Abstract
Background
Cardiac Bridging Integrator 1 (cBIN1) is a membrane deformation protein that generates calcium microdomains at cardiomyocyte t-tubules, whose transcription is reduced in heart failure, and is released into blood. cBIN1 score (CS), an inverse index of plasma cBIN1, measures cellular myocardial remodeling. In patients with heart failure with preserved ejection fraction (HFpEF), CS diagnoses ambulatory heart failure and prognosticates hospitalization. The performance of CS has not been tested in patients with heart failure with reduced ejection fraction (HFrEF).
Methods and Results
CS was determined from plasma of patients recruited in a prospective study. Two comparative cohorts consisted of 158 ambulatory HFrEF patients (left ventricular ejection fraction (LVEF) ≤ 40%, 57 ± 10 years, 80% men) and 115 age and sex matched volunteers with no known history of HF. N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were also analyzed for comparison. CS follows a normal distribution with a median of 0 in the controls, which increases to a median of 1.9 (p < 0.0001) in HFrEF patients. CS correlates with clinically assessed New York Heart Association Class (p = 0.007). During 1-year follow-up, a high CS (≥ 1.9) in patients predicts increased cardiovascular events (43% vs. 26%, p = 0.01, hazard ratio 1.9). Compared to a model with demographics, clinical risk factors, and NT-proBNP, adding CS to the model improved the overall continuous net reclassification improvement (NRI 0.64; 95% CI 0.18-1.10; p = 0.006). Although performance for diagnosis and prognosis was similar to CS, NT-proBNP did not prognosticate between patients whose NT-proBNP values were > 400 pg/ml.
Conclusion
CS, which is mechanistically distinct from NT-proBNP, successfully differentiates myocardial health between patients with HFrEF and matched controls. A high CS reflects advanced NYHA stage, pathologic cardiac muscle remodeling, and predicts 1-year risk of cardiovascular events in ambulatory HFrEF patients. CS is a marker of myocardial remodeling in HFrEF patients, independent of volume status.
Keywords: heart failure, cardiac muscle remodeling, ion channels, calcium handling, cBIN1 score
Introduction
Heart failure (HF) is currently one of the most burdensome global public health problems. This complex, progressive condition is caused by a cascade of pathophysiologic insults that lead to abnormally functioning myocardium, which at the myocyte level is associated with disturbed intracellular calcium homeostasis and impaired mitochondrial function and cellular metabolism. The current prevalence of HF among adults in the United States is 6.2 million and is projected to keep increasing over the next 10 years (Virani et al., 2020). Half of this population have reduced ejection fraction (HFrEF) with high morbidity and mortality mainly caused by cardiac pump failure and sudden cardiac death. Clinical management remains challenging, with a total cost of care greater than $30 billion per year. HF hospitalizations are already the highest single cost to Medicare for Americans over the age of 65, and this expense is expected to increase (Go et al., 2014). The substantial burden of this disease represents a healthcare and economic imperative to assess the health of cardiac muscle and use that information to limit future hospitalizations.
Unlike with most cancers which have advanced tissue based molecular diagnostics, our current methods at assessing prognosis in heart failure are more limited to status quo hemodynamics and organ function assessments (Murray et al., 2005; Chow and Senderovich, 2018; Zadikany et al., 2019). Current guidelines define HFrEF patients as having transthoracic echocardiogram obtained left ventricular ejection fraction (LVEF) of less than 40% (Yancy et al., 2013). Traditional diagnostic tools for assessing the severity of HFrEF include not only the transthoracic echocardiogram (Loeppky et al., 1984), but New York Heart Association (NYHA) assessment (Dickstein et al., 2008), cardiopulmonary exercise testing (Weber and Janicki, 1985; Corra et al., 2002), and cardiac index obtained from right heart catheterization (Ganz et al., 1971). These assessments provide valuable information regarding overall cardiac function (Ponikowski et al., 2016). However, because these assessments will vary with loading conditions, they do not necessarily correlate with intrinsic myocardial health. Furthermore, current cardiac assessment tools often require specialized equipment and highly trained medical staff, limiting accessibility for patients being seen in a general clinician’s office. Clinicians could be well served with a quantitative blood-based tool that is able to effectively assess the molecular health and reserve of cardiac muscle to assist with critical clinical decision making such as the need for left ventricular assist device (LVAD) and implantable cardioverter defibrillator (ICD), as well as heart transplant priorities.
The gold standard biomarkers to assess patients with HF symptoms are the brain natriuretic peptide (BNP) family (Sun et al., 2014). Both BNP and its more stable and non-active version, N-terminal prohormone BNP (NT-proBNP) are secreted by cardiomyocytes in response to pressure and stretch. Active BNP results in a downstream natriuresis, diuresis, and vasodilatation (Lee and Daniels, 2016). As stretch-response molecules, blood available BNP biomarkers help assess fluid status and assist in evaluating patients with acute dyspnea (Januzzi et al., 2005). However, NT-proBNP is not more effective than a usual care strategy in high-risk patients with HFrEF (Felker et al., 2017). Furthermore, BNP values are affected by renal function and body mass index (BMI) (Myhre et al., 2018). Taken together, HF diagnosis and management of ambulatory patients could benefit from a quantitative assessment of the health of cardiac muscle that is insensitive to volume, BMI, or non-cardiac organ function.
