Abstract
Introduction:
Traffic-related air pollution has been shown to be neurotoxic to the developing fetus and in term-born infants during early childhood. It is unknown whether there is an increased risk of adverse neurobehavioral outcome in preterm infants exposed to higher levels of air pollution during the fetal period.
Objective:
To assess the association between prenatal exposure to traffic-related air pollution on early preterm infant neurobehavior.
Methods:
Air pollution exposure was estimated by two methods: density of major roads and density of vehicle-miles traveled (VMT), each at multiple buffering areas around residential addresses. We examined the association between prenatal exposure to traffic-related air pollution and performance on the Neonate Intensive Care Unit (NICU) Network Behavioral Scale (NNNS), a measure of neurobehavioral outcome in infancy for 240 preterm neonates enrolled in the NICU-Hospital Exposures and Long-Term Health cohort. Linear regression analysis was conducted for exposure and individual NNNS subscales. Latent profile analysis (LPA) was applied to classify infants into distinct NNNS phenotypes. Multinomial logistic regression analysis was conducted between exposure and LPA groups. Covariates included gestational age, birth weight z-score, post-menstrual age at NNNS assessment, socioeconomic status, race, delivery type, maternal smoking status, and medical morbidities during the NICU stay.
Results:
Among all 13 NNNS subscales, hypotonia was significantly associated with VMT (104 vehiclemile/km2) in 150m (β=0.01, P-value<0.001), 300m (β=0.01, P-value=0.003), and 500m (β=0.01, P-value=0.002) buffering areas, as well as with road density in a 500m buffering area (β=0.03, P-value=0.03). We identified three NNNS phenotypes by LPA. Among them, high density of major roads within 150m, 300m, and 500m buffers of the residential address was significantly associated with the same phenotype (P<0.05).
Conclusion:
Prenatal exposure to intensive air pollution emitted from major roads may impact early neurodevelopment of preterm infants. Motor development may be particularly sensitive to air pollution-related toxicity.
Keywords: Traffic-related air pollution, NNNS, latent profile analysis
Introduction
Traffic-related air pollution (TRAP) is a mixture of ubiquitous toxic chemicals generated by the combustion of fossil fuels. TRAP is known to be neurotoxic [1], likely via oxidative stress and neuroinflammation caused by particulate matter [2, 3], polycyclic aromatic hydrocarbons [4], or other chemicals in vehicle exhaust [5–7]. Epidemiologic studies have reported that exposure to TRAP can adversely affect children’s cognitive function [8–10], executive function [11], and behavioral development [12]. Our existing understanding of perinatal TRAP neurotoxicity comes from studies of young or school age children. Evidence for the impact of TRAP on infant development is lacking.
Many known components of traffic-related air pollution can cross the placental barrier and enter the fetal circulation [13–15]. The current epidemiological evidence for prenatal or perinatal exposure to TRAP and neurobehavioral function during infancy is mixed. One recent study suggested that living close to a major road during pregnancy may be adversely associated with behavioral development in infants ranging from 8 to 36 months age, but did not find a statistically significant association [16]. Other studies reported that prenatal exposure to traffic-related air pollution (e.g., suspended particulate matter, NO2, SO2, and benzene) may adversely affect infants’ cognitive, verbal, and motor development [12, 17].
Neurobehavioral disorders are prevalent [18], with significantly increased rates among preterm infants [19]. The Neonatal Intensive Care Unit (NICU) Network Neurobehavioral Scale (NNNS) is a measurement of neurobehavioral outcome in infancy, which was developed to detect infants at high risk of neurobehavioral disorders early in life [20, 21]. The NNNS consists of 13 individual subscales that grade an infant’s resting tone, basic reflexes, motor function, attention, social reactivity, and stress response [20]. The NNNS has been used in numerous studies as a valid indicator of infants’ neurodevelopment [22–24].
Previous work has shown associations between NNNS performance and different types of exposure, including environmental chemicals [25–29], maternal psychological conditions [25, 30], and health conditions [23]. One conclusion is that NNNS is highly sensitive to both internal and external exposure during the prenatal and perinatal period. To our knowledge, the only literature linking prenatal TRAP exposure to NNNS performance is a single conference abstract that reported that prenatal exposure to traffic-related air pollution was associated with high arousal in a term birth cohort [31]. There are no prior studies of the impact of TRAP on early neurobehavioral performance among preterm infants. We hypothesized that preterm infants, a sensitive population for neurobehavioral toxicity [32] may demonstrate neurobehavioral impairment associated with perinatal TRAP exposure.
Methods
NICU-HEALTH
The NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) cohort [33] is part of the DINE (Developmental Impact of NICU Exposures) cohort of the ECHO (Environmental influences on Child Health Outcomes) program (ClinicalTrials.gov NCT01420029, NCT01963065, NCT03061890; http://www.nih.gov/echo). It is a cohort study aimed at evaluating environmental exposures and associated neurobehavioral development in preterm infants. NICU-HEALTH enrolls infants born at birth weight <1500 g or with gestational age less than 33 weeks at the Mount Sinai Hospital. Enrollment has occurred in two phases thus far. Phase I enrolled preterm infants between 2011 to 2013; phase II launched in 2015 and enrolls to the present. Through July 2019, NICU-HEALTH has enrolled 284 preterm infants and collected biospecimens including urine, stool, hair, saliva, and blood from infants and their mothers during the infants’ NICU stay. We have previously examined the association between NICU-based phthalate exposure to and neurologic function among NICU-HEALTH participants [22, 29]. NICU-HEALTH was approved by the Mount Sinai Program for the Protection of Human Subjects.
