Abstract
Introduction
Neuropeptide Y (NPY) acts directly on the vasculature as a co-transmitter with norepinephrine for an augmented contraction. Little, however, is known about the effects of NPY on the microvasculature of human skeletal muscle. NPY signaling has not been studied in the setting of cardiac surgery and cardiopulmonary bypass (CPB). We investigated the role of NPY signaling on vasomotor tone in the microvessels of human skeletal muscle, as well as the effect of CPB on NPY-induced responsiveness.
Methods
Specimens taken from intercostal muscles were collected from patients pre- and post-CPB undergoing coronary artery bypass grafting or cardiac valve surgery (N=8/group). Microvessels (157 ± 47 microns) were isolated in vitro in a no-flow state. Arterial microvascular responses to an NPY agonist, a Y1 receptor antagonist, phenylephrine, and the co-administration of NPY and phenylephrine were examined. The abundance and localization of the Y1 receptor were measured using Western blot and immunofluorescence, respectively.
Results
Arterial microvessels showed responsiveness to the NPY agonist (10−9-4×10−7 M) both before and after CPB, reaching a 12.5% vasoconstriction from the baseline luminal diameter. With administration of the Y1 receptor antagonist after NPY, the contractile response was eliminated (N=3/group, p=.04). No difference in vasoconstriction was observed between pre- and post-CPB groups (p=.73). The co-administration of NPY and phenylephrine (10−9–10−4 M) elicited no difference in vasoconstriction (N=7/group, p=.06 both pre- and post-CPB) when compared with phenylephrine alone (10−9-10−4 M). No change in the protein expression or localization of the Y1 receptor was detected by Western blotting (N=6/group, p=.44) or immunofluorescence (N=6/group, p=.13).
Conclusions
NPY induced vasoconstriction, suggesting that NPY may play an important role in the regulation of the peripheral microvasculature. There was no change in microvascular responsiveness to NPY after CPB, nor were there any synergistic effects of NPY on phenylephrine-induced vasoconstriction in the skeletal muscle microvasculature.
TOC Statement- 20-sus-106
Neuropeptide Y (NPY) has vasoactive effects on the vasculature of human peripheral skeletal muscle microvasculature via the Y1 receptor, but its effects were not decreased with the use of cardiopulmonary bypass (CPB) in cardiac surgery. The importance of this study is two-fold: first, this it is the first study to analyze the effects of NPY in the peripheral microvasculature and, second, there was no change after CPB, a deviation from prior vasoactive mediators studied within this framework, which helps to more accurately map the deleterious downstream effects of CPB.
INTRODUCTION
The use of cardiopulmonary bypass (CPB) in cardiac surgery has been linked to challenges in the management of perioperative blood pressure.1 Vasoplegia, a syndrome characterized by a pathologically low systemic vascular resistance has been shown to cause decreased vascular tone after CPB occurring in up to 25% of patients and may lead to end-organ damage and mortality,2 especially when vasopressor drugs are required. In ex vivo studies of microvessels isolated from human tissue, serotonin,3 phenylephrine,2 β-agonists,4 and endothelin-15 have all been shown to elicit decreased effects on vessel tone after CPB, while vasopressin has been found to elicit an increased contractile response.6 The effects of Neuropeptide Y (NPY), a sympathetic co-transmitter with norepinephrine which induces a greater, more prolonged contraction7, has not been studied in the microvasculature of human skeletal muscle in the context of CPB or otherwise. Because sympathetic neural output is a major driver of vasomotor tone, the peripheral vascular effects of NPY may have considerable impact on vasomotor tone and its regulation. Given the aforementioned incidence of hypotension and vasoplegia in the setting of CPB, it is possible that the mechanism for decreased vascular tone is due to decreased sensitivity of vasomodulatory receptors, in this case, possibly via Y1 receptors, which NPY acts on in the peripheral vasculature.7 Alternatively, it is also possible that the decreased microvessel tone is due to a change in the expression of receptors.
Here, we explored the role that NPY plays in the microvasculature of human peripheral skeletal muscle in cardiac surgery patients. We investigated the role of NPY signaling on the vasomotor tone of human skeletal muscle microvessels, and interrogated the effects of CPB on this NPY-mediated tone. We also looked for concomitant changes in the relative abundance of NPY Y1 receptors using Western blotting and immunofluorescence.