Cardiac bridging integrator 1 (cBIN1) score (CS), a novel biomarker of HF, has recently been introduced as a diagnostic and prognostic test in patients with heart failure with preserved ejection fraction (HFpEF) (Nikolova et al., 2018). cBIN1 is a cardiac-specific transverse tubule (t-tubule) membrane sculpting protein, functioning to organize microdomains responsible for calcium release and excitation-contraction (EC) coupling (Hong et al., 2014), as well as efficient diastolic calcium reuptake (Liu et al., in press). In doing so, cBIN1 helps maintain intracellular calcium homeostasis particularly at functionally important microenvironment adjoining t-tubule, junctional sarcoplasmic reticulum, and mitochondria. As a result, low cardiomyocyte cBIN1, which is also measurable at the plasma level (Xu et al., 2017), is intrinsically linked to cardiac inotropy (Hong et al., 2012), lusitropy (Liu et al., in press), and arrhythmia risks (Hong et al., 2014) particularly during stress response (Fu et al., 2016). Derived from the inverse of plasma cBIN1 level (Nikolova et al., 2018), CS rises with worsening HF. In this study, we explored whether CS has the potential to diagnose HFrEF when compared with matched controls. We also explored CS’s ability, within the HFrEF cohort, to accurately predict future cardiac events.
Materials and Methods
Study Design
All human studies were approved by the institutional review board at Cedars-Sinai Medical Center. Full informed consent was obtained from all subjects prior to participation in the study. The study involved two human populations, including patients with documented HFrEF and volunteers with no known history of HF.
The HFrEF cohort, followed longitudinally in the Advanced Heart Failure clinic at Cedars-Sinai Smidt Heart Institute, consisted of those in the clinic with a known diagnosis of HFrEF (left ventricular ejection fraction (LVEF) ≤ 40% and history of HF). From July 2014–November 2015, 158 patients were enrolled and a blood sample was obtained from patients at the time of clinic-scheduled phlebotomy. Patients with LVEF > 40% at the time of enrollment were excluded. Patient demographics, clinical information, medications, and laboratory and diagnostic test results were gathered from the hospital electronic medical records into a secure de-identified database. Subsequent clinical information was updated from chart review occurring every 3 months for 1 year.
The comparison cohort, consisting of 115 age and sex matched volunteers with no known history of HF, was obtained from the Cedars-Sinai MIRIAD IBD Consortium and Innovative Research, under similar plasma collection and preparation as the HFrEF samples. A clinical history, including patient demographics, medical history, and current medications was obtained from each volunteer.
Sample Processing and CS Determination
Whole venous blood was drawn into EDTA lavender tubes, stored immediately at 4°C for less than 4 h, and then processed to plasma and flash frozen for storage at −80°C prior to use, as previously described (Nikolova et al., 2018). The concentration of cBIN1 was determined using a cBIN1 specific sandwich-ELISA assay provided by Sarcotein Diagnostics, as previously described (Xu et al., 2017). Findings are reported using CS, the natural log of the inverse of cBIN1 plasma concentration (Nikolova et al., 2018), which increases as cBIN1 decreases.
NT-proBNP Assay
NT-proBNP values were obtained from the plasma of control and HFrEF patients. The Cedars-Sinai Medical Center clinical laboratory referred samples to Quest Diagnostics Laboratory to perform the NT-proBNP assay using electrochemiluminescence.
Cardiac Event Defined as the Primary Outcome During Follow-Up
Cardiac events were predefined as any HF or cardiac hospitalization, left ventricular assist device (LVAD) or mechanical circulatory support (MCS) placement, orthotopic heart transplantation (OHT), or death within the 1 year after time of blood draw. All chart review and adjudication were done by a two-physician panel, who were not involved in the clinical care of the patients (YX and RZ).
Statistical Analysis
Data distributions were assessed for normality by the Kolmogorov-Smirnov test. Continuous variables with normal distributions were expressed as means and standard deviations and compared using two-sided t-tests. Continuous variables with non-normal distributions were analyzed with medians and interquartile ranges (IQR) and compared using Mann–Whitney U and Kruskal–Wallis tests. Categorical variables were compared using Fisher’s exact or chi-square test. Receiver operating characteristic (ROC) analyses were performed to determine the sensitivity and specificity of CS and NT-proBNP to diagnose HF. Kaplan-Meier and Cox-proportional hazard analyses were used to compare the differences in event-free survival rates between patients with high and low values of CS. Model one included age, sex, BMI, NYHA class, LVEF, estimated glomular filtration rate (eGFR), and NT-proBNP. Model two included the same covariants with CS added to it. To determine the increase in discriminative value to predict 1 year outcomes following the addition of CS as a covariant in the survival model, we evaluated continuous net reclassification improvement (NRI). Two-sided p-values were reported and a p < 0.05 was considered statistically significant. Statistical analyses were conducted using SAS Version 9.3.1 software (SAS Institute, Inc.), RStudio Version 1.0.143 (RStudio, Inc.), and GraphPad Prism Version 6 (GraphPad Software).
