Abstract
Metabolic disorders, such as obesity, elevated blood pressure, dyslipidemias, insulin resistance, hyperglycemia, and hyperuricemia have all been identified as risk factors for an epidemic of important and widespread chronic-degenerative diseases, such as type 2 diabetes and cardiovascular disease, that constitute some of the world's most important public health challenges. Their increasing prevalence can be associated with an aging population and to lifestyles within an obesogenic environment. Taking educational level as a proxy for lifestyle, and using both logistic and linear regressions, we study the relation between a wide set of metabolic biomarkers, and educational level, body mass index (BMI), age, and sex as correlates, in a population of 1,073 students, academic and non-academic staff at Mexico's largest university (UNAM). Controlling for BMI and sex, we consider educational level and age as complementary measures—degree and duration—of exposure to metabolic insults. Analyzing the role of education across a wide spectrum of educational levels (from primary school to doctoral degree), we show that higher education correlates to significantly better metabolic health when compared to lower levels, and is associated with significantly less risk for waist circumference, systolic blood pressure, glucose, glycosylated hemoglobin, triglycerides, high density lipoprotein and metabolic syndrome (all p < 0.05); but not for diastolic blood pressure, basal insulin, uric acid, low density lipoprotein, and total cholesterol. We classify each biomarker, and corresponding metabolic disorder, by its associated set of statistically significant correlates. Differences among the sets of significant correlates indicate various aetiologies and the need for targeted population-specific interventions. Thus, variables strongly linked to educational level are candidates for lifestyle change interventions. Hence, public policy efforts should be focused on those metabolic biomarkers strongly linked to education, while adopting a different approach for those biomarkers not linked as they may be poor targets for educational campaigns.
Keywords: metabolic syndrome, obesity, cross-sectional population study, education, age, body mass index (BMI), fasting blood test analysis
1. Introduction
In the last few decades, there has been a significant increase in the incidence of metabolic disorders, including disturbed glucose metabolism, general and abdominal obesity, elevated blood pressure, dyslipidemias, insulin resistance, hyperglycemia, and hyperuricemia that are all risk factors for several serious diseases, such as type 2 Diabetes Mellitus (DM2), cardiovascular disease (CVD), and stroke (1–4). Although these risk factors have a genetic component (5, 6), it is generally accepted that their current elevated incidence in most developed countries is due to a greatly increased exposure to metabolic insults that has at least two major sources: an obesogenic environment that may facilitate overconsumption, poor nutrition, and sedentarism, i.e., “life-style” (behavioral) factors (7–10); and aging populations, wherein the gradual appearance of metabolic disorders can occur due to longer lifespans (11, 12). Thus, aging can be thought of as a measure of the duration of metabolic insults and lifestyle a measure of their degree.
Metabolic biomarkers, as well as anthropometric and physiological variables, such as body mass index (BMI) and blood pressure, should reflect not only intrinsic genetic and physiological dependencies but also, importantly, metabolic insults due to the interaction between an individual and their environment, as intermediated by nutrition, activity, stress, and a host of other factors, which may differ between the sexes (13, 14). Indeed, risk factors for chronic diseases are widely classified as either modifiable or non-modifiable, with the former having been proven to cluster, as they are influenced by common sources, thus providing new insights to better target demographic profiles and inform public policies (15, 16). The complexity of human physiology, however, is such that the impact of a particular environmental interaction may be quite heterogeneous across different metabolic subsystems.
In terms of intervention and prevention, unlike age or sex, it is to modifiable risk factors, associated with behavior and lifestyle, that we must look to in order to make changes that will generate a positive impact on health status (17–19). Unfortunately, behavior and lifestyle, are highly complex and multi-factorial. To analyze them, various correlated, single variable proxies, such as educational level or socio-economic status, have been used in many studies. Educational level may be the more appropriate, being objective, easy to quantify, and capturing multiple facets of the social determinants of health, including personal behavior, living and working conditions, economic and social opportunities and resources (20). There is ample evidence that higher educational level is generally correlated with better health outcomes (20–26). In most studies, however, the spectrum of educational level in the associated populations is somewhat restricted, with either few participants at one extreme or the other. Additionally, most studies that have focused on the relation between health status and education, have also restricted attention to only one, or a few, health measures or biomarkers, such as BMI. For example, relevant to the context of the present study, the relation between BMI and educational level has been investigated in multiple studies (27–29), where it has been established that, in general, BMI is inversely related to educational level. Various studies have concluded that a more modest level of education is associated with a poorer general state of health (22–24).
Even though distinct metabolic factors have been individually linked to many diseases and, in particular, to CVD and DM2, overall metabolic risk has principally been addressed in the context of metabolic syndrome (MS) (30), which has been shown to be a useful tool for identifying individuals at risk of atherosclerotic CVD and DM2 (31–33), although there remains some controversy about its global significance (7, 34, 35), and as to whether its contribution to risk is equal or larger than the sum of its parts (36). While there exist different operational definitions of MS (30, 35, 37–39), that have been updated over time (13), most recent ones comprise a subset of the following (37): BMI, waist circumference (WC), total cholesterol, high density lipoprotein (HDL), triglycerides, glycemia, and high blood pressure—with specific thresholds for defining abnormal values. As well as the risk factors directly associated with MS, other metabolic biomarkers have been found to be of interest (40), such as glycosylated hemoglobin (Hb A1c) (41), homeostatic model assessment of insulin resistance (HOMA-IR) (42, 43), basal insulin (44, 45), and uric acid (46).
In this paper, we examine the dependence of a wide set of metabolic risk factors and biomarkers on educational level, BMI, age, and sex, within a unique Mexican university-based population that exhibits a particularly wide range of educational levels and ages. The overall aim of the study is to understand and predict obesity, and metabolic diseases over time by accumulating and analyzing a highly multi-factorial, multi-scale data set covering genetic, physiological, anthropometric, social, epidemiological, and psychological variables. However, in this paper we will use only transverse data to determine risk profiles for the considered metabolic variables, including anthropometric, blood pressure, and fasting blood test parameters, with special emphasis on how educational level and age are linked to metabolic risk. In particular, we will consider the impact of education beyond the undergraduate level, noting that it leads to additional health benefits. We explore several variables considered to be modifiable factors for MS (WC, HDL, blood pressure, triglycerides, and glucose) for which behavioral life-style changes are considered as first-line treatment (47). Although educational level has been found as a predictor variable associated with the odds of meeting healthy life-style guidelines, both in adults and senior populations (48), it is not clear if it is equally predictive for any metabolic biomarker and associated disorder.