Geocoding
Families of 263 NICU-HEALTH mothers reported their residential address at the time of their preterm infants’ birth. This was used as the prenatal residential address. Residential addresses were geocoded using a method developed by the United States (U.S) Census Bureau [34]. Briefly, geocoding was conducted using the geocoding tool in ArcGIS Pro software (ESRI, Redlands, CA) and the U.S. Topologically Integrated Geographic Encoding and Referencing (TIGER)/Line 2017 database. TIGER/Line files are structured with street segments, usually from intersection to intersection, such that a range of addresses is coded for each side of the street. The results of geocoding were the latitude and longitude of residential addresses.
Exposure
TRAP is highly concentrated near major roads [35]. Most of our study participants were living near road. As such, proximity to major road, which is a commonly used measure of traffic-related air pollution, was not applicable to our analysis due to its small variation among our study participants. We estimated TRAP by calculating the density of roads and vehicle-miles traveled (VMT) within 150m, 300m, and 500m of a participants’ home. Traffic density and VMT data in our study area was obtained from the U.S. Department of Transportation’s Highway Performance and Monitoring System (HPMS) in Shapefile format [36], which has been used as a valid provider of traffic volume and road network data in previous studies [37–40]. The Shapefile compiled the annual average daily traffic (AADT) for all highways and roads maintained by the HPMS. AADT represents the average number of vehicles traveled in both directions on a road in a single day. In this study, exposure assessment was restricted to roads with more than 10,000 AADT in the study area. We obtained the Shapefiles for the years 2011–2017 to match the birth years of NICU-HEALTH participants. Vehicle classification data were not available for all years, so were not included in analyses. Geospatial analyses were conducted in the ArcGIS Pro software (ESRI, Redlands, CA).
Road density was calculated in 100 meter per square kilometer units. Conceptually, a circular buffer was drawn around each participant’ geocoded residential address. The length of roads that fell within the buffer was calculated. The road density was calculated as the sum of all linear roads (m) divided by the area of buffer (km2). The unit of road density was scaled to 103 m/km2 in statistical analysis.
VMT density was calculated in VMT per square kilometer units. The VMT of each buffer was calculated as the sum of “AADT x length of road” for all road segments located inside the buffering area [40]. The VMT density (vehicle-mile/km2) was calculated as the sum of VMTs divided by the area of the buffer (km2). The unit of VMT density was scaled to 104 vehicle-mile/km2 for statistical analysis.
Neurobehavioral Assessment
Participants underwent neurobehavioral assessment prior to NICU discharge with the NICU Network Neurobehavioral Scale (NNNS) administered by a certified examiner. Exams were performed between 34 and 37 weeks postmenstrual age (PMA).
Statistical analysis
Descriptive statistics were calculated for exposure variables, subscales of NNNS, and covariates. Linear regression models were used to estimate the association between each exposure and each subscale of the NNNS, adjusting for gestational age (GA) at birth, birth weight (BW) z-score, PMA at NNNS assessment, insurance type (Medicaid or private insurance), neighborhood socioeconomic (SES) z-score race (Caucasian, African American, or other), ethnicity (Hispanic or not), maternal smoking status (smoking or non-smoking during pregnancy), delivery type (vaginal or Cesarean), NICU morbidity, and base deficit at birth (a surrogate biomarker of degree of illness at birth). All of those adjusted covariates were collected by the NICU-HEALTH cohort except for the variable of neighborhood SES z-score. The calculation of neighborhood SES z-score was referred to the methods used by large population studies [41, 42]. The neighborhood SES z-score is a sum of the z-scores of six 2015 US American Community Survey [43] measures in our study area: median household income, % of households receiving interest, dividend, or net rental income, % of adults 25+ with high school degree, % of adults 25+ with a college degree, % of individuals ages 16+ in professional, managerial, or executive occupations, and % of people with income in the past 12 months below poverty level. Individuals were assigned to the neighborhood SES z-score of the block group they resident in.
We further identified NNNS phenotypes and the association of phenotype with prenatal exposure to TRAP based on a multi-step approach. Prior to undertaking this analysis, we examined the missing rate for all 13 subscales of NNNS in our dataset. The majority (56.7%) of participants were missing data for the “habituation” subscale as this subscale requires the infant to be deeply asleep at the beginning of the exam. We thus excluded “habituation” from the multi-step analysis.
We identified NNNS phenotypes among study participants by completing a latent profile analysis (LPA). In the first step, the optimal number of latent profiles was chosen by Akaike information criterion (AIC), Bayesian information criterion (BIC), Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (LMRT), and Bootstrap Likelihood Ratio Test (BLRT). For LMRT and BLRT, we compared the likelihood between models with k to (k-1) classes. The null hypothesis was that the k-1 class model would be adequate to describe the latent profiles of this population, thus a significant result would suggest that a k class model could rather necessary. In the second step, the conditional posterior probability of being one profile was calculated. In the third step, the association between identified latent profile subgroup and exposure was investigated by introducing covariates into a multinomial logistic regression model with the conditional posterior probabilities as the dependent variable. Covariates included in the multinomial logistic model were the same to those used in the linear regression models fitted previously. The LPA and multinomial logistic regressions were conducted using R3STEP function in Mplus Version 8.2 software [44].
Sensitivity analysis
NNNS performance is strongly affected by the PMA of preterm infants at the time of the examination due to the rapid growth and development of the central nervous system during the preterm period. To avoid misclassification of NNNS phenotype caused by chronological or gestational age, we additionally compared PMA and GA variables between different latent profiles groups. If the difference of PMA and gestational age was not statistically significant, then the LPA result was considered to be valid and not influenced by the developmental stage of the infant at the time of the exam.
