Abstract
Our understanding of the molecular mechanisms underlying adaptations to resistance exercise remains elusive despite the significant biological and clinical relevance. We developed a novel voluntary mouse weightlifting model, which elicits squat-like activities against adjustable load during feeding, to investigate the resistance exercise-induced contractile and metabolic adaptations. RNAseq analysis revealed that a single bout of weightlifting induced significant transcriptome responses of genes that function in post-translational modification, metabolism and muscle differentiation in recruited skeletal muscles, which were confirmed by increased expression of fibroblast growth factor-inducible 14 (Fn14), Down syndrome critical region 1 (Dscr1) and Nuclear receptor subfamily 4, group A, member 3 (Nr4a3) genes. Long-term (8 weeks) voluntary weightlifting training resulted in significantly increases of muscle mass, protein synthesis (puromycin incorporation in SUnSET assay) and mTOR pathway protein expression (raptor, 4e-bp-1 and p70S6K proteins) along with enhanced muscle power (specific torque and contraction speed), but not endurance capacity, mitochondrial biogenesis and fiber type transformation. Importantly, weightlifting training profound improved whole-body glucose clearance and skeletal muscle insulin sensitivity along with enhanced autophagy (increased LC3 and LC3-II/I ratio, and decreased p62/Sqstm1). These data suggest that resistance training in mice promotes muscle adaptation and insulin sensitivity with simultaneous enhancement of autophagy and mTOR pathway.
Keywords: Resistance exercise, hypertrophy, muscle power, contractile adaptation, metabolic adaptation
Introduction
Skeletal muscle makes up approximately 40% of body weight and plays an essential role daily life, metabolism and other physiological functions (1). It is well known that aging and physical inactivity cause loss of muscle mass and function, compromising mobility and life quality. Importantly, skeletal muscle abnormalities increase the propensity of co-morbidities, contributing significantly to the overall mortality under various diseased conditions. On the contrary, regular exercise improves muscle and whole-body function with many health benefits (2, 3). In particular, resistance exercise, a type of exercise that employs resistive load during muscle contraction, is the most effective in building up muscle mass and strength as well as preventing muscle atrophy and frailty in humans (4). Furthermore, resistance exercise promotes metabolic function across all age groups under various conditions (5–7). It is for this reason that muscle-strengthening activities that work all major muscle groups in addition to aerobic exercise are highly recommended for the general population (5). However, we still have a poor understanding of the molecular mechanism underlying resistance exercise.
Much of our knowledge about the mechanisms of resistance exercise has come from studies using animal models, including surgical ablation of synergistic muscles (8, 9 ), ladder climbing (10), passive stretching (11), and electric stimulation against load or with a stimulation frequency that mimics resistance exercise (12, 13 ). The invasive nature of these models causes inevitable injury and inflammation (14). A model of voluntary weightlifting was developed in rats in 1998 by Klitgaard et al., showing significant increases of plantar flexor muscle weight and strength after a very long duration of training (36 weeks) (15). Their model requires human handling during each session of the whole training period and, as a result, has not been employed widely by the research community. Thus, the pre-existing animal models of resistance training in animals either involve invasive procedures, which will inevitably confound the physiological relevance of the findings due to injury and inflammation, or require constant human handling during the intervention, which preclude large-scale, in depth mechanistic studies. There is an urgent need of developing a physiological animal model of resistance exercise.
Current research findings in human and animals support a potent anabolic effect of resistance exercise. Mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), a highly conserved, serine/threonine kinase of the phosphatidylinositol kinase-related kinase family, is a central node in this process (16). mTORC1 and its upstream regulators and downstream effectors as well as protein synthesis can all be activated by an acute bout of isometric contractions induced by high-frequency (100 Hz) electrical stimulation that mimics resistance exercise in rat skeletal muscle (13 ). Electrical stimulation-induced muscle contractions also stimulate glucose transporter Glut4 translocation to plasma membrane (17), which promotes glucose uptake. Pharmacological and genetic interventions revealed that mTORC1 is not only required for the maintenance of insulin sensitivity and intramyocellular lipid content (18) but also for increased protein synthesis and muscle hypertrophy induced by high-frequency electrical stimulation (19). Whether mTORC1 signaling plays a central role in the anabolic and metabolic adaptations in physiological model of resistance exercise has yet to be resolved.
Recent evidence show that resistance training also promotes autophagy, a highly conserved cellular catabolic process for aggregate proteins and dysfunctional organelles. Resistance exercise in humans led to increased autophagosome content (20)(21). Findings of resistance exercise in animal models is inconclusive, due to multiple confounding factors. For example, ladder-climbing in rats was reported to have increased autophagy along with increased expression of autophagy related proteins in one study (22), but decrease autophagy in another (23). Since autophagy/mitophagy is required for metabolic adaptation to endurance exercise training (24–26), and resistance training improves insulin sensitivity and glucose metabolism in skeletal muscle (26), it is conceivable that resistance training may promote insulin sensitivity and glucose metabolism in skeletal muscle by promoting autophagy/mitophagy.
We developed a novel model of voluntary weightlifting in mice that does not involve human handling during training. This model employs a cage top on a regular mouse cage where an individually housed mouse performs squat-like activity against controlled load during feeding, recapitulating many of the known responses in humans to a single bout, short-term and long-term resistance exercise training. Voluntary weightlifting had effects on transcriptional, biochemical, cellular, contractile and metabolic adaptations. In addition, orchestrated, concurrent regulation of autophagy and mTOR signaling pathway was associated with physiological contractile and metabolic adaptations. These findings provide a research platform to provide interventions to combat muscle atrophy, frailty and other muscle related disease conditions.
Materials and Methods
Animals
Male C57BL/6J mice (male, 26~28 g, 10~14 weeks old) were obtained from Jackson Laboratory and housed in the animal facility at 22–24°C with a 12 h/12 h light/dark cycle and with access to food and water ad libitum. The Institutional Animal Care and Use Committee at the University of Virginia approved all animal procedures. All samples were harvested after mice were euthanized under anesthesia following an overnight fasting at least 48 hours after the last bout of weightlifting.
Voluntary weightlifting exercise
For acute weightlifting exercise and long-term weightlifting training, we replaced the regular cage with the newly designed weightlifting cage top at the beginning of the dark cycle (18:00) and switched to regular cage top next morning (9:00). This was to minimize the potential impact of limited food intake during the dark cycle. We confirmed that neither acute weightlifting nor long-term weightlifting training resulted in significant loss of body weight comparing with mice housed in regular cages. Following two days of acclimatization with elevated lever plate with elevated ramp (mice can reach food without additional load to the hindlimbs), mice in the acute weightlifting group were allowed to push against 150%-body weight load for one night. Mice in the long-term (8 weeks) weightlifting group were allowed to push against resistance 100% body weight load on the first day with incremental increase of load by 20% body weight each day until reaching 240% of body weight load. The training includes 5 sessions/week with 1-day rest after 3 days of training and another one after another 2 days of training.
Magnetic resonance imaging (MRI)
MRI was performed under anesthesia using 1.25% isoflurane in oxygen as previously describe (27). Imaging was performed on a 7.0T MR system (Clinscan, Bruker Biospin, Ettlingen, Germany) using a 35mm volume coil and an MR-compatible physiological monitoring and gating system for mice (SA Instruments, Inc., Stony Brook, NY). Maximum gradient strength of the system was 600 mT/m and the peak slew rate achievable was 6000 mT/m/ms.
Glucose tolerance test
This test was performed at least 24 hours after the last bout of weightlifting training to avoid acute effects of exercise as described previously (26).
In vivo muscle function
Muscle contractile function of hindlimb plantar flexion muscles was measured in vivo under anesthesia by using the Aurora Dual-Mode Lever System (Model 300C-LR; Aurora Scientific, Aurora, Canada) at least 24 hours after the last bout of weightlifting activities as described previously (28).
Treadmill running test
We performed this procedure, which allows measurement of endurance capacity in mice, as described previously (26).
Echocardiography
Echo was performed with measurements of LV end-diastolic and end-systolic diameters, end-diastolic and end-systolic wall thicknesses of interventricular septum, end-diastolic and end-systolic LV posterior wall thickness. Fractional shortening and ejection fraction were calculated as described (29).
Insulin sensitivity in skeletal muscle
This analysis of insulin stimulated Akt phosphorylation in skeletal muscle provides direct evidence of insulin sensitivity in living mice. Briefly, following an overnight fasting, a muscle biopsy (gastrocnemius muscle) was taken from a hindlimb of the mouse under anesthesia induced by isoflurane. Another biopsy was taken from gastrocnemius muscle of the other hindlimb 10 min after a single bolus injection of insulin (5 U/kg body weight). Western blot analysis was then performed for phosphorylated (S473) and total Akt, and increased ratio of p-Akt (S473) to total Akt was calculated as insulin sensitivity in skeletal muscle. A polit study with a range between 1 and 20 U/kg body weight showed that 5 U/kg body weight elicited an optimal stimulation of Akt phosphorylation in skeletal muscle.