METHODS
Human subjects and tissue harvesting
Patients undergoing cardiac surgery (CABG or cardiac valve surgery) utilizing CPB at Rhode Island Hospital were recruited. Exclusion criteria included patients with aortic cross clamp time greater than 120 min, over 180 min on CPB, or patients whose operations involved repair of an aortic aneurysm. Before enrollment into the study, informed consent was obtained consistent with protocols from the Institutional Review Board of Rhode Island Hospital and the Warren Alpert Medical School of Brown University.
Skeletal muscle specimens were harvested from intercostal muscles adjacent to the left internal mammary artery before the initiation of CPB. After CPB, skeletal muscle samples were again acquired from intercostal muscles. Samples were either fixed in 10% formalin for 24 h followed by paraffinization and sectioning (6 mm) for immunohistochemistry, snap frozen in liquid nitrogen for Western blotting, or stored in Krebs buffer solution for ex vivo microvessel analysis, as conducted and described in prior microvessel studies.2,3,4 The CPB circuit was composed of a Medtronic Affinity integrated hollow fiber oxygenator/cardiotomy reservoir with trillium coating, and an arterial 38 mg-filter with trillium coating (Medtronic, Minneapolis, MN). The cardioplegia perfusion system (Medtronic Myotherm 4:1 system) with trillium coating was used. The heart was arrested with a 4:1 hyperkalemic, hypothermic, blood-based cardioplegic solution. Systemic cooling to 30–32 degrees C was performed on CPB. After completion of the operation, the cross clamp was removed and the patients were separated from CPB after rewarming to 37 degrees C.
Ex vivo skeletal muscle microvessel analysis
Microvessels (157 ± 47 microns) isolated from pre- and post-CPB skeletal muscle were dissected using a 40 X dissecting microscope and cannulated with glass micropipettes on either end (40 to 80 micrometers in diameter) and secured with 10–0 monofilament polypropylene suture. The microvessels were placed in a microvascular organ bath chamber with continuously circulating, oxygenated (95% oxygen, 5% CO2) Krebs buffer at physiologic temperature (37°C). To pressurize microvessels, a burette was filled with Krebs buffer and an attached manometer was used to regulate the pressure. An inverted microscope (40 to 200X, Olympus CK2, Olympus Optical, Tokyo. Japan) connected to a video camera was used to display the vessel image onto a monitor. The internal luminal diameter of the vessels was measured using an electronic dimension analyzer (Living Systems, Burlington, VT). Vessels were allowed to equilibrate at physiologic conditions in the microvessel chamber for 60 min before applying vasoactive reagents. Increasing concentrations of NPY agonist (Sigma-Aldrich, St. Louis, MO) starting at 10−9 M and increasing to 4×10−7 M were applied to the vessels (ELSEVEIR ASK THE AUTHORS IF THE AGENTS WERE APPLEIED INTRALUMINALLY OR IN THE BATH SOLUTION) and luminal diameter wasmeasured at each concentration. Phenylephrine (10−9 to10−4 M) and co-treatment of phenylephrine (10−9 to10−4 M) with an initial dose of NPY agonist (3×10−7M) were also applied to the vessels, and the diameter was again measured at each concentration. Additional experiments were performed with the NPY Y1 blocking agent BIBP-3226 (10−5 M), and this procedure was performed as described in preceding literature.2,3,8–10
Western blotting
Pre- and post-CPB skeletal muscle specimens were collected from 6 patients and samples were snap frozen. Tissue samples were then dissolved in SDS-PAGE (Thermo-Fisher, Waltham, MA). After dissolution, protein was fractionated on a graduated SDS-PAGE gel (8% to16%) and transferred to a polyvinylidene difluoride membrane (Millipore, Burlington, MA).4,11,12 Membranes were incubated for 1 h in a 1:900 dilution of anti-NPY1 R antibodies as well as a 1:500 dilution of GAPDH (AbCam, Cambridge, MA). Densitometric analysis to calculate the band intensity of the protein type was performed using ImageJ.