Results
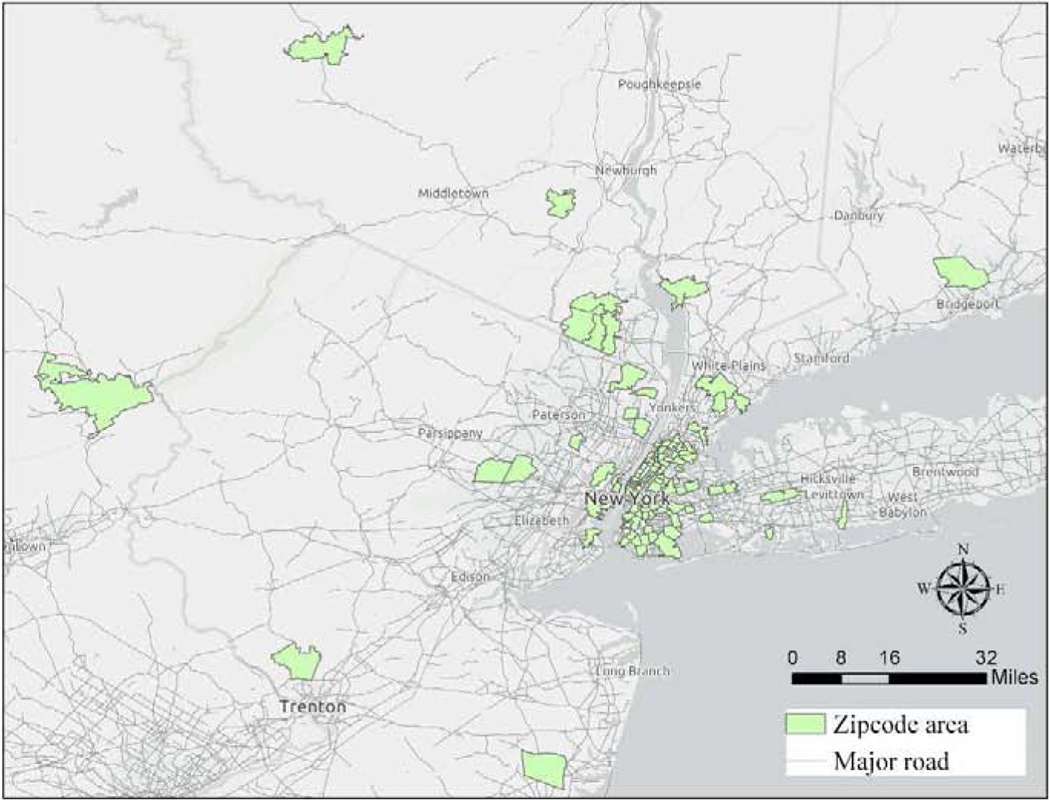
Geocoding yielded to 96.7% completion rate, identifying the coordinates of 255 families’ residential addresses. Figure 1 shows the geographic distribution of all geocoded participants. To protect participants’ identifying information, only the zipcode areas of each home is shown on the map. Among those whose addresses were geocoded, 82.7% lived in four of the five boroughs of New York City: Manhattan, Queens, Brooklyn, and the Bronx. Table 1 shows the demographic and clinical characteristics of the study population. The average gestational age of included preterm infants was 30.1 (±2.1) weeks. The characteristics of preterm infants enrolled in this study did not differ significantly from the complete population of the NICU-HEALTH cohort. The inclusion and exclusion of study participants in each step of analysis is shown in Figure S1 (Supplementary Material).
Figure 1.
Geographic Distribution of NICU-HEALTH participants. The highlighted areas are the zipcodes lived by NICU-HEALTH participants. The grey lines show the roads with more than 10,000 annual average daily traffic (AADT). Road data were obtained from the Highway Performance and Monitoring System (HPMS) representing the study year of 2012.
Table 1.
Characteristics of Participants.
| Included (N=228)a | NICU-HEALTH (N=284) | P-value of Comparison | ||||
|---|---|---|---|---|---|---|
| No. ( %) | Mean (SD) | No. (%) | Mean (SD) | |||
| Sex | ||||||
| Boy | 112 (49.1) | 144 (50.7) | 0.72 | |||
| Girl | 116 (50.9) | 140 (49.3) | ||||
| Gestational Age (week) | 30.1 (2.1) | 30.2 (2.0) | 0.37 | |||
| Birth weight z-score | −0.1 (0.8) | −0.2 (0.9) | 0.79 | |||
| Race | ||||||
| Caucasian | 135 (59.2) | 162 (57.0) | 0.71 | |||
| African American | 57 (25.0) | 61 (21.5) | ||||
| Other | 36 (15.8) | 61 (21.5) | ||||
| Ethnicity | ||||||
| Hispanic | 21 (9.2) | 25 (8.8) | 0.83 | |||
| Other | 207 (90.8) | 263 (92.6) | ||||
| Delivery Type | ||||||
| Vaginal | 105 (46.1) | 123 (43.3) | 0.53 | |||
| Cesarean | 123 (53.9) | 161 (56.7) | ||||
| Insurance type | ||||||
| Medicaid | 64 (28.1) | 81 (28.5) | 0.91 | |||
| Private Insurance | 164 (71.9) | 203 (71.5) | ||||
| Neighborhood SES Z-score | 0.00 (3.75) | -b | - | - | ||
| Maternal smoking | ||||||
| Yes | 47 (20.6) | 54 (19.0) | 0.48 | |||
| No | 171 (79.4) | 230 (81.0) | ||||
| NICU morbidityc | ||||||
| Yes | 53 (23.3) | 69 (25.0) | 0.67 | |||
| No | 174 (76.7) | 207 (75.0) | ||||
| Base deficit (mEq/L) | −3.6 (3.6) | −3.6 (3.5) | ||||
Criteria of inclusion: whose addresses were geocoded and NNNS were measured.
