorescent in situ hybridization (smFISH)
A mix of commercially-available Stellaris smFISH probes was used to label individual mRNAs (Key resources table) (Trcek et al., 2015, Trcek et al., 2017). The probes were designed as 20 nucleotides long DNA primers and covalently coupled to a CAL Fluor 590 dye (https://www.biosearchtech.com/stellarisdesigner). To prepare Alexa488-labeled smFISH probes, the probes were AmMC12 modified at the 5′ end (IDT Technologies), labelled using the AlexaFluor 488 oligonucleotide Amine labeling kit and purified using the MicroSpin G-25 Columns (Trcek et al., 2015, Trcek et al., 2017). Hybridization of mRNAs with smFISH probes was carried out as described before (Trcek et al., 2015, Trcek et al., 2017).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| TRIzol reagent | Invitrogen | 15596018 |
| Acid-phenol:chloroform | Invitrogen | AM9720 |
| Chloroform | Fisher | AC40463–5000 |
| Isopropanol | Fisher Scientific | A416–500 |
| RQ1 RNase-Free DNase | Promega | M6101 |
| Oligo dT(20) primer | Thermo Fisher Scientific | 18418020 |
| SuperScript® III Reverse Transcriptase | Life Technologies | 18080–044 |
| YBR Green reporter dye | Roche | 04707516001 |
| Tissue freezing medium | General Data | TFM-C |
| Poly-L-lysine solution | Sigma | P4707–50ml |
| Epoxy | Devcon | 00470740 |
| Catalase | Sigma-Aldrich | C3115–50MG |
| Glucose Oxidase | Sigma-Aldrich | G2133–10KU |
| IRF670 plasmid | Addgene | 45457 |
| Critical Commercial Assays | ||
| AlexaFluor 488 oligonucleotide Amine labeling kit | Life Technologies | A-20191 |
| MicroSpin G-25 Columns | GE Healthcare | 27-5325-01 |
| 384-well qRT-PCR plates | Roche | 04729749001 |
| Plastic disposable molds | Fisherbrand | 22-363-552 |
| Cryostat | Leica | CM 3050 S |
| Low profile blades | Accu-Edge | 4689 |
| Experimental Models: Organisms/Strains | ||
| w1118 (“wild type”) | Bloomington Drosophila Stock Center | 5905 |
| pFlyFos-Osk:sfGFP | (Jambor et al., 2015, Trcek et al., 2015) | N/A |
| y,w; P[E GFP-vas w+]cyIII | (Trcek et al., 2015) | N/A |
| UASp-GFP-Aub | (Webster et al., 2015) | N/A |
| GFP-Tud | (Zheng et al., 2016) | N/A |
| ;;P{GAL4::VP16-nos.UTR}/TM3Ser | (Pae et al., 2017) | N/A |
| w; matα-gal4;PrDr/TM3 | (Pae et al., 2017) | N/A |
| w; P(EPgy2)gclEY09611/CyO; nosGal4VP16 (w+)/TM3 | (Cinalli and Lehmann, 2013) | N/A |
| w; P(EPgy2)gclEY09611/CyO; nosGal4VP16 (w+)/TM3 | (Cinalli and Lehmann, 2013) | N/A |
| nos-(MS2)18 | (Sinsimer et al., 2013) | N/A |
| hsp83-MCP-GFP | (Forrest and Gavis, 2003) | N/A |
| nos-tub B,C, D chimeras | (Gavis et al., 1996) | N/A |
| nos RNAi y1 sc* v1; P{TRiP.HMS00930}attP2 | Bloomington Drosophila Stock Center | 33973 |
| CycB RNAi y1 sc* v1; P{TRiP.HMS02163}attP2/TM3, Sb1 | Bloomington Drosophila Stock Center | 40915 |
| mCherry RNAi y1 sc*v1 sev21; P{y[+t7.7] v[+t1.8]=UAS-mCherry.VALIUM10}attP2 | Bloomington Drosophila Stock Center | 35787 |
| w[*]; P{w[+mC]=GAL4-slbo.2.6}1206 P{w[+mC]=UAS-GFP.S65T}T2/CyO | Kyoto Stock Center | 108–787 |
| ry nosBN e / TM3 Sb e | (Wang et al., 1994) | N/A |
| sp/CyO; nosBN,Vasa:GFP/TM3 Ser | This study | N/A |
| nosDef (Df(3R)DlFx3/TM3 Sb, Ser) | (Forbes and Lehmann, 1998) | N/A |
| moesin-GFP ORF tagged with nos 3′UTR | (Rangan et al., 2009) | N/A |
| EGFP ORF-tagged with gcl 3′UTR | (Rangan et al., 2009) | N/A |
| yw; P(w+EGFP-nos)11; P(w+EGFP-nos)5 | (Forrest et al., 2004) | N/A |
| nosp-5′gcl-EGFP-3′gcl and nosp-5′pgc-EGFP-3′pgc | (Eagle et al., 2018) | N/A |
| smFISH Probes (5′ to 3′) | ||
| aret cgtatccagacggaagcagg, aacatcttgatgttgtccgg, cgtactcctcgaacatctcg, cgcagaacgttgatcgagtg, aaagcagcaacccttgctaa, gcggcgtgtctcgtataaaa, attatgcaaagcatcctggg, acatcccatttagcgtctta, gggcttcatttgaatgggat, cattgcgattctcgctatcg, atacccacaaagagtttgcg, ctcgttcagcttcttgttta, ccgtggacttcaaatagctt, ttggtggcgaatgtgacgaa, ctttaatggccgaaatggca, atgatcttgttctggctcag, gtatcggcgaacttgacgac, ttccagagattggcctgaat, aggtatgttgatgttgctgg, aatggcatctgcgccgagaa, ctgcagcagttgaatggagg, gtgagggcttgcaacaactg, catcggggtcaaaagtcctg, aatggccgctagattctgga, cattcgagagactcggctgc, gatgccattggattcggatc, ggcggctgttgacatatatg, gcgaagtggacaatgcactg, gcggcagatggtagatgaaa, agatccgtatcggtgaactc, cgagatcacattgccgaagg, ctcgtctgtttgtcgatgaa, aaacccgaagcacttcgaca, agtccggattgtcgaaggag, ttcttcagctgcaccttgag | Stellaris smFISH probes | N/A |
| tao1 ttgtagccggacatctgttc, taaagttgtggagcagcgtg, tgctgatgcttcgtctcaag, tttgtgtagtttcttctcgg, ttctcctgctttagcgtaat, gtcgactcatccatggagag, tgcgattgcagggtaagatc, agctcgatgtactgcttctg, atcatccgtttgcgcttgaa, gtggtgcttaagaagcatgg, tgttgcttgtttatctgctc, ttttgattatgcaactcggt, tcttgatgcggtccatgtag, cttttgcttcaagctcttcg, aactgcttgcgtatctggag, tgtagcgtttgtattgcttc, tttggtgtcgtctgcagtac, ttaatgacctccttctgttg, cgatgcttctcttccttcag, atgctttgctcgtactgttc, ctgactttggaacatgtccg, ggctttcgtctagtttgtag, gtacgttggcactcaatcac, ttcttgttcttgttctggta, cgattctcgagctcacgacg, tccatcttattctcgagcag, ctggttgaattgctgcaact, tagtatgcttctcgtgtttc, ttcgttatcaaatgcttcca, tgctgaaacctagggcaatt, tcgggatacctcaatcagag, attgaaccagacagacttcc, gttgaactattgctatgggc, atagcgaaccagccggaaaa, tggcattgttgttgtctatg, actaaacacttgttgtgggg, tcgcactgttggctttataa, gatttctgttgcttgtgtcg, gctctattgatgtcttagcg, tgtttctgctgctattgttt, aaacattgctagctagccat, gtgtgtcttctttctagttc, catgtgtgttttgggttgtg, ccaatccgataattgatcgc, ggttctcatgttttcctaag, tattgcagctttagcttgtt, ccgatcgagtttcctttaat, atgttagtatcttgttgcca | Stellaris smFISH probes | N/A |
| pum ctgcgagtagagattctgtt, tcaacgagctcgtgttcaag, tccgaacatattgttggcag, gggattcgagaagatcgagc, cttcgagaaggcgagatctt, gagatcgcgtagctgaagat, cctgtgagaactccacaatg, ttctgttggataaaccgcga, atctcgctgaacaccatttg, ctggatgacatagttgccaa, ttgacgcatttcagcacatg, cacatgattgccattctgat, acgcactcaatgcacttttg, ttgatgatgaactgcagcgc, tgggtgcttagcgagtaaac, ctcaaggattctctggatca, ctcatgcagttcgtccaaaa, acatagttgccatattggtc, gtgttcaagcacatgctgaa, agaatcgacttatcctcctg, tgatagcaccagaactttgc, acgtttgaggcgaacttgtg, catgggtaacacatttctcc, cttcatcatcacgtgcaacg, accacatagttggcatactg, aacttggcattgatgtgctt, gggattggttatcttcatgt, accgaacaaatggtgggtga, gcctggcttaatttgttatt, tgttaagctctacagcttct, ttatcgctgcttttccaata, caaatcttcgagtgcagctc, tgtgtgttttactatcctct, aagctcttttgcttttttgc, ttatgcgagcgcattttaca, tttcatctttgcggttcatg, gatcgcgatttactacgcat, tttcttttttttcagctcgc, tcgtaggctaattcatgcag, aatggttcctctgtctgaac, gttatccctcgtatatatgt, ctgctggctccaaaaacatt, tctctgttttcattgtgtct, attgtgtgggtgtatgcaac, tgaatgggctgaagagtcgt, gtttgttgtttggggttttt, gaccaatatccatgcagatc, tttggcttacttggctaaca | Stellaris smFISH probes | N/A |