Results
Study Cohorts
158 HFrEF patients (57 ± 10 years old, 80% men) were age and sex matched to 115 volunteers with no known history of HF (54 ± 6 years old, 80% men) (Table 1). The HFrEF cohort consisted of 55 (35%) ischemic and 101 (64%) non-ischemic patients. Most of the non-ischemic patients have idiopathic cardiomyopathy (68%), with the remainder due to valvular disease, toxin-mediated disease, or infiltrative disease. Most patients were classified as New York Heart Association (NYHA) II or III (35 and 48%, respectively) and the prevalence of comorbidities was 45% hypertension, 37% diabetes, and 23% chronic kidney disease. The baseline median LVEF on transthoracic echocardiography was 24 ± 8%. Patients were treated with guideline-directed medical therapy by the Cedars-Sinai Advanced Heart Disease group.
TABLE 1.
Baseline characteristics of HFrEF and matched controls.
| Characteristics (%) | Heart failure with reduced EF (n = 158) | Matched controls (n = 115) | p-value |
| Age (SD) | 57 ± 10.4 | 54 ± 6.1 | <0.001 |
| ≤ 50 years | 39 (25) | 29 (25) | NS |
| > 50 years | 119 (75) | 86 (75) | |
| Male | 126 (80) | 92 (80) | NS |
| White | 93 (59) | 67 (58) | NS |
| BMI, kg/m2 (SD) | 29 ± 6.1 | 29 ± 5.6 | NS |
| Hypertension | 71 (45) | 13 (11) | <0.001 |
| Diabetes | 59 (37) | 8 (7) | <0.001 |
| CKD | 36 (23) | ||
| LVEF | 24 ± 8.1 | ||
| Medications | |||
| Beta-Blockers | 139 (88) | ||
| ACE-I/ARB | 120 (76) | ||
| Diuretics | 125 (79) | ||
| NYHA | |||
| I | 21 (13) | ||
| II | 55 (35) | ||
| III | 76 (48) | ||
| IV | 6 (4) | ||
| Subtype of heart failure | |||
| Ischemic | 55 (35) | ||
| Non-Ischemic | 101 (64) | ||
| Valvular | 10 (10) | ||
| Dilated | 11 (11) | ||
| Toxin | 9 (9) | ||
| Infiltrative | 2 (2) | ||
| Other | 69 (68) |
HFrEF, heart failure with reduced ejection fraction; SD, standard deviation; BMI, body mass index; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; NYHA, New York Heart Association.
CS Is Elevated in Patients With HFrEF
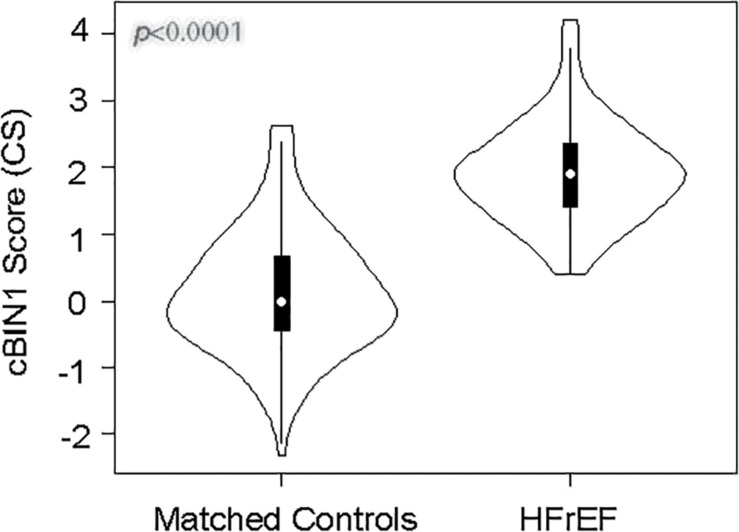
Violin plots of CS in HFrEF and matched controls are shown in Figure 1. The HFrEF cohort median CS is elevated relative to the controls, 1.9 (IQR 1.4–2.4; mean ± SD 1.9 ± 0.7) compared to 0 (IQR -0.5–0.7; mean ± SD 0.1 ± 0.9), respectively (p < 0.0001), with an approximately normal distribution in both cohorts. The HFrEF cohort median of ln NT-proBNP level, 7.0 (IQR 6.0–8.1), is also significantly elevated compared to the median level of the control cohort, 3.3 (IQR 2.7–4.1), with p < 0.0001 (Supplementary Figure S1). NT-proBNP levels (pg/ml) have a median of 28 (IQR 15–60) in controls and a median of 1,081 (IQR 409–3,419) in HFrEF patients.