Neighborhood SES was not available for whose residential addresses were not geocoded.
NICU morbidity has missing rates.
Table S1 (Supplementary Material) shows coefficients and P-values for traffic exposure regressing on each subscale of the NNNS, with covariate adjustment. Among all 13 subscales, hypotonia was significantly associated with VMT density (104 vehicle-mile/km2) in 150m (β=0.01, P-value<0.0001), 300m (β=0.01, P-value=0.003), and 500m (β=0.01, P-value=0.002) buffering areas, as well as with road density (103m/km2) in the 500m buffering area (β=0.03, P-value=0.03).
We analyzed the latent profiles of 214 preterm infants who had complete data for 12 NNNS subscales (habituation was excluded). In model fitting, we tested 2–4 profiles. A 3-profile solution was selected as the best model (Table 2). The change of bootstrap P-value from a 3-profile to a 4-profile model indicated that the 3-profile model was better than the 4-profile model (P-value < 0.001 compared to P value = 1.00). The AIC and BIC also suggested that a 3-profile model fit better than the other tested models. The P-values for LMR were not statistically significant in all tested models.
Table 2.
The diagnosis of latent profile analysis given two-four number of profiles.
| N of Profiles | Two | Three | Four |
|---|---|---|---|
| Profile 1 | 35 | 13 | 36 |
| Profile 2 | 179 | 35 | 0 |
| Profile 3 | 166 | 0 | |
| Profile 4 | 177 | ||
| Model Diagnosis | |||
| AIC | 3408.54 | 3196.57 | 3460.54 |
| BIC | 3533.08 | 3364.87 | 3672.60 |
| Sample-Size Adjusted BIC | 3415.84 | 3206.43 | 3472.96 |
| Entropy | 0.92 | 0.96 | 0.96 |
| LMR P-value | 0.17 | 0.21 | 0.50 |
| LMR Adjusted P-value | 0.17 | 0.22 | 0.50 |
| Bootstrap P-value | 0.00 | 0.00 | 1.00 |
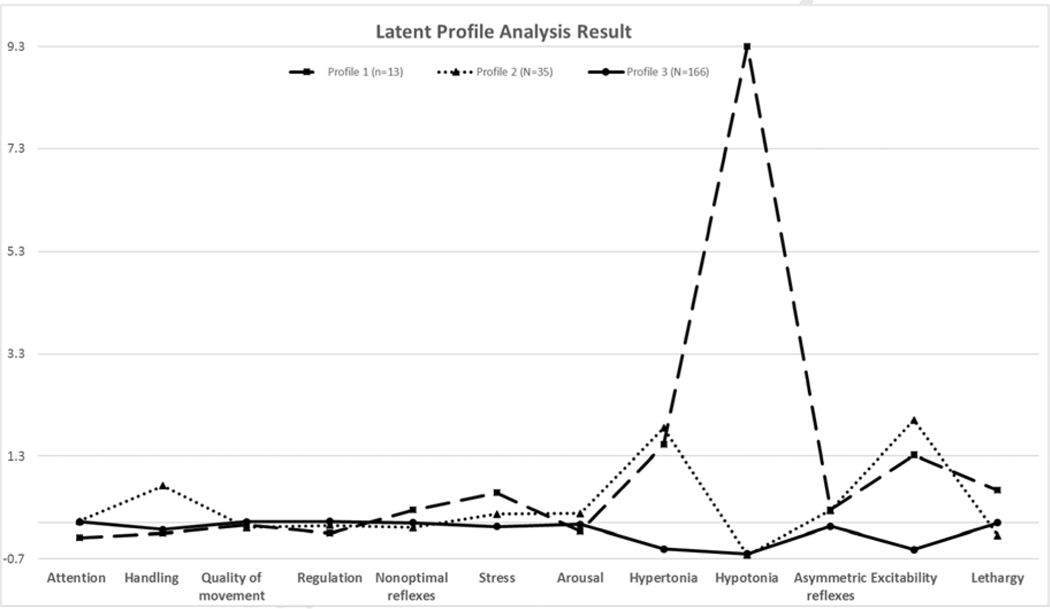
Preterm infants were grouped into three identified latent profiles of NNNS performance, which were considered as three distinct neurobehavioral phenotypes. The heterogeneity of the three phenotypes is shown in Table 3 and Figure 2. The percentage of preterm infants in profiles 1, 2, and 3 were 6.1%, 16.4%, and 77.6%, respectively. Preterm infants in profile 3had the average scores for all 12 analyzed NNNS subscales, representing typical development. Preterm infants in profile 1 had a higher average score for the hypotonia and lethargy subscales than those in other two groups. Preterm infants in profile 2 had higher scores for hypertonia and excitability subscales than those in the other two groups. The comparisons of PMA and gestational age between phenotype groups were not statistically significant.
Table 3.