| eIF4G2 attgttgaaagtggggtggt, cgttagtgggaaaggtcaca, aagctgtttctgttgtggag, actgggaatgccaaagttgt, ccaaaataggggtcgtcatc, caagtgcggagcaaatgtgt, ctgttgttactgacggagtt, ggtactattgtcatccagag, acaataacgatgaccccgag, ctgatctgatctttatccgt, ctttgtttgttggccaattt, agctgtggtaactatcgact, aatactcgacttcctgttgg, gtggtaatgctggagttgat, ttctgttttttcttctccag, gcattgttgctatttgtgtt, gaattgaagctgttgcgtct, gagctgcatatgaaacctgt, agcgatagtttttgggaacc, ggcacatagtccaatgattc, cttaagcagcacctcaaagt, catttgtcattttagcctca, ctgaatgcgggtgatcaacg, tttgcgttggcatcatttac, cgactgcagcttgaaaagct, aacaaatgacccactgtggt, tcttgcatcataaaccgcac, tttggcatcttctgcatgaa, atctatgcttagggtctgat, agcaattgaatcgtcggtgg, aacaaggatgtaggcggagg, gaatgcgtcatttacttcca, gtgcaaatagtccgtgagta, ccaaacatcttttaggtcca, ctgtacaaactcccgatact, gagtgcaacagctacggaag, tcgatacacagtcgcagtag, tctatgcaagcaatctccag, tacagaacgctgaacgtgct, tagcttataccacttgtcga, tcaatgaatgggcgacggta, agtttgtggttggttgtttg, tggtttcaaacgatgttgcc, ctgatctagtcatcgtgcaa, tctatacccagttcacattg, ttcgtatttgttttgagcca, gctctttgtttttgcttttt, ttgcattgggttttcattgt | Stellaris smFISH probes | N/A |
| orb tgcaaattctgcagtttggc, aggagctttagcgtgttgat, ttgatcagcggcatttgttg, cgctcatcaagttaccaaga, ccatttctgtcatcagtgat, ccatcgttggtggttataat, gcagccgaactcttatagaa, ttgagcatacggtgcgaacg, cgaggaagaccttgggagaa, ctcactaatatcccagggaa, gcttgaagatctggatgagc, gccactccactttaatagat, taaacataacccttgggctg, gcttgtccgattcaaagatt, gccgaaagtaatgccttgac, agaatcatccacctgaagca, agaagtagttcctaccacag, atacgccgcgaagagatttt, tgacttcaacatccttggac, gagtcagcgataatccaagg, ggagctggatcgcacaaaat, caaacacggttttcgttggg, atccattatatttcccaagc, atctataccagcatacagca, cgatcgggtacttgtacttg, ttgctaaatgtcacacgtcc, cagctttcatataggagcga, taatctcgataaaggcggcc, cttcttggtgaacttcgtgg, caccgcatatggaacatagg, attccctacaatagtaggga, gcagaagtatcggaagcacg, ttgacgatgtcacagctgtg, acttggagttgcgagtcaag, tcgacgaagatggtccgatg, ggccagagaagggtaacgaa, cattctgttgtccctgatac, catcacgttgagactgtggg, ttaccattgatgtagcagcg, tgaatttgccgctgctgaag, atgctgttgacgctgtattc, agccgtaacagtctagatcg, catccgttaaaacggtgtgg, gtccaaagtctcacacatta, catatttctacgtcgcctac, ccatcggctgcaagaaaact, taatgacgatgatgagcccg, agcttcatattgtaccgata | Stellaris smFISH probes | N/A |
| CG18446 acgtttctctgcggactaaa, ttaaattcactagggtggcc, caactgttctgtaggatcgc, ctcttcggttggtggaatta, tatgatctccacatcgctat, gtggtgatgatggttcatga, ggtatggcggatggaatttg, cagtgggtgaaaatggtggg, gttccttcgggaacatttat, aggtagcacggaacacagag, atcattgaagggctgtttgc, gtgttattccaggcgttaat, acaaagggtgttatcgtcgg, ataagtgctgatctgtggga, attgtgatggtgatccatgt, cgagcggcggtgagtaaaaa, atgaaccgcaggcatttgaa, ggtggtatctgaatcggatg, tatgctgagcggtcatatac, gccgatgacctaaaaactgc, aggagattgcggtgacattg, attggatgaccagaaggctc, tgttggttacttggaatcgc, aggttttgcatttcaagcga, atcctgaatcggatcttgtg, aagccaaggatttggcattt, aattgcactcgaactgacct, gcttggtcaaattcttctct, ttgcatgtgaggcacttatg, tcatgtgcatcaggaggaag, aaagcaatcccgtgtttgac, tttggatattcgggatcagc, cggatacaggcgaatctaca, cgacattggggagacaggaa, agcgttgaatattcgtgtgc, cgtaaaacccgattccaact, aagtggatatgtccgtttgg, agatccggattgaagtaggg, gccaagttcaacctataagt, gataccccaagcttcttaaa, tggctatcttattagctcga, catagtcagaagctccatga, cttgcgagtgcgatatgtat, tcgagatccagatccaaagt, caagaagtttggggctttgg, atgctcagcgtttttagttt, agcgtccgagtgcttaaaat, gcgtttctttatttccattt | Stellaris smFISH probes | N/A |
| Shu gcgtttgagaaaagagcttt, agttggcgctattcttttgg, aaacgaattccggaatgcgc, gttttcttccattatcgcaa, ttgagcaactgtggggtgta, gatccgaataggacaaggga, aactcaacgccctttttcac, catggttctgctgcgaattg, tatgtccacggtgaacgtag, cgcacgcagttcgtcgaatg, tttcgtcgatctcgctcatc, cgagtgatccgcttgtaaat, cagtagccactgtaccgaac, cagggaggagtcgaagggag, tggccagtttcaaacacgaa, taaggacgcatgctgcggac, ggagattataaactcggcct, aaccgagttctccgaaaagc, cactttaaagagcgcatccg, cctatgagcgagtagtcaat, cttgggggatagcatcgatg, cacacaaaacttgtcgcggt, atgtaggtgcaagtcgacag, cagttgctgcactctgatag, cagtagttcagtgagctcac, agtaagcagttcagtttgct, gatcatcaggttttggttca, gcttgttcatcttgttgtaa, gtaaggccttcatcatgatg, gaagagcgccttgcaagacg, caagttgtactcgcccagag, ataatctcgtcgctgatctc, ctcatacttgctgattctct, tttaacgagaaggcacgtgc, tttgcggacgtctgactttg, ttgaaatcctgttcctttgc, gttcttaaaccgcctaatca, aagctcacttgttgatctga, gtcgaactgagcgttcgaat, ttccttggccaatttgcaag, gggagacaaagttagcttca, atgtcagaacatcctcttgg, aagcgaacttaacatccggc, tactggggcttcttagtaag, ctgctcttctagaacttgga, taacattcgtctgggcttac, acttaaggtctacgttgctc, gccgtgcattacttttattg | Stellaris smFISH probes | N/A |
| sra acgcattgttgttggacttg, gtcaggagtggtcggatcag, ttgtcggagtggctgcattg, cagtttgttcttgcttctgc, cactgctgttctgtgtcgac, cgacagcttgtctatgctgc, ggcggcgttgatgaaaatgt, ggagcgacggatgctgattg, tcgtcgaacgagtctgcatc, cacgatgattgaggtgggta, aaacacctcggagtggatgt, cgtgcttcagctctggattg, gaatgtgcggaataactcct, cactggaacgtagccgattc, aacacgcaggcgacggaagc, ccgcaatggcattgtcatag, cagcttgattcgcgcatttg, ccgtcttcttgttaaactcg, tgggcaaagtagcaggtgat, tgcaggttcttgttgctgac, ggggagatgaggaactgttt, caacgcggccaaaagatcat, atggccgtgtgcacaataat, cacttggtctggacaattgg, tcaccaccttatgattatgc, caggttttcagctttagttt, ccgatcgtttggttttacaa, aaatactcaatcgaagcccc, gttcgagtgcctattaatac, ggaaagtatgtcgggtatgg, atgctacgcttgaaggaagg, tgtatcagaagccaactggg, tgcggttgttagccaatgaa, atcgcgagataaacacatct, tcttacacatagacacactc, ttgcgatggcttaacatcaa, tgtagatattgttagcaggt, cgtcgaaggtacacagagat, atatctgggatttcggatcg, tcgctggcttagaattacat, gatgcgatatcgttacacga, gtatgtatgtgtatgccgat, actacacgtaggcatataga, ttcattttgttttgtttggt | Stellaris smFISH probes | N/A |
| Pi3K21B aagcccatctgcaatatcat,tcataaagttgctgccactt, tttagcaaccagagagcttc, ctctcgctcgaatcaggaag, atggacagagcatagtgacc, agtgctgcacgatgttcttg, ggtgctcgtctcgtaaataa, gtaaggggcagcaaatccaa, tcttcagcgtggcatatata, gaattgttggcatagtgctc, ttcttccagtacaagacagg, gatcatctgaacttgcagcg, tcgtactccagatccatttc, catctacgacgtcatgttca, agttggagggcgaaatggag, ttagcggcaatttgatgtgc, gagatagatagctagcctga, gagttagggctttagagcaa, cagccgtgtttatatctaga, cttttgacagagcctgcaaa, tactggcatatccagcttaa, gtctgactttcgagtcattc, cgctttactagcttgatgtt, atctgaatacatgttagggt, attggcagttcggacactaa, ttgtgacaaggagccttttt, ctttggctctcattactcaa, taacactaggtaacgcgtgt, ggccgaaataatagcattgc, tgctgatgtacacaatcggc, gccatccatcacacacaaaa, aattcttgaggggatagcgg, ttttcttttgtttctggagc, atcaccgaaaacgttttccc, agcttctagcaagctgttaa, atcacgcgaaatgcagtatt, acagatagctcgacttatca, atacgggatagggcgttgat, aatttcttcacgatcctacc, acacgacgaaatgtgctgaa, tgcttctttgtttcgttttt, tcgcaaacgttttgcaagcg, cggctaggttatcaaaacct, atattagagcaaggggtggg, acgtacgtagatttcactta, atcaactgatcacatcgagt, tttgttccgactgagtgtat, tctgcgtgtaaagttctctt | Stellaris smFISH probes | N/A |
