Abstract
Background: Growth hormone (GH) stimulates the production of insulin-like growth factor 1 (IGF-1) in most tissues and together GH and IGF-1 profoundly impact adipose tissue deposition, glucose metabolism and cardiovascular function. A low serum IGF-I level has been reported as being associated with obstructive sleep apnea (OSA) and might be one of the mechanisms underlying cardio-metabolic risk in OSA patients.
Methods: In a multicenter national study, 817 patients consulting for suspicion of OSA (OSA confirmed for 567 patients) underwent serum IGF-1 measurements. We analyzed the association between an IGF-1 level below the median value of the population and variables related to cardio-metabolic risk: body mass index (BMI) and waist circumference, apnea hypopnea index (AHI), cholesterol and triglycerides (expressed as median and divided into quartiles for continuous variables).
Results: After adjustment for age and gender, low IGF-1 levels were associated with increased BMI and AHI (Odds ratios (OR) = 2.83; p < 0.0001 and OR = 3.03, p < 0.0001 for Quartile 4 vs. Quartile1, respectively), with elevated cholesterol levels (OR = 1.36, p = 0.0444), and elevated triglyceride levels (OR = 1.36; p = 0.0008).
Conclusions: Both adiposity and sleep apnea synergistically predict low levels of IGF-1 and thus could together contribute toward cardio-metabolic risk. Further work are needed to confirm whether IGF-1 levels allow grading severity and predicting response to treatments to aim at a personalized medicine for patients suffering from OSA.
Keywords: obstructive sleep apnea, abdominal obesity, insulin-like growth factor, biomarker, cardiovascular risk
Introduction
Growth hormone (GH) stimulates the production of insulin-like growth factor 1 (IGF-1) in most tissues and together GH and IGF-1 profoundly impact adipose tissue deposition, glucose metabolism and cardiovascular function (1, 2). IGF-1 binds to the insulin receptor due to its high homology with insulin, but with a lower affinity, inducing “insulin-like” metabolic functions (3). GH deficiency induces low IGF-1 levels and is associated with cardio-vascular functional and morphological dysfunction, leading a higher mortality risk by cardiovascular disease (4–7). IGF-1 appears to be cardioprotective through maintenance of the functional and structural integrity of the microcirculation, increasing NO bioavailability, decreasing reactive oxygen species (ROS) production, and exerting anti-inflammatory, anti-apoptotic, and proangiogenic effects (3). It has also been demonstrated that low IGF-1 levels correlate with insulin resistance, atherogenic dyslipidemia, and increased blood pressure that constitute the three pillars of metabolic syndrome (8, 9). Moreover, low levels of IGF-1 have been found to be linked with the occurrence of late cardiovascular events and mortality (10, 11). Abnormalities of GH/IGF-1 axis with GH deficiency, are associated with cardio-vascular functional and morphological dysfunction and leads a higher mortality risk by cardiovascular disease.
Obstructive sleep apnea (OSA) is a highly prevalent condition that affects 4% of men and 2% of women in the general population, but is much more frequent in those with abdominal obesity (12). Sleep apnea is characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep, resulting in apneas and hypopneas with intermittent hypoxia, sleep fragmentation and a reduction in slow wave sleep (SWS) (13). The pituitary secretion of GH occurs preferentially at night (14) and IGF-1 levels positively correlate with the amount of SWS. Conversely, a decrease in sleep duration and an increase in indices of sleep apnea severity as measured by the apnea + hypopnea index (AHI) and the degree of oxygen desaturation, are associated with lower levels of GH/IGF-1. Obstructive sleep apnea alters insulin sensitivity and is independently associated with hypertension, coronary heart disease, stroke, arrhythmias, and all-cause mortality (13).
In a previous study by our team (13), IGF-1 was systematically measured in more than 800 men and women attending sleep clinics due to a suspicion of OSA. We reported that acromegaly, which consists in the abnormal excretion of GH by a pituitary adenoma, was more frequent in this population (15). In the present study, we took advantage of this well characterized prospective cohort to assess the GH/IGF-1 axis in the sleep apnea-obesity comorbid couple. We hypothesized that an OSA-related alteration of the somatotropic axis might be an independent mechanism underlying OSA-related insulin resistance and its cardio-metabolic consequences (16, 17).
Materials and Methods
Study Population
Between November 2013 and October 2014, patients referred for OSA to ten sleep centers in France were prospectively included. Inclusion criteria were age 18–75 years, and having a first assessment for OSA diagnosis. Exclusion criteria were any previously diagnosed sleep disorder, pregnant, or lactating women or patients under legal protection. After oral information about the study, each participant signed a written consent. The study was approved by the Grenoble Alpes University Hospital ethics committee (IRB: 13-CHUG-37) and registered in clinicaltrials.gov (NCT02789696). Eligible patients (1285) were asked to participate; 412 declined to do so or had exclusion criteria; 873 were included and 817 had complete laboratory data and were included in the analysis (15).