2. Methods
2.1. Selection and Description of Participants
The present work is based on a cross-sectional, community-based health study in a population of students, academics and supporting staff of the Universidad Nacional Autónoma de México (UNAM), which is Mexico's largest university. Although the present study is part of a larger prospective investigation, here we will analyze the data of the initial baseline stage, taken in 2014, which consisted of 1,073 participants who were voluntarily enrolled into the study. Each participant provided a written informed consent. Besides filling in a substantial questionnaire, each participant had various vital signs [systolic blood pressure (SBP), diastolic blood pressure (DBP)] and anthropometric measurements (weight, height, WC) taken by trained medical staff using standard procedures. Additionally, they underwent a laboratory fasting blood test.
2.1.1. Ethical and Human Research Considerations
This study was carried out in accordance with the recommendations of the Ethics Committee of the Facultad de Medicina of the UNAM, which approved the procedures and protocols for this study under project FM/DI/023/2014. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
2.1.2. Database
Study data is available in the UNAM repository at the web page: http://www.c3.unam.mx/health/, in either csv or database formats, and includes:
Demographic information: sex, age, occupation (student, academic, or supporting staff), educational level, marital status, number of children, and siblings.
Anthropometric measures: height, weight, and WC.
Blood pressure: single (one-time) measurements of SBP and DBP.
Metabolic factors determined by laboratory results of a fasting blood test: glucose, glycosylated hemoglobin (Hb A1c), basal insulin, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, and uric acid levels.
From the original data set, 14 particular values associated with distinct variables were excluded, based on two main criteria:
outliers based on physiologically improbable or impossibly low values. This included removing from further analysis two glucose measurements, five values of HbA1c, and one each of insulin, HOMA, triglycerides, and uric acid;
values of HDL or LDL which were inconsistent with a given total cholesterol value. Three such values of LDL were removed from further analysis.
2.1.3. Study Groups
The population was divided into five groups based on their “educational level,” as seen in Table 1:
Table 1.
General description of the demographic data, anthropometric and blood pressure measurements of the UNAM study population.
| Descriptor | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|
| n | 48 | 285 | 405 | 181 | 154 | 1,073 |
| 4.5% | 26.6% | 37.6% | 16.9% | 14.4% | 100% | |
| Women n | 35 | 190 | 264 | 116 | 84 | 689 |
| (%) | (73%) | (67%) | (65%) | (64%) | (54%) | (64%) |
| Men n | 13 | 95 | 141 | 65 | 70 | 384 |
| (%) | (27%) | (33%) | (35%) | (36%) | (46%) | (36%) |
| Age | 50 ± 13 | 43 ± 13 | 41 ± 12 | 42 ± 12 | 49 ± 13 | 43 ± 13 |
| (years) | (24–77) | (19–74) | (20–80) | (24–67) | (29–81) | (19–81) |
| Women age | 51 ± 13 | 43 ± 12 | 41 ± 12 | 42 ± 11 | 47 ± 13 | 43 ± 12 |
| (years) | (24–77) | (20–74) | (20–80) | (24–67) | (29–81) | (20–81) |
| Men age | 45 ± 14 | 42 ± 14 | 40 ± 12 | 41 ± 12 | 51 ± 12 | 43 ± 13 |
| (years) | (25–62) | (19–72) | (21–74) | (27–67) | (29–76) | (19–76) |
| Weight | 70 ± 14 | 71 ± 14 | 68 ± 15 | 66 ± 12 | 69 ± 14 | 69 ± 14 |
| (kg) | (41–120) | (38–149) | (35–142) | (45–106) | (41–108) | (35–149) |
| Women weight | 67 ± 11 | 67 ± 13 | 64 ± 13 | 62 ± 11 | 61 ± 10 | 64 ± 12 |
| (kg) | (41–90) | (38–112) | (35–121) | (45–100) | (41–99) | (35–121) |
| Men weight | 79 ± 17 | 78 ± 15 | 77 ± 16 | 74 ± 12 | 79 ± 10 | 77 ± 14 |
| (kg) | (60–120) | (52–149) | (47–142) | (56–106) | (57–108) | (47–149) |
| Height | 1.6 ± 0.1 | 1.59 ± 0.09 | 1.61 ± 0.09 | 1.62 ± 0.09 | 1.65 ± 0.09 | 1.61 ± 0.09 |
| (m) | (1.41–1.90) | (1.39–1.96) | (1.24–1.97) | (1.42–1.89) | (1.44–1.90) | (1.24–1.97) |
| Women height | 1.52 ± 0.06 | 1.55 ± 0.07 | 1.57 ± 0.07 | 1.58 ± 0.07 | 1.58 ± 0.06 | 1.56 ± 0.07 |
| (m) | (1.41–1.65) | (1.39–1.90) | (1.42–1.97) | (1.42–1.89) | (1.44–1.70) | (1.39–1.97) |
| Men height | 1.67 ± 0.09 | 1.68 ± 0.07 | 1.69 ± 0.08 | 1.71 ± 0.06 | 1.72 ± 0.06 | 1.69 ± 0.07 |
| (m) | (1.54–1.90) | (1.48–1.96) | (1.24–1.90) | (1.60–1.84) | (1.60–1.90) | (1.24–1.96) |
| BMI | 29 ± 4 | 28 ± 5 | 26 ± 5 | 25 ± 4 | 25 ± 4 | 26 ± 5 |
| (kg/m2) | (17.1–39.6) | (15.6–51.8) | (13.3–50.6) | (15.1–38.5) | (16.5–35.9) | (13.3–51.8) |
| Women BMI | 29 ± 4 | 28 ± 6 | 26 ± 5 | 25 ± 4 | 24 ± 4 | 26 ± 5 |
| (kg/m2) | (17.1–37.5) | (15.6–51.8) | (13.3–46.7) | (15.1–38.5) | (16.5–35.9) | (13.3–51.8) |
| Men BMI | 28 ± 4 | 28 ± 5 | 27 ± 6 | 25 ± 4 | 27 ± 3 | 27 ± 5 |
| (kg/m2) | (22.3–39.6) | (18.3–42.6) | (15.2–50.6) | (18.2–38.0) | (21.4–34.9) | (15.1–50.6) |
| WC | 96 ± 9 | 94 ± 12 | 92 ± 13 | 89 ± 12 | 92 ± 12 | 92 ± 12 |
| (cm) | (71–116) | (63–134) | (60–153) | (64–162) | (64–167) | (60–167) |
| Women WC | 95 ± 10 | 93 ± 12 | 90 ± 13 | 87 ± 13 | 87 ± 10 | 90 ± 12 |
| (cm) | (71–115) | (63–134) | (60–149) | (64–162) | (64–112) | (60–162) |
| Men WC | 97 ± 8 | 97 ± 11 | 95 ± 13 | 91 ± 11 | 98 ± 12 | 96 ± 12 |
| (cm) | (85–116) | (73–128) | (70–153) | (66–119) | (78–167) | (66–167) |
| SBP | 117 ± 17 | 115 ± 15 | 111 ± 15 | 112 ± 15 | 112 ± 13 | 113 ± 15 |
| (mmHg) | (90–160) | (80–180) | (70–180) | (80–180) | (90–160) | (70–180) |
| DBP | 76 ± 13 | 75 ± 11 | 73 ± 11 | 73 ± 11 | 74 ± 10 | 74 ± 11 |
| (mmHg) | (60–100) | (50–110) | (50–110) | (50–110) | (60–90) | (50–110) |
| Systemic PP | 41 ± 10 | 40 ± 10 | 38 ± 10 | 39 ± 9 | 39 ± 9 | 38 ± 10 |
| (mmHg) | (20–70) | (20–100) | (10–90) | (20–70) | (10–90) | (10–100) |
Reported values for each group are mean ± standard deviation, and ranges (minimum–maximum).