| IRFP670-ORF cgatcacaggatgtgaggtc, tggaacctggtatgtggata, taatgcgtactgcctgtgca, cctgcattttctgtgatgcg, tgtctcccttccaaagaatg, cgagcaattcgccaacacgg, tccgtctcgccaaaataatc, agtgcgttacggagagcgtg, cagccaaatatgagggcagg, aaaagtcctgccagtcaatc, catggcggtgcaaactgatg, cggctcaaactctatgatgc, caatgatttgccgagtcagc, atctcctccaacgacttgag, catagcctggagatagcgtg, cgaagcgatagagcatcacg, cttttagcttcgccgattac, caggaacgattccaggtcac, ttcaaatacagcagacgggc, tgtcggaaaccacacggatc, acaatgcgcgaactgattcc, aactcaaatccagagctgcg, ccaaatgacacgggcttatc, tacgcccatatttcgcaaaa, tatggacagggacatgcttg, cccacaaggtaccatctata, gttcgtagtgatggcatatt, tacacgttgcgccattggaa, aaatccgcaaacatctctgc, ggccgtgaaatgcaaggaca | Stellaris smFISH probes | N/A |
| MS2 catgaggatcacccatgtct, agtattcccgggttcattag, cctaaggtacctaattgcct | Stellaris smFISH probes | N/A |
| EGFP tcctcgcccttgctcaccat, gggcaccaccccggtgaaca, cgccgtccagctcgaccagg, ctgaacttgtggccgtttac, gccctcgccctcgccggaca, tcagcttgccgtaggtggca, gtggtgcagatgaacttcag, ccagggcacgggcagcttgc, tcagggtggtcacgagggtg, ctgaagcactgcacgccgta, cttcatgtggtcggggtagc, cggacttgaagaagtcgtgc, tggacgtagccttcgggcat, cttgaagaagatggtgcgct, gggtcttgtagttgccgtcg, ccctcgaacttcacctcggc, gatgcggttcaccagggtgt, ttgaagtcgatgcccttcag, ccccaggatgttgccgtcct, tgtagttgtactccagcttg, catgatatagacgttgtggc, tgccgttcttctgcttgtcg, cggatcttgaagttcacctt, gctgccgtcctcgatgttgt, tggtagtggtcggcgagctg, gtcgccgatgggggtgttct, ttgtcgggcagcagcacggg, ggactgggtgctcaggtagt, cgttggggtctttgctcagg, aggaccatgtgatcgcgctt, ggcggcggtcacgaactcca, tcgtccatgccgagagtgat | Stellaris smFISH probes | N/A |
| osk, nos, CycB, gcl and GFP probes were described previously | (Trcek et al., 2015) | |
| STORM smFISH Probes (5′ to 3′) | ||
| nos dSTORM3:ggtaaagctacgcgccaact (Alexa647; 5′UTR) | IDT Technologies | N/A |
| nos dSTORM4: aagcacagtttattcaactg (Alexa647; 5′UTR) | IDT Technologies | N/A |
| nos dSTORM5,7: ccaggcgctatttaaacgtt (Alexa 647; 3′UTR) | IDT Technologies | N/A |
| nos dSTORM6, 8: ttttcagaatatgtgtacac (Alexa647; 3′UTR) | IDT Technologies | N/A |
| nos dSTORM9, 10: cgagattggtggacacagtg (Alexa568; middle ORF) | IDT Technologies | N/A |
| nos dSTORM11:gtttccctttcacagaaaca (Alexa647; 3′UTR) | IDT Technologies | N/A |
| nos dSTORM12: tgatacgattgacagttcga(Alexa647; 3′UTR) | IDT Technologies | N/A |
| gcl dSTORM1: gggacagtaattacatgcgt (Alexa568; 5′UTR) (cross-correlation) | IDT Technologies | N/A |
| gcl dSTORM4: acttgtaaaactgcagttac (Alexa647; 5′UTR) | IDT Technologies | N/A |
| gcl dSTORM6: gcggatatgcttatactcga (Alexa647; 3′UTR) | IDT Technologies | N/A |
| gcl dSTORM7: cgaactgctgcgggtaaatg (Alexa647; 3′UTR) | IDT Technologies | N/A |
| gcl dSTORM8: ccgacgaatgttcagtctac (Alexa647; middle ORF) | IDT Technologies | N/A |
| gcl dSTORM9: tggaaccaaagacagcatcc (Alexa647; beginning ORF) | IDT Technologies | N/A |
| osk dSTORM1: aatcgcgcaaatgcttcact (Alexa647; 3′UTR, next to the stem loop) | IDT Technologies | N/A |
| osk dSTORM2: caataacttgcagtacgcgc (Alexa647; end of ORF) | IDT Technologies | N/A |
| osk dSTORM3: gatctgaaccaaaggcttgc (Alexa568; middle ORF) | IDT Technologies | N/A |
| osk dSTORM4: ggaattcacttgtgactgcg (Alexa568; 5′UTR) | IDT Technologies | N/A |
| osk dSTORM5: caggaaatccgtcacgttgt (Alexa647; beginning ORF) | IDT Technologies | N/A |
| osk dSTORM6: attacggccaaaatgcagca (Alexa647; 3′UTR next to the stem loop) | IDT Technologies | N/A |
| Osk dSTORM7: tacacagcttttgggatagc (Alexa647; 3′UTR) | IDT Technologies | N/A |
| CycB dSTORM3: gtttttgtatgaatgtgcga (Alexa647; 3′UTR) (cross-correlation) | IDT Technologies | N/A |
| qRT-PCR oligonucleotides (5′ to 3′) | ||
| nos Fw: acctacgtgtgccccatctg | IDT Technologies | N/A |
| nos Rv: ttccgccttgatcgcatcct | IDT Technologies | N/A |
| gcl Fw: tgactctgagccttcgacgc | IDT Technologies | N/A |
| gcl Rv: cgccagttgtccgcagattg | IDT Technologies | N/A |
| CycB Fw: cgcccactctgaccttctac | IDT Technologies | N/A |
| CycB Rv: ttggaccgcactatttcctc | IDT Technologies | N/A |
| DMN Fw: agacgcctggaagtaagcag | IDT Technologies | N/A |
| DMN Rv: gtaaggcggctcaacttgtc | IDT Technologies | N/A |
| Recombinant DNA | ||
| IRF670 | Addgene | 45457 |
| Gal4-responsive UASp promoter plasmid | (Rorth, 1998) | N/A |
| Software and Algorithms | ||
| https://www.biosearchtech.com/stellarisdesigner | LGC Biosearch technologies | N/A |
| Airlocalize spot detection algorithm | (Trcek et al., 2017, Lionnet et al., 2011) | |
| PCC(Costes) co-localization ImageJ plugin | (Bolte and Cordelieres, 2006) | JACoP ImageJ |
| Sigmaplot | www.systatsoftware.com | N/A |
| Script for pair-correlation analysis | (Veatch et al., 2012) | Matlab |
| Script for auto-correlation analysis | (Sengupta et al., 2011) | Matlab |
Dataset has been submitted: Trcek, Tatjana (2020), “nanos mRNA and Vasa:GFP”, Mendeley Data, V1, http://dx.doi.org/10.17632/h4wfnfsgw8.1
mRNA concentration and cluster abundance measurements using smFISH
Somatic mRNA concentrations were quantified as described previously (Trcek et al., 2015). In short, using iSIM, the images of smFISH-labeled embryos were acquired in 3D with a 150 nm Z step. A 3D ROI with known spatial dimensions was then chosen outside of the Vasa:GFP stain (Figure S1A,B, yellow square labeled “Soma”), which demarcated the germ plasm boundaries. The absolute number of smFISH-labeled mRNAs within the 3D ROI was determined and finally, the nM concentration of mRNAs in the 3D ROI and hence in the embryo calculated (Trcek et al., 2015, Trcek et al., 2017). We observed a high correlation between the relative expression levels determined by RNA-sequencing(mod et al., 2010) and the somatic concentration determined by smFISH for the mRNAs tested (R2=0.85; Figure S1E,F), indicating that smFISH quantified mRNA concentrations over a wide gene expression range, as demonstrated previously (Trcek et al., 2015, Trcek et al., 2017).