Study Design
This was a prospective, cross-sectional, multicenter observational study.
Data Collection
Each investigator completed the electronic record of the French sleep registry: the “Observatoire du sommeil de la fédération de Pneumologie” (OSFP). This electronic health database, created by the French Federation of Pneumology, is a standardized electronic register used by sleep physicians in France to collect anthropometric characteristics, symptoms through validated scales for sleepiness, fatigue and depression, and medical history (smoking, hypertension, diabetes, hypercholesterolemia, hypertriglyceridemia, coronary heart disease, arrhythmias, stroke, heart failure, gastroesophageal reflux, depression, peripheral arteriopathy, glaucoma, and thyroid disease). Ethical committee approval for setting up the database was obtained from “Le Comité consultatif sur le traitement de l'information en matière de recherche en santé” (C.C.T.I.R.S n° 09.521) and authorization from the “Commission Nationale Informatique et Liberté” (C.N.I.L), the French information technology and personal data protection authority. The OSFP Independent Scientific Advisory Committee approved data use for this study. Beyond usual data items, a specific electronic file was created, including specific laboratory data (IGF-1, fasting lipid profile, fasting glucose) that were specifically measured for this study.
Hormone Assays and Blood Tests
Blood samples were collected by the patient's closest medical analysis laboratory, at the patient's convenience. Serum IGF-1, fasting plasma glucose and fasting plasma lipid profile (total cholesterol, LDL cholesterol, HDL-cholesterol, and triglyceride levels) were measured with commercial kits available in each laboratory where a blood sample was collected.
Nocturnal Poly(Somno)Graphy Data
Nocturnal polysomnography or polygraphy was performed using the routine clinical procedure of each participating sleep center. The recordings were scored according to standardized methods, described in American Academy of Sleep Medicine (AASM) Manual for Scoring Sleep and Associated Events (18). Patients with an AHI >15 apnea + hypopnea events per hour were considered as suffering from moderate to severe OSA.
Statistical Analysis
Descriptive data are reported as median [interquartile range] for continuous variables and as n (%) for qualitative variables.
To assess which factors associated with low serum IGF-1 levels (defined as a level below the median value for the overall cohort), conditional univariate logistic regressions were performed since IGF-1 levels were not normally distributed. Univariate regressions were systematically adjusted on age and sex. Concerning continuous variables, if log-linearity was not respected, the variables were divided into quartiles, or recoded based on the median according to the Akaike information criteria (AIC) methodology. If log-linearity was respected the analysis was performed with continuous variables.
To look for the respective contributions of obesity and OSA to low IGF-1 levels, a multivariable logistic regression was performed with age, sex, body mass index (BMI), and AHI as independent variables and with IGF-1 level as a dependent variable. The same multivariable logistic regression was performed with waist circumference instead of BMI.
There was a maximum of 7% missing data for all data presented, except for 16% of missing data for the waist circumference. The missing data were not replaced for the univariate analysis. For the multivariate analysis, missing data were replaced by the median for the population for age, BMI and AHI, and the median according to sex for waist circumference.
Results
The study population characteristics are shown in Table 1. The whole group was middle-aged, predominantly male, obese and suffering from the usual co-morbidities associated with sleep apnea.
Table 1.
Characteristics of patients.
| N = 817 | |
|---|---|
| Anthropometric characteristics | |
| Age, years | 53 [44; 61] |
| Male gender, % | 63.9 |
| BMI, kg/m2 | 29.8 [26.1; 34.5] |
| Waist Circumference, cm | 106 [97; 116] |
| Sleep apnea | |
| AHI, events/h | 25.3 [15.0; 41.0] |
| AHI <5 events/h, % | 7.2 |
| AHI 5-15 events/h, % | 17.7 |
| AHI 15-30 events/h, % | 32.8 |
| AHI >30 events/h, % | 42.2 |
| Epworth Sleepiness score, /24 | 9 [5; 13] |
| Laboratory measurements | |
| IGF-1, ng/ml | 138 [109; 176] |
| Fasting blood glucose, mg/dL | 98 [91; 109] |
| Total cholesterol, g/l | 2.04 [1.75; 2.30] |
| LDL cholesterol, g/l | 1.24 [1.00; 1.46] |
| HDL cholesterol, g/l | 0.51 [0.42; 0.61] |
| Triglycerides, g/l | 1.21 [0.86; 1.74] |
| Tabacco consumption | |
| Former smokers, % | 31.5 |
| Current smokers, % | 18.8 |
| Hypertension, % | 39.2 |
| Diabetes, % | |
| All diabetes | 7,6 |
| type 1 | 2.9 |
| type 2 | 4.7 |
| Dyslipidemia | |
| Hypercholesterolemia, % | 21.4 |
| Hypertriglyceridemia, % | 3.8 |
| Cardiovascular diseases | |
| Coronary heart disease, % | 2.6 |
| Arrhythmias, % | 4.8 |
| Stroke, % | 2.9 |
| Heart failure, % | 2.0 |
| Other comorbidities | |
| Gastroesophageal reflux, % | 18.3 |
| Depression, % | 11.0 |
| Peripheral arteriopathy, % | 1.7 |
| Glaucoma, % | 2.2 |
| Thyroid disease, % | 6.0 |
Data are presented as median [interquartile range] for continuous variables and as percentage for qualitative variables. BMI, body mass index; AHI, apnea+hypopnea index; IGF-1 = Insulin-like growth factor-1.