Immunofluorescence
Samples of the skeletal muscle fixed with formalin were deparaffinized in xylene. Ethanol was diluted with phosphate buffered saline (PBS) solution and tissue samples were soaked subsequently in increasing concentrations of the diluted ethanol. Antigen retrieval was performed using Dako Target Retrieval Solution at a temperature of 95°C (Thermo-Fisher), rinsed in PBS, incubated in 3% hydrogen peroxide, washed once more with PBS, and blocked with 5% goat serum in PBS for 2 h at room temperature. FAfter blocking, samples were washed with PBS and incubated overnight at 4°C with rabbit polyclonal primary antibodies to Y1 receptors (AbCam, Cambridge, MA) and with associated goat primary antibodies to alpha smooth muscle actin (αSMA) (AbCam). Dilutions of 1:500 in 5% goat serum were used for Y1 antibodies and a 1:250 dilution of αSMA was used. Negative controls of αSMA with respective secondary antibodies were also performed. Samples were washed in PBS and incubated with Alexa fluor secondary antibodies (AbCam). Red fluorophores were used to label Y1, and green fluorophores were used to label αSMA. Fluorescent microscopy was performed using a Zeiss LSM510 confocal system (Carl Zeiss AG, Oberkochen, Germany) following methods of studies reported elsewhere.2,3,11,12
Chemicals
Neuropeptide Y and BIBP 3226 trifluoroacetate (Y1 receptor antagonist) were purchased from Tocris soaked (ELSEVIER THEY NEED TO ADD THE CITY AND STATE OF THE COMPANY). Phenylephrine was purchased from Sigma Aldrich.
Data analysis
Data are presented as the mean ± standard deviation or the mean ± standard error of the mean. Patients are used as their own controls pre- versus post-CPB as performed in previous studies.2,3,4 Microvessel reactivity is measured as a percent change compared to its baseline diameter pre- versus post-CPB. The same method was used for the comparative experiments of phenylephrine to NPY + phenylephrine pre- vs. post CPB. A 2-factor analysis of variance for repeated measures was performed to assess vessel reactivity to NPY agonist pre- versus post-CPB. The same test was performed for NPY agonist versus NPY agonist treated with Y1 blocker BIBP 3226 trifluoroacetate pre-CPB as well as comparative experiments of phenylephrine to NPY + phenylephrine pre- vs. post CPB. To assess changes in receptor abundance by Western blot and immunofluorescence in pre- versus post-CPB, a paired t-test was used.
RESULTS
Patient characteristics
Patient characteristics are presented in Table 1. Samples from 8 patients were collected for the analysis of microvessel reactivity. Patient demographics are reported as mean ± standard deviation. Mean age was 62.9 y ± 10.7 y 2 of the 8 patients were female, mean body mass index was 31.9 ± 6.7, 62.5%, and 5 of the 8 patients had a diagnosis of hypertension. Mean HbA1c was 7.35 ± 2.16. Duration of CPB was 86 min ± 35 min, and cross clamp time was 62 min ± 27 min. Four of the 8 patients underwent valve repair or replacement, and the other four underwent coronary artery bypass grafting.