Because of the high somatic CycB expression, smFISH could not be used to quantify somatic concentration of this mRNA reliably and we instead estimated it. As noted above, we observed a linear relationship between the mRNA concentration determined by smFISH and the mRNA levels determined by RNA-seq (Figure S1E, formula). Using this linear relationship and CycB mRNA levels determined by RNA-seq (Flybase (mod et al., 2010)), we extrapolated the somatic concentration of CycB mRNA to be 12.46 nM (Table S1, marked as *).
To determine the mRNA concentration in the germ plasm (GP), we analyzed a 3D ROI with known spatial dimensions demarcated by the Vasa:GFP stain, which identified the germ granule-bound mRNAs (Figure S1A,B, yellow square labeled “GP” (germ plasm)). The number of smFISH-labeled spots per 3D GP ROI was then determined and their intensity normalized by the intensity of a single smFISH-labeled mRNA located in the soma to determine the absolute number of transcripts per 3D ROI (Trcek et al., 2015, Trcek et al., 2017). The spatial dimensions of the 3D ROI were then used to calculate the nM mRNA concentration in GP (Table S1).
To determine the abundance of mRNA clusters (average number of mRNAs per homotypic cluster) in germ granules, we analyzed a 3D ROI as described above. For each embryo, we measured the number of smFISH-labeled mRNA clusters within the 3D GP ROI and afterwards determined the number of mRNAs in each cluster by normalizing its fluorescent intensity to the intensity of a single smFISH-labeled mRNA located in the somatic regions of the embryo (Figure S1A,B, yellow square labeled “Soma”) (Trcek et al., 2015, Trcek et al., 2017). At least three embryos per mRNA were analyzed this way, and the average mRNA cluster abundance among all analyzed clusters determined.
Importantly, we found that up to the periphery of the germ plasm, where germ granules meet the somatic regions of the embryo, the number of Vasa:GFP granules increased linearly with the size of 3D ROIs (Figure S1C,D, magenta dotted line in D) indicating that germ granules are homogenously distributed within the germ plasm. Therefore, the mRNA concentration in germ plasm and the average abundance of mRNA clusters were invariant of the size of the 3D ROIs.
To determine the abundance of CycB clusters in GP, we first estimated the total fluorescence intensity of a single somatic CycB mRNA. We calculated the somatic concentration of CycB as described above and afterwards estimated the number of CycB molecules per 3D ROI of known spatial dimensions in the soma. We then quantified the total fluorescence intensity of somatic CycB within a 3D ROI and divided this number by the number of CycB within this ROI to obtain an estimate for the fluorescence intensity of a single somatic CycB. This value was then used to normalize the fluorescence intensity of CycB smFISH labeled mRNA spots in the GP to determine the concentration of CycB and CycB cluster abundance in the germ plasm.
Correlating mRNA properties with the position of mRNA clusters in germ granules
To determine which mRNA property best predicted the position of clusters within granules, we correlated several mRNA parameters with the spatial position of homotypic clusters within Vasa:GFP-labeled granules, we used the PCC(Costes) approach (Trcek et al., 2015). We found that timing of translational onset in germ granules (Rangan et al., 2009), susceptibility to mRNA decay in the somatic regions of the embryo (Thomsen et al., 2010), and length of their 3′UTR (mod et al., 2010) had little effect on cluster positioning. For example, mRNAs that were translationally repressed in granules were as likely positioned at the edge of the granule (gcl) as in the center of the granules (CycB) ((Trcek et al., 2015). To directly address whether the translational status of a localized mRNA alters its position in the granule, we made use of the known function of Nanos protein as a translational repressor of CycB mRNA translation. In the absence of Nos, localized CycB becomes precociously translated (Kadyrova et al., 2007, Sonoda and Wharton, 2001), however, the position of CycB clusters in Vasa:GFP-labeled granules did not change (Figure S2C,D). This supports the conclusion that the translational status of mRNAs within granules does not inform cluster position. This result also indicates that the removal of even abundant RNA clusters such as nos did not affect the spatial position of other mRNAs clusters.
Next we found that an mRNA’s translational efficiency in the cytoplasm of oocytes, during the period when mRNAs enrich into granules, and at the end of oogenesis, when mRNA enrichment to granules is complete, is correlated negatively with the positioning of mRNA clusters (Kronja et al., 2014, Little et al., 2015). These measurements of mRNAs in heavy polysomal fractions largely account for the RNA pool that will end up in the somatic region of the embryo as germ granule mRNAs only account for approximately 3% of total mRNA (Trcek et al., 2015, Bergsten and Gavis, 1999) and suggested that mRNAs that translated less efficiently in the oocyte cytoplasm tend to position more centrally within granules, while those that translated more efficiently positioned towards the granule periphery (Figure S2A). Similarly, mRNA length had a moderately negative effect on position, with longer mRNAs showing a preference to be positioned at the edge of the granule (Figure S2A). Finally, we found that the best predictor of mRNA cluster position in Drosophila germ granules were the somatic concentration of a transcript in the embryo, its germ plasm (GP) concentration in the embryo, fold enrichment (the ratio between the GP and the somatic mRNA concentration in the embryo), and mRNA cluster abundance (see main text and Figure 1G, S2A,B).
Correlating mRNA cluster abundance with the abundance of granule proteins
To generate correlations between smFISH-labeled mRNAs and fluorescently-tagged proteins, two-dimensional ROIs within germ plasm from each fluorescent channel were analyzed using Airlocalize, which generated an array of XY coordinates and the fluorescent intensity occurring at each coordinate for both channels (two arrays total, one per channel) (Trcek et al., 2017). The distances between all coordinates across these two arrays were calculated, and coordinates that occurred within 400nm of each other were identified as occurring within the same granule. The intensities of any two coordinates co-localizing to the same granule were paired together. Coordinates that did not co-localize to the same granule with any spot from the alternative channel were paired with a zero intensity value for the other channel. Finally, a Pearson’s correlation was calculated between all paired intensity values between the two channels, producing a measure of how granule components correlated with each other per granule across all granules within a germ plasm ROI. Our analysis revealed that only 44.2% of the variability in the abundance of nos clusters could be explained by the variability in Osk protein abundance (Figure 1E; S1G), an RBP previously implicated in recruitment of nos to germ granules (Yang et al., 2015, Jeske et al., 2015). This correlation was even smaller when the nos cluster abundance was correlated with the abundance of Vasa and Tud and negative when correlated with the abundance of Aub (Figure 1F; S1G). Notably, the correlation between nos and Vasa was nearly equivalent to the correlation between pgc and gcl mRNA clusters, two mRNAs that enrich in granules independently of one another (Figure S1G).