Participants are grouped by educational level: 1, primary; 2, secondary; 3, undergraduate; 4, master; 5, doctorate.
Primary or elementary education, at most 6 years of school attendance (which in Mexico corresponds to the level called “primaria”).
Secondary or high-school, between 6 and 12 years of school attendance (which in Mexico includes “secundaria” and “preparatoria” levels).
Undergraduate, from 2 to 5 years of study after secondary level (which in Mexico includes “licenciatura” and “carrera técnica”).
Master, from 1 to 3 years of study after undergraduate level (which in Mexico comprises “maestría” and “especialidad”).
Doctorate, corresponds to the doctorate and postdoctoral levels, more than 4 years of study after master's level (which in Mexico are “doctorado” (Ph.D.) and “postdoc” levels).
2.2. Laboratory Procedures
Blood samples were obtained by trained medical professionals from participants who had fasted for at least 8 h and at most 12 h. Samples were stored at 4–5°C, and submitted for chemical analysis to obtain glucose, Hb A1c, basal insulin, total cholesterol, HDL, LDL, triglycerides, and uric acid levels. The analysis was performed at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.”
Fasting plasma glucose was measured using spectrophotometry and potentiometry with a hexokinase kit (amorting PIPES, NAD, Hexokinase, ATP, Mg2+, G6P-DH; AU 2700 Beckman Coulter®). Hb A1c was measured with High Performance Liquid Chromatography (HPLC) analysis with the Variant® Turbo kit 2.0, which consisted of 2 buffers and 1 wash solution. Fasting plasma insulin concentrations were determined using Chemiluminescence (Access Ultrasensitive Insulin, Unicell Dxl 800 Beckman Coulter®, Sensitivity: 0.03–300 U/mL). The lipid profile was obtained with enzymatic colorimetric assay (glycerol phosphate oxidase, cholesterol oxidase, accelerator-selective, detergent, and liquid-selective detergent). Uric acid was measured using the colorimetric method with uricase enzymatic OSR6698, system AU2700/5400, Beckmann Coulter®.
2.3. Derived Variables
Database information was used to calculate several derived parameters that are standard in clinical practice (49–51), such as BMI (52):
| (1) |
systemic pulse pressure (PP) (53):
| (2) |
and the homeostasis model assessment insulin resistance (HOMA-IR) index (43, 54):
| (3) |
2.4. Healthy Parameter Ranges
For the anthropometric and biomarker measurements that are involved in the definition of MS by the International Diabetes Federation we used the cutoffs from Alberti et al. (55). For WC, we employed the suggested cutoffs for Ethnic South and Central Americans suggested in the same definition of MS (55) which have been used in other studies on Mexican populations (56). For PP, BMI, HOMA-IR, basal insulin, total cholesterol, LDL, Hb A1c, and uric acid we used cutoffs taken from the literature. These healthy norm value ranges are given in Table S1, where we also show the percentage of the studied population that exhibits abnormal values. MS was defined using the International Diabetes Federation worldwide definition (55) where central obesity was obligatory plus any two of:
Raised triglycerides ≥ 150 mg/dl.
Reduced HDL cholesterol < 40 mg/dl in men and < 50 mg/dl in women.
Raised blood pressure—SBP ≥ 130 mmHg or DBP ≥ 85 mmHg.
Raised fasting plasma glucose ≥ 100 mg/dl.
2.5. Statistics
The statistical analysis used in this study consisted first of simple population level diagnostics—means and standard deviations—as summary statistics of different subgroups. For analyzing the relation between the metabolic biomarkers and the correlates—educational level, age, BMI, and sex—we used both logistic and linear regressions (57, 58). Each measured biomarker was taken individually as the dependent variable. Logistic and linear regressions were performed for each one in relation to the independent variables: “educational level,” “BMI,” “sex,” and “age.” In the case of WC, BMI was not included as an independent variable due to its very high degree of collinearity with WC. Regression coefficients and their corresponding p-values were calculated, where we take the standard p < 0.05 as indicating that the relation does not support the null hypothesis that the independent and dependent variables are unrelated. However, given the recent debate and later position statement by the American Statistical Association (59) on statistical results based on p-values alone, we will not make definitive statements in our conclusions based only on such values. The class variable for the logistic regressions is the class of abnormal values associated with the thresholds seen in Table S1 which have been taken from the literature cited in the table. For example, for glucose, values above 100 mg/dL indicate the non-healthy class. For the linear regressions, in the case of age, due to observed non-linearities in the relations we also included a quadratic term and its corresponding regression coefficient to determine if this offered a better fit to the data. We also checked the variance inflation factors between educational level, age, and sex to check any degree of collinearity. All factors were very close to one.
We included both types of regressions as they offer complementary perspectives of the relations between the dependent and independent variables and, as such, significance in one does not necessarily imply significance in the other. The principal purpose of the logistic regressions is to determine those factors that are linked to metabolic disorders by calculating the odds ratios of the variables, whereas the linear regression is to determine to what extent the average level of a given metabolic factor is a function of age, BMI, and educational level, without emphasizing any relation to any definition of metabolic disorder.
3. Results
A general description of the study population is given in Table 1 (demographic, anthropometric measures, and blood pressure), and Table 2 (laboratory fasting blood analysis), where the population is divided into five groups according to their educational level (as described in section 2.1). Reported results include anthropometric measures, single (one-time) measurements of blood pressure, and the laboratory chemical analysis of the fasting blood test.
Table 2.