Table 1.
Patient Characteristics
| Age, y | 62.9 ± 10.7 |
| Female | 2 (25%) |
| BMI | 31.9 ± 6.7 |
| Hypertension | 5 (62.5%) |
| HbA1c | 7.35 ± 2.16 |
| Duration of cross clamp (min) | 61.9 ± 27.1 |
| Duration of CPB (min) | 86.1 ± 43.8 |
| Valve repair/replace | 4 (50%) |
| CABG | 4 (50%) |
BMI, body mass index; CABG, coronary artery bypass graft; HbAlc, hemoglobin A1c; mean ± standard deviation
Microvascular reactivity
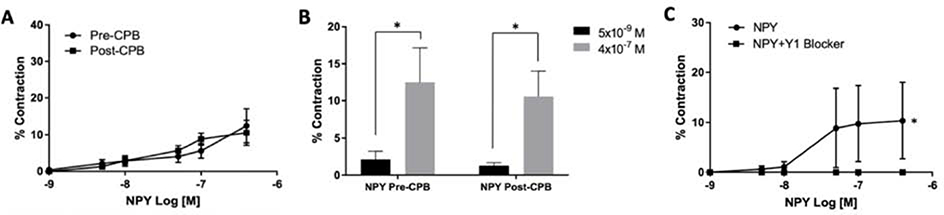
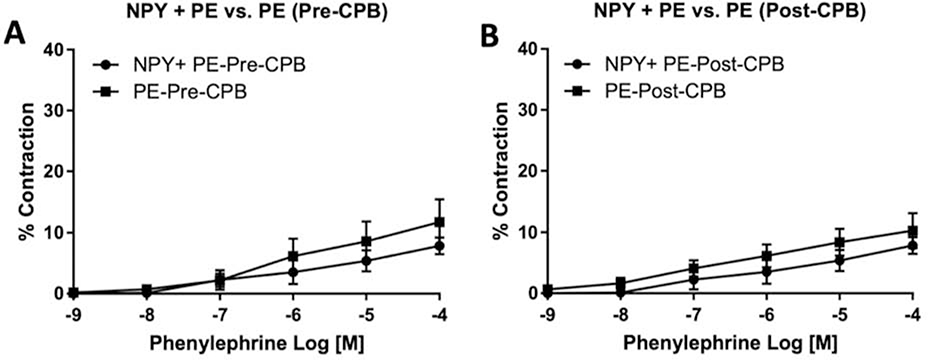
Microvessels responded to the NPY agonist (10−9 to 4×10−7 M) both pre- and post-CPB, reaching up to a 12.5% increase in vasoconstriction from baseline lumen diameter. Comparison of select concentrations of NPY agonist (5×10−9 M and 4×10−7 M) both pre- and post-CPB showed a statistically significant increase in contractile capacity (N = 8/group, p = .05 pre-CPB and p=, Figure 1, B). Administering a Y1 receptor antagonist to pre-CPB vessels after treatment with NPY agonist statistically significantly abolished the NPY-induced contraction in a dose-dependent manner (N=3/group, p = .04, Figure 1, C). No difference was observed in dose-dependent NPY-induced vasoconstriction between pre- and post-CPB groups (N = 8/group, p = .73, Figure 1, A). The co-treatment of phenylephrine (10−9 to 10−4 M) with an initial dose of NPY agonist (3×10−7M) when compared with phenylephrine alone (10−9 to 10−4 M) showed no enhancement in vasoconstriction both pre- and post-CPB (N = 7/group, p = .06 pre- and post-CPB, Figure 2).
Figure 1.
(A) Dose-dependent contractile response to NPY agonist (10−9 to 4×10−7 M) pre- and post-CPB. The N = 8/group, p = .73 versus pre-CPB; mean ± standard error of the mean. (B) Response to NPY agonist comparing select concentrations (5×10−9 M and 4×10−7 M) both pre- and post-CPB, demonstrating NPY contractile effects on skeletal muscle microvessels. The N = 8/group, *p = .04 for pre-CPB and *p = .04 for post- CPB versus lesser concentration; mean ± standard error of the mean. (C) Dose-dependent contractile response to NPY agonist in the presence or absence of the selective Y1 NPY receptor antagonist BIBP-3226 (10−5 M), pre-CPB. The N = 3/group, *p = .04 versus pre-CPB alone; mean ± standard error of the mean.
Figure 2:
(A) Dose-dependent contractile response to the administration of phenylephrine (10−9 to 10−4 M) with an initial dose of NPY agonist (3×10−7M) compared to phenylephrine alone (10−9 to 10−4 M), pre-CPB. The N = 7/group, p = .06; mean ± standard error of the mean. (B) Dose-dependent response to the administration of phenylephrine (10−9 to 10−4 M) with an initial dose of NPY agonist (3×10−7M) compared to phenylephrine alone (10−9 to 10−4 M), post-CPB. The N = 7/group, p = .06; mean ± standard error of the mean.