FIGURE 1.
Distribution of CS among HFrEF and matched controls. The vertical axis is the cBIN1 score (CS), and the width of each violin graph depicts the density plot at each measured value. The HFrEF cohort median CS is elevated relative to the controls, 1.9 (IQR 1.4–2.4) compared to 0 (IQR -0.5–0.7), respectively (p < 0.0001).
Median CS (Table 2) and NT-proBNP (Supplementary Table S1) values were analyzed among different subgroups of demographics and clinical parameters. CS and NT-proBNP do not vary with sex or age. Unlike NT-proBNP, CS does not differ among BMI categories (normal, overweight, and obese) in controls or HFrEF patients. Among HFrEF patients with normal vs. decreased estimated glomerular filtration rate (eGFR < 60 ml/min/m2), CS does not differ (1.8 and 1.9, respectively), while patients with worsening eGFR had increased NT-proBNP levels (p = 0.01). CS an NT-proBNP correlate with clinically assessed New York Heart Association Class (p = 0.007 and p < 0.001, respectively). The lack of association with CS and obesity or renal function is consistent with results previously reported in patients with HFpEF and matched controls (Nikolova et al., 2018).
TABLE 2.
CS among subgroups in matched controls and HFrEF patients.
| Characteristics | N | Matched controls | IQR (Q1-Q3) | p-value | N | HFrEF | IQR (Q1-Q3) | p-value |
| All patients | 115 | 0.0 | −0.5–0.7 | – | 158 | 1.9 | 1.4–2.4 | – |
| Sex | NS | NS | ||||||
| Men | 92 | 0.0 | −0.5–0.8 | 126 | 1.9 | 1.4–2.3 | ||
| Women | 23 | 0.0 | −0.3–0.4 | 32 | 1.9 | 1.3–2.4 | ||
| Age (years) | NS | NS | ||||||
| < 55 | 67 | −0.1 | −0.5–0.7 | 54 | 1.8 | 1.5–2.1 | ||
| ≥ 55 | 48 | 0.1 | −0.4–0.7 | 104 | 2.0 | 1.4–2.4 | ||
| Race/Ethnicity | NS | 0.03 | ||||||
| White | 67 | 0.1 | −0.6–0.8 | 93 | 1.8 | 1.3–2.1 | ||
| Black | 24 | 0.0 | −0.5–0.9 | 26 | 2.1 | 1.8–2.6 | ||
| Hispanic | 24 | −0.1 | −0.3–0.4 | 28 | 2.0 | 1.5–2.5 | ||
| Asian | 0 | – | – | 9 | 1.6 | 1.3–2.3 | ||
| BMI (kg/m2) | NS | NS | ||||||
| Normal (<25) | 32 | 0.0 | −0.5–0.7 | 29 | 1.9 | 1.4–2.4 | ||
| Overweight (25–29.9) | 34 | 0.0 | −0.4–0.7 | 65 | 1.9 | 1.4–2.4 | ||
| Obese (30–34.9) | 25 | −0.1 | −1.0–0.4 | 38 | 2.0 | 1.7–2.5 | ||
| Morbid Obesity (≥35) | 14 | −0.2 | −0.4–0.1 | 25 | 1.7 | 1.1–2.1 | ||
| Etiology | NS | |||||||
| Ischemic HFrEF | 55 | 2.0 | 1.5–2.5 | |||||
| Non-ischemic HFrEF | 101 | 1.9 | 1.4–2.3 | |||||
| eGFR (ml/min/m2) | NS | |||||||
| <60 | 94 | 1.9 | 1.5–2.2 | |||||
| >60 | 64 | 1.8 | 1.4–2.5 | |||||
| NYHA | 0.007 | |||||||
| I | 21 | 1.6 | 1.3–2.0 | |||||
| II | 55 | 1.8 | 1.5–2.3 | |||||
| III | 76 | 2.0 | 1.4–2.5 | |||||
| IV | 6 | 2.6 | 2.1–3.0 | |||||
| OHT | 0.0006 | |||||||
| No | 127 | 1.8 | 1.4–2.2 | |||||
| Yes | 31 | 2.3 | 1.7–3.0 |
HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; Q1, quartile 1; Q3, quartile 3; BMI, body mass index; eGFR, estimated glomular filtration rate; NYHA, New York Heart Association; OHT, orthotopic heart transplant.