General description of the laboratory fasting blood analysis of the UNAM study population.
| Descriptor | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|
| Glucose | 114 ± 61 | 98 ± 36 | 97 ± 33 | 91 ± 14 | 98 ± 35 | 97 ± 34 |
| (mg/dL) | (69–357) | (64–418) | (66–359) | (64–167) | (70–325) | (64–418) |
| Hb A1c | 6 ± 2 | 6 ± 1 | 5 ± 1 | 5.1 ± 0.6 | 5 ± 1 | 5 ± 1 |
| (%) | (4.4–15.0) | (4.0–14.4) | (3.7–15.2) | (4.0–9.8) | (4.0–12.8) | (3.7–15.2) |
| Basal insulin | 10 ± 4 | 10 ± 8 | 8 ± 6 | 7 ± 5 | 7 ± 6 | 8 ± 6 |
| (units/mL) | (2.8–21.8) | (1.7–63.2) | (1.1–55.8) | (0.9–33.3) | (1.4–45.2) | (0.9–63.2) |
| HOMA-IR | 3 ± 1 | 3 ± 3 | 2 ± 2 | 2 ± 1 | 2 ± 2 | 2 ± 2 |
| (0.5–7.7) | (0.3–23.2) | (0.2–28.5) | (0.2–10.8) | (0.3–10.3) | (0.2–28.5) | |
| Uric acid | 6 ± 2 | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| (mg/dL) | (2.4–10.5) | (2.4–9.7) | (1.6–9.7) | (2.4–10.8) | (2.7–9.1) | (1.6–10.8) |
| Women | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| (2.4–7.5) | (2.4–7.5) | (1.6–8.4) | (2.4–8.0) | (2.7–8.3) | (1.6–8.4) | |
| Men | 7 ± 1 | 7 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 |
| (5.4–10.5) | (4.2–9.7) | (2.7–9.7) | (3.3–10.8) | (3.0–9.1) | (2.7–10.8) | |
| Triglycerides | 190 ± 110 | 180 ± 130 | 160 ± 94 | 150 ± 91 | 155 ± 99 | 160 ± 110 |
| (mg/dL) | (60–565) | (38–988) | (27–642) | (38–695) | (42–766) | (27–988) |
| Women | 175 ± 110 | 170 ± 110 | 140 ± 80 | 140 ± 80 | 129 ± 68 | 150 ± 92 |
| (60–565) | (42–988) | (27–606) | (38–695) | (42–379) | (27–988) | |
| Men | 240 ± 100 | 210 ± 150 | 180 ± 110 | 170 ± 99 | 190 ± 120 | 190 ± 122 |
| (87–408) | (38–845) | (41–642) | (40–535) | (51–766) | (38–845) | |
| Cholesterol | 210 ± 45 | 200 ± 46 | 200 ± 40 | 200 ± 42 | 210 ± 38 | 200 ± 42 |
| (mg/dL) | (65–300) | (81–527) | (61–389) | (114–328) | (101–333) | (61–527) |
| Women | 200 ± 48 | 200 ± 48 | 200 ± 40 | 200 ± 42 | 200 ± 40 | 200 ± 43 |
| (65–300) | (81–527) | (61–354) | (114–328) | (101–307) | (61–527) | |
| Men | 220 ± 38 | 200 ± 42 | 200 ± 42 | 200 ± 40 | 210 ± 36 | 200 ± 41 |
| (148–268) | (100–285) | (107–389) | (126–328) | (147–333) | (100–389) | |
| HDL | 45 ± 10 | 45 ± 11 | 47 ± 12 | 49 ± 13 | 52 ± 14 | 48 ± 12 |
| (mg/dL) | (27–71) | (24–93) | (27–91) | (26–98) | (27–94) | (24–98) |
| Women | 47 ± 9 | 47 ± 11 | 51 ± 13 | 52 ± 13 | 57 ± 15 | 51 ± 13 |
| (27–65) | (26–93) | (28–91) | (26–98) | (34–94) | (26–98) | |
| Men | 40 ± 10 | 40 ± 9 | 41 ± 8 | 44 ± 10 | 45 ± 10 | 42 ± 9 |
| (32–71) | (24–70) | (27–65) | (28–77) | (27–74) | (24–77) | |
| LDL | 127 ± 34 | 117 ± 36 | 120 ± 34 | 122 ± 34 | 127 ± 32 | 120 ± 35 |
| (mg/dL) | (60.2–198.8) | (3.0–279.4) | (6.2–259.8) | (16.6–233.8) | (11.4–220.8) | (3.0–279.4) |
| Women | 126 ± 33 | 118 ± 37 | 120 ± 33 | 120 ± 36 | 124 ± 31 | 120 ± 34 |
| (60.2–198.8) | (11.0–279.4) | (6.2–259.8) | (16.6–233.8) | (68.6–220.8) | (6.2–279.4) | |
| Men | 132 ± 38 | 120 ± 36 | 118 ± 37 | 125 ± 30 | 130 ± 34 | 120 ± 35 |
| (69.0–178.0) | (3.0–198.4) | (11.8–217.4) | (48.2–211.2) | (11.4–207.0) | (3.0–217.4) |
Reported values for each group are mean ± standard deviation, and ranges (minimum–maximum).
Participants are grouped by educational level: 1, primary; 2, secondary; 3, undergraduate; 4, master; 5, doctorate.
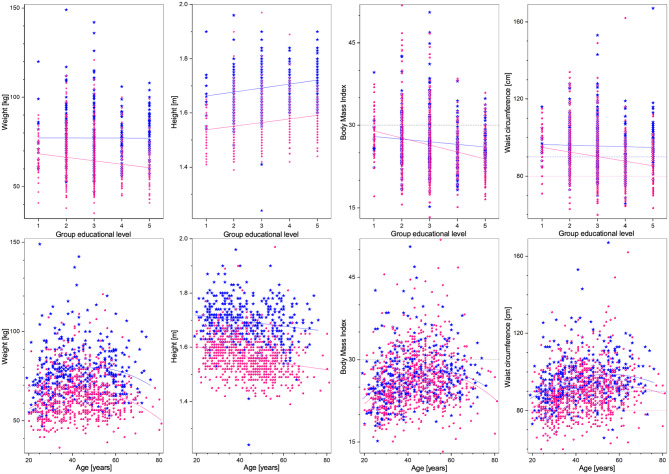
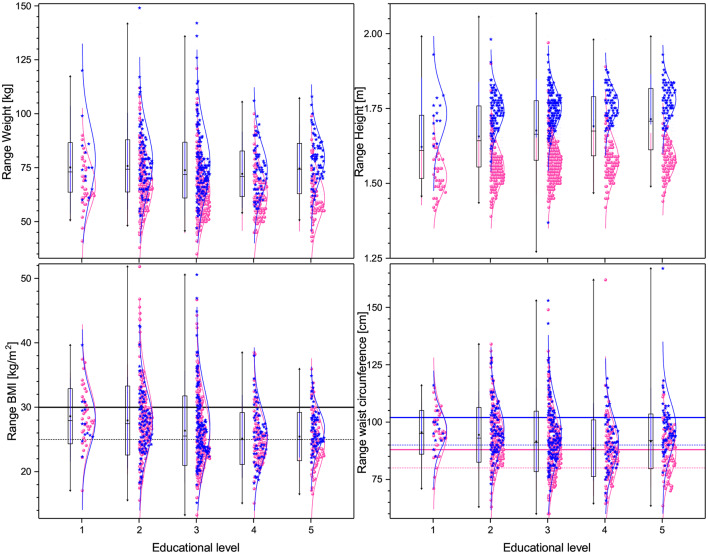
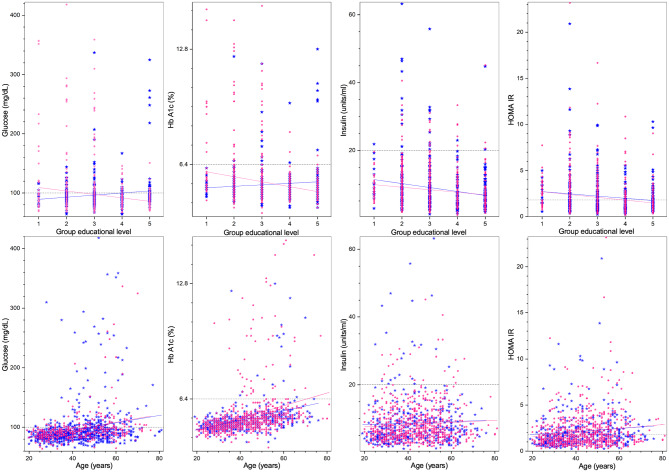
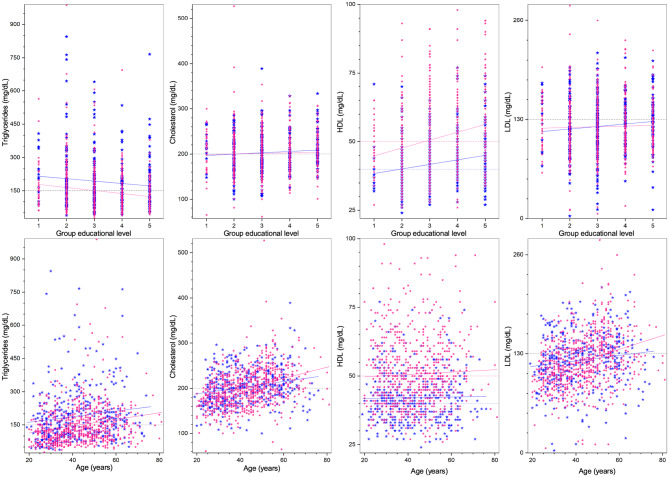