Surface sensing of translation assay (SUnSET)
SUnSET assay allows estimation of global protein synthesis in cultured mammalian cells and in tissues of living animals (30). We performed this assay according to previously described method (30). Briefly, we injected mice under anesthesia with puromycin (AG Scientific, San Diego, CA) at 0.04 μmol/g body weight (i.p.) 30 min before sample harvesting. Western blot was then performed with a mouse anti-puromycin antibody (Kerafast, Boston, MA) and FITC-conjugated anti-mouse secondary antibody. Puromycin incorporation was measured by using LI-COR system with both ponceau S staining and Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) quantity as loading control.
RNAseq and semi-quantitative PCR
RNA was isolated by using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNAseq library construction and analysis were performed as previously described (31). Semi-quantitative PCR was performed as described previously (32). Additional primers were used including: Tweak forward primer AGTGAGTGGCTGGGAAGAGA, reverse primer CAGCCTTAAGATGAGCCCAG; Cytb forward primer ATATACACGCAAACGGAGCC, reverse primer CTGTTTCGTGGAGGAAGAGG; 18S rRNA forward primer AAACGGCTACCACATCCAAG, reverse primer CCCTCTTAATCATGGCCTCA.
Western blot analysis
Muscle sample preparation and western blot analysis were performed as described previously (26). The following primary antibodies were used: LC3 (Novus Biological NB100–2220), p62/SQSTM1 (sigma P0067), Atg6 (Novus Biological NB500–249), Atg7 (R&D systems MAB6608), Puromycin (Kerafast EQ001), Akt (Cell signaling CST9272), p-Akt (S473) (Cell signaling CST9271), Raptor (Cell signaling CST2280), p-p70S6k (T389) (Cell signaling CST9205), p70S6k (Abcam AB37699), p-4e-bp-1 (S65) (Cell signaling CST9451), 4e-bp-1 (Cell signaling CST9452), Cox4 (Abcam AB33985), OXPHOS cocktail (Thermo PA5–28220), and Gapdh (Cell signaling CST2118).
Statistical analysis
All data were presented as mean ± standard error (SEM). Unpaired t-test or Two-Way ANOVA was used for comparisons between groups or among groups using GraphPad Prism 7.0 with Sidak’s multiple comparisons post hoc analysis. A statistically significant difference was achieved when p < 0.05.
Results
Establishment of voluntary weightlifting model in mice
Back squat is a common, compound movement in human resistance exercise that engages a large group of lower extremity muscles, including quadriceps, gastrocnemius, plantaris and soleus muscle (33). This exercise mode leads to functional improvements, such as balance, strength, mobility, and glucose metabolism. An animal model that recapitulates this resistance exercise would permit understanding the underlying cellular and molecular adaptations. We developed a mouse cage environment that is conducive to voluntary hindlimb plantar flexion movement against shoulder-loaded resistance during feeding. A mouse in the exercise group wore a neck collar (Fig. S1B) and extended hips, knees and raised heels from a height-adjustable metal ramp to push a weight-loaded lever plate in order to access food in a container through a hole in the lever plate (Fig. 1A). The regular cage top was replaced with a weightlifting cage top during the dark cycle, so the weightlifting activities were voluntary without the need of human handling. A magnet was attached to the lever plate, and weightlifting activities were recorded by a magnetic sensor connecting to a computer recording system (DSI). Each cage top was calibrated by measurement of the force generated at the hole of the lever plate when different weights were added on the extended arm of the lever plate (Fig. S1A).
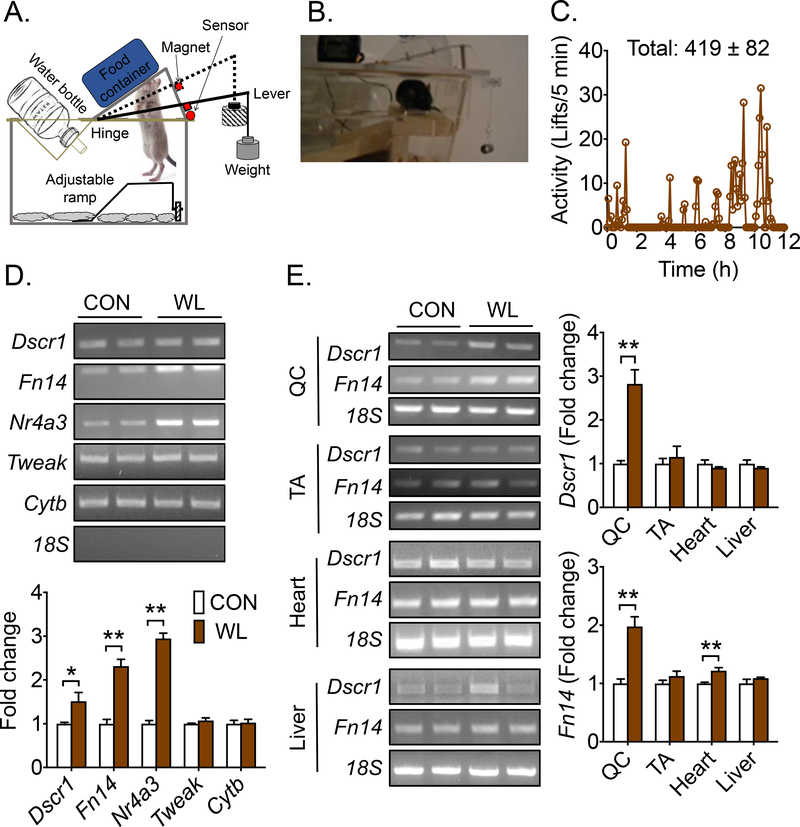
Fig. 1. A single bout of voluntary weightlifting activates recruited muscles.
Mice were subjected a single bout of overnight voluntary weightlifting (WL) in cages equipped with newly invented weightlifting cage tops with mice subjected to sedentary cage activity as control (CON) followed by semi-quantitative RT-PCR analysis of mRNA expression in recruited muscles and other tissues. (A) A cartoon illustrating the design of the weightlifting cage top, depicting a mouse performing squat-like movement against a weighted lever to access food; (B) A video recording of weightlifting activity of a mouse; (C) A representative record of weightlifting activity with quantification for 9 mice over the 12-hour dark cycle (419 ± 82); (D) Semi-quantitative RT-PCR analysis of Dscr1 (n = 5), Fn14 (n = 5), Nr4a3 (n = 5), Tweak (n = 3–4), and Cytb (n = 3–4) mRNA in gastrocnemius muscle using 18S RNA as reference control; and (E) Semi-quantitative RT-PCR analysis of Dscr1, Fn14 mRNA in quadriceps (QC; n = 5), tibial anterior muscle (TA; n = 5), heart (n = 3–4), and liver (n = 3–4). Bars represent mean ± SE. * and ** denote p<0.05 and p<0.01, respectively.
Based on pilot studies, the height of the metal ramp was adjusted such that the distance from the top of the ramp to the food in the food container is 0.7 cm shorter than the distance from the nose to the heels of the mouse (please see the Methods section). This setting ensured food accessibility as well as full hindlimb extension during weightlifting verified by video recording (Fig. 1B). The sedentary control mice were housed in the same cage environment except that the lever plate was fixed at the lifted position, and the metal ramp was adjusted at an elevated position such that the mouse did not have to lift weight to access food. This weightlifting model mimics the heel raised, squat-like task to recruit multiple muscle groups by flexing and extending at the hip, knee, and ankle joints in a single maneuver, recapitulating the ascent phase of the human heel raised squat with loading.
Validation of weightlifting activity was made by subjecting mice to an overnight acute bout of weightlifting against 150%-body weight load followed in assessment of mRNA expression. Our pilot study results instructed us to use this workload for untrained mice to have reliable weightlifting activity. Mice in the weightlifting group lifted the plates 419 ± 82 times (Fig. 1C) with no aberrant effect on body weight (Supplemental Fig. S1C). At the end of the dark cycle, all mice were euthanized, and the hindlimb muscles, including quadriceps, gastrocnemius and tibialis anterior muscles, as well as the heart and liver were harvested. Three genes were chosen to validate the transcriptional responses by semi-quantitative RT-PCR: Down syndrome critical region gene 1 (Dscr1 or Rcan1), fibroblast growth factor-inducible 14 (Fn14 or Tnfrsf12a) and Nuclear receptor subfamily 4, group A, member 3 (Nr4a3 or Nor-1) mRNA. These three genes have been shown to be responsive to acute exercise (32, 34). mRNA was measured for tumor necrosis factor superfamily, member 12 (Tweak) that encodes the ligand for Fn14 and mitochondrial cytochrome b (Cytb) that encodes a mitochondrial respiratory protein. Significantly increased mRNA for Dscr1 (1.5-fold; p < 0.01), Fn14 (2.3-fold; p < 0.01) and Nr4a3 (3.0-fold; p < 0.01) was observed in gastrocnemius muscle of weightlifting mice compared with the sedentary control mice, but not for Tweak and Fn14 mRNA (Fig. 1D), consistent with the transcriptional responses being gene-specific. We measured Fn14 and Dscr1 mRNA in quadriceps and tibialis anterior muscles, heart and liver. Significantly induced expression was observed only in recruited quadriceps, but not in antagonistic tibialis anterior muscle. A subtle, but statistically significant, increase in Fn14 mRNA was observed in the heart, but none of these changes were observed in the liver (Fig 1E). Collectively, these findings demonstrate that acute weightlifting activity induces specific transcriptional responses in recruited skeletal muscles.