CS Diagnoses Failing Heart Muscle
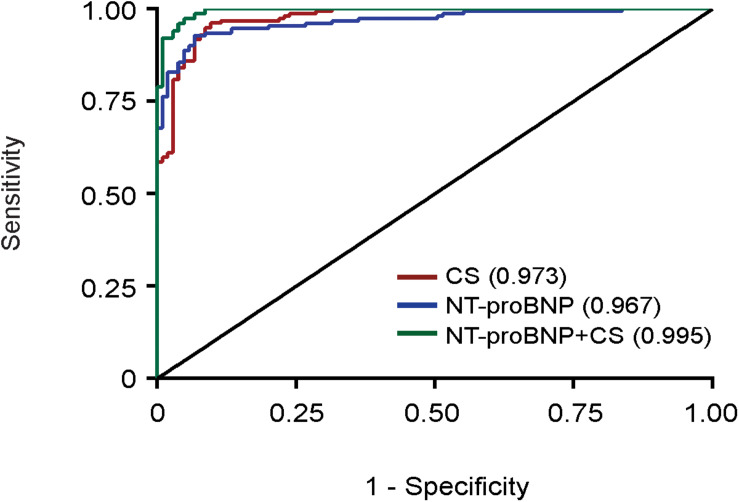
Since CS is higher in HFrEF patients than controls, we generated receiver operating characteristic (ROC) curves for CS and NT-proBNP, adjusting for age, sex, and BMI (Figure 2). The area under the curve (AUC) of the ROC indicates that both CS (AUC = 0.973, red) and NT-proBNP (AUC = 0.967, blue) distinguish patients with HF from the control population. When CS and NT-proBNP were combined, the ability to determine HF from the control population, while already strong, still improved with statistical significance (AUC = 0.995, green, p = 0.01) suggesting that CS (muscle health) and NT-proBNP (intracardiac volume) can be complementary.
FIGURE 2.
Receiver operating characteristic (ROC) curves. ROC curve containing the various sensitivities and specificities of CS (AUC = 0.973, red), NT-proBNP (AUC = 0.967, blue), and combined NT-proBNP and CS (AUC = 0.995, green) to diagnose disease in our control and HFrEF cohorts.
Elevated CS Prognosticates Cardiovascular Hospitalization in HFrEF Patients
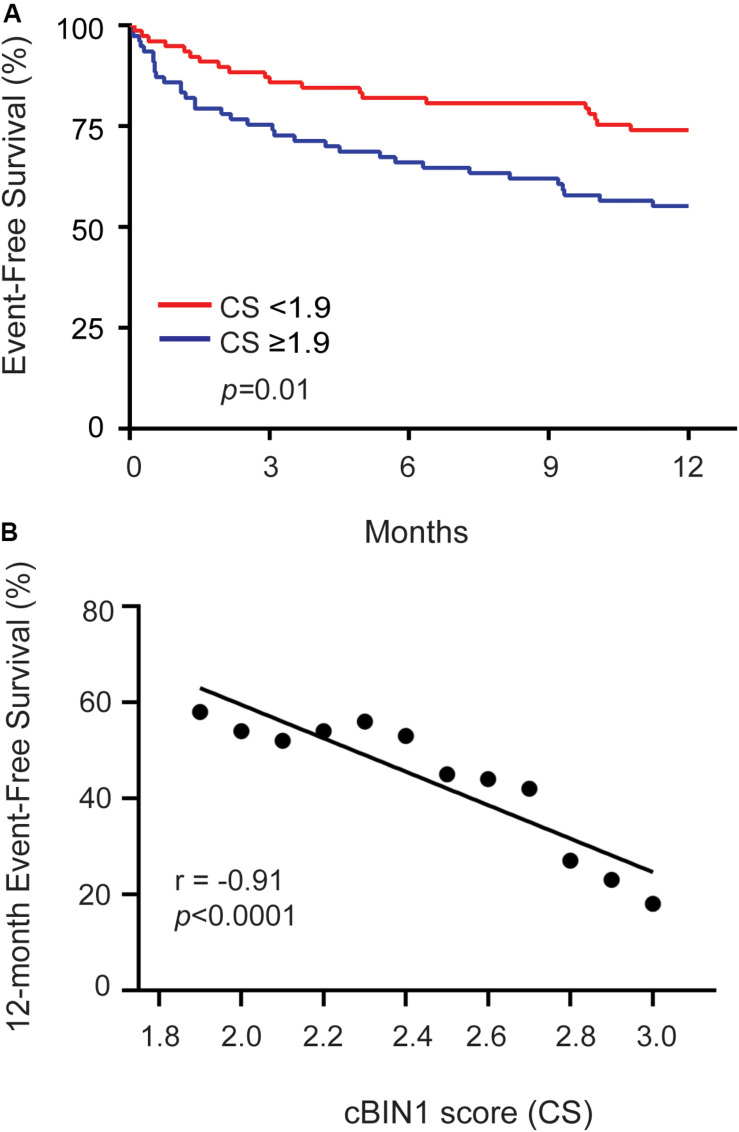
Next, we explored whether, in addition to its diagnostic value, CS can serve as a prognostic marker in predicting future clinical outcomes in patients with HFrEF. During the 12-month follow-up period, we found that 66 patients (42%) had at least one cardiovascular (CV) event: 41 (26%) patients had a HF-related hospitalization, 16 (10%) patients with a hospitalization diagnosis that was cardiac, but not HF, in origin, 31 (20%) patients had OHT, 4 (3%) went onto MCS or LVAD, and 9 (6%) patients died (6 of which were due to cardiac etiology). Kaplan-Meier survival curves were generated (Figure 3A). The median CS level of 1.9 (drawn at the initial visit) was used as a cutoff to differentiate high verse low CS (Nikolova et al., 2018). A cutoff higher than the median of 1.9 further increases specificity of 12-month event free survival (Figure 3B). Using a model including age, sex, BMI, NYHA class, LVEF, eGFR, and NT-proBNP (Table 3), the hazard ratio (HR) of a high CS ≥ 1.9 predicting CV event rate among HFrEF patients during 1 year of follow-up was 1.9 (43 vs. 26%, p = 0.03). The addition of CS ≥ 1.9 significantly improved continuous net reclassification improvement (NRI 0.64; 95% CI 0.18-1.10; p = 0.006).