The mean (standard deviation) of NNNS subscales in each of the three latent profile groups.
| Profile 1 (N=13, 6.1%) | Profile 2 (N=35, 16.4%) | Profile 3 (N=166, 77.6%) | Mean | ANOVA P-value | |
|---|---|---|---|---|---|
| Attention | 3.29 (0.29) | 4.75 (0.16) | 4.68 (0.06) | 4.60 (0.68) | <0.0001 |
| Handling | 0.12 (0.06) | 0.27 (0.04) | 0.13 (0.01) | 0.15 (0.03) | 0.03 |
| Quality of movement | 4.55 (0.10) | 4.32 (0.8) | 4.86 (0.03) | 4.76 (0.14) | <0.0001 |
| Regulation | 4.69 (0.16) | 5.56 (0.09) | 5.98 (0.04) | 5.84 (0.29) | <0.0001 |
| Non-optimal reflexes | 6.93 (0.65) | 5.03 (0.29) | 5.53 (0.16) | 5.54 (3.80) | 0.23 |
| Stress | 0.15 (0.01) | 0.11 (0.01) | 0.09 (0.002) | 0.09 (0.001) | <0.0001 |
| Arousal | 2.85 (0.09) | 4.02 (0.09) | 3.30 (0.03) | 3.39 (0.19) | 0.02 |
| Hypertonia | 0.15 (0.11) | 0.17 (0.07) | 0.03 (0.01) | 0.06 (0.06) | 0.001 |
| Hypotonia | 0.77 (0.31) | 0.03 (0.03) | 0.03 (0.01) | 0.08 (0.11) | <0.0001 |
| Asymmetric reflexes | 1.20 (0.39) | 1.20 (0.20) | 0.89 (0.08) | 0.96 (1.14) | 0.103 |
| Excitability | 2.32 (0.35) | 3.00 (0.30) | 0.48 (0.07) | 1.00 (1.62) | <0.0001 |
| Lethargy | 8.46 (0.72) | 3.92 (0.29) | 5.17 (5.17) | 5.19 (3.69) | 0.009 |
Figure 2.
The three profiles calculated from latent profile analysis with 12 NNNS subscales. Y axis is calculated as the ratio of “mean (profile) – mean (all participants)” and “mean (all participants)” for each subscale.
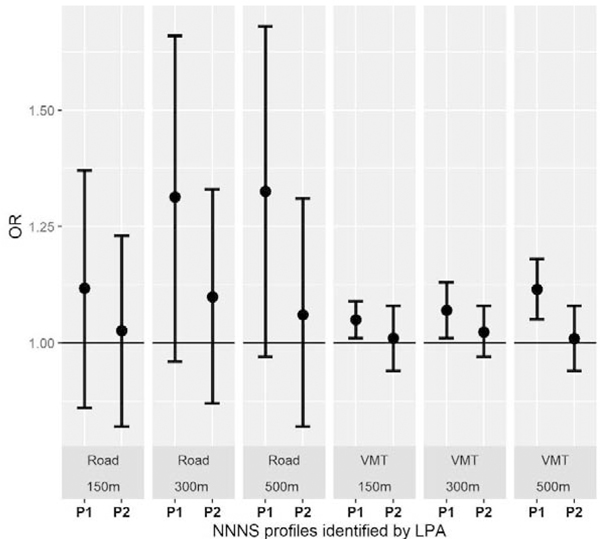
The associations between TRAP exposure and the posterior probability of phenotypes were examined using multinomial logistic regression modeling. Preterm infants in profile 3 were modeled as the reference. Figure 2 shows the adjusted odds ratio (OR) and 95% confidence interval (CI) for each of the exposure variables by phenotype and by buffering areas. Exposure to higher intensity of traffic was associated with profiles 1 and 2. Specifically, compared with the reference group, those who demonstrated a profile 1 phenotype had higher exposure to VMT (104 vehicle-mile/km2) during the prenatal period in all study buffering areas (for 150m buffer: OR=1.05, 95% CI=1.01, 1.09; for 300m buffer: OR=1.07, 95% CI=1.01, 1.13; for 500m buffer: OR=1.12, 95% CI=1.05, 1.18). The association between road density (103m/km2) and profile 1 was positive but not statistically significant. Associations between road density, VMT density, and the profile 2 phenotype was found to be positive in all models, but their 95% CIs still overlapped the null.
Discussions
Prenatal exposure to traffic density was associated with characteristic motor phenotypes in our cohort of hospitalized preterm infants. This association was consistently found for exposures measured in 150m, 300m, and 500m buffers around homes and the effect was stronger when roads inside a larger buffering area were included. As our entire study population was born in the late second or early third trimester, our study suggests that the impact of prenatal TRAP exposure on neurobehavioral development begins early in gestation. We also found that prenatal TRAP exposure could affect infants’ NNNS profile over a moderate distance. Larger road buffers included more roads than smaller buffers such that traffic-related air pollution in larger buffers was more strongly associated with our outcome of interest. The odds of demonstrating profile 1 performance were 1.05 to 1.12 times higher than the reference group given a one unit (104 vehicle-mile/km2) increase of VMT. The area of a 500m buffer is 0.79 km2. A unit increase of VMT in a 500m buffer represents approximately 1256 additional vehicles passing the residence in a day. Based on the average AADT of different types of road, adding a road with 1256 AADT means adding a urban boulevard into the buffer [45]. We thus found a strong association of TRAP with the neurobehavioral profile of preterm infants.
The NNNS was developed as an assessment for infants at risk for abnormal neurodevelopment. We observed an association between TRAP exposure and the hypotonia subscale of the NNNS in linear regression analysis. There are some challenges to using the scores of NNNS in epidemiological research. The 13 NNNS subscales are quite heterogeneous; a higher score indicates an optimal result on one subscale but adverse performance on another subscale. Many subscales are not independent; they can be correlated directly or inversely. These obstacles can be addressed by LPA [46]. LPA is an unsupervised clustering approach which calculates a reduced-dimension indicator of NNNS, where individuals group into a range of latent profiles based on their scores of all available subscales. This approach has been applied to identify NNNS phenotypes in high-risk births [47], healthy births [23, 24], and preterm births [48] in epidemiologic studies.