Transcriptome responses to a single bout of weightlifting activity
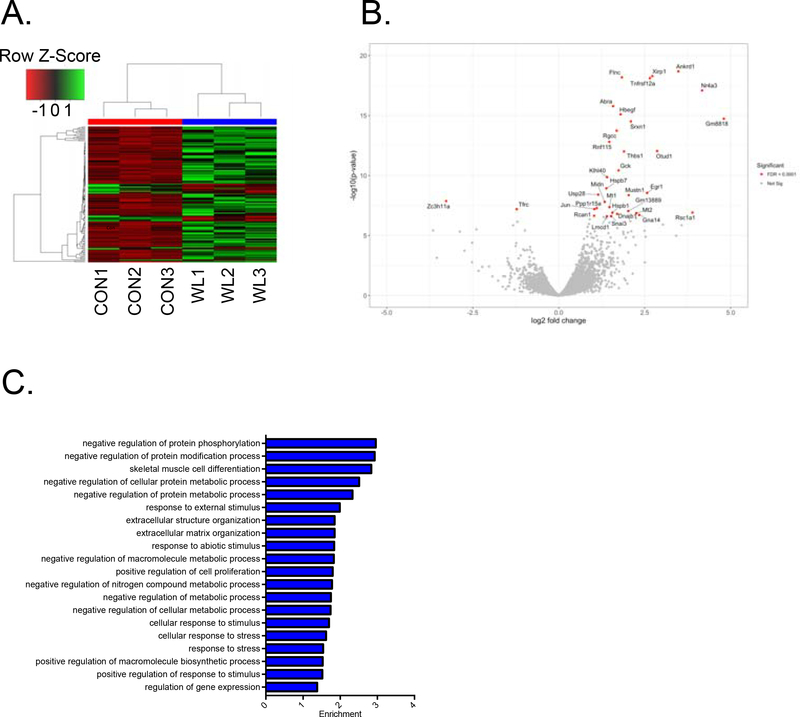
Culmination of transcriptome responses to single bouts of exercise underscores skeletal muscle adaptations induced by training. RNA sequencing of recruited gastrocnemius muscle showed significantly increased (341 genes) and suppressed (149 genes) mRNA expression compared with sedentary mice (Fig. 2A). The 30 most highly responsive genes from differential expression (DE) analysis include Dscr1 and Fn14 and Nr4a3 (Fig. 2B), indicating a high degree of muscle plasticity to resistance exercise at the transcriptome level. PANTHER gene ontology was used to analyze and identify the highly regulated biological processes. Based upon gene ontology (PANTHER) analysis, 490 genes are classified into 34 biological processes (top 20 shown in Fig. 2C). The biological processes with highest enrichment were protein phosphorylation, modification, metabolism process, and skeletal muscle cell differentiation (top 20 up-regulated and top 20 down-regulated genes, Table 1 and 2). These findings are consistent with the importance of transcriptional control of protein turnover, muscle differentiation and extracellular matrix remodeling in the process of adaptations to resistance exercise in mice. It is important to note that our analysis following an acute bout of weightlifting may elicit many stress responses; not all the regulated genes are directly involved in the muscle adaptations.
Fig. 2. A single bout of weightlifting activity induces transcriptome regulation.
Total RNA was isolated from gastrocnemius muscles in sedentary control mice (CON) and acutely (overnight) weightlifting exercised mice (WL) and used for RNAseq analysis. (A) A transcriptome heatmap of RNA sequencing data of significantly changed genes in gastrocnemius muscles of CON and WL mice (n = 3 for each group); (B) A volcano plot of all the genes highlighting the 30 top responsive genes (red spots); (C) Top 20 biological processes of gene ontology analysis; and (D) A list of top 20 upregulated and downregulated genes.
Table 1.
Top 20 up-regulated genes by an acute bout of weightlifting
| Gene ID | Gene Name | Log2 (Fold change) |
|---|---|---|
| XLOC_014928 | Nr4a3 | 4.14 |
| XLOC_011380 | Ankrd1 | 3.48 |
| XLOC_001742 | Unknown | 3.11 |
| XLOC_001384 | Atf3 | 3.04 |
| XLOC_011579 | Otud1 | 2.76 |
| XLOC_023770 | Xirp1 | 2.65 |
| XLOC_021891 | Unknown | 2.63 |
| XLOC_021639 | Mt2 | 2.45 |
| XLOC_002378 | Unknown | 2.40 |
| XLOC_009593 | Tnfrsf12a | 2.30 |
| XLOC_016477 | Uchl1 | 2.30 |
| XLOC_017518 | Serpine1 | 2.28 |
| XLOC_010181 | Egr1 | 2.18 |
| XLOC_012311 | Srxn1 | 2.15 |
| XLOC_004693 | Fos | 2.14 |
| XLOC_023769 | Csrnp1 | 2.11 |
| XLOC_017298 | Unknown | 2.08 |
| XLOC_009776 | Hspa1b | 2.04 |
| XLOC_022720 | Sln | 1.99 |
| XLOC_009777 | Hspa1a | 1.96 |
Table 2.
Top 20 down-regulated genes by an acute bout of weightlifting
| Gene ID | Gene Name | Log2 (Fold change) |
|---|---|---|
| XLOC_004993 | Unknown | −2.33 |
| XLOC_013177 | Actc1 | −2.04 |
| XLOC_012015 | Unknown | −1.73 |
| XLOC_000988 | Unknown | −1.61 |
| XLOC_010450 | Aqp4 | −1.56 |
| XLOC_007275 | Unknown | −1.50 |
| XLOC_015924 | Foxo6 | −1.42 |
| XLOC_009700 | Mdga1 | −1.35 |
| XLOC_015188 | Cited4 | −1.35 |
| XLOC_010486 | Nrep | −1.32 |
| XLOC_001313 | Grem2 | −1.31 |
| XLOC_011439 | Pdzd7 | −1.30 |
| XLOC_015378 | Lrrc38 | −1.29 |
| XLOC_008241 | Tfrc | −1.24 |
| XLOC_001389 | Unknown | −1.21 |
| XLOC_009154 | Col11a2 | −1.20 |
| XLOC_008805 | Tiam1 | −1.19 |
| XLOC_019299 | Dpf1 | −1.17 |
| XLOC_022995 | 6430571L13Rik | −1.09 |
| XLOC_008102 | Rmi2 | −1.08 |
Long-term weightlifting training induces skeletal muscle hypertrophy with enhanced expression mTOR pathway
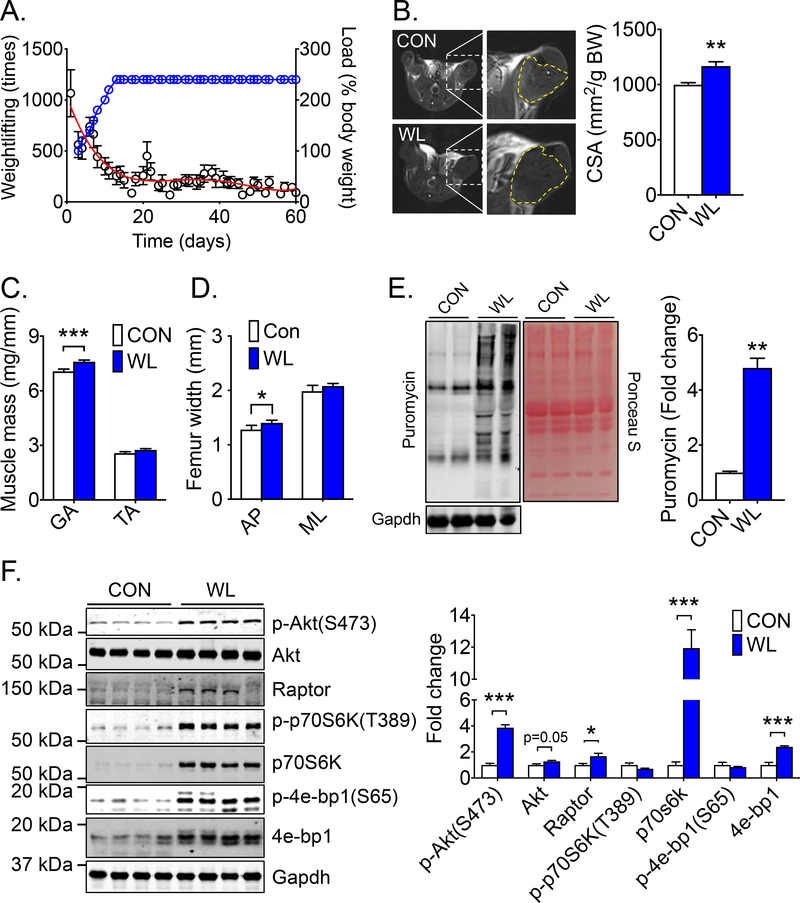
To ascertain the impact of weightlifting training on muscle mass, mice were subjected to voluntary weightlifting for 8 weeks (5 days/week). The training protocol began with no addition of weight on the plate lever (a workload equivalent to ~100% body weight) and gradually increased (20% increment on each training day) to 240% body weight in 10 days (referred to as staging phase). As the workload increased, there was a progressive decline of weightlifting activity (repetition per night) (Fig. 3A). The workload was kept at 240% of body weight for the remainder of the training period (referred to as steady phase). The weightlifting activity per night reached and remained at about 200 repeats during this phase (Fig. 3A). After 8 weeks of training, the cross-sectional area (CSA) of the lower hindlimb was assessed using magnetic resonance imaging (MRI). A moderate but significant increase of CSA by 14% (p < 0.05) was observed in weightlifting trained mice compared with sedentary control mice (Fig 3B). Hypertrophy of recruited skeletal muscle was further confirmed by increased wet weight of soleus, plantaris, and gastrocnemius muscles, but not that of tibialis anterior muscle (Fig. 3C and Supplemental Fig. S2A). There was no change of heart weight and any of the cardiac functional parameters assessed by electrocardiogram and echocardiogram (Supplemental Fig. S2A and Supplemental Table S1 and S2). Long-term voluntary weightlifting resulted in increased anteroposterior (AP) femur width, but not medial-lateral (ML) femur width, tibia and femur lengths and bone mineral density assessed by dual-energy X-ray absorptiometry (DEXA) (Fig. 3D and Supplemental Fig. S2B). These findings revealed adaptations to the musculoskeletal system of the weightbearing hindlimbs.