Univariate Logistic Regressions
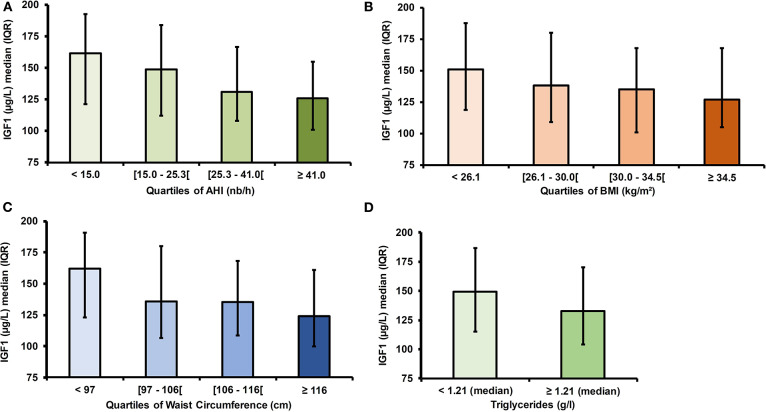
Higher age, BMI, waist circumference and AHI were found to be associated with IGF-1 levels below the median. Elevated triglycerides and total cholesterol levels were linked to low IGF-1 levels whereas fasting glucose levels, LDL-cholesterol and HDL-cholesterol were not (Table 2 and Figure 1).
Table 2.
Factors associated with low IGF-1 levels (below the median) in univariate logistic regressions.
| IGF-1 levels | IGF-1 levels | OR (95% CI) | p value | |
|---|---|---|---|---|
| below the median | above the median | |||
| Age (years) | 56 [48; 64] | 49 [41; 59] | <0.0001 | |
| Q2 vs. Q1 | 1.70 (1.11; 2.61) | 0.0143 | ||
| Q3 vs. Q1 | 3.50 (2.30; 5.33) | <0.0001 | ||
| Q4 vs. Q1 | 4.23 (2.76; 6.48) | <0.0001 | ||
| Male gender (%) | 62.5 | 65.2 | 0.90 (0.67; 1.21) | 0.5003 |
| Anthropometric markers | ||||
| BMI (kg/m2)* | 30.8 [27.0; 34.9] | 29.1 [25.1; 33.9] | <0.0001 | |
| Q2 vs. Q1 | 1.66 (1.08; 2.55) | 0.0199 | ||
| Q3 vs. Q1 | 1.83 (1.20; 2.80) | 0.0051 | ||
| Q4 vs. Q1 | 2.83 (1.82; 4.41) | <0.0001 | ||
| Waist circumference (cm)* | 109.0 [100.0; 119.0] | 104.0 [93.0; 114.0] | <0.0001 | |
| Q2 vs. Q1 | 2.09 (1.29; 3.38) | 0.0026 | ||
| Q3 vs. Q1 | 2.25 (1.40; 3.63) | 0.0009 | ||
| Q4 vs. Q1 | 3.01 (1.85; 4.89) | <0.0001 | ||
| Sleep apnea severity | ||||
| AHI (events/hour)* | 30.0 [18.0; 49.0] | 22.0 [10.7; 35.0] | <0.0001 | |
| Q2 vs. Q1 | 1.02 (0.65; 1.61) | 0.9304 | ||
| Q3 vs. Q1 | 1.80 (1.14; 2.84) | 0.0116 | ||
| Q4 vs. Q1 | 3.03 (1.88; 4.88) | <0.0001 | ||
| Epworth score* | 9 [5; 12] | 9 [6; 13] | 0.7535 | |
| Q2 vs. Q1 | 1.20 (0.77; 1.87) | 0.4168 | ||
| Q3 vs. Q1 | 1.28 (0.82; 2.00) | 0.2843 | ||
| Q4 vs. Q1 | 1.18 (0.75; 1.86) | 0.4658 | ||
| Plasma glucose and lipid profile | ||||
| Glucose (mg/dL) | 101 [92; 114] | 96 [90; 105] | 1.33 (0.97; 1.82) | 0.0775 |
| Total cholesterol (g/L) | 2.08 [1.78; 2.34] | 2.01 [1.73; 2.26] | 1.36 (1.01; 1.84) | 0.0444 |
| HDL-c (g/L) | 0.51 [0.42; 0.61] | 0.50 [0.42; 0.61] | 0.68 (0.26; 1.77) | 0.4286 |
| LDL-c (g/L) | 1.25 [0.98; 1.48] | 1.22 [1.01; 1.44] | 1.30 (0.96; 1.75) | 0.0928 |
| Triglycerides (g/L) | 1.35 [0.95; 1.91] | 1.14 [0.79; 1.60] | 1.36 (1.13; 1.62) | 0.0008 |
Data are reported as median [interquartile range] for continuous variables and by percentage for qualitative variables in the subgroups of participants having IGF-1 below or above the median. Univariate logistic regressions with IGF-1 levels as dependent variable are reported by Odd ratios (95% CI) and are adjusted for age and gender. BMI, body mass index; Q, quartile; AHI, apnea+hypopnea index.