Quantifying co-localization using PCC(Costes)
Co-localization between overlapping mRNA clusters and Vasa:GFP granules and mRNA clusters labeled with spectrally-distinct fluorophores was quantified in 3D ROIs using the Pearson Correlation Coefficient-Costes approach (here termed PCC(Costes)), as previously described (Trcek et al., 2015). PCC(Costes) measures co-localization by determining how fluorescence intensities of labeled objects spatially correlate with each other within the overlap and afterwards statistically evaluates the significance of this co-localization (Costes et al., 2004, Bolte and Cordelieres, 2006). PCC(Costes) of 1.0 indicates perfect, non-random co-localization while PCC(Costes) of ~0.0 indicates that two objects labeled with spectrally-distinct dyes co-localize with each other by chance (Costes et al., 2004, Bolte and Cordelieres, 2006). PCC(Costes) is insensitive to the frequency and the duration of overlap between objects and therefore measures co-localization even in crowded cellular environments such as germ plasm (Trcek et al., 2015, Costes et al., 2004, Bolte and Cordelieres, 2006). To establish PCC(Costes) as a method to analyze co-localization further, we have previously employed a spot detection algorithm to quantify nm distance between the centers of mass of overlapping objects labeled with spectrally distinct fluorophores (Trcek et al., 2015). We demonstrated that as the PCC(Costes) coefficient decreases, the distance between overlapping objects labeled with spectrally distinct colors increases indicating that the two objects co-localize at a greater yet fixed spatial distance relative to each other (Trcek et al., 2015, Bolte and Cordelieres, 2006, Costes et al., 2004). Additionally, we have previously determined the upper limit of co-localization detection using the double-labeled pgc mRNA and measured a high PPC(Costes) of 0.90±0.004 and a distance of 33.1 ± 5.1 nm (Trcek et al., 2015). We detected a similar co-localization for Osk:GFP and Vasa:KuOr proteins (PCC(Costs) of 0.93±0.01; distance of 30.6±4.3 nm(Trcek et al., 2015)) and therefore concluded that Oskar and Vasa were homogeneously mixed within granules. In contrast, a non-granule-enriched ccr4 mRNA randomly co-localized with Vasa:GFP-labeled germ granules with a PCC(Costs) of 0.04±0.02 and at a spatial distance of 408.5 ± 31.4 nm (Table S1 (Trcek et al., 2015)), indicating that ccr4 randomly co-localizes with germ granules and was not a granule component (Trcek et al., 2015).
To determine how endogenous nos co-localizes with itself and determine the upper limit of co-localization detection using PCC(Costes) in Figure 3A, we prepared two sets of nos probes that had the same sequences but were labeled with either green or red probes. We mixed them together and labeled the nos mRNA clusters concurrently and therefore variably. This approach best simulated the variability that can arise due to different mRNA composition of different chimeras in vivo. Nevertheless, in this control, we achieved high PCC(Costes) of 0.84+/−0.01 indicating that the variability in probe hybridization or probe on and off rates add minimally to the noise of the measurements and do not obscure the underlying spatial organization of the molecules within clusters.
Quantifying total embryonic mRNA levels using qRT-PCR
To determine total embryonic mRNA levels using qRT-PCR, caged flies were provided with a fresh apple juice plate containing a dollop of fresh yeast paste and allowed to lay eggs for 1.5h hours. After dechorionation, the embryos were resuspended in 100 μl of 1XPBS and 800 μl TRIzol reagent (Invitrogen; 15596018) and broken up using a pellet pestle motor (Kimble Kontes). The RNA was then extracted with the acid-phenol:chloroform (Invitrogen; AM9720), followed by one extraction with chloroform (Fisher; AC40463–5000) and one extraction with isopropanol (Fisher Scientific; A416–500) (Kohrer and Domdey, 1991). The RNA was precipitated to the bottom of the tube during a 15 min spin at maximal speed at 4°C. Isopropanol was then removed, the RNA was washed once with 70% ethanol, air-dried and resuspended in nuclease free water. DNA contaminations were removed with RQ1 RNase-Free DNase (Promega; M6101). Afterwards, the RNA was re-purified using the acid-phenol:chloroform extraction, as described above. 5 μg of total RNA was transcribed into cDNA in the reverse transcriptase reaction using Oligo dT(20) primer (Thermo Fisher Scientific; 18418020) and the SuperScript® III Reverse Transcriptase (Life Technologies; 18080–044). Relative differences in mRNA concentrations we determined using a comparative CT method for relative quantification by RT-PCR (Livak and Schmittgen, 2001). SYBR Green was used as a reporter dye (Roche; 04707516001). The reactions were set up in 384-well qRT-PCR plates (Roche; 04729749001) in three biological and four technical replicates for each RNAi condition and for each gene. qRT-PCR was performed using the Roche LightCycler® 480 II system and a standard qRT-PCR program. The reaction was carried out using 1 μl of cDNA (1:3 dilution), and 300 nM of Fw and Rv and 5 μl SYBR Green PCR mix. Dissociation curves generated through a thermal denaturation step were used to verify amplification specificity. A sample with no reverse transcriptase was used as a negative control. DMN gene was used to normalize RNA levels. The gene specific primers used in the qRT-PCR reaction are provided in the Key resources table. Transcription of the GAL4 inducer, which triggered expression of the RNAi against CycB, nos or mCherry, was achieved by the maternal alpha tubulin promoter (mat-alpha) (Staller et al., 2013).
Fluorescence recovery after photobleaching (FRAP)
FRAP was used to evaluate the kinetics of exchange of fluorescently-tagged granule components Osk, Vasa, Aub and Tud and nos mRNA with the intergranular space. To detect nos mRNA in FRAP experiments, we used a nos transgene that was genetically engineered with 18 tandemly-repeated RNA loops derived from the MS2 bacteriophage (Brechbiel and Gavis, 2008). These repeats accommodate co-transcriptional labeling of the RNA with the GFP-tagged MS2 coat protein (MCP-GFP) (Bertrand et al., 1998, Tutucci et al., 2018), allowing nos detection in live embryos (Figure 2B,C) (Sinsimer et al., 2013). nos-MS2-MCP-GFP mRNA forms clusters of similar abundance as WT nos (Figure S3A) and rescues the nos null phenotype (see below) (Forrest et al., 2004) indicating that the essential features of nos regulation are recapitulated on this chimeric mRNA. Live embryos were prepared and mounted onto an imaging chamber as described before (Kistler et al., 2018). Per embryo, a 3μm × 3μm 2D ROI was photo-bleached in the middle of the germ plasm, where germ granules were homogeneously distributed within the germ plasm (Figure S1C,D). Since germ granules are too small to allow photo-bleaching of individual granules, we instead photo-bleached a larger ROI in the middle of the germ plasm, where germ granules are homogeneously distributed within the germ plasm (Figure S1C,D). We have previously shown that up to 7% of Osk and Vasa resides in the intergranular space, that approximately 40% of Osk and Vasa found in granules exchange with the intergranular space and that the rest does not exchange and remains associated within granules (Kistler et al., 2018). Thus, we anticipated capturing the behavior of at least three populations of fluorescently-tagged molecules with distinct kinetics of fluorescence recovery (Figure S3B); population P1 located in the intergranular space with fast mobility (M1), population P2 located within granules that is exchanging with the intergranular space with a slow mobility (M2) and population P3 found within granules that does not exchange with the intergranular space (immobile fraction). To capture the kinetic of exchange of three populations of fluorescently-tagged molecules with distinct kinetics of fluorescence recovery (Figure 3B), the FRAP recovery curves were first normalized as described before (Kistler et al., 2018, Brangwynne et al., 2009, Rapsomaniki et al., 2012). Afterwards, the recovery curves were fit to a two-term exponential equation (f(t) = a*(1-exp(−b*t))+ c*(1-exp(−d*t))) using Sigmaplot (www.systatsoftware.com), where a and c represented the percent mobile fractions (Figure S3B, Table S2, population P1 and P2), and b and d represented the rate constants of fluorescence recovery. b and d were then used to calculate the half time to full fluorescence recovery t1/2 (s) using the equation b=ln(2)/t1/2 (Figure S3B, Table S2, mobility M1 and M2) (Rapsomaniki et al., 2012, Brangwynne et al., 2009, Kistler et al., 2018). To statistically evaluate, whether a two-term exponential equation was more appropriate to describe our FRAP data than a single-term exponential equation ((f(t) = a*(1-exp(−b*t))) (Kistler et al., 2018), we used both the F-test and the Normality Test (Shapiro-Wilk). By doing so, we determined that the FRAP recoveries of Osk:GFP, Vasa:GFP, Aub:GFP, Tud:GFP and nos-MS2-MCP-GFP were best described using a two-term exponential equation while the FRAP recoveries of a GFP and MCP-GFP expressed in the absence of the MS2-tagged nos, were best described using a single-term exponential equation (Table S2).