Regarding demographic characteristics, the split by sex is 64% women to 36% men, which is not uncommon in studies in Mexico (60, 61). As seen in Table 1, the highest (lowest) educational level groups have a higher (lower) proportion of men. With respect to the anthropometric measures (see Table 1, Figures 1, 4), we observe that those with higher educational level are generally taller, weigh less, have a smaller BMI, and smaller WC. The non-linear parabolic nature of the relation between weight, BMI, WC, and age is clearly visible in Figure 1, where we see that BMI, WC and weight tend to increase for older participants, with its highest value around 55 years old, while height decreases slightly. As a function of sex, females have a smaller WC, less weight, less BMI, and are shorter. Similar plots of the laboratory fasting blood test associated with metabolism (glucose, Hb A1c, basal insulin, and HOMA-IR) are seen in Figure 2 and associated with dyslipidemias (triglycerides, total cholesterol, LDL, and HDL) in Figure 3. Each plot shows the best fit regression for each relation. Further results using box-whisker plots, where the corresponding probability distributions are given as a function of educational level are shown in Supplementary Material for the anthropometric measures in Figure 4, blood pressure in Figure 5, and for the laboratory chemical analysis of the fasting blood test in Figure 6.
Figure 1.
Anthropometric measures (from left to right: weight, height, BMI, and WC) as a function of educational level (top), and age (bottom). Distribution blue stars correspond to men, pink dots to women, continuous lines correspond to the best minimum least squares adjustment of the data, and dashed lines to cutoff values.
Figure 4.
Box plots and distributions of the anthropometric measures for the different educational levels. (Top) panels show weight (left), height (right), while (bottom) panels show BMI (left) and waist circumference (right). Each box corresponds to one standard deviation around the mean (star). Distribution dots correspond to each subject, (pink for female and blue for male), the curve is the best Gaussian fit of the data, and horizontal lines correspond to thresholds defining health.
Figure 2.
Laboratory fasting blood test associated with metabolism (from left to right: glucose, Hb A1c, insulin, HOMA-IR) as a function of educational level (top), and age (bottom). Distribution blue stars correspond to men, pink dots to women, continuous lines correspond to the best minimum least squares adjustment of the data, and dashed lines to cutoff values.
Figure 3.
Laboratory fasting blood test associated with dyslipidemias (from left to right: total cholesterol, HDL, LDL, and triglycerides) as a function of educational level (top), and age (bottom). Distribution blue stars correspond to men, pink dots to women, continuous lines correspond to the best minimum least squares adjustment of the data, and dashed lines to cutoff values.
Figure 5.
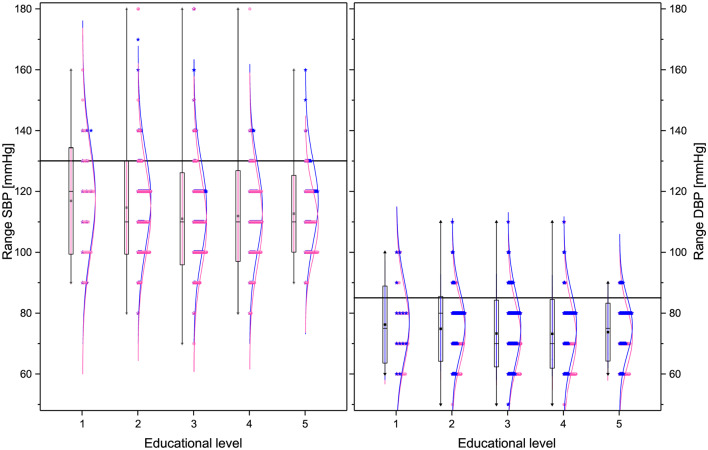
Box plots and distributions of the blood pressure parameters for the different educational levels. From left to right: SBP, and DBP. Each box corresponds to standard deviation around the mean (star). Distribution dots correspond to each subject (pink for female and blue for male), the curve is the best Gaussian fit of the data, and horizontal lines correspond to thresholds defining health.
Figure 6.