Figure 1.
IGF-1 levels according to abdominal obesity and obstructive sleep apnea severity. (A) IGF-1 levels according to AHI quartiles. (B) IGF-1 levels according to BMI quartiles. (C) IGF-1 levels according to waist circumference quartiles. (D) IGF-1 levels according to triglyceride levels. IGF-1, Insulin Growth factor 1; AHI, Apnea Hypopnea Index; OR, Odds Ratio; IQR, interquartile range.
Multivariate Analysis
A multivariable regression analysis was performed with IGF-1 levels as dependent variable and age, sex, BMI and AHI as independent variables. Age, BMI and AHI were independently associated with IGF-1 levels (Table 3).
Table 3.
Respective contribution of age, gender, BMI, and apnea severity to low IGF-1 levels in multivariate analysis (below the median).
| OR (95%CI) | p value | |
|---|---|---|
| Age | <0.0001 | |
| Q2 vs. Q1 | 1.65 (1.06; 2.58) | 0.0269 |
| Q3 vs. Q1 | 3.46 (2.23; 5.37) | <0.0001 |
| Q4 vs. Q1 | 4.04 (2.59; 6.33) | <0.0001 |
| Male gender | 0.76 (0.55; 1.06) | 0.1065 |
| BMI | 0.0022 | |
| Q2 vs. Q1 | 1.58 (1.03; 2.42) | 0.0383 |
| Q3 vs. Q1 | 1.75 (1.14; 2.69) | 0.01 |
| Q4 vs. Q1 | 2.36 (1.51; 3.69) | 0.0002 |
| AHI | 0.0003 | |
| Q2 vs. Q1 | 0.99 (0.63; 1.56) | 0.9609 |
| Q3 vs. Q1 | 1.59 (1.04; 2.43) | 0.0331 |
| Q4 vs. Q1 | 2.39 (1.51; 3.79) | 0.0002 |
A multivariable logistic regression model was built with IGF-1 levels as dependent variable and age, gender, BMI, AHI as independent variables. Results are reported by odds ratios (95% CI). BMI, body mass index; AHI, apnea+hypopnea index; Q, quartile. The bold values indicate p value corresponding to the variable as a whole.
Sleep apnea syndrome is associated with a central distribution of adiposity that was not captured by BMI. To take into account this potential confounding factor in the association between AHI and IGF-1 levels, we repeated the multivariable model including waist circumference instead of BMI among the independent variables. Results were similar to the previous model (Table 4).
Table 4.
Respective contribution of age, gender, waist circumference, and apnea severity to low IGF-1 levels (below the median).
| OR (95%CI) | p value | |
|---|---|---|
| Age | <0.0001 | |
| Q2 vs. Q1 | 1.6 (1.03; 2.49) | 0.0375 |
| Q3 vs. Q1 | 3.23 (2.09; 4.98) | <0.0001 |
| Q4 vs. Q1 | 3.61 (2.33; 5.58) | <0.0001 |
| Male gender | 0.71 (0.51; 0.99) | 0.0419 |
| Waist circumference | 0.0006 | |
| Q2 vs. Q1 | 1.98 (1.28, 3.06) | 0.0023 |
| Q3 vs. Q1 | 1.5 (0.97; 2.33) | 0.0719 |
| Q4 vs. Q1 | 2.42 (1.55; 3.77) | 0.0001 |
| AHI | <0.0001 | |
| Q2 vs. Q1 | 1.04 (0.66; 1.64) | 0.8581 |
| Q3 vs. Q1 | 1.65 (1.08; 2.53) | 0.0199 |
| Q4 vs. Q1 | 2.57 (1.62; 4.06) | <0.0001 |
A multivariable logistic regression model was built with IGF-1 levels as dependent variable and age, gender, waist circumference, AHI as independent variables. Results are reported by odds ratios (95% CI). AHI, apnea+hypopnea index. The bold values indicate p value corresponding to the variable as a whole.