Hybridizing cut embryos with smFISH probes for imaging with STORM
Vasa:GFP-expressing embryos were fixed, devitellinized and stored in 100% methanol at 4°C until needed, as described previously (Trcek et al., 2017). Afterwards, embryos were rehydrated as described before (Trcek et al., 2017). Afterwards they were resuspended in 1X phosphate-buffered saline (PBS) and laid into plastic disposable molds (Fisherbrand; 22-363-552) to achieve a single and uniform layer of embryos at the bottom of the mold. The buffer was then removed and the molds with the embryos filled with the tissue freezing medium (General Data; TFM-C). Embryos were frozen immediately on dry ice. Frozen and embedded embryos were then cut with a 10 μm Z step using a cryostat (Leica; CM 3050 S) at −19°C using pre-chilled low profile blades (Accu-Edge; 4689). Each cut slice was affixed onto microscope coverslips coated with the Poly-L-lysine solution (Sigma; P4707–50ml) and steeped in 1XPBS for 5 min. Afterwards, the cut embryos were post-fixed with 4% paraformaldehyde (Trcek et al., 2017) for 5 minutes, washed once with 1XPBS (5 min) and hybridized with smFISH probes or stored O/N at 4°C and used the next day. Freezing and cutting of the embryos did not alter how CycB and gcl mRNA clusters co-localized with Vasa:GFP granules (Figure S4F), indicating that the spatial dimensions of germ granules were preserved during sample preparation. Cut embryo slices attached onto the coverslips were steeped into a pre-hybridization solution containing 10% deionized formamide and 1X saline-sodium citrate (SSC) buffer (Trcek et al., 2017) for 15 min. Afterwards, coverslips were placed face-down onto 30 μl of hybridization solution containing smFISH probes (see below) within the hybridization chamber. This chamber comprised of a 10 cm petri dish, a parafilm strip placed into the petri dish and a falcon tube cap filled with the pre-hybridization solution to humidify the chamber (Trcek et al., 2012). The chamber was sealed with parafilm, and into a 37°C incubator for 3h shielded from light. Afterwards, the coverslips were washed twice with pre-hybridization solution pre-warmed to 37°C for 15 min, followed by two 5 min washes with 1XPBS at RT.
To prepare the smFISH-containing hybridization solution for STORM imaging, the same protocol was used as described previously (Trcek et al., 2017), but instead of a mix of probes designed to hybridize along the entire length of the mRNA target, a single smFISH probe covalently modified at its 5′ end with Alexa 568 or Alexa647 photoswitchable dye was used per mRNA. These probes were custom design using the Stellaris designer (Biosearch Technologies) and then fluorescently-modified and purchased form Integrated DNA Technologies (Key resources table).
Imaging of smFISH-labeled samples using STORM
Hybridized samples were mounted onto a perforated microscope slide and sealed with Epoxy (Devcon; 00470740). Samples were then perfused with 100 μl of 1XPBS containing 10% glucose and 1 μl of Gloxy anti-bleach solution (100 μl of Gloxy contains 80 μl 1XPBS, 20 μl Catalase (Sigma-Aldrich; C3115–50MG) and 10 mg Glucose Oxidase (Sigma-Aldrich; G2133–10KU)). Per embryo, 2000 images were acquired in 2D at the rate of 30 Hertz.
We individually hybridized mRNAs with smFISH probes coupled with photoswitchable Alexa568 or Alexa647 dyes (Key resources table), while Vasa:GFP continued to demarcate the position of germ granule-bound mRNA clusters (Figure S4A–D, magenta ROI). After image acquisition (Video S1), particles were detected and images reconstructed (Figure S4E). Our imaging approach revealed bright, distinct and frequent photoswitchable events of fluorescently-labeled probes (Video S1) indicating that after the initial inactivation of fluorophores, a sparse subset of dyes was activated into a fluorescent state thereby markedly increasing our ability to determine precise positions of smFISH-hybridized mRNAs with low localization uncertainty. An ROI coinciding with the Vasa:GFP label was cropped from reconstructed images (Figure S4E, magenta ROI) and analyzed to plot the distances among all detected fluorescently-labeled mRNAs (Figure S4D,E, black dots). Since clusters do not form outside of germ granules and the vast majority of clusters contained more than one mRNA (Table S1,6), the plot of distances represented the spatial dimensions of homotypic mRNA clusters. By fitting this data to an auto-correlation function (Sengupta et al., 2011) (Figure S4E, violet line), we extracted the radii (in nm) of mRNA clusters reported by a particular smFISH probe. Notably, the radii represented the physical size of an mRNA cluster rather than the physical dimension of a trans RNA:RNA interaction. Finally, we further validate STORM as a method to detect the spatial organization of clustered mRNAs by investigated the co-localization of CycB clusters with gcl clusters (Figure S4J–L). Using cross-correlation analysis (Veatch et al., 2012) we determined that the mean distance between CycB and gcl clusters in germ granules to be 134.9±90.3 nm (Figure S4L), a distance similar to our previous measurement of 78.9±8.1 obtained with smFISH and iSIM approach (Trcek et al., 2015), confirming that CycB and gcl mRNAs organized homotypic clusters within the same germ granule.
Importantly, this distance was shorter than the 250 nm radius of the granule observed by EM (Arkov et al., 2006, Mahowald, 2001), yet longer than the average radius of 62.8±2.3 nm and 35.4±2.3 nm for CycB and gcl mRNA clusters, respectively (Figure S4L). This result indicated that CycB and gcl mRNAs do not mix with each other within the same granule, but instead occupy distinct positions, confirming our previous measurements (Figure 1G) (Trcek et al., 2015). Finally, we observed a flat cross-correlation curve for CycB and gcl mRNAs in the somatic regions of the embryo indicating that in the soma, all detected distances among these two mRNAs were equally likely (Figure S4K). Therefore, outside of germ granules, CycB and gcl randomly co-localized with each other, consistent with previous observations (Trcek et al., 2015).
Single-Molecule Localization
For STORM taken via the sCMOS camera, the single-molecule localization procedure was carried out as previously described (Yin et al., 2019). Briefly, raw STORM images were box-filtered with a box size of 4x FWHM of a 2D PSF, and with each pixel within the box weighted by the inverse of its variance (Huang et al., 2013). A 9×9 pixel regions around each local maximum of the filtered images were then submitted for single-molecule localization. The single-molecule localization was achieved by fitting each single PSF into a 2D Gaussian profile via Maximum Likelihood Estimation (MLE), and the fitting accuracy was determined by the Cramér-Rao Lower Bound (CRLB) when the fitting reached the termination criteria (p = 0.05). Note that the read-out noise of each pixel was considered as a normal distribution with its offset, variance, and gain pre-calibrated (Huang et al., 2013). The centers of the single-molecule localizations that appeared in consecutive frames within 2.5x localization precisions were considered as one blinking event. The resulting coordinates of the single-molecule centers were then submitted for Auto-Pair-Correlation analyses.
Pair-Correlation Analysis
Binary images ICHx(r) were submitted to a custom-written Matlab script for pair-correlation analysis. The pair-correlation function was computed as (Veatch et al., 2012):
where and denotes Fourier and inverse Fourier transform, respectively. * denotes complex conjugation; ρCHx denotes the average density (number of localizations / image size) from channel x; and N is the normalization factor (Veatch et al., 2012). Note that the auto-correlation was computed by taking CH1 and CH2 as the same channel.
The auto-correlation was fitted as (Sengupta et al., 2011)
where σ denotes the localization accuracy; 〈ρ〉 denotes the average density of molecules after over-counting correction; rapp denotes the apparent average radius of the clusters; and A denotes the compaction factor. The average molecular content of each cluster 〈N〉 was calculated as
The cross-correlation was fitted as
where dapp denotes the apparent average distance between correlation pairs.
QUANTIFICATION AND STATISTICAL ANALYSIS
Representative images are shown for all experiments. All experiments were repeated at least three independent times unless indicated otherwise. Statistical analysis was performed using Sigmaplot. Two-tailed Student t-test was used to determine significance.
Supplementary Material
Highlights.
Germ granule-enriched mRNA organization was probed by super-resolution imaging in vivo
Many mRNAs from the same gene self-assemble into homotypic clusters in germ granules
Homotypic self-assembly relies on properties of an mRNP but not specific RNA sequences
Sequence-specific trans RNA:RNA interactions among clustered mRNAs are not detected
ACKNOWLEDGEMENTS
We thank Alexey Soshnev for preparation of the graphical abstract, Harshad Vishwasrao and Jiji Chen of the trans-NIH advanced imaging and microscopy facility for their assistance with iSIM and Benjamin Lin for help with cloning. This work was supported by the Howard Hughes Medical Institute, where RL is an Investigator, by K99HD088675 awarded to TT, the Intramural Research Programs of the US NIBIB, by R01 GM067758 awarded to E.R.G, and NIH training grant T32 GM007388 awarded to W.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
HS receives royalties, as co-inventor, from sales of instant SIM by Visitech. The NIH, its officers, and staff, do not recommend or endorse any company, product, or service.