Western blotting and Immunohistochemistry
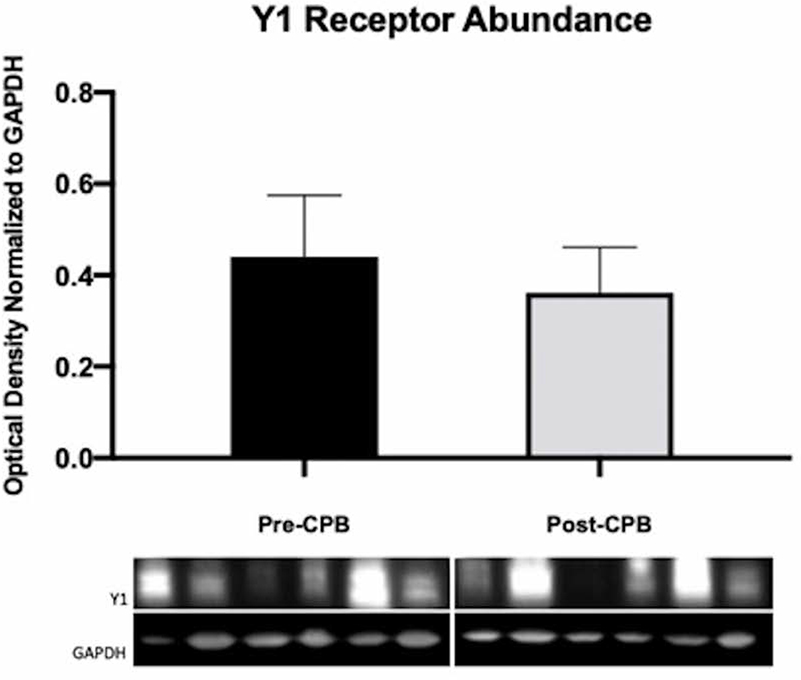
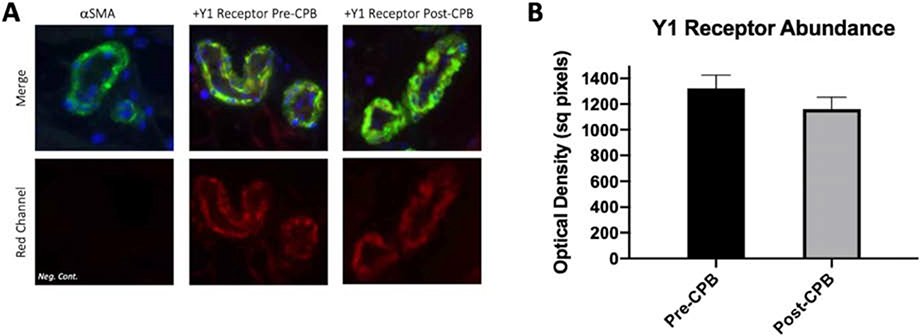
We observed no difference in the abundance of NPY Y1 receptors as assayed by Western blotting (N = 6/group, p = .44, Figure 3). Similarly, there was no change in abundance as assayed by immunofluorescence, nor did we observe a change in receptor localization to vessels detected by colocalization analysis to αSMA (N = 6/group, p = .13, Figure 4).
Figure 3.
Immunoblots of human skeletal muscle for pre-CPB and post-CPB Y1 receptors. Densitometric analysis shows no difference between pre- and post-CPB groups. The N = 6/group, p > .05; mean ± standard deviation.
Figure 4.
(A) Representative immunofluorescence of human skeletal muscle for Y1 receptors, alpha smooth-muscle actin as a marker of smooth muscle, 40,6-diamidino-2- phenylindole for nuclear staining (DAPI). (B) Optical density of fluorescence. No significant difference between pre- and post-CPB groups. The N = 6/group, p > 0.05; mean ± standard deviation.
DISCUSSION
In previous studies, we demonstrated that CPB alters the responsiveness of the microvasculature to various vasoactive mediators, such as phenylephrine,2 endothelin-1,5 serotonin,3 and isoproterenol.4 Here, we showed a deviation from this pattern with the administration of NPY using an ex vivo model (Figure 1, A). Importantly, we first demonstrated that microvessels of human skeletal muscle are capable of responding to an NPY agonist in a dose-dependent manner (Figure 1, B). While NPY-signaling has been well-studied in other domains of the human body and in other animals, such as the gut-brain axis,13,14 the myocardium,15,16,17, and the saphenous vein,18 h NPY-signaling as yet to be studied in the human skeletal muscle microcirculation. In the myocardium of pig models, NPY improves myocardial perfusion in the setting of hypercholesterolemia and chronic myocardial ischemia.16 In the human myocardium, NPY has demonstrated its effects on cardiac remodeling and its differentially altered expression in the serum and myocardium in type 2 diabetics, suggestive of a role in coronary artery disease pathogenesis.17 Of particular pertinence, NPY has also been studied in mesenteric vessel beds in rats as a cotransmitter with norepinephrine, decreasing the EC50 of norepinephrine.19
The same study also found that NPY acts via Y1 receptors in the rat mesenteric vessel beds.19 Similarly, we found that NPY acts predominantly via Y1 receptors in the microcirculation of human skeletal muscle. Of note, our study demonstrated albeit under ex vivo conditions that NPY acts on the microvasculature of human skeletal muscle by eliciting a vasoconstrictor response via Y1 receptors, because this response was abolished in the presence of the selective Y1 receptor blocker BIBP 3226 trifluoroacetate.