Discussion
In this large prospective cohort, low IGF-1 levels were independently associated with increasing age, waist circumference or BMI and AHI. The originality of our data is to demonstrate that severe sleep apnea was the single comorbidity associated with low IGF-1 values, independent of BMI or waist circumference. Thus, both adiposity and sleep apnea seem to synergistically predict low levels of IGF-1 and thus could participate together toward cardio-metabolic risk.
IGF-1 Levels in Obesity, Metabolic Syndrome and Cardiovascular Diseases
Plasma IGF-1 levels are known to be inversely correlated with BMI particularly in individuals with central obesity (1). The novelty of our work is to demonstrate that sleep apnea is a key player in the GH/IGF-1 axis dysfunction occurring in obesity. Low IGF-1 levels were also found to be associated with the different components of metabolic syndrome, type 2 diabetes, and cardiovascular diseases (1, 9, 19). Results from the Framingham heart study showed a dose-response relationship between lowering in IGF-1 values and the number of abnormalities of metabolic syndrome factors (8, 20). Accordingly, normal IGF-1 levels were linked with a lower prevalence of metabolic syndrome (21). The results of the present study are in agreement with these previous results since in univariate analysis increased levels of triglycerides were associated with low IGF-1 levels.
Low IGF-1 levels have previously been linked with reduced insulin sensitivity, and with a higher risk of glucose intolerance and type 2 diabetes (22–24). Low IGF-1 levels were linked to coronary atherosclerosis and re-stenosis (25–27), ischemic heart disease (11, 28, 29), congestive heart failure (30), and stroke (31–33). In the current study, we found no significant association between low IGF-1 and fasting glucose, type 2 diabetes, hypertension or previous cardiovascular events. However, the prevalence of previous cardiovascular diseases was relatively low among included patients and the absence of a significant association might be explained by lack of power.
IGF-1 and OSA
As well as chronic intermittent hypoxia, OSA also induces sleep fragmentation and an inability to achieve deep sleep that alter circadian hormone regulation, impacting the corticotropic and somatotropic axis. Somatotropic cells of the anterior pituitary secrete GH mostly during SWS (14, 34, 35). Thus, longer SWS has been associated with increased IGF-I levels (36) whereas poorer sleep quality or OSA, by reducing SWS, inhibits the somatotropic axis (37). IGF-1 levels vary with age and the picture of the relationship is further complicated by the fact that both OSA and age are impacting the amount of SWS. The specific role of somatotropic axis dysfunction in OSA deserves attention since part of the OSA related cardiometabolic risk might be explained by lower IGF-1 levels in apneic patients. An association between OSA and lower GH and IGF-1 levels has been previously reported (38–42), independent of obesity (43, 44). However, the sample size of previous studies generally did not allow adjustment for confounders and multivariate analysis. In contrast, our large sample made it possible to demonstrate an inverse dose-response relationship between IGF-1 levels and the severity of AHI, independently of age, BMI or waist circumference. Circulating IGF-I and its interaction with IGFBP-1 is crucial for glucose homoeostasis and exert a protective effect against the development of glucose intolerance (22). On the other hand, there is a bidirectional association between both type 1 and 2 diabetes and obstructive sleep apnea (45–48). Mechanistic studies in rodent exposed to intermittent hypoxia have demonstrated organ specific insulin resistance related to intermittent hypoxia (49–51). Namely, we have evidenced that IH induces a pro-inflammatory phenotype of the adipose tissue, which may be a crucial link between OSA, central obesity and the development of insulin resistance (51). The association in human literature between IGF-1 and insulin resistance is more conflicting but seem to exist in non-diabetic patients with sleep apnea (52). The interaction between IGF-1, central obesity and insulin resistance is difficult to dissect precisely in clinical studies but is certainly playing a major role in OSA-related cardio metabolic co-morbidities (43, 53). Thus, low IGF-1 levels could be considered as a biomarker of OSA severity toward cardiometabolic health, being specifically related to OSA and associated with an increased cardiometabolic risk.
The standard therapy for OSA is continuous positive airway pressure (CPAP), but responses are highly variable, with some patients showing large treatment effects and others little to none with no way for clinicians to distinguish between those who will and will not respond. A biomarker predictive of cardio-metabolic response would be particularly helpful for minimally symptomatic OSA patients who will not accept CPAP treatment unless some benefits in terms of risk reduction can be monitored. It has been reported in cohort studies that CPAP treatment is associated with an increase in IGF-1 levels (42, 44, 54) and is related to adherence to CPAP therapy (55); however, in a parallel randomized sham-placebo controlled 1-month trial, both therapeutic and sub-therapeutic nasal CPAP improved IGF-1 without differences between the groups (56). Therefore, whether CPAP is able to restore the somatotropic axis and IGF-1 levels need further controlled studies and whether an increase in IGF-1 under CPAP is related to long-term cardiovascular protection remains to be established.