REFERENCES
- ARKOV AL, WANG JY, RAMOS A & LEHMANN R 2006. The role of Tudor domains in germline development and polar granule architecture. Development, 133, 4053–62. [DOI] [PubMed] [Google Scholar]
- BERGSTEN SE & GAVIS ER 1999. Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development, 126, 659–69. [DOI] [PubMed] [Google Scholar]
- BERTRAND E, CHARTRAND P, SCHAEFER M, SHENOY SM, SINGER RH & LONG RM 1998. Localization of ASH1 mRNA particles in living yeast. Mol Cell, 2, 437–45. [DOI] [PubMed] [Google Scholar]
- BOLTE S & CORDELIERES FP 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc, 224, 213–32. [DOI] [PubMed] [Google Scholar]
- BRANGWYNNE CP, ECKMANN CR, COURSON DS, RYBARSKA A, HOEGE C, GHARAKHANI J, JULICHER F & HYMAN AA 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science, 324, 1729–32. [DOI] [PubMed] [Google Scholar]
- BRECHBIEL JL & GAVIS ER 2008. Spatial regulation of nanos is required for its function in dendrite morphogenesis. Curr Biol, 18, 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREITWIESER W, MARKUSSEN FH, HORSTMANN H & EPHRUSSI A 1996. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev, 10, 2179–88. [DOI] [PubMed] [Google Scholar]
- CINALLI RM & LEHMANN R 2013. A spindle-independent cleavage pathway controls germ cell formation in Drosophila. Nat Cell Biol, 15, 839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEVER JL, WONG ML & PARSLOW TG 1996. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J Virol, 70, 5902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTES SV, DAELEMANS D, CHO EH, DOBBIN Z, PAVLAKIS G & LOCKETT S 2004. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J, 86, 3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURD A, CLEASBY A, MAKOWSKA K, YORK A, SHROFF H & PECKHAM M 2015. Construction of an instant structured illumination microscope. Methods, 88, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE WVI, YEBOAH-KORDIEH DK, NIEPIELKO MG & GAVIS ER 2018. Distinct cis-acting elements mediate targeting and clustering of Drosophila polar granule mRNAs. Development, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS KA, DEMSKY M, MONTAGUE RA, WEYMOUTH N & KIEHART DP 1997. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol, 191, 103–17. [DOI] [PubMed] [Google Scholar]
- EISENBERG H & FELSENFELD G 1967. Studies of the temperature-dependent conformation and phase separation of polyriboadenylic acid solutions at neutral pH. J Mol Biol, 30, 17–37. [DOI] [PubMed] [Google Scholar]
- ENO C, HANSEN CL & PELEGRI F 2019. Aggregation, segregation, and dispersal of homotypic germ plasm RNPs in the early zebrafish embryo. Dev Dyn, 248, 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRANDON D, KOCH I, WESTHOF E & NUSSLEIN-VOLHARD C 1997. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3’ UTR-STAUFEN ribonucleoprotein particles. EMBO J, 16, 1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES A & LEHMANN R 1998. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development, 125, 679–90. [DOI] [PubMed] [Google Scholar]
- FORREST KM, CLARK IE, JAIN RA & GAVIS ER 2004. Temporal complexity within a translational control element in the nanos mRNA. Development, 131, 5849–5857. [DOI] [PubMed] [Google Scholar]
- FORREST KM & GAVIS ER 2003. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol, 13, 1159–68. [DOI] [PubMed] [Google Scholar]
- GAVIS ER, CURTIS D & LEHMANN R 1996. Identification of cis-acting sequences that control nanos RNA localization. Dev Biol, 176, 36–50. [DOI] [PubMed] [Google Scholar]
- GUNKEL N, YANO T, MARKUSSEN FH, OLSEN LC & EPHRUSSI A 1998. Localization-dependent translation requires a functional interaction between the 5 ‘ and 3 ‘ ends of oskar mRNA. Genes & Development, 12, 1652–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG F, HARTWICH TM, RIVERA-MOLINA FE, LIN Y, DUIM WC, LONG JJ, UCHIL PD, MYERS JR, BAIRD MA, MOTHES W, DAVIDSON MW, TOOMRE D & BEWERSDORF J 2013. Video-rate nanoscopy using sCMOS camera-specific single-molecule localization algorithms. Nat Methods, 10, 653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIN A & VALE RD 2017. RNA phase transitions in repeat expansion disorders. Nature, 546, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMBOR H, BRUNEL C & EPHRUSSI A 2011. Dimerization of oskar 3’ UTRs promotes hitchhiking for RNA localization in the Drosophila oocyte. RNA, 17, 2049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMBOR H, SURENDRANATH V, KALINKA AT, MEJSTRIK P, SAALFELD S & TOMANCAK P 2015. Systematic imaging reveals features and changing localization of mRNAs in Drosophila development. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESKE M, BORDI M, GLATT S, MULLER S, RYBIN V, MULLER CW & EPHRUSSI A 2015. The Crystal Structure of the Drosophila Germline Inducer Oskar Identifies Two Domains with Distinct Vasa Helicase- and RNA-Binding Activities. Cell Rep, 12, 587–98. [DOI] [PubMed] [Google Scholar]
- KADYROVA LY, HABARA Y, LEE TH & WHARTON RP 2007. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development, 134, 1519–27. [DOI] [PubMed] [Google Scholar]
- KHONG A, MATHENY T, JAIN S, MITCHELL SF, WHEELER JR & PARKER R 2017. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol Cell, 68, 808–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMHA J, KERR K & MACDONALD PM 1995. Translational Regulation of Oskar Messenger-Rna by Bruno, an Ovarian Rna-Binding Protein, Is Essential. Cell, 81, 403–412. [DOI] [PubMed] [Google Scholar]
- KIRINO Y, VOUREKAS A, SAYED N, DE LIMA ALVES F, THOMSON T, LASKO P, RAPPSILBER J, JONGENS TA & MOURELATOS Z 2010. Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. RNA, 16, 70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISTLER KE, TRCEK T, HURD TR, CHEN R, LIANG FX, SALL J, KATO M & LEHMANN R 2018. Phase transitioned nuclear Oskar promotes cell division of Drosophila primordial germ cells. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHRER K & DOMDEY H 1991. Preparation of high molecular weight RNA. Methods Enzymol, 194, 398–405. [DOI] [PubMed] [Google Scholar]
- KRONJA I, YUAN B, EICHHORN SW, DZEYK K, KRIJGSVELD J, BARTEL DP & ORR-WEAVER TL 2014. Widespread changes in the posttranscriptional landscape at the Drosophila oocyte-to-embryo transition. Cell Rep, 7, 1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGDON EM, QIU Y, GHANBARI NIAKI A, MCLAUGHLIN GA, WEIDMANN CA, GERBICH TM, SMITH JA, CRUTCHLEY JM, TERMINI CM, WEEKS KM, MYONG S & GLADFELTER AS 2018. mRNA structure determines specificity of a polyQ-driven phase separation. Science, 360, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMANN R 2016. Germ Plasm Biogenesis-An Oskar-Centric Perspective. Essays on Developmental Biology, Pt A, 116, 679-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIONNET T, CZAPLINSKI K, DARZACQ X, SHAV-TAL Y, WELLS AL, CHAO JA, PARK HY, DE TURRIS V, LOPEZ-JONES M & SINGER RH 2011. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods, 8, 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE SC, SINSIMER KS, LEE JJ, WIESCHAUS EF & GAVIS ER 2015. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat Cell Biol, 17, 558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU H, WANG JY, HUANG Y, LI Z, GONG W, LEHMANN R & XU RM 2010. Structural basis for methylarginine-dependent recognition of Aubergine by Tudor. Genes Dev, 24, 1876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVAK KJ & SCHMITTGEN TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402–8. [DOI] [PubMed] [Google Scholar]
- MAHOWALD AP 2001. Assembly of the Drosophila germ plasm. Int Rev Cytol, 203, 187–213. [DOI] [PubMed] [Google Scholar]