FIGURE 3.
Cardiovascular event free survival of HFrEF patients during 12-month follow-up. (A) Kaplain-Meier survival curve is shown here for all HFrEF patients. The red line demonstrates patients with CS < 1.9 and the blue line demonstrate patients with CS ≥ 1.9. Event free survival is defined as patients who did not have a cardiovascular (CV) event (HF-related hospitalization, cardiac hospitalization, LVAD, OHT, or death) during 12-months follow-up. A low CS (<1.9) predicted a higher event-free survival among all HFrEF patients (p = 0.01). (B) Scatterplot of 12-month event-free survival vs. CS for all CS ≥ 1.9 indicates a negative correlation (Pearsons’s correlation coefficient −0.91, p < 0.0001).
TABLE 3.
Risk of CV Event in HFrEF patients with multivariate Cox regression analysis.
| Variables | Model without CS |
Model with CS |
||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age, > 55 vs. < 55 years | 0.88 | 0.45–1.72 | 0.71 | 0.95 | 0.49–1.86 | 0.88 |
| Sex, female vs. male | 0.45 | 0.20–1.03 | 0.06 | 0.47 | 0.20–1.07 | 0.07 |
| BMI, kg/m2 | 1.0 | 0.98–1.09 | 0.25 | 1.04 | 0.98–1.1 | 0.19 |
| NYHA class, III-IV vs. I-II | 2.75 | 1.39–5.43 | <0.01 | 2.59 | 1.33–5.06 | 0.01 |
| LVEF% | 1.03 | 0.99–1.07 | 0.14 | 1.04 | 1.00–1.08 | 0.07 |
| eGFR, ml/min/m2 | 1.00 | 0.98–1.01 | 0.53 | 0.99 | 0.98–1.01 | 0.36 |
| NT-proBNP, Q4 vs. Q1 | 3.05 | 1.00–9.30 | 0.05 | 3.30 | 1.1–10.0 | 0.03 |
| CS, >1.9 vs. <1.9 | – | – | – | 1.93 | 1.07–3.48 | 0.03 |
CV, cardiovascular; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; CI, confidence interval; eGFR, estimated glomular filtration rate; BMI, body mass index; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction.
For NT-proBNP, the Kaplan-Meier survival curve using the median NT-proBNP cutoff value of 1078 pg/ml, was not quite significant in predicting 1-year CV event (40 vs. 28%, p = 0.06). Interestingly, the prognostic power of NT-proBNP was in the low values (<409 pg/ml in the 1st quartile) (Supplementary Figure S2). A low NT-proBNP predicted CV events with a HR of 3.3 (40 vs. 19%, p = 0.03). There was no significant prognostication in patients with NT-proBNP greater than 409 pg/ml.
Discussion
The prevalence of HFrEF is increasing over time as our population continues to age, making this clinical syndrome a significant public health concern. The diagnosis of HF remains complex, involving a combination of history taking, physical examination, labs, imaging, and functional studies. Among the existing armamentarium of HF diagnostics, there is currently no invasive or non-invasive tool to measure intrinsic cardiomyocyte remodeling. CS can be used as a blood available biomarker to assist in this clinical need.
In pre-clinical animal models, pathophysiological changes in cardiomyocytes of failing hearts such as t-tubule remodeling is considered the transition point from functional compensation to decompensated heart failure (Wei et al., 2010). cBIN1 reductions are associated with t-tubule remodeling and the progression of heart failure (Hong et al., 2012, 2014; Xu et al., 2017). With recovery of muscle, cBIN1 levels also recover (Lyon et al., 2012). Given that cBIN1 is also blood available, CS provides a blood available “liquid biopsy” of cardiac muscle.
HF progression can be reflected by different biomarkers, with robust data demonstrating the ability of natriuretic peptides (BNP and NT-proBNP) to reflect myocardial wall stress (Iwanaga et al., 2006). As a marker of acute volume overload, the diagnostic utility of BNP peptides is well-validated in detecting acute decompensated HF (Januzzi et al., 2005). However, blood BNP values are affected by obesity and renal dysfunction, in addition to requiring adjustments for age and sex (Myhre et al., 2018). Furthermore, because natriuretic peptides are reflective of volume status, it can be difficult to use BNP as a marker to distinguish between patients with severe HFrEF and patients with non-cardiac origin volume overload, such as renal failure.