The three NNNS profiles identified by LPA were consistent with the three profiles found in Sucharew et al [24], a study of healthy infants selected from low exposure areas. Sucharew et al. [24] considered infants in their hypotonic group, similar to our profile 1, having unfavorable development because they later demonstrated worse psychomotor development and lower externalizing scores on the Bayley Scales of Infant and Toddler Development and the Behavior Assessment System for Children – 2 [24]. In our study, preterm infants in profile 1 had experienced higher prenatal exposure to TRAP. All mothers of preterm infants with profile 1 phenotype lived in New York City while pregnant, where a large volume of gasoline-powered vehicles likely contributed the traffic-related air pollution. The links found between TRAP and profile 1 mirror problems with TRAP-associated motor development in other studies of older infants and children [16, 49]. However, our findings are in contrast findings in the Health Outcomes and Measures of the Environment (HOME) study where TRAP was associated with higher arousal scores but not motor abnormalities [31]. Given the differences between the hospitalized preterm NICU-HEALTH cohort and the community-based term-born HOME cohort, we cannot make a direct comparison for NNNS performance between the two. HOME participants of were healthy infants with NNNS completed approximately 5 weeks after birth (PMA 42–47 weeks), while the majority of NICU-HEALTH study participants had not reached full-term equivalent (37–40 weeks) when their NNNS was completed. Our study population had lower average arousal scores at baseline compared to term births [23, 24], thereby the power to detect an association between TRAP and arousability might be hindered.
Several biological mechanisms could explain the association between hypotonia and perinatal TRAP exposure. Hypotonia can be caused by damages at many levels of the nervous and musculoskeletal systems, from the brain to peripheral nerves to muscles [50]. Inhalation of air pollutants could cause neuroinflammation and has been see in both human and animal models reflected in increased cytokine production and reactive oxygen species in the brain [51]. Laboratory studies found that levels of proinflammatory cytokines (IL-1α, TNF-α) and immune-related transcription factors (NF-kB and AP-1) were increased in mouse’s brain after exposure to inhaled particulate matter [52]. In a study examining neurogenesis in relation to nano-sized traffic-related air pollutant exposure, male rats exposed prenatally had 70% fewer newly generated neurons in the dentate gyrus of the hippocampus at 5 months of age (equivalent to human adolescence) than rats without prenatal TRAP exposure [53]. Several MAP kinase pathways seem to link TRAP exposure and neuroinflammation in the brain [3]. Although a single biological mechanism for autism spectrum disorder (ASD) has not been established, studies suggest that the MAPK pathway may play a key role in regulating genetic variants in relation to ASD [54]. In addition to direct effects on CNS development, vehicle exhaust contains several endocrine disruptors that could disrupt thyroid balance in infants [55]. One early study found increased risk of hypotonia among infants prenatally exposed to polychlorinated biphenyls (PCBs) [56], which likely act by interrupting thyroid function [57]. Additionally, exposure to particulate matter during the third trimester has been associated with reduced free thyroid hormone (FT3, FT4) and thyroid-stimulating hormone (TSH) in cord blood samples [58].
Our study has some limitations. Given the unique geographic location of this single-center study, our study population over-sampled people living in the dense urban New York City tristate area, where usually higher level of TRAP is the norm. Second, the cross-sectional study design is not adequate to infer causality between prenatal TRAP and preterm infants’ neurobehavioral performance. In addition, the traffic data we obtained were the daily average traffic volume that not change within a calendar year. We cannot adjust for the seasonal variation of traffic [39] thereby the study exposure may had been misclassified to a certain extent. Third, in a survey conducted by the NICU-HEALTH study, 20% of families reported moving during pregnancy. Unfortunately, neither the specific time of the move nor the previous address was available for those who moved during pregnancy. Moving during pregnancy could cause misclassification of environmental exposure if the move was far and the exposure had a great spatial heterogeneity. For movers, our study-generated TRAP exposure would mainly represent the exposure level in a period prior to birth. Fourth, only TRAP exposure was considered, other air pollution sources like cooking gas, poor ventilation, and indoor dust were not addressed in our analyses. Last, the sample size of this study was small and the study population is specific. The NNNS phenotypes and their relationship to TRAP requires further investigation in large population-based studies.
Prenatal exposure to TRAP studied with multiple adverse health outcomes in child health, but collecting data representing the exposure exclusively for prenatal period is often difficult. One strength of our study is that the exposure to TRAP presumably stopped at birth, with postnatal exposure being exactly same to all participants. Thus, our finding indicates a sensitive window of TRAP exposure. Exposure to high levels of TRAP is an important influential factor of neurodevelopment during fetal stage, and this effect could be narrowed to the period before the late-third trimester, when neurodevelopment is particularly rapid. Future work to determine the contribution of the various constituents of TRAP to specific health effects will aid in risk reduction.
Conclusion
Our study suggests a link between prenatal exposure to intensive traffic-related air pollution and neurobehavioral development among preterm infants, especially for the development of motor function during infancy. As we look towards solutions for morbidities related to preterm birth, it is becoming increasingly clear that exposures during the prenatal period may be more important than previously thought. Our study suggests that TRAP exposure during pregnancy may impact all births, term or preterm.
Supplementary Material
Figure 3.