Fig. 3. Long-term weightlifting training induces skeletal muscle hypertrophy and skeletal adaptations along with increased protein synthesis and enhanced Akt-mTOR pathway in skeletal muscle.
Mice were subjected to 8 weeks of voluntary weightlifting (WL) with sedentary mice as control (CON) followed MRI imaging, SUnSET and western blot analyses of the recruited muscles. (A) Weightlifting training activity in WL mice, displaying the weightlifting repeats (dots in black) (left Y axis) with a red trending curve for % body weight load (dot in blue) (right Y axis, n = 9); (B) Representative MRI and quantification of hindlimb muscle cross-sectional area normalized by body weight (n = 4–5); (C) Muscle wet weight of gastrocnemius and tibialis anterior muscles (n = 12 for each group); (D) Anteroposterior (AP) and medial-lateral (ML) femur width (n = 5 for each group); (E) Representative SUnSET image with ponceau S staining and Gapdh as loading control (n = 4–5); and (F) Representative western blot images and quantification of Akt-mTOR pathway proteins (p-Akt (S473), Akt, Raptor, p-p70S6k (T389), p70S6k, p-4e-Bp-1 (S65), and 4e-Bp-1) in gastrocnemius muscle with Gapdh served loading control (n = 4). Bars represent mean ± SE. *, ** and *** denote p < 0.05, p < 0.01, and p < 0.001, respectively.
Skeletal muscle hypertrophy is thought to be caused by increased protein synthesis rather than decreased protein degradation, where protein synthesis is primarily controlled by mTORC1 (13). Surface sensing of translation (SUnSET) assay (35) was employed, in which a structural analogue of the tyroslyl-tRNA (antibiotic puromycin) is injected in vivo to label nascent peptide chains for a fixed duration before sample harvesting. A significant increase of puromycin incorporation in gastrocnemius, plantaris and quadriceps muscles was detected, as well as a moderate increase in the heart in weightlifting trained mice, but not in the liver (Fig. 3E and supplemental Fig. S2C). Similar findings were observed after 4 weeks of training in an independent series of mice (Supplemental Fig. S2D). To investigate the role of mTORC1 in resistance training, phosphorylation and content of key signaling molecules were measured in this pathway. A moderate, but significant, increase of Akt (1.27-fold; p = 0.05) and p-Akt (3.87-fold; p < 0.001) was observed, as well as a significant increase of regulatory-associated protein of mTOR (raptor) (1.67-fold; p < 0.05) (Fig. 3F). Importantly, mTORC1 targets ribosomal protein S6 kinase 1 (p70S6K) (12.0-fold; p < 0.001) and eukaryotic translation initiation factor 4E binding protein 1 (4e-Bp1) (2.40-fold; p < 0.001) showed profound increases with no significant increases of phosphorylation state (ratio of phosphorylated protein to total protein) at T389 and S65, respectively (Fig. 3F). For the first time, these data reveal profoundly enhanced protein expression of mTORC1 pathway as a possible mechanism for driving protein synthesis following weightlifting training.
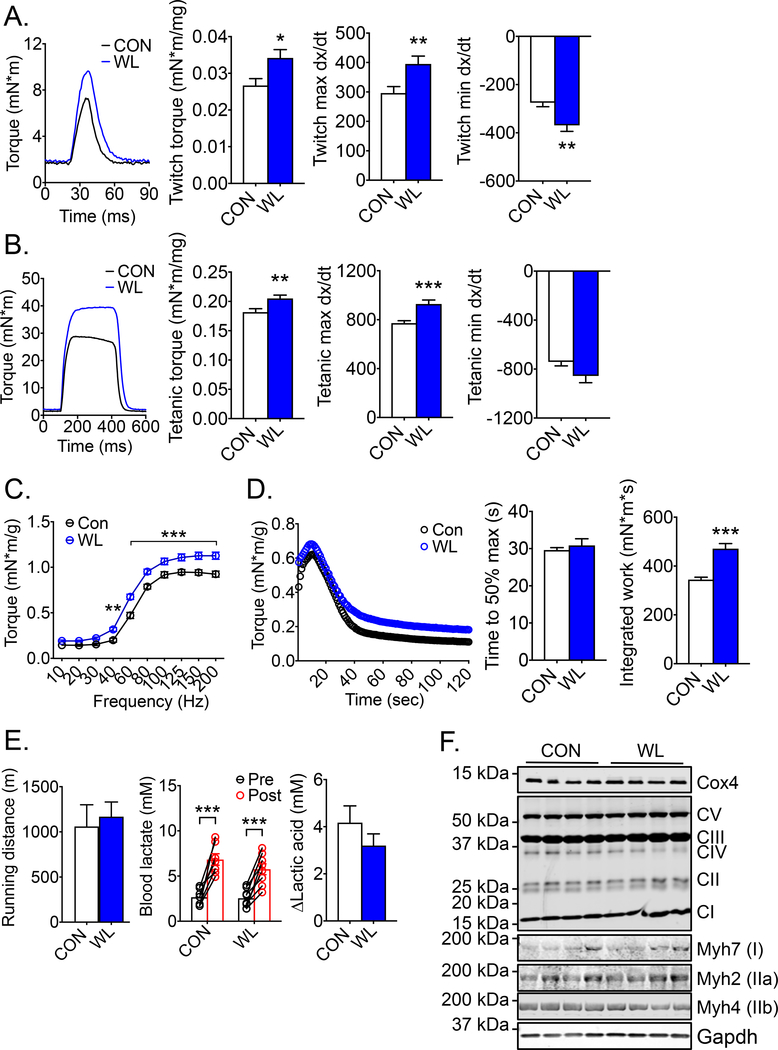
Long-term voluntary weightlifting training promotes muscle contractile function
Resistance training is not only effective in building up muscle mass, but also in promoting muscle power (36, 37). We performed non-invasive muscle contractile function assay for plantar flexor muscles in the hindlimb in vivo via sciatic nerve stimulation. Weightlifting trained mice showed significantly increased specific twitch (1.28-fold; p < 0.05) and tetanic torque (1.13-fold; p < 0.01) in comparison with sedentary control mice (Fig. 4A and 4B). Weightlifting trained muscles had significantly greater contraction (max dx/dt) (1.33-fold; p < 0.01) and relaxation (min dx/dt) speed (1.34-fold; p < 0.01) during twitch contraction with similar trends in tetanic contraction (Fig. 4A and 4B). When the motor nerve was stimulated with electrical pulses of increasing frequencies (force-frequency curve), a significant change in the force frequency curve was not observed, but there was an upshift of the curve in weightlifting trained mice (Fig. 4C). Repeated tetanic contraction induced by pulses at physiological frequency (60 Hz) (fatigability test) showed no significant change in time to 50% maximal contraction but a significantly increased integrated work (1.37-fold; p < 0.001) in weightlifting trained mice compared with sedentary control mice (Fig. 4D) (Table 3). Similar but slightly variable findings were observed following 4 weeks of training (Supplemental Fig. S3A–D, Supplemental Table S3). It is well known that voluntary running in mice improves endurance capacity primarily through adaptations, including mitochondrial biogenesis, angiogenesis and fiber type transformation (25). There were no differences in running distance and in induction of blood lactic acid level in exhaustive treadmill running test between weightlifting trained and sedentary control mice (Fig. 4E). Consistent with these findings, there were no significant changes in mitochondrial electron transport chain protein Cox4, proteins in the electron transport chain protein complex I, II, III, IV and V, or fiber type composition, based on the expression of myosin heavy chain proteins Mhy7 (type I), Myh2 (type IIa) and Myh4 (type IIb) (Fig. 4F and Supplemental Fig. S3E). Together, these findings demonstrate that voluntary weightlifting training in mice specifically leads to musculoskeletal adaptations with improved strength as well as contraction and relaxation speed in recruited muscles.