There is a need for additional HF biomarkers that can help identify the intrinsic health of cardiac muscle cells independent of volume status. Because CS reflects cardiac muscle cell health, it is stable and independent of fluctuations induced by intracardiac volume, inflammatory state, or body habitus (Nikolova et al., 2018). The characteristic performance of CS observed in the current HFrEF cohort indicates that a positive CS can help accurately identify failing heart muscle and is not sensitive to comorbid conditions. Thus, CS provides an unprecedent and non-invasive tool to help with evaluation of myocyte health from EC-coupling efficiency to electrical stability, determining individual’s risks of pump failure and arrhythmias. As a signature footprint from myocytes, CS is therefore particularly important at many critical clinical decision-making points for HFrEF patients, guiding medical treatment choices, clinical surveillance intervals, criteria determination for LVAD and ICD implant, and evaluation of the need and the recovery potential of a heart transplant.
In this report we find that within a HFrEF cohort, a CS cutoff value of greater than 1.9 accurately predicts cardiac hospitalization during a 1 year follow-up period (Figure 3), as it did in a HFpEF cohort (Nikolova et al., 2018). In addition, our findings indicate that the addition of CS, to a model with established risk predictors, significantly improved risk classification for CV events in HFrEF patients. In an ambulatory clinic, a high CS may provide the added prognostic information needed to help sway the pendulum of clinical care toward more aggressive surveillance or escalating care to more advanced therapies. Conversely, an advanced HFrEF patient with a low, even normal CS, may indicate an ability to postpone advanced therapies and continue monitoring with periodic clinic visits. Clinical decompensation with a high CS would indicate failing heart, whereas clinical decompensation with a low CS could suggest extracardiac factors such as renal failure or medical non-compliance are dominating the clinical decline.
Limitations
This is a real-world single center clinical patient population with no exclusion criteria and the HFrEF patients had follow up at the discretion of clinical providers. We would like to reproduce these findings in a prospective multicenter HFrEF cohort. In keeping with our prior study, we used a CS cutoff of the median (1.9). Based on the data in Figure 3B, investigators may choose to use a higher cutoff than the median CS, which would improve test specificity.
Conclusion
A protein involved in cardiomyocyte t-tubule remodeling is blood available and can function as a biomarker that helps diagnose and prognosticate ambulatory HFrEF patients. The potential advantages of CS include early pre-clinical diagnosis in asymptomatic patients, differentiating cardiac origin volume overload from extracardiac source, prognosticating outcomes in stable ambulatory patients, and evaluating myocardial recovery once on a successful therapeutic regimen. In an era of ballooning health care costs, CS is a blood-available test that substantially adds to the assessment of ambulatory HFrEF.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RMS, upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Cedars-Sinai Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TCH, TTH, and RS contributed to conception and design of the study. TCH, YX, and RZ organized the database. TCH performed the statistical analysis. YX wrote the first draft of the manuscript. TCH, YX, RZ, AN, MK, MH, AW, TTH, and RS wrote sections of the manuscript. GM, DM, DG, DC, JM, AH, BA, LC, MK, JP, JK, and MH consented patients and collected patient samples. RB, A-MC, and SA ran ELISA assays.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Sarcotein Diagnostics (www.sarcotein.com) for providing the ELISA-based CS assay (Nikolova et al., 2018). Sarcotein Diagnostics has recently generated a standardized version of the CS assay for general use. To avoid confusion in CS values, future studies should use new standardized assay.