Study Strengths and Limitations
Our study was by far the largest in the field including in 10 sleep centers in private and academic practices more than 800 patients referred for OSA suspicion. We acknowledge the heterogeneity in methods for IGF-1 assays but this was mandatory for assuring the feasibility of the study and the assessment of a unique large sample of patients. The major study strength was the capability to assess an unbiased population but this is balanced by a restricted dataset regarding the respective distribution of the different sleep stages namely slow wave sleep which is classically related to IGF-1 levels. IGF-1 levels vary with age but we have adjusted the multivariate analysis with age. Data are also limited regarding indices of insulin resistance as circulating insulin or HBA1C were not systematically assessed. Further studies are needed to assess IGF-1 changes after OSA treatment. The reliability of IGF-1 as a potential marker of cardiovascular risk in OSA patients remains to be validated in longitudinal studies.
Conclusion
In a large multicenter OSA population, Low IGF-1 levels were associated with severity of apnea+ hypopnea index, measures of adiposity and lipids abnormalities. Both adiposity and sleep apnea could synergistically contribute toward higher cardio-metabolic risk. The risk of low IGF-1 levels increased with OSA severity. We suggest that IGF-/1 might be helpful in OSA personalized medicine for grading severity cardio-metabolic risk in OSA and guiding specific interventions.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Grenoble Alpes University Hospital ethics committee (IRB: 13-CHUG-37). The database was approved by “Le Comité consultatif sur le traitement de l'information en matière de recherche en santé” (C.C.T.I.R.S n° 09.521) with authorization from the “Commission Nationale Informatique et Liberté” (C.N.I.L), the French information technology and personal data protection authority. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
L-MG and A-LB analyzed the data and wrote the manuscript. J-LP designed the study, analyzed data, and critically reviewed the present work. OC, PC, MS, BS, JG-R, and RT participated in the conception of the study design and critically reviewed the manuscript.
Conflict of Interest
OC has received invitations from IPSEN to scientific meetings. J-LP has received a lecture fee from IPSEN. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MB declared a past co-authorship with the authors L-MG, RT, and J-LP to the handling editor.
Acknowledgments
We thank Alison Foote (Grenoble Alpes University Hospital) for critically editing the manuscript.
Footnotes
Funding. This work was supported by an unrestricted grant from IPSEN. J-LP and RT were supported by a research grant from the French National Research Agency (ANR-12-TECS-0010) in the framework of the Investissements d'avenir program (ANR-15-IDEX-02) and the e-health and integrated care and Trajectories Medicine [MIAI @ Grenoble Alpes (ANR-19-P3IA-0003)] Chairs of excellence of the University Grenoble Alpes.
References
- 1.Berryman DE, Glad CAM, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. (2013) 9:346–56. 10.1038/nrendo.2013.64 [DOI] [PubMed] [Google Scholar]
- 2.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. (1978) 253:2769–76. [PubMed] [Google Scholar]
- 3.Puche JE, Castilla-Cortázar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J Transl Med. (2012) 10:224. 10.1186/1479-5876-10-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Somma C, Scarano E, Savastano S, Savanelli MC, Pivonello R, Colao A. Cardiovascular alterations in adult GH deficiency. Best Pract Res Clin Endocrinol Metab. (2017) 31:25–34. 10.1016/j.beem.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Isgaard J, Arcopinto M, Karason K, Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine. (2015) 48:25–35. 10.1007/s12020-014-0327-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherlock M, Ayuk J, Tomlinson JW, Toogood AA, Aragon-Alonso A, Sheppard MC, et al. Mortality in patients with pituitary disease. Endocr Rev. (2010) 31:301–42. 10.1210/er.2009-0033 [DOI] [PubMed] [Google Scholar]
- 7.Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf). (2008) 69:347–58. 10.1111/j.1365-2265.2008.03292.x [DOI] [PubMed] [Google Scholar]
- 8.Akanji AO, Smith RJ. The insulin-like growth factor system, metabolic syndrome, and cardiovascular disease risk. Metab Syndr Relat Disord. (2012) 10:3–13. 10.1089/met.2011.0083 [DOI] [PubMed] [Google Scholar]
- 9.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. (2016) 14:3. 10.1186/s12967-015-0762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, et al. Insulin-like growth factor-1 as a vascular protective factor. Circulation. (2004) 110:2260–5. 10.1161/01.CIR.0000144309.87183.FB [DOI] [PubMed] [Google Scholar]