Odds ratio and 95% confidence interval of traffic exposures and identified NNNS phenotypes. Road: road density exposure (103m/km2); VMT: VMT density exposure (104 vehicle-mile/km2). P1: profile 1; P2: profile 2; P3: profile 3. ORs and 95% CIs were calculated using multinomial logistic regression. Covariates: gender, gestational age at birth, birth weight z-score, postmenstrual age at NNNS assessment (PMA), delivery type, insurance type, neighborhood SES z-score, race, ethnicity, maternal smoking status, NICU morbidity, and base deficit at birth.
Highlights.
Three phenotypes of neurobehavior were identified based on NNNS and LPA.
Hypotonia was significantly associated with prenatal exposure to road traffic.
The first two trimesters could be a sensitive window of air pollution exposure.
Acknowledgments
Funding and competing interests: The NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) study is supported by a cooperative agreement, UH3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional past funding for this cohort came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, a National Institute of Environmental Health Sciences (NIEHS) mentored award K23ES022268 to Dr. Annemarie Stroustrup, and the primary phase of the ECHO program UG3OD02332. Dr. Xueying Zhang is funded by the Environmental Medicine and Public Health Fellowship of Icahn School of Medicine at Mount Sinai.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costa LG, et al. , Neurotoxicity of traffic-related air pollution. NeuroToxicology, 2017. 59: p. 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook RD, et al. , Particulate Matter Air Pollution and Cardiovascular Disease. 2010. 121(21): p. 2331–2378. [DOI] [PubMed] [Google Scholar]
- 3.Kleinman MT, et al. , Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicology Letters, 2008. 178(2): p. 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera FP, et al. , Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. 2006. 114(8): p. 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hougaard KS, et al. , Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Particle and fibre toxicology, 2008. 5: p. 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LNA, et al. , Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. NeuroToxicology, 2007. 28(5): p. 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton JL, et al. , Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. 2017. 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basagaña X, et al. , Neurodevelopmental Deceleration by Urban Fine Particles from Different Emission Sources: A Longitudinal Observational Study. 2016. 124(10): p. 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, et al. , Association of Traffic-Related Air Pollution with Children’s Neurobehavioral Functions in Quanzhou, China. 2009. 117(10): p. 1612–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suglia SF, et al. , Association of Black Carbon with Cognition among Children in a Prospective Birth Cohort Study. American Journal of Epidemiology, 2007. 167(3): p. 280–286. [DOI] [PubMed] [Google Scholar]
- 11.Harris MH, et al. , Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicology and Teratology, 2016. 57: p. 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yorifuji T, et al. , Prenatal Exposure to Traffic-related Air Pollution and Child Behavioral Development Milestone Delays in Japan. Epidemiology, 2016. 27(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 13.Wick P, et al. , Barrier Capacity of Human Placenta for Nanosized Materials. 2010. 118(3): p. 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavan N and Naidu K, Polycyclic aromatic hydrocarbons in placenta, maternal blood, umbilical cord blood and milk of Indian women. 1995. 14(6): p. 503–506. [DOI] [PubMed] [Google Scholar]
- 15.Dejmek J, et al. , The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. 2000. 108(12): p. 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha S, et al. , Prenatal and early life exposures to ambient air pollution and development. Environmental Research, 2019. 174: p. 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guxens M, et al. , Prenatal Exposure to Residential Air Pollution and Infant Mental Development: Modulation by Antioxidants and Detoxification Factors. 2012. 120(1): p. 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandjean P and Landrigan PJ, Neurobehavioural effects of developmental toxicity. The Lancet Neurology, 2014. 13(3): p. 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheong JL, et al. , Association Between Moderate and Late Preterm Birth and Neurodevelopment and Social-Emotional Development at Age 2 YearsAssociation Between Preterm Birth and Neurodevelopment, Social-Emotional Development at 2 YearsAssociation Between Preterm Birth and Neurodevelopment, Social-Emotional Development at 2 Years. JAMA Pediatrics, 2017. 171(4): p. e164805–e164805. [DOI] [PubMed] [Google Scholar]
- 20.Lester BM and Tronick EZ, The Neonatal Intensive Care Unit Network Neurobehavioral Scale Procedures. 2004. 113(Supplement 2): p. 641–667. [PubMed] [Google Scholar]
- 21.Tronick E and Lester BM, Grandchild of the NBAS: The NICU Network Neurobehavioral Scale (NNNS). 2013. 26(3): p. 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroustrup A, et al. , Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLOS ONE, 2018. 13(3): p. e0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appleton AA, et al. , Prenatal Programming of Infant Neurobehaviour in a Healthy Population. 2016. 30(4): p. 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sucharew H, et al. , NICU Network Neurobehavioral Scale Profiles Predict Developmental Outcomes in a Low-Risk Sample. 2012. 26(4): p. 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salisbury AL, et al. , Prenatal cocaine use and maternal depression: Effects on infant neurobehavior. Neurotoxicology and Teratology, 2007. 29(3): p. 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyle MG, et al. , Neurobehavioral effects of treatment for opiate withdrawal. 2005. 90(1): p. F73–F74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donauer S, et al. , Prenatal Exposure to Polybrominated Diphenyl Ethers and Polyfluoroalkyl Chemicals and Infant Neurobehavior. The Journal of Pediatrics, 2015. 166(3): p. 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law KL, et al. , Smoking During Pregnancy and Newborn Neurobehavior. 2003. 111(6): p. 1318–1323. [DOI] [PubMed] [Google Scholar]