Once established that there was indeed a dose-dependent response to NPY treatment, we found no attenuation of this response after CPB. Further ex vivo trials comparing the co-administration of NPY and phenylephrine to phenylephrine alone showed no difference both pre- and post-CPB (Figure 2). This part of the study attempted to mimic the in vivo augmentative effects of NPY on norepinephrine. It is possible that the lack of potentiation when compared to phenylephrine alone was due to the ex vivo model to which it was restricted. It is also conceivable that, while norepinephrine and phenylephrine are structurally and functionally almost identical, they interact with NPY differently and therefore, do not effectively characterize the cotransmitter effects of NPY and norepinephrine in the human skeletal muscle. Finally, one must consider the possibility that the NPY potentiation of norepinephrine may not be very strong in the microvessels of human intercostal muscle.
In addition, we observed no change in Y1 receptor abundance in the microvessels from Western blotting or immunofluorescence studies, which suggests that the NPY signaling pathway is unaffected by CPB at the receptor level, as well.
The study is limited by its small sample size, as is a common limitation in research involving collection of human tissue. Typically, it is often difficult to draw conclusions from studies with small sample size, because there can be a wide array of patient factors that play a role in the results. Given that our study uses the same patients in pre- versus post-CPB analysis, however, this helps to mitigate substantially these confounding factors. Additionally, another potential limitation would be that the patients enrolled in the study have substantial comorbidities, a legitimate concern in a study with a small sample size4. Nevertheless, this sample is representative of patients who undergo cardiac surgery, a population whose comorbidities are what directly contribute to their need for operative intervention. With this mind, our study appears to be generalizable and has a high degree of external validity. This is not to say with certainty that particular, isolated comorbidities may not cause decreases in NPY-signaling after CPB, even if we found no change on the whole. We have found previously, for example, that a decrease in contractile response to phenylephrine was more prominent in vessels from diabetic patients compared to nondiabetics2; a more specific line of inquiry such as this could be applied to NPY to further understand how it is affected by CPB, if at all. Finally, while the vasoconstrictive effects were minimal in comparison to the effects of phenylephrine, given the lack of response attenuation with CPB and unaffected receptor expression, NPY could be considered as a potential drug in the therapy of perioperative blood pressure management to combat the pathologically low systemic vascular resistance that is seen all too often with cardiac surgery.
Microvascular vasomotor tone and regulation are the main determinants of tissue perfusion. Alterations in blood pressure and EKG changes occur in most patients after cardiac surgery;1 most of these cause no or few clinical issues, but some can lead to tissue malperfusion. The sympathetic nervous system and circulating and neuronally released levels of norepinephrine and epinephrine have a major component to this regulation. Because NPY is co-released with NE and has been reported to modulate the actions of catecholamines, the findings have comsoderable physiologic and clinical relevance.
In conclusion, NPY causes vasoconstriction in the peripheral microvasculature, but its effects are unchanged after cardiac surgery utilizing CPB. Importantly, this is the first study to demonstrate its effects in human skeletal muscle vessels, and it is the first vasoactive mediator that we have worked with that is unaffected by CPB, suggesting that not all pathways are affected equally. Understanding the underlying mechanisms of vasoplegia and the downstream, deleterious effects of CPB are important for guiding management of abnormalities of perioperative blood pressure. This deviation from previous patterns demonstrates that there is still much work to be done within this framework.
Acknowledgements
We would like to thank the many nurses, physician’s assistants, perfusionists, and other members of the operating room staff for facilitating the collection of patient samples.