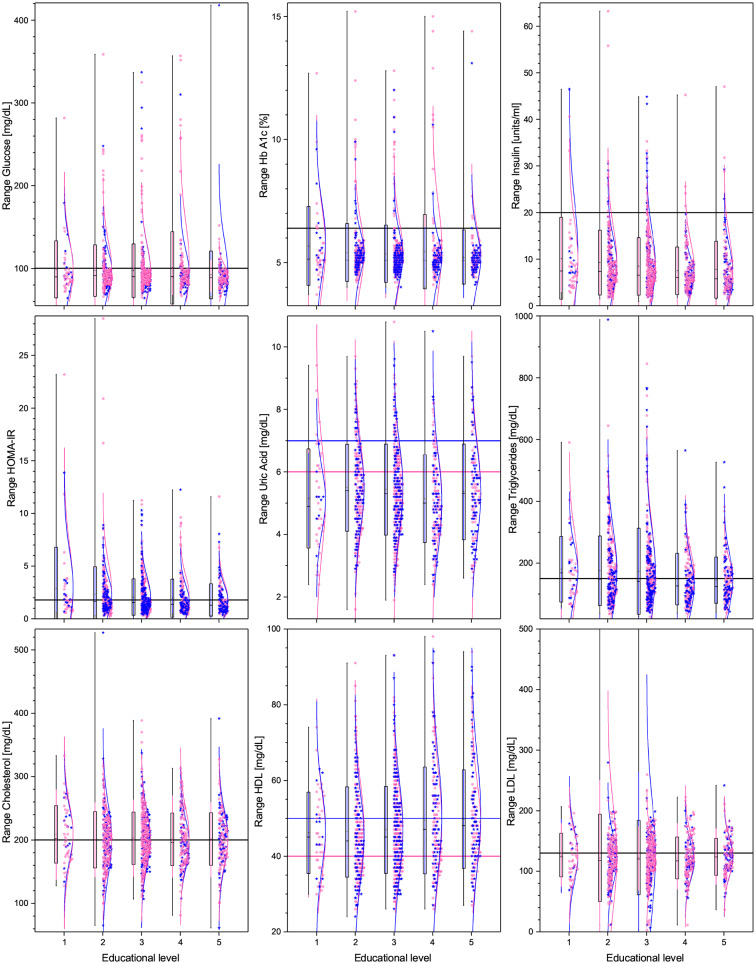
Box plots and distributions of the laboratory chemical analysis of the fasting blood test for the different educational levels. From top right to bottom left are: glucose, Hb A1c, insulin, total cholesterol, HDL, LDL, triglycerides, and uric acid levels. Each box corresponds to one standard deviation around the mean (star). Distribution dots correspond to each subject (pink for female and blue for male), the curve is the best Gaussian fit of the data, and horizontal lines correspond to thresholds defining health.
Results for the binomial regression taking educational level, BMI, age, and sex as independent variables are shown in Table 3, where we note that all abnormal class probabilities are decreasing functions of educational level and increasing functions of age, although in the case of WC for men the relation is not statistically significant at the 95% confidence level. In the case of blood pressure, Table 3 shows that the probability of abnormal values of SBP and DBP exhibits a strong dependence on educational level, with a decreasing odds ratio of 66% for every increase in educational level (p < 0.0005), while for DBP the corresponding decrease in odds ratio is 80% (p = 0.01). PP, on the other hand, is independent of educational level at the p < 0.05 level, but dependent on age (p = 0.007). As a function of age, we see that both SBP and DBP are affected, with odds ratio increases of 6.2 and 4.1% per year, respectively. Turning now to the metabolic parameters: we see that glucose (p = 0.013), Hb A1c (p = 0.003), HOMA-IR (p < 0.0005) all depend on educational level and on age (p < 0.0005 for all three factors), with odds ratios < 1 for educational level and >1 for age. Basal insulin depends neither on educational level (p = 0.23) nor on age (p = 0.975). For the lipid analysis, from Table 3 we see that triglycerides (p < 0.0005), and HDL for women (p < 0.0005) and for men (p < 0.0005), depend on educational level, whereas total cholesterol (p = 0.781) and LDL (p = 0.677) do not. On the contrary, although triglycerides (p < 0.0005) also depend on age, along with total cholesterol (p < 0.0005) and LDL (p < 0.0005), HDL for women (p = 0.190) and men (p = 0.383) do not. For uric acid, in Table 3 we see that it is independent of educational level for women (p = 0.285) and dependent for men (p = 0.032), and dependent on age for women (p = 0.024), and independent for men (p = 0.868).
Table 3.
Binomial logistic regressions of anthropometric, blood pressure, and fasting blood test variables taking as class variable the at risk population using the cutoffs of Table S1, for the independent variables education (Edu), BMI, age, and sex.
| Variable | N | Edu | BMI | Age | Sex | ||||
|---|---|---|---|---|---|---|---|---|---|
| exp(b) | p | exp(b) | p | exp(b) | p | exp(b) | p | ||
| BMI | 1,073 | 0.681 | 0.000** | 1.021 | 0.001* | 0.946 | 0.726 | ||
| WC (Women) | 689 | 0.700 | 0.000** | 1.042 | 0.000** | ||||
| WC (Men) | 384 | 0.912 | 0.384 | 1.047 | 0.000** | ||||
| SBP | 1,073 | 0.760 | 0.022* | 1.190 | 0.000** | 1.065 | 0.000** | 0.393 | 0.000** |
| DBP | 1,073 | 0.939 | 0.491 | 1.167 | 0.000** | 1.038 | 0.000** | 0.614 | 0.016* |
| PP | 1,073 | 0.848 | 0.507 | 1.129 | 0.004* | 1.061 | 0.011* | 0.382 | 0.087 |
| Glucose | 1,072 | 0.926 | 0.306 | 1.101 | 0.000** | 1.055 | 0.000** | 0.910 | 0.580 |
| Hb A1c | 1,068 | 0.767 | 0.034* | 1.095 | 0.000** | 1.064 | 0.000** | 1.544 | 0.150 |
| Insulin | 1,072 | 1.068 | 0.679 | 1.203 | 0.000** | 0.985 | 0.276 | 0.609 | 0.125 |
| HOMA-IR | 1,071 | 0.846 | 0.011* | 1.240 | 0.000** | 1.013 | 0.022* | 1.075 | 0.624 |
| Uric acid (Women) | 689 | 1.025 | 0.832 | 1.106 | 0.000** | 1.016 | 0.115 | ||
| Uric acid (Men) | 383 | 0.856 | 0.140 | 1.110 | 0.000** | 0.994 | 0.480 | ||
| Triglycerides | 1,072 | 0.859 | 0.014* | 1.091 | 0.000** | 1.025 | 0.000** | 0.469 | 0.000** |
| Total cholesterol | 1,073 | 0.993 | 0.907 | 1.008 | 0.549 | 1.041 | 0.000** | 0.880 | 0.335 |
| HDL (Women) | 689 | 0.770 | 0.001* | 1.116 | 0.000** | 0.983 | 0.012* | ||
| HDL (Men) | 384 | 0.737 | 0.002* | 1.065 | 0.007* | 1.002 | 0.797 | ||
| LDL | 1,070 | 0.986 | 0.813 | 1.009 | 0.489 | 1.037 | 0.000** | 0.805 | 0.109 |
| Metabolic syndrome | 1,073 | 0.827 | 0.005* | 1.202 | 0.000** | 1.036 | 0.000** | 0.960 | 0.789 |
Indicates statistically significant at the p < 0.05 level.
Indicates statistically significant at the p < 0.001 level.