Fig. 4. Long-term weightlifting training enhances skeletal muscle power.
Mice were subjected to 8 weeks of voluntary weightlifting (WL) with sedentary mice as control (CON) followed by measurements of contractile function and endurance exercise capacity in vivo as well as measurements of mitochondrial and contractile proteins in skeletal muscle. (A) Representative twitch force of planta flexor muscles of the hindlimbs with quantification for twitch torque normalized by muscle mass, twitch max dx/dt and min dx/dt (n = 12 for each group); (B) Representative tetanic force of planta flexor muscles of the hindlimbs with quantification for tetanic torque normalized by muscle mass, tetanic max dx/dt and min dx/dt (n = 12 for each group); (C) Force-frequency curve, displaying torque normalized by body weight (n = 6–7); (D) Fatigability curve, display torque normalized by body weight with quantification of time to 50% maximal force and integrated work performed during the test (n = 6–7); (E) Running distance and blood lactic acid level pre- and post-test during treadmill running test (n = 7–8); and (F) Representative images and quantification of mitochondrial (Cox4 and Complex I-V) and contractile proteins (MHC I, IIa and IIb) in gastrocnemius muscles with Gapdh as loading control (n = 4–6); Bars and dots represent mean ± SE. *, ** and *** denote p < 0.05, p < 0.01, and p < 0.001, respectively.
Table 3.
In vivo muscle function analysis at 8 weeks of weightlifting training
| CON (n = 12) | WL (n = 12) | |
|---|---|---|
| Twitch torque (mN*m/g) | 0.14 ± 0.01 | 0.19 ± 0.01* |
| Twitch max dx/dt | 296 ± 22 | 395 ± 26** |
| Twitch min dx/dt | −275 ± 17 | −369 ± 25** |
| 1/2RT twitch (s) | 0.015 ± 0.000 | 0.014 ± 0.000 |
| Tetanic torque (mN*m/g) | 0.96 ± 0.02 | 1.15 ± 0.03*** |
| Tetanic max dx/dt | 772 ± 19 | 928 ± 33*** |
| Tetatnic min dx/dt | −741 ± 33 | −856 ± 55 |
| 1/2RT tetanic (s) | 0.219 ± 0.006 | 0.174 ± 0.014** |
| Time to 50% max (s) | 29.6 ± 0.6 | 30.9 ± 1.8 |
| Integrated work (mN*m*s) | 344 ± 10 | 471 ± 21*** |
Values are presented as mean ± SE. *, ** and *** denote p < 0.05, p < 0.01 and p < 0.001, respectively, in comparison with CON mice.
Long-term voluntary weightlifting training improves metabolic function and insulin signaling in skeletal muscle along with enhanced autophagy
Endurance exercise training is one of the most efficient interventions for improving metabolic function along with reduced adiposity in mice and humans (38). Weightlifting did not lead a significant change in body weight. In contrast, sedentary mice showed a slight decline of body weight in the first week after being housed individually (Fig. 5A). These results suggest a negative impact of social separation on body weight, which could be mitigated by weightlifting. Consistent with increased energy expenditure with weightlifting activity, mice in the weightlifting group had greater daily food consumption (~16%) than the sedentary mice when they reached the workload of 240% body weight resistance (Supplemental Fig. S4).
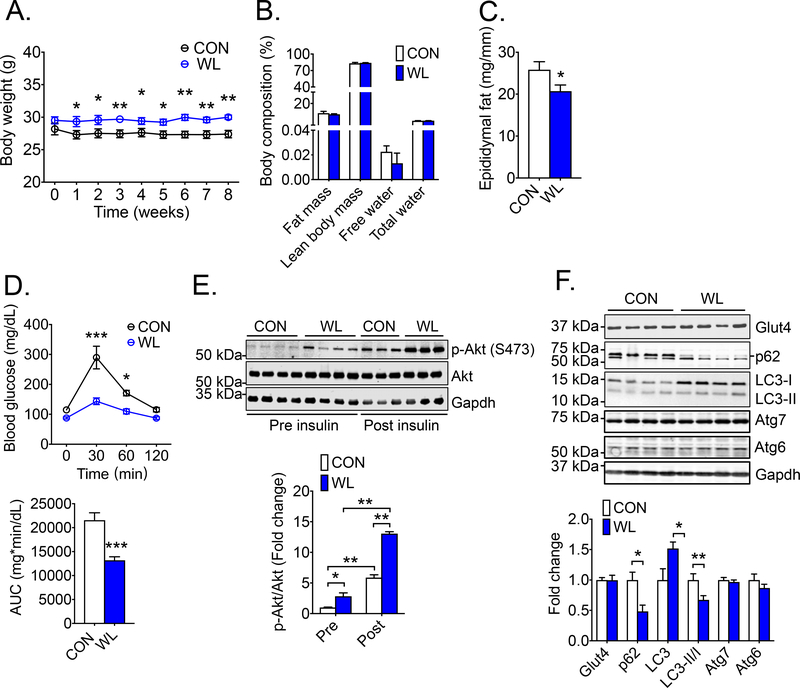
Fig. 5. Long-term weightlifting training enhances metabolic function with increased insulin sensitivity in skeletal muscle along with increased autophagy.
Mice were subjected to 8 weeks of voluntary weightlifting (WL) with sedentary mice as control (CON) followed by measurements of body composition, epididymal fat mass, whole-body glucose tolerance, insulin stimulated Akt phosphorylation as well as autophagy in gastrocnemius muscles. (A) Body weight during 8 weeks of weightlifting training; (B) MRI-based measurements of body composition (n = 4 for each group); (C) Epididymal fat mass normalized by tibia length (n = 12); (D) Blood glucose detected by tail vein blood during glucose tolerance test before and at 30, 60, and 120 min after intraperitoneal injection of glucose with quantification of the area under curve (AUC) (n = 12); (E) Representative image and quantification of p-Akt (S473)/Akt in gastrocnemius muscle pre- and 10 min post-intraperitoneal injection of insulin with Gapdh as loading control (n = 3–4); and (F) Representative images and quantification of glucose transporter (Glut4) (n = 4) and autophagy proteins (p62, LC3, Atg7 and Atg6) (n = 6–11) in gastrocnemius muscles with Gapdh as loading control; Bars and dots represent mean ± SE. *, ** and *** denote p < 0.05, p < 0.01, and p < 0.001, respectively.
MRI-based body composition analysis showed no significant changes in total fat mass, lean body mass, free water content and total water content between the two groups (Fig. 5B). Weightlifting training led to a 19.7% (p < 0.05) reduction of epididymal fat mass (Fig. 5C). These findings showed a moderate impact of resistance training on visceral fat with no major impact on the body composition in mice on normal diet. In contrast, there was a profound improvement of whole-body glucose clearance by glucose tolerance test (GTT) with a 38% decrease (p < 0.01) in area under curve (AUC) (Fig 5D). To determine whether the improved glucose clearance in resistance exercise-trained mice was due to increased insulin sensitivity in skeletal muscle, Akt phosphorylation was measured in gastrocnemius muscle pre- and 10 min post-insulin injection (5 unit/kg, i.p.). An elevated baseline (2.84-fold; p < 0.05) and a more robust responsiveness of Akt phosphorylation at S473 was detected in weightlifting-trained mice (13.1-fold) compared with sedentary control mice (5.89-fold; p < 0.01) (Fig. 5E). Glut4 is a key glucose transporter responsible for insulin- and exercise-stimulated glucose transport in skeletal muscle (39), and increased Glut4 expression has been reported in endurance exercise training (39). No increased Glut4 protein expression was observed in gastrocnemius muscle (Fig. 5F), a result different from the findings in endurance exercise models.