- MARQUET R, BAUDIN F, GABUS C, DARLIX JL, MOUGEL M, EHRESMANN C & EHRESMANN B 1991. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res, 19, 2349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOD EC, ROY S, ERNST J, KHARCHENKO PV, KHERADPOUR P, NEGRE N, EATON ML, LANDOLIN JM, BRISTOW CA, MA L, LIN MF, WASHIETL S, ARSHINOFF BI, AY F, MEYER PE, ROBINE N, WASHINGTON NL, DI STEFANO L, BEREZIKOV E, BROWN CD, CANDEIAS R, CARLSON JW, CARR A, JUNGREIS I, MARBACH D, SEALFON R, TOLSTORUKOV MY, WILL S, ALEKSEYENKO AA, ARTIERI C, BOOTH BW, BROOKS AN, DAI Q, DAVIS CA, DUFF MO, FENG X, GORCHAKOV AA, GU T, HENIKOFF JG, KAPRANOV P, LI R, MACALPINE HK, MALONE J, MINODA A, NORDMAN J, OKAMURA K, PERRY M, POWELL SK, RIDDLE NC, SAKAI A, SAMSONOVA A, SANDLER JE, SCHWARTZ YB, SHER N, SPOKONY R, STURGILL D, VAN BAREN M, WAN KH, YANG L, YU C, FEINGOLD E, GOOD P, GUYER M, LOWDON R, AHMAD K, ANDREWS J, BERGER B, BRENNER SE, BRENT MR, CHERBAS L, ELGIN SC, GINGERAS TR, GROSSMAN R, HOSKINS RA, KAUFMAN TC, KENT W, KURODA MI, ORR-WEAVER T, PERRIMON N, PIRROTTA V, POSAKONY JW, REN B, RUSSELL S, CHERBAS P, GRAVELEY BR, LEWIS S, MICKLEM G, OLIVER B, PARK PJ, CELNIKER SE, HENIKOFF S, KARPEN GH, LAI EC, MACALPINE DM, STEIN LD, WHITE KP & KELLIS M 2010. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science, 330, 1787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE MD & HU WS 2009. HIV-1 RNA dimerization: It takes two to tango. AIDS Rev, 11, 91–102. [PMC free article] [PubMed] [Google Scholar]
- NEMOZ C, ROPARS V, FRIT P, GONTIER A, DREVET P, YU J, GUEROIS R, PITOIS A, COMTE A, DELTEIL C, BARBOULE N, LEGRAND P, BACONNAIS S, YIN Y, TADI S, BARBET-MASSIN E, BERGER I, LE CAM E, MODESTI M, ROTHENBERG E, CALSOU P & CHARBONNIER JB 2018. XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat Struct Mol Biol, 25, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEPIELKO MG, EAGLE WVI & GAVIS ER 2018. Stochastic Seeding Coupled with mRNA Self-Recruitment Generates Heterogeneous Drosophila Germ Granules. Curr Biol, 28, 1872–1881 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAE J, CINALLI RM, MARZIO A, PAGANO M & LEHMANN R 2017. GCL and CUL3 Control the Switch between Cell Lineages by Mediating Localized Degradation of an RTK. Dev Cell, 42, 130–142 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITCHIAYA S, MOURAO MDA, JALIHAL AP, XIAO L, JIANG X, CHINNAIYAN AM, SCHNELL S & WALTER NG 2019. Dynamic Recruitment of Single RNAs to Processing Bodies Depends on RNA Functionality. Mol Cell, 74, 521–533 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANGAN P, DEGENNARO M, JAIME-BUSTAMANTE K, COUX RX, MARTINHO RG & LEHMANN R 2009. Temporal and spatial control of germ-plasm RNAs. Curr Biol, 19, 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPSOMANIKI MA, KOTSANTIS P, SYMEONIDOU IE, GIAKOUMAKIS NN, TARAVIRAS S & LYGEROU Z 2012. easyFRAP: an interactive, easy-to-use tool for qualitative and quantitative analysis of FRAP data. Bioinformatics, 28, 1800–1. [DOI] [PubMed] [Google Scholar]
- ROOVERS EF, KAAIJ LJT, REDL S, BRONKHORST AW, WIEBRANDS K, DE JESUS DOMINGUES AM, HUANG HY, HAN CT, RIEMER S, DOSCH R, SALVENMOSER W, GRUN D, BUTTER F, VAN OUDENAARDEN A & KETTING RF 2018. Tdrd6a Regulates the Aggregation of Buc into Functional Subcellular Compartments that Drive Germ Cell Specification. Dev Cell, 46, 285–301 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RORTH P 1998. Gal4 in the Drosophila female germline. Mech Dev, 78, 113–8. [DOI] [PubMed] [Google Scholar]
- ROUNDTREE IA, EVANS ME, PAN T & HE C 2017. Dynamic RNA Modifications in Gene Expression Regulation. Cell, 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUST MJ, BATES M & ZHUANG X 2006. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods, 3, 793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGHAVI P, LAXANI S, LI X, BULLOCK SL & GONSALVEZ GB 2013. Dynein Associates with oskar mRNPs and Is Required For Their Efficient Net Plus-End Localization in Drosophila Oocytes. Plos One, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENGUPTA P, JOVANOVIC-TALISMAN T, SKOKO D, RENZ M, VEATCH SL & LIPPINCOTT-SCHWARTZ J 2011. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods, 8, 969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSIMER KS, LEE JJ, THIBERGE SY & GAVIS ER 2013. Germ plasm anchoring is a dynamic state that requires persistent trafficking. Cell Rep, 5, 1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONODA J & WHARTON RP 2001. Drosophila Brain Tumor is a translational repressor. Genes Dev, 15, 762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALLER MV, YAN D, RANDKLEV S, BRAGDON MD, WUNDERLICH ZB, TAO R, PERKINS LA, DEPACE AH & PERRIMON N 2013. Depleting gene activities in early Drosophila embryos with the “maternal-Gal4-shRNA” system. Genetics, 193, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBER D, TAUBER G, KHONG A, VAN TREECK B, PELLETIER J & PARKER R 2020. Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSEN S, ANDERS S, JANGA SC, HUBER W & ALONSO CR 2010. Genome-wide analysis of mRNA decay patterns during early Drosophila development. Genome Biol, 11, R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRCEK T, CHAO JA, LARSON DR, PARK HY, ZENKLUSEN D, SHENOY SM & SINGER RH 2012. Single-mRNA counting using fluorescent in situ hybridization in budding yeast. Nat Protoc, 7, 408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRCEK T, GROSCH M, YORK A, SHROFF H, LIONNET T & LEHMANN R 2015. Drosophila germ granules are structured and contain homotypic mRNA clusters. Nat Commun, 6, 7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRCEK T, LIONNET T, SHROFF H & LEHMANN R 2017. mRNA quantification using single-molecule FISH in Drosophila embryos. Nat Protoc, 12, 1326–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUTUCCI E, VERA M, BISWAS J, GARCIA J, PARKER R & SINGER RH 2018. An improved MS2 system for accurate reporting of the mRNA life cycle. Nat Methods, 15, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TREECK B, PROTTER DSW, MATHENY T, KHONG A, LINK CD & PARKER R 2018. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc Natl Acad Sci U S A, 115, 2734–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEATCH SL, MACHTA BB, SHELBY SA, CHIANG EN, HOLOWKA DA & BAIRD BA 2012. Correlation functions quantify super-resolution images and estimate apparent clustering due to over-counting. PLoS One, 7, e31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C, DICKINSON LK & LEHMANN R 1994. Genetics of nanos localization in Drosophila. Dev Dyn, 199, 103–15. [DOI] [PubMed] [Google Scholar]
- WEBSTER A, LI S, HUR JK, WACHSMUTH M, BOIS JS, PERKINS EM, PATEL DJ & ARAVIN AA 2015. Aub and Ago3 Are Recruited to Nuage through Two Mechanisms to Form a Ping-Pong Complex Assembled by Krimper. Mol Cell, 59, 564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN DR, LEE WTC, YIN Y, OFRI DM, BERMUDEZ-HERNANDEZ K, KEEGAN S, FENYO D & ROTHENBERG E 2018. Spatiotemporal dynamics of homologous recombination repair at single collapsed replication forks. Nat Commun, 9, 3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG N, YU Z, HU M, WANG M, LEHMANN R & XU RM 2015. Structure of Drosophila Oskar reveals a novel RNA binding protein. Proc Natl Acad Sci U S A, 112, 11541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN Y, LEE WTC & ROTHENBERG E 2019. Ultrafast data mining of molecular assemblies in multiplexed high-density super-resolution images. Nat Commun, 10, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YORK AG, CHANDRIS P, NOGARE DD, HEAD J, WAWRZUSIN P, FISCHER RS, CHITNIS A & SHROFF H 2013. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat Methods, 10, 1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG J, GAO M, HUYNH N, TINDELL SJ, VO HD, MCDONALD WH & ARKOV AL 2016. In vivo mapping of a dynamic ribonucleoprotein granule interactome in early Drosophila embryos. FEBS Open Bio, 6, 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data and codes generated in this study should be directed and will be fulfilled by the lead contact, Ruth Lehmann (ruth.lehmann@med.nyu.edu) and co-corresponding author Tatjana Trcek (ttrcekp1@jhu.edu).