Finally, for MS, the odds ratio for educational level is 0.83 (p = 0.005), for BMI it is 1.20 (p < 0.0005), and for age 1.04 (p < 0.0005) but not on sex (p = 0.532). In Table S2, we show the incidence of MS in the different educational subgroups as well as the number and proportion in each group that have high WC but no other MS condition, using this as an indicator of better metabolic health. The importance of educational level is evident in the incidence of MS: 52% (Primary), 41% (Secondary), 32% (Undergraduate), 24% (Masters), and 26% (Doctorate) with an overall average of 33%. Taking the undergraduate level as reference, the difference between undergraduate and higher educational levels is significant, and the difference between undergraduate and lower levels is also significant (p = 0.005). Other risk measures follow a similar pattern. For example, for those at risk with a high WC, the percentages associated with presenting no other MS risk factor are: 16.3% (Primary), 17.0% (Secondary), 24.2% (Undergraduate), 29.6% (Masters), and 32.5% (Doctorate), where, again, the differences between undergraduate and higher levels are statistically significant (p = 0.006).
The results for the linear regressions are seen in Table 4. These results in general confirm the results determined using the logistic regression. For instance, we note that BMI and WC in women are very significant (p < 0.0005), whereas WC in men is not (p = 0.141), while the components of BMI—weight and height—are both significant, p < 0.0005 and p < 0.0005, respectively. As a function of sex, although, naturally, females have a smaller WC, less weight, and are shorter, their BMI for a given age is similar to that of men, with no statistically significant difference (p = 0.726). For blood pressure, Table 4 confirms that SBP depends on educational level (p = 0.001) and age (p < 0.0005), while for DBP the dependence on educational level is slightly weaker (p = 0.005). We also note there is a statistically significant difference between men and women for both SBP (p < 0.0005) and DBP (p = 0.005). Table 4 also confirms the dependence of glucose, Hb A1c, and HOMA-IR on educational level (p = 0.002, p < 0.0005, and p < 0.0005, respectively) and also on age (p < 0.0005 for all three factors). In this case, basal insulin is also dependent on educational level (p < 0.0005) and age (p = 0.033). Note that there are no significant differences between female and male subjects. Similarly, for lipids we see from Table 4, we see that triglycerides (p < 0.0005), HDL for women (p < 0.0005) and for men (p < 0.0005) are all dependent on educational level while total cholesterol (p = 0.357) and LDL (p = 0.261) are not. Similarly, age is significant for triglycerides (p < 0.0005), total cholesterol (p < 0.0005), and LDL (p < 0.0005) but not for HDL for women (p = 0.266) or for men (p = 0.738). Note that there is also a statistically significant difference between men and women for triglycerides (p < 0.0005). Finally, for uric acid, the linear regression yields a dependence on educational level for men (p = 0.002), but not for women (p = 0.170), and independence from age for both women (p = 0.254) and for men (p = 0.812).
Table 4.
Multiple linear regressions of anthropometric, blood pressure, and fasting blood test variables using education (Edu), BMI, age, and sex as independent variables.
| Variable | N | Edu | p | BMI | p | Age | p | Sex | p |
|---|---|---|---|---|---|---|---|---|---|
| Height | 1,073 | 0.015 | 0.000** | −0.001 | 0.000** | −0.127 | 0.000** | ||
| Weight | 1,073 | −1.287 | 0.000** | 0.077 | 0.014* | −13.127 | 0.000** | ||
| BMI | 1,073 | −1.03 | 0.000** | 0.076 | 0.000** | −0.758 | 0.014* | ||
| WC (Women) | 689 | −2.389 | 0.000** | 0.206 | 0.000** | ||||
| WC (Men) | 384 | −0.804 | 0.141 | 0.207 | 0.000** | ||||
| SBP | 1,073 | −0.331 | 0.383 | 1.009 | 0.000** | 0.315 | 0.000** | −4.493 | 0.000** |
| DBP | 1,073 | −0.099 | 0.727 | 0.707 | 0.000** | 0.164 | 0.000** | −3.047 | 0.000** |
| PP | 1,073 | −0.232 | 0.387 | 0.302 | 0.000** | 0.150 | 0.000** | −1.446 | 0.015* |
| Glucose | 1,072 | −2.068 | 0.028* | 0.733 | 0.000** | 0.592 | 0.000** | −0.255 | 0.902 |
| Hb A1c | 1068 | -0.137 | 0.000 ** | 0.030 | 0.000 ** | 0.030 | 0.000 ** | 0.119 | 0.107 |
| Insulin | 1,072 | −0.320 | 0.051 | 0.594 | 0.000** | −0.012 | 0.371 | −0.175 | 0.628 |
| HOMA-IR | 1,071 | −0.115 | 0.036* | 0.177 | 0.000** | 0.008 | 0.096 | −0.029 | 0.813 |
| Uric acid (Women) | 689 | 0.039 | 0.317 | 0.073 | 0.000** | −0.001 | 0.668 | ||
| Uric acid (Men) | 383 | −0.128 | 0.028* | 0.090 | 0.000** | −0.006 | 0.256 | ||
| Triglycerides | 1,072 | −9.909 | 0.001* | 3.827 | 0.000** | 1.183 | 0.000** | −41.313 | 0.000** |
| Total cholesterol | 1,073 | 1.061 | 0.363 | −0.005 | 0.983 | 1.004 | 0.000** | −2.428 | 0.346 |
| HDL (Women) | 689 | 1.926 | 0.000** | −0.733 | 0.000** | 0.097 | 0.011* | ||
| HDL (men) | 384 | 1.428 | 0.001 * | -0.408 | 0.000 ** | 0.018 | 0.613 | ||
| LDL | 1,070 | 0.973 | 0.323 | −0.114 | 0.594 | 0.677 | 0.000** | −1.520 | 0.483 |
Indicates statistically significant at the p < 0.05 level.
Indicates statistically significant at the p < 0.001 level.
As a means of comparing the very similar results between the two types of regressions in Table 5 we show the relation between each metabolic biomarker and its corresponding set of statistically significant correlates, from among educational level (E), BMI (B), age (A), and sex (S), where for each metabolic factor, where a risk factor appears if it is significantly related at the p < 0.05 level. The asterisk denotes that the corresponding factor exhibits the same relation (increasing/decreasing risk) but is not significant at the p < 0.05 level with the sample size associated with the present study. Boldface denotes those variables involved in the definition of MS.
Table 5.
Significant correlates for each metabolic biomarker from: Education (E), BMI (B), Age (A), and Sex (S), as determined from the odds ratios of the logistic regressions using the thresholds of Table S1, and multiple linear regressions.
| Metabolic biomarker | Significant correlates | Significant correlates |
|---|---|---|
| logistic | linear | |
| WC–women | EA | EA |
| WC–men | E*A | E*A |
| SBP | EBAS | E*BAS |
| DBP | BAS | BAS |
| Glucose | E*BA | EBA |
| Hb A1c | EBA | EBA |
| Insulin | B | B |
| HOMA-IR | EBA | EBA* |
| Uric acid–women | B | B |
| Uric acid–men | E*B | EB |
| Triglycerides | EBAS | EBAS |
| Total cholesterol | A | A |
| HDL–women | EBA | EBA |
| HDL–men | EB | EB |
| LDL | A | A |
Denotes that the corresponding factor indicates the same relation but is not significant at the 95% confidence level with this sample size. Boldface indicates those variables that enter in the definition of MS.