It has been shown that an acute bout of resistance exercise in humans reduces microtubule-associated proteins 1A/1B light chain 3B (LC3)-II/I ratio, an index of autophagy activation (21). Autophagy is important for endurance exercise-induced adaptations in different tissues/organs (40, 41), but its role in resistance training is not clear. Significantly increased LC3 content (sum of LC3-I and LC3-II) and decreased LC3-II/I ratio were observed in weightlifting trained muscle along with reduced level of p62/Sequestosome 1 (p62/sqstm1) (Fig. 5F), a cargo protein for autophagy that is degraded upon incorporation into the autolysosome (23). There was no evidence of increased autophagy-related 6 (Atg6) and autophagy-related (Atg7), both previously shown to be induced by endurance exercise (25). These findings suggest that resistance exercise training leads to increases of autophagy in skeletal muscle. Therefore, long-term weightlifting training improves whole-body glucose clearance along with improved insulin responses in skeletal muscle in the absence of enhanced mitochondrial biogenesis and glucose transporter expression; but it increases autophagy, which may underlie improved metabolic functions.
Discussion
Skeletal muscle is a very malleable organ that is responsive to a variety of cues, including physical activity, nutrients, aging, and diseases. Resistance training promotes skeletal muscle hypertrophy and improves cardiovascular and metabolic functions (5). However, the underlying molecular mechanism(s) for the myriad benefits of resistance training remains elusive. Several existing experimental animal models mimic human resistance training (8, 12, 15, 42); however, the invasive and/or the labor-intensive features of these models have limited them being widely used for mechanistic studies (43). Here, we developed a novel resistance exercise model in mice that involves voluntary recruitment of a large group hindlimb muscles against adjustable load during feeding. This human labor-free, non-invasive model makes large scale mechanistic studies a reality.
A single bout of weightlifting activity in this model led to increased mRNA of Dscr1, Fn14 and Nr4a3, but not that of Tweak and Cytb, in recruited gastrocnemius muscle (Fig. 1D), demonstrate gene specificity of impact of exercise. Further, highly induced Dscr1 and Fn14 mRNA in recruited quadriceps muscle, but not in antagonist tibialis anterior muscle, heart and liver, reveals the tissue specificity of the transcriptional responses (Fig. 1E), consistent with the electromyography (EMG) findings in human that ascent squat movement results in robust EMG activity in gastrocnemius and quadriceps muscles with little in antagonist tibialis anterior muscle (44). The lack of gene responses in tibialis anterior muscle was further corroborated by the lack of significant hypertrophy following long-term training (Fig. 3C).
RNAseq analysis revealed 341 up-regulated and 149 down-regulated genes in gastrocnemius muscle following a single bout overnight weightlifting. These are mainly functional genes in protein phosphorylation, protein modification, skeletal muscle cell differentiation, cellular protein metabolism and other cellular responses to extracellular stimuli, supporting the importance of these processes in early remodeling induced by resistance exercise. Increased Dscr1 (or Rcan1) mRNA is consistent with previous findings in skeletal muscle following eccentric contractions for promoting hypertrophy (45). Increased Fn14 mRNA is also in line with findings in human resistance exercise (34). Interestingly, Nr4a3 (also known as Nor1) gene was the most robustly (17-fold) induced in this study (Fig. 2D). Nr4a3 gene is highly responsive to endurance exercise (46) and has been shown to promote oxidative phenotype, autophagy and hypertrophy (47). Ankrd1 (also known as Carp) mRNA showed a 11-fold increase following overnight weightlifting, which has previously been shown to be induced by eccentric contractions in mice (48) and by exhaustive jumping exercise in humans (49). Finally, a robust upregulation of Atf3 mRNA (8-fold) was observed following weightlifting (Fig. 2D). Upregulation of Atf3 gene, which was induced by eccentric contractions (50), has anti-inflammatory roles by inhibiting chemokine and cytokine expression (51). These findings support that acute weightlifting elicits transcriptome responses as an early step in skeletal muscle adaptation to resistance exercise, laying a foundation for future dissection of molecular mechanisms underlying structural and functional adaptations to resistance training.
Resistance exercise promotes muscle hypertrophy and strength by enhancing anabolic processes in humans (52). Our long-term weightlifting training (8 weeks) resulted in moderate, but significant, hypertrophy in recruited muscles as indicated by increased CSA of plantar flexor muscles measured by MRI (17%) and muscle wet mass (14%) with no significant changes in tibialis anterior muscle and heart weight. The negative findings in antagonist tibialis anterior muscle and heart suggest that the increased muscle mass in recruited muscles (gastrocnemius, soleus and plantaris muscles) in weightlifting trained mice are not due to a general effect of moderately reduced body weight in the sedentary control mice. We did not observe changes of any of the parameters of cardiac function assessed by electrocardiogram and echocardiogram (Supplemental Fig. S2A and Supplemental Table S1 and S2). These findings are consistent with findings in human high-volume resistance exercise training (53). The degree of muscle hypertrophy in this study is highly consistent with that (8–12%) observed in humans (54). This is clearly different from the dramatic hypertrophy observed following surgical ablation of synergistic muscle, which reaches ~50% hypertrophy within a few days (8). The relatively moderate degree and slow process of muscle hypertrophy in our model suggest a very different underlying regulatory mechanisms compared with invasive muscle hypertrophy model, such as synergic muscle ablation.
Organ/tissue mass is determined by a balance between protein synthesis and degradation (i.e. protein turnover), and resistance training promotes anabolism in skeletal muscle, favoring protein synthesis over degradation (52). As a nodal sensor and integrator of environmental cues for organismal growth and homeostasis, the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) is particularly relevant since an acute muscle contraction increases phosphorylation of mTOR at Ser-2448, downstream p70S6 kinase-1 (S6K1) at Thr-389 and rpS6 at Ser-240/244 (13) along with increased protein synthesis (13). Importantly, mTORC1, but not mTORC2, is critical for maintaining muscle mass and metabolic function (55), and activation of mTORC1 contributes significantly to increased protein synthesis and hypertrophy induced by resistance exercise (19, 56). We speculated that resistance training induces both catabolic and metabolic adaptations in skeletal muscle through mTORC1 pathway for the following reasons: 1) Both mTOR-dependent and -independent mechanisms contribute to increased protein synthesis and muscle hypertrophy induced by resistance exercise-mimicking contractions (19); 2) mTORC1 in skeletal muscle is required for normal insulin sensitivity and intramyocellular lipid content (18), and 3) Isometric contractions increase Akt phosphorylation and Glut4 translocation to plasma membrane (17). Here, we showed significant increases of protein synthesis as measured by puromycin incorporation in hypertrophied muscle after 8 weeks of weightlifting training (Fig. 3E and Supplemental Fig. S2C) and at early time (Supplemental Fig. S2D) prior to detectable changes in muscle mass, consistent with the notion the increased protein synthesis is primarily responsible for skeletal muscle hypertrophy. To investigate the signaling pathway that is potentially involved in hypertrophic adaptation, we measured mTORC1 protein phosphorylation and expression in recruited skeletal muscle and observed enhanced protein expression of this pathway in the absence of profound changes in phosphorylation state (Fig. 3F), suggesting that enhanced protein expression as a novel mechanism of enhanced mTORC1 signaling capacity in promoting hypertrophy. Since the samples were harvested after overnight fast, this enhanced expression of mTORC1 proteins along with increased insulin response (Fig. 5E) may lead to a more dramatic increase of protein synthesis. This enhanced anabolic response may play a very important role in promoting skeletal muscle hypertrophy in these mice. To our knowledge, this is the first study that showed clear evidence of enhanced protein expression of mTORC1 pathway in promoting muscle hypertrophy following resistance exercise training.