- 11.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. (2004) 89:114–20. 10.1210/jc.2003-030967 [DOI] [PubMed] [Google Scholar]
- 12.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. (1993) 328:1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 13.Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primer. (2015) 2015:15015. 10.1038/nrdp.2015.24 [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E. Slow wave sleep and release of growth hormone. JAMA. (2000) 284:2717–8. 10.1001/jama.284.7.861 [DOI] [PubMed] [Google Scholar]
- 15.Galerneau L-M, Pépin J-L, Borel A-L, Chabre O, Sapene M, Stach B, et al. Acromegaly in sleep apnoea patients: a large observational study of 755 patients. Eur Respir J. (2016) 48:1489–92. 10.1183/13993003.01229-2016 [DOI] [PubMed] [Google Scholar]
- 16.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. (2010) 17:11–21. 10.1159/000262524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. (2014) 1311:151–73. 10.1111/nyas.12355 [DOI] [PubMed] [Google Scholar]
- 18.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. (2012) 8:597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Anversa P. The insulin-like growth factor I system: physiological and pathophysiological implication in cardiovascular diseases associated with metabolic syndrome. Biochem Pharmacol. (2015) 93:409–17. 10.1016/j.bcp.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Lam CSP, Chen M-H, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, et al. Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. (2010) 30:1479–84. 10.1161/ATVBAHA.110.203943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh J, Kim J-Y, Park S, Youn J-C, Son NH, Shin D-J, et al. The relationship between insulin-like growth factor-1 and metabolic syndrome, independent of adiponectin. Clin Chim Acta Int J Clin Chem. (2012) 413:506–10. 10.1016/j.cca.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 22.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet Lond Engl. (2002) 359:1740–5. 10.1016/S0140-6736(02)08655-5 [DOI] [PubMed] [Google Scholar]
- 23.Sesti G, Sciacqua A, Cardellini M, Marini MA, Maio R, Vatrano M, et al. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. (2005) 28:120–5. 10.2337/diacare.28.1.120 [DOI] [PubMed] [Google Scholar]
- 24.Mannino GC, Greco A, De Lorenzo C, Andreozzi F, Marini MA, Perticone F, et al. A fasting insulin-raising allele at IGF1 locus is associated with circulating levels of IGF-1 and insulin sensitivity. PLoS ONE. (2013) 8:e85483. 10.1371/journal.pone.0085483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. (2000) 86:125–30. 10.1161/01.RES.86.2.125 [DOI] [PubMed] [Google Scholar]
- 26.Cercek B, Sharifi B, Barath P, Bailey L, Forrester JS. Growth factors in pathogenesis of coronary arterial restenosis. Am J Cardiol. (1991) 68:24C−33C. 10.1016/0002-9149(91)90220-F [DOI] [PubMed] [Google Scholar]
- 27.van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJH, Lips P, Drent ML. The association of serum insulin-like growth factor-I with mortality, cardiovascular disease, and cancer in the elderly: a population-based study. J Clin Endocrinol Metab. (2010) 95:4616–24. 10.1210/jc.2010-0940 [DOI] [PubMed] [Google Scholar]
- 28.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. (2002) 106:939–44. 10.1161/01.CIR.0000027563.44593.CC [DOI] [PubMed] [Google Scholar]
- 29.Conti E, Andreotti F, Sciahbasi A, Riccardi P, Marra G, Menini E, et al. Markedly reduced insulin-like growth factor-1 in the acute phase of myocardial infarction. J Am Coll Cardiol. (2001) 38:26–32. 10.1016/S0735-1097(01)01367-5 [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Sullivan LM, D'Agostino RB, Roubenoff R, Harris T, Sawyer DB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. (2003) 139:642–8. 10.7326/0003-4819-139-8-200310210-00007 [DOI] [PubMed] [Google Scholar]
- 31.Johnsen SP, Hundborg HH, Sørensen HT, Orskov H, Tjønneland A, Overvad K, et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. (2005) 90:5937–41. 10.1210/jc.2004-2088 [DOI] [PubMed] [Google Scholar]
- 32.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. (2004) 117:312–7. 10.1016/j.amjmed.2004.02.049 [DOI] [PubMed] [Google Scholar]
- 33.Dong X, Chang G, Ji X-F, Tao D-B, Wang Y-X. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS ONE. (2014) 9:e94845. 10.1371/journal.pone.0094845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Van Cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. (2000) 10 Suppl B:S57–62. 10.1016/S1096-6374(00)80011-8 [DOI] [PubMed] [Google Scholar]
- 35.Van Cauter E, Latta F, Nedeltcheva A, Spiegel K, Leproult R, Vandenbril C, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. (2004) 14 Suppl A:S10–7. 10.1016/j.ghir.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 36.Prinz PN, Moe KE, Dulberg EM, Larsen LH, Vitiello MV, Toivola B, et al. Higher plasma IGF-1 levels are associated with increased delta sleep in healthy older men. J Gerontol A Biol Sci Med Sci. (1995) 50:M222–6. 10.1093/gerona/50A.4.M222 [DOI] [PubMed] [Google Scholar]