FUNDING/SUPPORT
This research project was supported by the National Institutes of Health (NIH) R01-HL46716 and RO1HL128831 to F.W.S; This work was supported in part by 1R01HL127072–01A1, 1R01 HL136347–01, National Institute of General Medical Sciences (NIGMS) of the NIH [5P20- GM103652 (Pilot Project and CORE)] and AHA-Grant-in-Aid (#15GRNT25710105) to J.F.; NHLBI T35 training grant: T5T34HL09430809 through Brown University to B.M.
Footnotes
COI/DISCLOSURES: The authors have indicated that they have no conflicts of interest regarding the content of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruel M, Khan T, Voisine P, Bianchi C, Sellke F. Vasomotor dysfunction after cardiac surgery. European Journal of Cardio-Thoracic Surgery. 26(5):1002–1014. [DOI] [PubMed] [Google Scholar]
- 2.Sellke N, Gordon C, Lawandy I, et al. Impaired coronary contraction to phenylephrine after cardioplegic arrest in diabetic patients. J Surg Res. 2018;230: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabe SA, Feng J, Liu Y, Scrimgeour LA, Ehsan A, Sellke FW. Decreased contractile response of peripheral arterioles to serotonin after CPB in patients with diabetes. Surgery. 2018;164:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler O, Anderson K, Liu Y, et al. Skeletal muscle microvasculature response to β-adrenergic stimuli is diminished with cardiac surgery. Surgery. 2020; 167(2):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng J, Liu Y, Khabbaz KR, et al. Endothelin-1-induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-a signaling pathways. Surgery. 2010;147:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellke N, Kuczmarski A, Lawandy I, Cole VL, Ehsan A, Singh AK et al. Enhanced coronary arteriolar contraction to vasopressin in patients with diabetes after cardiac surgery. J Thorac Cardiovasc Surg. 2018; 156: 2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan CMJ, Green P, Tapoulal N, Lewandowski AJ, Leeson P and Herring N (2018) The Role of Neuropeptide Y in Cardiovascular Health and Disease . Front. Physiol. 9:1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Chu LM, Dobrilovic N, Liu Y, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J, Chu LM, Robich MP, et al. Effects of cardiopulmonary bypass on endothelin-1-induced contraction and signaling in human skeletal muscle microcirculation. Circulation. 2010;122(11 Suppl):S150–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng J, Liu Y, Chu LM, et al. Thromboxane-induced contractile response of human coronary arterioles is diminished after cardioplegic arrest. Ann Thorac Surg. 2011;92:829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamler A, Wang SY, Aguirre DE, Johnson RG, Sellke FW. Cardiopulmonary bypass alters vasomotor regulation of the skeletal muscle microcirculation. Ann Thorac Surg. 1997;64:460–465 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Sellke EW, Feng J, et al. Calcium activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass, Surgery. 2008;144:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Valpuesta FJ, Nyce JW, Griffin-Biggs TA, Ice JC, Myers RD Antisense to NPY-Y1 Demonstrates that Y1 Receptors in the Hypothalamus Underlie NPY Hypothermia and Feeding in Rats. Proceedings: Biological Sciences. 1996;263(1372):881. [DOI] [PubMed] [Google Scholar]
- 14.Kohno D, Gao H, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus Ca2+ signalling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 52(4):948–956. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Zhang Q, Qi H, Shi P, Song C, Liu Y and Sun H. Deletion of Neuropeptide Y Attenssuates Cardiac Dysfunction and Apoptosis During Acute Myocardial Infarction. Front. Pharmacol. 10:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robich MP, Matyal R, Chu LM, et al. Effects of neuropeptide Y on collateral development in a swine model of chronic myocardial ischemia. Journal of Molecular and Cellular Cardiology. 2010;49(6):1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matyal R, Mahmood F, Robich M, et al. Chronic type II diabetes mellitus leads to changes in neuropeptide Y receptor expression and distribution in human myocardial tissue. European Journal of Pharmacology. 2011;665(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoso MV, Miranda R, Briones R, Irarrazaval MJ, Huidobro-Toro JP. Release and functional role of neuropeptide Y as a sympathetic modulator in human saphenous vein biopsies. Peptides. 2004;25:53–64. [DOI] [PubMed] [Google Scholar]
- 19.Fetscher HC, Schäfers RF, Wambach G, Philipp T, Michel MC. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn-Schmiedeberg’s Archives Of Pharmacology. 1996;353(3):314–323. [DOI] [PubMed] [Google Scholar]