Resistance training in humans increases the force and velocity of muscle contraction, collectively called muscle power (work/time). In this study, 8 weeks of weightlifting training resulted in significant increases of maximal force of twitch and tetanic contractions (Fig. 4A and 4B) similar to what was observed in humans (36, 37). Weightlifting training in mice also resulted in moderate increases of velocity of both twitch and tetanic contractions (Fig. 4A and 4B) as additional evidence of increased muscle power. Our measurements of maximal twitch and tetanic contractions were normalized by muscle mass, demonstrating improved contractile function beyond hypertrophy. This is highly consistent with findings in humans where improved maximal force by resistance training exceeded the increases of muscle cross-sectional area (36, 37). Since the measurements of muscle contractions were performed in vivo by motor nerve stimulation for isometric contractions with the same preload and maximal stimuli (currency and frequency) for all groups of mice, the differences in magnitude of torque generation are unlikely due to muscle mechanics, morphological factors and neural factors. We measured myosin heavy chain (MHC) proteins in the recruited gastrocnemius muscles and found no evidence of fiber type transformation (Fig. 4F and Supplemental Fig. S3E). The fact that the overall increases of muscle tetanic contraction without major impacts on the shape of force-frequency curve (Fig. 4C and Supplemental Fig. S3C) suggests that the improved muscle power is not due to alterations of calcium handling, i.e. release of Ca2+ from SR and/or restoration of Ca2+ level in the cytosol. While a previous study has shown progressive resistance exercise training increased cross-sectional area and peak Ca2+-activated force of all three types of isolated fiber ex vivo without affecting specific force (57), more recently studies have shown increased in vivo muscle specific force and myofibrillar ATPase activity following resistance training (58). The mechanisms underlying the improved muscle power, particularly specific force and velocity of muscle contraction, remain to be elucidated.
Autophagy is an important cellular process that facilitates unnecessary or toxic cargos removal in muscle quality control (59), and both insufficient and excessive autophagy contribute to muscle atrophy (60). Aerobic exercise promotes autophagy to clear toxic components, balance redox state and maintain cellular homeostasis (25, 26), whereas the effect of resistance exercise is unclear. It was reported that progressive resistance exercise-induced muscle hypertrophy was associated with reduced autophagy as indicated by decreased in LC3-II/I ratio and an increase in p62 level (23). In this study, we observed increased autophagy capacity (increased total LC3) and flux (decreased LC3-II/I ratio and p62 level) with no changes in Atg7 and Atg6 proteins (Fig. 5F), which is consistent with findings in humans (20). Autophagy is a catabolic process, which is unlikely to be directly related to hypertrophy. However, its impact on metabolic adaptation in endurance exercise is well known (24–26). In this study, weightlifting training led to enhanced autophagy concurrent with improved clearance of blood glucose in GTT (Fig. 5D) and increased Akt phosphorylation after insulin injection (i.p.) (Fig. 5E), recapitulating the findings in humans (61). To the best of our knowledge, this is the first study showing a profound positive impact of resistance exercise on whole body glucose metabolism with direct evidence of improved insulin sensitivity in skeletal muscle in an animal model. It is of note that the moderate muscle hypertrophy in recruited muscles alone does not appear to be responsible for the profound improvement of glucose metabolism. Future studies should focus on the signaling mechanism(s) by which resistance training promotes insulin sensitivity in skeletal muscle.
mTOR activates anabolic process under the condition of great availability of nutrient supply, including promoting protein synthesis and inhibition of catabolic autophagy through activating phosphorylation of p70S6K at threonine 389 (T389) and inhibitory phosphorylation of Ulk1 at serine 757 (S757) (62). On the contrary, energetic stress, such as exercise, stimulates catabolic processes of autophagy/mitophagy through activation of AMP-activated protein kinase (AMPK) and consequent activating phosphorylation of Unc-51 like autophagy activating kinase 1 (Ulk1) at serine 555 (S555) (24, 26). These two processes are typically considered antagonizing signaling events. However, these signaling pathways can be activated simultaneously as long as the activation mechanisms do not overlap. For example, system biology studies have shown that amino acid can acutely activate AMPK and Ulk1 through Ca2+/calmodulin-dependent protein kinase kinase (CaMKKβ) concurrently with activation of mTOR (63). We observed that weightlifting training stimulates autophagy flux and concurrently promotes protein expression in mTOR pathway along with enhanced responsiveness to insulin-stimulated Akt. These findings demonstrate that these two pathways can be enhanced through different mechanisms in physiological model of resistance exercise in mice.
In summary, we have developed a novel voluntary weightlifting model in mice, mimicking physiological adaptation similar to back squat exercise in humans. Using this model, we observed significant increased mRNA of genes that function in post-translational modification, metabolic control and muscle differentiation following a single bout exercise. We also observed that improved skeletal muscle power along with increased protein synthesis, muscle mass, and expression of proteins in the mTOR pathway following long-term (8 weeks) training. Weightlifting training resulted in profound improvement of whole-body glucose metabolism and skeletal muscle insulin sensitivity along with enhanced autophagy in recruited skeletal muscles. We conclude that resistance training promotes skeletal muscle adaptation and insulin sensitivity with simultaneous enhancement of autophagy and mTOR pathway. We believe that this study has paved the way for future studies to mechanistically dissect the underlying mechanisms of the physiological adaptations and their impact on physiology, health and disease prevention.
Supplementary Material
Acknowledgments
We thank Mr. R. Jack Roy at the Molecular Imaging Core of University of Virginia for the technical support for MRI imaging. The study was supported by U.S. National Institutes of Health (NIH) grant AR050429 (Z.Y.).
Nonstandard Abbreviations
- 4e-bp-1
Eukaryotic translation initiation factor 4E-binding protein 1
- AMPK
AMP-activated protein kinase
- Atg6
Autophagy-related 6
- Atg7
Autophagy-related 7
- CSA
cross-sectional area
- DEXA
dual-energy X-ray absorptiometry
- Dscr1
Down syndrome critical region 1
- EMG
electromyography
- Fn14
Fibroblast growth factor-inducible 14
- Gapdh
Glyceraldehyde 3-phosphate dehydrogenase
- GTT
Glucose tolerance test
- LC3
Microtubule-associated proteins 1A/1B light chain 3B
- MRI
Magnetic resonance imaging
- mTOR
Mechanistic target of rapamycin
- Nr4a3
Nuclear receptor subfamily 4, group A, member 3
- p62/Sqstm1
p62/Sequestosome 1
- p70S6K
p70S6 kinase
- Raptor
Regulatory-associated protein of mTOR
- SUnSET
Surface sensing of translation
- Ulk1
Unc-51 like autophagy activating kinase 1
References
- 1.Hawley JA, Hargreaves M, Joyner MJ, and Zierath JR (2014) Integrative biology of exercise. Cell 159, 738–749 [DOI] [PubMed] [Google Scholar]
- 2.Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, Broccatelli M, Savera G, D’Elia M, Pahor M, Bernabei R, Landi F, and Consortium S (2017) Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 29, 35–42 [DOI] [PubMed] [Google Scholar]
- 3.Safdar A, Saleem A, and Tarnopolsky MA (2016) The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol 12, 504–517 [DOI] [PubMed] [Google Scholar]
- 4.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, and Evans WJ (1990) High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263, 3029–3034 [PubMed] [Google Scholar]
- 5.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, and Bazzarre T (2000) AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 101, 828–833 [DOI] [PubMed] [Google Scholar]
- 6.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, and Ross R (2009) Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 169, 122–131 [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, and Arslanian S (2012) Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 61, 2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, and Hornberger TA (2011) The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589, 5485–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, and Peterson CA Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Barton ER, Sweeney HL, and Farrar RP (2004) Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol (1985) 96, 1097–1104 [DOI] [PubMed] [Google Scholar]
- 11.Sylow L, Moller LL, Kleinert M, Richter EA, and Jensen TE (2015) Stretch-stimulated glucose transport in skeletal muscle is regulated by Rac1. J Physiol 593, 645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong TS, and Booth FW (1990) Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol (1985) 69, 1718–1724 [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara R, Arihara Y, Takegaki J, Nakazato K, and Ishii N (2017) Relationship between exercise volume and muscle protein synthesis in a rat model of resistance exercise. J Appl Physiol (1985) 123, 710–716 [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RB, Marum P, Tullson P, and Saubert C. W. t. (1979) Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol Respir Environ Exerc Physiol 46, 835–842 [DOI] [PubMed] [Google Scholar]
- 15.Klitgaard H (1988) A model for quantitative strength training of hindlimb muscles of the rat. J Appl Physiol (1985) 64, 1740–1745 [DOI] [PubMed] [Google Scholar]
- 16.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, and Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 17.Kido K, Yokokawa T, Ato S, Sato K, and Fujita S (2017) Effect of resistance exercise under conditions of reduced blood insulin on AMPKalpha Ser485/491 inhibitory phosphorylation and AMPK pathway activation. Am J Physiol Regul Integr Comp Physiol 313, R110–R119 [DOI] [PubMed] [Google Scholar]