Footnotes
Funding. Funding for this research including NIH/NHLBI HL133286, HL152691, and HL138577. The MIRIAD IBD Biobank is supported by the Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, NIH/NIDDK Grant No. P01DK046763, The European Union Grant No. 305479, NIH/NIDDK Grant No. DK062413, and The Leona M. and Harry B. Helmsley Charitable Trust.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00503/full#supplementary-material
References
- Chow J., Senderovich H. (2018). it’s time to talk: challenges in providing integrated palliative care in advanced congestive Heart failure. a narrative review. Curr. Cardiol. Rev. 14 128–137. 10.2174/1573403X14666180123165203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corra U., Mezzani A., Bosimini E., Scapellato F., Imparato A., Giannuzzi P. (2002). Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am. Heart J. 143 418–426. 10.1067/mhj.2002.120772 [DOI] [PubMed] [Google Scholar]
- Dickstein K., Cohen-Solal A., Filippatos G., McMurray J. J., Ponikowski P., Poole-Wilson P. A., et al. (2008). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart failure association of the ESC (HFA) and endorsed by the European Society of intensive care medicine (ESICM). Eur. J. Heart Fail. 10 933–989. [DOI] [PubMed] [Google Scholar]
- Felker G. M., Anstrom K. J., Adams K. F., Ezekowitz J. A., Fiuzat M., Houston-Miller N., et al. (2017). Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 318 713–720. 10.1001/jama.2017.10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Shaw S. A., Naami R., Vuong C. L., Basheer W. A., Guo X., et al. (2016). Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation 133 388–397. 10.1161/CIRCULATIONAHA.115.018535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz W., Donoso R., Marcus H. S., Forrester J. S., Swan H. J. (1971). A new technique for measurement of cardiac output by thermodilution in man. Am. J. Cardiol. 27 392–396. [DOI] [PubMed] [Google Scholar]
- Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Blaha M. J., et al. (2014). Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129 e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T., Yang H., Zhang S. S., Cho H. C., Kalashnikova M., Sun B., et al. (2014). Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat. Med. 20 624–632. 10.1038/nm.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T. T., Smyth J. W., Chu K. Y., Vogan J. M., Fong T. S., Jensen B. C., et al. (2012). BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 9 812–820. 10.1016/j.hrthm.2011.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga Y., Nishi I., Furuichi S., Noguchi T., Sase K., Kihara Y., et al. (2006). B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 47 742–748. 10.1016/j.jacc.2005.11.030 [DOI] [PubMed] [Google Scholar]
- Januzzi J. L., Jr., Camargo C. A., Anwaruddin S., Baggish A. L., Chen A. A., Krauser D. G., et al. (2005). The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95 948–954. 10.1016/j.amjcard.2004.12.032 [DOI] [PubMed] [Google Scholar]
- Lee N. S., Daniels L. B. (2016). Current understanding of the compensatory actions of cardiac natriuretic peptides in cardiac failure: a clinical perspective. Card. Fail. Rev. 2 14–19. 10.15420/cfr.2016:4:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- >Liu Y. Z. K., Li J., Agvanian S., Caldaruse A. M., Shaw S., Hitzeman T. C., et al. (in press). In mice subjected to chronic stress, exogenous cBIN1 preserves calcium handling machinery and cardiac function. JACC Basic Transl. Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeppky J. A., Hoekenga D. E., Greene E. R., Luft U. C. (1984). Comparison of noninvasive pulsed Doppler and Fick measurements of stroke volume in cardiac patients. Am. Heart J. 107 339–346. 10.1016/0002-8703(84)90384-3 [DOI] [PubMed] [Google Scholar]
- Lyon A. R., Nikolaev V. O., Miragoli M., Sikkel M. B., Paur H., Benard L., et al. (2012). Plasticity of surface structures and beta2-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail. 5 357–365. 10.1161/circheartfailure.111.964692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S. A., Kendall M., Boyd K., Sheikh A. (2005). Illness trajectories and palliative care. BMJ 330 1007–1011. 10.1136/bmj.330.7498.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre P. L., Vaduganathan M., Claggett B. L., I, Anand S., Sweitzer N. K., Fang J. C., et al. (2018). Association of natriuretic peptides with cardiovascular prognosis in heart failure with preserved ejection fraction: secondary analysis of the TOPCAT randomized clinical trial. JAMA Cardiol. 3 1000–1005. 10.1001/jamacardio.2018.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova A. P., Hitzeman T. C., Baum R., Caldaruse A. M., Agvanian S., Xie Y., et al. (2018). Association of a novel diagnostic biomarker, the plasma cardiac bridging integrator 1 score, with heart failure with preserved ejection fraction and cardiovascular hospitalization. JAMA Cardiol. 3 1206–1210. 10.1001/jamacardio.2018.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P., Voors A. A., Anker S. D., Bueno H., Cleland J. G. F., Coats A. J. S., et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37 2129–2200. [DOI] [PubMed] [Google Scholar]
- Sun R. R., Lu L., Liu M., Cao Y., Li X. C., Liu H., et al. (2014). Biomarkers and heart disease. Eur. Rev. Med. Pharmacol. Sci. 18 2927–2935. [PubMed] [Google Scholar]
- Virani S. S., Alonso A., Benjamin E. J., Bittencourt M. S., Callaway C. W., Carson A. P., et al. (2020). Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 141 e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Weber K. T., Janicki J. S. (1985). Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am. J. Cardiol. 55 22a–31a. [DOI] [PubMed] [Google Scholar]
- Wei S., Guo A., Chen B., Kutschke W., Xie Y. P., Zimmerman K., et al. (2010). T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 107 520–531. 10.1161/circresaha.109.212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Fu Y., Liu Y., Agvanian S., Wirka R. C., Baum R., et al. (2017). The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 15:e2002354. 10.1371/journal.pbio.2002354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E., Jr., Drazner M. H., et al. (2013). 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart association task force on practice guidelines. J. Am. Coll. Cardiol. 62 e147–e239. [DOI] [PubMed] [Google Scholar]
- Zadikany R. H., Hong T., Shaw R. M. (2019). Heart failure: distinguishing failing myocardium from volume overload. Biomark. Med. 13 697–700. 10.2217/bmm-2019-0168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RMS, upon reasonable request.