- 29.Stroustrup A, et al. , Sources of clinically significant neonatal intensive care unit phthalate exposure. Journal of Exposure Science & Environmental Epidemiology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conradt E, et al. , The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics, 2013. 8(12): p. 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephanie Donauer AK, Patrick Ryan, Yingying Xu, Heidi Sucharew, Kimberly Yolton, Prenatal Exposure To Traffic-Related Air Pollution And Infant Neurobehavior. ISEE Conference Abstract, 2015. 2015(1). [Google Scholar]
- 32.Stroustrup A, Teitelbaum SL, and Aschner JL, The Value of Preterm Infant Environmental Health Cohorts: The Canary in the Coal MineThe Value of Preterm Infant Environmental Health CohortsThe Value of Preterm Infant Environmental Health Cohorts. JAMA Pediatrics, 2017. 171(12): p. 1139–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroustrup A BJ, Spear EA, Aguiar A, Zimmerman E, Isler JR, et al. , Cohort Profile: The neonatal intensive care unit hospital exposures and long-term health (NICU-HEALTH) cohort. . BMJ Open (Accepted pending minor revisions), 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bureau, U.S.C., Geocoding using ArcGIS & TIGER/LINE Shapefiles. 2013. [Google Scholar]
- 35.Hoek G, et al. , Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. The Lancet, 2002. 360(9341): p. 1203–1209. [DOI] [PubMed] [Google Scholar]
- 36.Transportation, U.S.D.o., HPMS Public Release of Geospatial Data in Shapefile Format. [Google Scholar]
- 37.Tian N, Xue J, and Barzyk TM, Evaluating socioeconomic and racial differences in traffic-related metrics in the United States using a GIS approach. Journal of Exposure Science & Environmental Epidemiology, 2013. 23(2): p. 215–222. [DOI] [PubMed] [Google Scholar]
- 38.McGuinn LA, et al. , Residential proximity to traffic and female pubertal development. Environment International, 2016. 94: p. 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Craft E, and Zhang K, Characterizing spatial variability of air pollution from vehicle traffic around the Houston Ship Channel area. Atmospheric Environment, 2017. 161: p. 167–175. [Google Scholar]
- 40.Houston D, et al. , Structural Disparities of Urban Traffic in Southern California: Implications for Vehicle-Related Air Pollution Exposure in Minority and High-Poverty Neighborhoods. Journal of Urban Affairs, 2004. 26(5): p. 565–592. [Google Scholar]
- 41.Diez Roux AV, et al. , Area Characteristics, Individual-Level Socioeconomic Indicators, and Smoking in Young Adults: The Coronary Artery Disease Risk Development in Young Adults Study. American Journal of Epidemiology, 2003. 157(4): p. 315–326. [DOI] [PubMed] [Google Scholar]
- 42.Hajat A, et al. , Air Pollution and Individual and Neighborhood Socioeconomic Status: Evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environmental Health Perspectives, 2013. 121(11–12): p. 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bureau, U.C., American Community Survey 5-Year Estimates. 2015. [Google Scholar]
- 44.Muthén, B.J.T.S.h.o.q.m.f.t.s.s., Latent variable analysis. The Sage handbook of quantitative methodology for the social sciences, 2004. 345(368): p. 106–109. [Google Scholar]
- 45.USDOT, Highway Functional Classification Concepts, Criteria and Procedures. [Google Scholar]
- 46.Gibson WA, Three multivariate models: Factor analysis, latent structure analysis, and latent profile analysis. Psychometrika, 1959. 24(3): p. 229–252. [Google Scholar]
- 47.Liu J, et al. , Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics, 2010. 125(1): p. e90–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lester BM, et al. , Neurobehavior related to epigenetic differences in preterm infants. 2015. 7(7): p. 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris MH, et al. , Prenatal and Childhood Traffic-Related Pollution Exposure and Childhood Cognition in the Project Viva Cohort (Massachusetts, USA). 2015. 123(10): p. 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leyenaar J, Camfield P, and Camfield C, A schematic approach to hypotonia in infancy. Paediatrics & child health, 2005. 10(7): p. 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayaraj RL, et al. , Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Current Environmental Health Reports, 2017. 4(2): p. 166–179. [DOI] [PubMed] [Google Scholar]
- 52.Campbell A, et al. , Particulate Matter in Polluted Air May Increase Biomarkers of Inflammation in Mouse Brain. NeuroToxicology, 2005. 26(1): p. 133–140. [DOI] [PubMed] [Google Scholar]
- 53.Woodward NC, et al. , Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Translational Psychiatry, 2018. 8(1): p. 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vithayathil J, Pucilowska J, and Landreth GE, Chapter 3 - ERK/MAPK signaling and autism spectrum disorders, in Progress in Brain Research, Shekhar A, Editor. 2018, Elsevier; p. 63–112. [DOI] [PubMed] [Google Scholar]
- 55.Pearce EN and Braverman LE, Environmental pollutants and the thyroid. Best Practice & Research Clinical Endocrinology & Metabolism, 2009. 23(6): p. 801–813. [DOI] [PubMed] [Google Scholar]
- 56.Rogan WJ, et al. , Neonatal effects of transplacental exposure to PCBs and DDE. The Journal of Pediatrics, 1986. 109(2): p. 335–341. [DOI] [PubMed] [Google Scholar]
- 57.Koopman-Esseboom C, et al. , Effects of Dioxins and Polychlorinated Biphenyls on Thyroid Hormone Status of Pregnant Women and Their Infants. Pediatric Research, 1994. 36(4): p. 468–473. [DOI] [PubMed] [Google Scholar]
- 58.Janssen BG, et al. , Fetal Thyroid Function, Birth Weight, and in Utero Exposure to Fine Particle Air Pollution: A Birth Cohort Study. 2017. 125(4): p. 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.