- 18.Guridi M, Kupr B, Romanino K, Lin S, Falcetta D, Tintignac L, and Ruegg MA (2016) Alterations to mTORC1 signaling in the skeletal muscle differentially affect whole-body metabolism. Skelet Muscle 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogasawara R, Fujita S, Hornberger TA, Kitaoka Y, Makanae Y, Nakazato K, and Naokata I (2016) The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep 6, 31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hentila J, Ahtiainen JP, Paulsen G, Raastad T, Hakkinen K, Mero AA, and Hulmi JJ (2018) Autophagy is induced by resistance exercise in young men, but unfolded protein response is induced regardless of age. Acta Physiol (Oxf) 224, e13069. [DOI] [PubMed] [Google Scholar]
- 21.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, and Rasmussen BB (2013) Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, Li X, and Qin ZH (2013) Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol 48, 427–436 [DOI] [PubMed] [Google Scholar]
- 23.Kwon I, Jang Y, Cho JY, Jang YC, and Lee Y (2018) Long-term resistance exercise-induced muscular hypertrophy is associated with autophagy modulation in rats. J Physiol Sci 68, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C, Sumpter R Jr., and Levine B (2012) Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 8, 1548–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, and Yan Z (2013) Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27, 4184–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, and Yan Z (2017) Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia LG, Donnet C, Bogaev RC, Blatt RJ, McKinney CE, Day KH, Berr SS, Jones LR, Moorman JR, Sweadner KJ, and Tucker AL (2005) Hypertrophy, increased ejection fraction, and reduced Na-K-ATPase activity in phospholemman-deficient mice. Am J Physiol Heart Circ Physiol 288, H1982–1988 [DOI] [PubMed] [Google Scholar]
- 28.Wilson RJ, Drake JC, Cui D, Ritger ML, Guan Y, Call JA, Zhang M, Leitner LM, Godecke A, and Yan Z (2019) Voluntary running protects against neuromuscular dysfunction following hindlimb ischemia-reperfusion in mice. J Appl Physiol (1985) 126, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Call JA, Chain KH, Martin KS, Lira VA, Okutsu M, Zhang M, and Yan Z (2015) Enhanced skeletal muscle expression of extracellular superoxide dismutase mitigates streptozotocin-induced diabetic cardiomyopathy by reducing oxidative stress and aberrant cell signaling. Circ Heart Fail 8, 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman CA, and Hornberger TA (2013) Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 41, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganta VC, Choi M, Farber CR, and Annex BH (2019) Antiangiogenic VEGF165b Regulates Macrophage Polarization via S100A8/S100A9 in Peripheral Artery Disease. Circulation 139, 226–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi S, Liu X, Li P, Akimoto T, Lee SY, Zhang M, and Yan Z (2005) Transcriptional profiling in mouse skeletal muscle following a single bout of voluntary running: evidence of increased cell proliferation. J Appl Physiol (1985) 99, 2406–2415 [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld BJ (2010) Squatting kinematics and kinetics and their application to exercise performance. J Strength Cond Res 24, 3497–3506 [DOI] [PubMed] [Google Scholar]
- 34.Raue U, Jemiolo B, Yang Y, and Trappe S (2015) TWEAK-Fn14 pathway activation after exercise in human skeletal muscle: insights from two exercise modes and a time course investigation. J Appl Physiol (1985) 118, 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, and Hornberger TA (2011) Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25, 1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen LL, Andersen JL, Magnusson SP, Suetta C, Madsen JL, Christensen LR, and Aagaard P (2005) Changes in the human muscle force-velocity relationship in response to resistance training and subsequent detraining. J Appl Physiol (1985) 99, 87–94 [DOI] [PubMed] [Google Scholar]
- 37.Kanehisa H, Ikegawa S, and Fukunaga T (1997) Force-velocity relationships and fatiguability of strength and endurance-trained subjects. Int J Sports Med 18, 106–112 [DOI] [PubMed] [Google Scholar]
- 38.Yan L, Sundaram S, and Nielsen FH (2017) Voluntary running of defined distances reduces body adiposity and its associated inflammation in C57BL/6 mice fed a high-fat diet. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 42, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 39.Yaspelkis BB 3rd, Singh MK, Trevino B, Krisan AD, and Collins DE (2002) Resistance training increases glucose uptake and transport in rat skeletal muscle. Acta Physiol Scand 175, 315–323 [DOI] [PubMed] [Google Scholar]
- 40.Sandri M, Coletto L, Grumati P, and Bonaldo P (2013) Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci 126, 5325–5333 [DOI] [PubMed] [Google Scholar]
- 41.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jaattela M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C, Madeo F, Martens S, Martinez J, Melendez A, Mizushima N, Munz C, Murphy LO, Penninger JM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Santambrogio L, Scorrano L, Simon AK, Simon HU, Simonsen A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, and Kroemer G (2017) Molecular definitions of autophagy and related processes. EMBO J 36, 1811–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, and Peterson CA (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cholewa J, Guimaraes-Ferreira L, da Silva Teixeira T, Naimo MA, Zhi X, de Sa RB, Lodetti A, Cardozo MQ, and Zanchi NE (2014) Basic models modeling resistance training: an update for basic scientists interested in study skeletal muscle hypertrophy. J Cell Physiol 229, 1148–1156 [DOI] [PubMed] [Google Scholar]
- 44.Stylianou AP, Guess TM, and Kia M (2013) Multibody muscle driven model of an instrumented prosthetic knee during squat and toe rise motions. J Biomech Eng 135, 041008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitomi Y, Kizaki T, Katsumura T, Mizuno M, Itoh CE, Esaki K, Fujioka Y, Takemasa T, Haga S, and Ohno H (2003) Effect of moderate acute exercise on expression of mRNA involved in the calcineurin signaling pathway in human skeletal muscle. IUBMB Life 55, 409–413 [DOI] [PubMed] [Google Scholar]
- 46.Mahoney DJ, Parise G, Melov S, Safdar A, and Tarnopolsky MA (2005) Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19, 1498–1500 [DOI] [PubMed] [Google Scholar]
- 47.Pearen MA, Eriksson NA, Fitzsimmons RL, Goode JM, Martel N, Andrikopoulos S, and Muscat GE (2012) The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol Endocrinol 26, 372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, and Lieber RL (2007) Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 293, C218–227 [DOI] [PubMed] [Google Scholar]
- 49.Koskinen SOA, Kyrolainen H, Flink R, Selanne HP, Gagnon SS, Ahtiainen JP, Nindl BC, and Lehti M (2017) Human skeletal muscle type 1 fibre distribution and response of stress-sensing proteins along the titin molecule after submaximal exhaustive exercise. Histochem Cell Biol 148, 545–555 [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Verdejo R, Vanwynsberghe AM, Hai T, Deldicque L, and Francaux M (2017) Activating transcription factor 3 regulates chemokine expression in contracting C2C12 myotubes and in mouse skeletal muscle after eccentric exercise. Biochem Biophys Res Commun 492, 249–254 [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Verdejo R, Vanwynsberghe AM, Essaghir A, Demoulin JB, Hai T, Deldicque L, and Francaux M (2017) Activating transcription factor 3 attenuates chemokine and cytokine expression in mouse skeletal muscle after exercise and facilitates molecular adaptation to endurance training. FASEB J 31, 840–851 [DOI] [PubMed] [Google Scholar]
- 52.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, and Rasmussen BB (2010) Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299, R533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, and Green DJ (2011) A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol 589, 5443–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blazevich AJ, Cannavan D, Coleman DR, and Horne S (2007) Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol (1985) 103, 1565–1575 [DOI] [PubMed] [Google Scholar]
- 55.Ohanna M, Sobering A, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly P, Sotiropoulos A, and Pende M (2005) Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7, 286–294 [DOI] [PubMed] [Google Scholar]
- 56.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, Hornberger TA, Spriet LL, Heigenhauser GJ, and Philp A (2017) Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep 7, 5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widrick JJ, Stelzer JE, Shoepe TC, and Garner DP (2002) Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283, R408–416 [DOI] [PubMed] [Google Scholar]
- 58.Philippe AG, Lionne C, Sanchez AMJ, Pagano AF, and Candau R (2019) Increase in muscle power is associated with myofibrillar ATPase adaptations during resistance training. Exp Physiol 104, 1274–1285 [DOI] [PubMed] [Google Scholar]
- 59.Sandri M (2010) Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298, C1291–1297 [DOI] [PubMed] [Google Scholar]
- 60.Sandri M (2013) Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol 45, 2121–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russell RD, Nelson AG, and Kraemer RR (2014) Short bouts of high-intensity resistance-style training produce similar reductions in fasting blood glucose of diabetic offspring and controls. J Strength Cond Res 28, 2760–2767 [DOI] [PubMed] [Google Scholar]
- 62.Kim J, Kundu M, Viollet B, and Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalle Pezze P, Ruf S, Sonntag AG, Langelaar-Makkinje M, Hall P, Heberle AM, Razquin Navas P, van Eunen K, Tolle RC, Schwarz JJ, Wiese H, Warscheid B, Deitersen J, Stork B, Fassler E, Schauble S, Hahn U, Horvatovich P, Shanley DP, and Thedieck K (2016) A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nat Commun 7, 13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.