- 37.Schüssler P, Yassouridis A, Uhr M, Kluge M, Weikel J, Holsboer F, et al. Growth hormone-releasing hormone and corticotropin-releasing hormone enhance non-rapid-eye-movement sleep after sleep deprivation. Am J Physiol Endocrinol Metab. (2006) 291:E549–56. 10.1152/ajpendo.00641.2005 [DOI] [PubMed] [Google Scholar]
- 38.Lanfranco F, Motta G, Minetto MA, Ghigo E, Maccario M. Growth hormone/insulin-like growth factor-I axis in obstructive sleep apnea syndrome: an update. J Endocrinol Invest. (2010) 33:192–6. 10.1007/BF03346580 [DOI] [PubMed] [Google Scholar]
- 39.Barceló A, Barbé F, de la Peña M, Martinez P, Soriano JB, Piérola J, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. (2008) 63:946–50. 10.1136/thx.2007.093740 [DOI] [PubMed] [Google Scholar]
- 40.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. (2007) 175:190–5. 10.1164/rccm.200602-270OC [DOI] [PubMed] [Google Scholar]
- 41.Izumi S, Ribeiro-Filho FF, Carneiro G, Togeiro SM, Tufik S, Zanella MT. IGF-1 levels are inversely associated with metabolic syndrome in obstructive sleep apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. (2015) 12:487–93. 10.5664/jcsm.5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. (1989) 68:352–8. 10.1210/jcem-68-2-352 [DOI] [PubMed] [Google Scholar]
- 43.Ursavas A, Karadag M, Ilcol YO, Ercan I, Burgazlioglu B, Coskun F, et al. Low level of IGF-1 in obesity may be related to obstructive sleep apnea syndrome. Lung. (2007) 185:309–14. 10.1007/s00408-007-9026-x [DOI] [PubMed] [Google Scholar]
- 44.Makino S, Fujiwara M, Handa H, Fujie T, Aoki Y, Hashimoto K, et al. Plasma dehydroepiandrosterone sulphate and insulin-like growth factor I levels in obstructive sleep apnoea syndrome. Clin Endocrinol (Oxf). (2012) 76:593–601. 10.1111/j.1365-2265.2011.04237.x [DOI] [PubMed] [Google Scholar]
- 45.Ota H, Fujita Y, Yamauchi M, Muro S, Kimura H, Takasawa S. Relationship between intermittent hypoxia and type 2 diabetes in sleep apnea syndrome. Int J Mol Sci. (2019) 20:4756. 10.3390/ijms20194756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. (2013) 1:329–38. 10.1016/S2213-2600(13)70039-0 [DOI] [PubMed] [Google Scholar]
- 47.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. (2017) 152:1070–86. 10.1016/j.chest.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borel A-L, Tamisier R, Böhme P, Priou P, Avignon A, Benhamou P-Y, et al. Obstructive sleep apnoea syndrome in patients living with diabetes: Which patients should be screened? Diabetes Metab. (2019) 45:91–101. 10.1016/j.diabet.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 49.Thomas A, Belaidi E, Aron-Wisnewsky J, van der Zon GC, Levy P, Clement K, et al. Hypoxia-inducible factor prolyl hydroxylase 1 (PHD1) deficiency promotes hepatic steatosis and liver-specific insulin resistance in mice. Sci Rep. (2016) 6:24618. 10.1038/srep24618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas A, Belaidi E, Moulin S, Horman S, van der Zon GC, Viollet B, et al. Chronic intermittent hypoxia impairs insulin sensitivity but improves whole-body glucose tolerance by activating skeletal muscle AMPK. Diabetes. (2017) 66:2942–951. 10.2337/db17-0186 [DOI] [PubMed] [Google Scholar]
- 51.Murphy AM, Thomas A, Crinion SJ, Kent BD, Tambuwala MM, Fabre A, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. (2017) 49:1731. 10.1183/13993003.01731-2016 [DOI] [PubMed] [Google Scholar]
- 52.Damanti S, Bourron O, Doulazmi M, Mandengue Sosso A-L, Nguyen-Michel V-H, Mariani J, et al. Relationship between sleep parameters, insulin resistance and age-adjusted insulin like growth factor-1 score in non diabetic older patients. PLoS ONE. (2017) 12:e0174876. 10.1371/journal.pone.0174876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol. (2017) 595:2423–30. 10.1113/JP273312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tachikawa R, Ikeda K, Minami T, Matsumoto T, Hamada S, Murase K, et al. Changes in Energy Metabolism after Continuous Positive Airway Pressure for Obstructive Sleep Apnea. Am J Respir Crit Care Med. (2016) 194:729–38. 10.1164/rccm.201511-2314OC [DOI] [PubMed] [Google Scholar]
- 55.Palm A, Berne C, Igelström H, Åsenlöf P, Janson C, Lindberg E. The impact of continuous positive airway pressure on circulating IGF-1 in patients with obstructive sleep apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. (2018) 14:385–91. 10.5664/jcsm.6982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meston N, Davies RJO, Mullins R, Jenkinson C, Wass J a H, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. (2003) 254:447–54. 10.1046/j.1365-2796.2003.01212.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.