4. Discussion
The fact that the highest/lowest educational levels have a lower/higher proportion of women reflects the preponderance of male faculty members in the university and the fact that, in Mexico, women have less opportunities to obtain higher education (62). At the educational extremes, for those in the lowest and highest education groups, the average age is higher than in the intermediate groups. For the lowest educational level - primary education only—this reflects the improvement in access to secondary education and beyond in Mexico in the last couple of decades, while the higher average age for postgraduates reflects the fact that the minimum age to obtain a PhD in Mexico is more than 27 years old. At the same time, the population considered is relatively homogeneous with respect to job-related activity levels, so that any substantial differences in activity are probably associated with voluntary activities.
The values of the metabolic variables we have considered are highly complex, multi-factorial functions, dependent on the interaction between an individual's physiology and their environment. Two important dimensions of this interaction are age and lifestyle, where the latter is itself complex and multi-factorial. We have here taken educational level to be a single proxy measure of lifestyle. Hence, we model the interaction between metabolism and environment using educational level and age. Additionally, we include BMI and sex as two important potential confounders. As age is not a modifiable factor, it is to educational level we must look for insight into the possibility of reducing metabolic risk. As such, we must first determine which metabolic variables are subject to variation as a function of educational level.
We first note that metabolic risk, as determined from the odds ratios of the logistic regressions, using the thresholds given in Table S1 (see Table 3), are increasing functions of BMI and age, and decreasing functions of educational level for all variables considered where conclusions can be made at the p < 0.05 significance level. We also see that the results of the linear regressions are in almost complete agreement with those of the logistic regressions (Table 5). Thus, within the confines of our results, we see that there is no metabolic variable where higher educational level correlates with an unhealthier metabolic state and, similarly, there is no variable where older age and higher BMI correlate with a healthier metabolic state. However, there is a great deal of heterogeneity as to which risk factors are significantly associated with a given metabolic variable, and as to the magnitude of the relation. Thus, the metabolic variables studied in this population have quite distinct risk profiles, as can be seen in Table 5. The principal profiles are EA, EB, EBAS, BAS, EBA, B, and A, where the appearance of a risk factor in the profile indicates a dependence of the corresponding metabolic variable on that risk factor, either with respect to a logistic or linear regression, where we emphasize again that independent/dependent here is with respect to the standard p < 0.05 and is therefore dependent on the sample size. The fact that the risk profiles are so distinct between the different components of MS has potential implications for understanding both its etiology and its characterization as a syndrome (34). Furthermore, their heterogeneity also implies that interventions need to be specifically tailored for each metabolic disorder.
Although many of the relations (metabolic variable—risk factor) that we study have been considered in the literature, a full understanding of the multi-factorial origin of the observed heterogeneity in the risk profiles for each metabolic factor requires much further study. Here we will restrict attention to some observations that we believe to be the most novel and relevant, beginning with the anthropometric variables. That BMI itself decreases significantly as a function of educational level is principally due to the fact that, in the case of men, although weight only decreases weakly, those of higher educational level are significantly taller. The relation between height and educational level is evidently multi-factorial. However, several potential relevant factors are: socio-economic status, wherein the higher the educational level, the more likely that person came from a family background of higher socio-economic status/educational level, and therefore a more nutritionally secure upbringing (20, 63, 64); and genetic factors, both specific to a Mexican population, where people at higher educational levels tend to have more European ancestry (65, 66). Thus, the fact that WC does not decrease with educational level in men, although BMI does, can be explained by the fact that the more educated men are significantly taller but only slightly heavier.
In the case of women, the increase in height, and the corresponding decrease in weight, as a function of educational level, provide an even greater effect on BMI when compared to men. The relation between education and BMI, especially in the case of women, has been observed in several studies (27, 29, 67). Our results are consistent with these findings. The relation between height and BMI vs. educational and socio-economic status has been considered in Tyrrell et al. (66), where it has been shown that the genetic component of height differences is relevant for the educational level and socio-economic status for men, but not for women where the genetic component of BMI is relevant for her socio-economic status. Taking as an example, social cultivation theory, where body standards are quite different for men and women, there is evidence that, for women, socio-economic status is inversely proportional to BMI in a much more significant way than for men, with height being more important for the latter (68).
Given the ample discussion in the literature as to the advantages and disadvantages of BMI vs. WC as a component of MS (69–71), we believe that this fact at least illuminates a potential defect of WC relative to BMI, especially in populations where there is a strong heterogeneity in height as a function of other variables, such as educational level. The principal reason is that WC depends not only on body shape, for example the distribution of visceral fat, but also on overall body size, whereas BMI and waist to height ratios are more attuned to being measures of body shape (Figure 4). Thus, not only are different thresholds relevant for WC due to genetic differences when comparing, say, an Asian with a northern European population, but also, as shown here, when considering populations with different educational levels. Additionally, using the recommended cutoffs for waist measurement for an Ethnic South or Central American population (55, 56), we note that 78.4% of the female population and 66.2% of the male population are above this threshold, while only 21.2% are above the threshold for BMI. This calls into question the use of this threshold for a Mexican population, as if such a large fraction of the population is defined as at risk with respect to this, or any other, factor, then it is not useful for stratifying risk between different populations.
Weight, BMI, and WC are increasing functions of age, and usually their risk analysis has involved studying only a linear relation, however, a regression that includes a quadratic term in age offers a statistically significant better fit than with only a linear term. Thus, weight, BMI, and WC are parabolic functions of age, increasing as a function of age (72) up to a maximum of 55–60 years old, and then decreasing. This parabolic shape (see Figure 1) is consistent with the fact that the chief risk group for adults is early adulthood, where most weight gain occurs (73).
The risk profiles of SBP and DBP are EBAS and BAS, respectively, showing that educational level is more relevant for SBP than DBP (see Figure 5, Table 5). The former depends on systolic debt (ventricular ejection volume), the surrounding vascular volume, and the distensibility of the arterial wall, whereas the latter only depends on the peripheral vascular resistance. Therefore, lifestyle habits, such as exercise and sodium consumption would be expected to affect more directly SBP (74). Indeed, a dependence on educational level has been noted in several studies in different countries, generally with the observation that higher levels of hypertension are associated with lower educational level (75–77). In the case of educational level, the unadjusted odds ratios for SBP and DBP, without controlling for BMI are 0.66 (p < 0.005) and 0.80 (p = 0.01), respectively. However, adjusting for BMI the corresponding odds ratios are 0.76 (p = 0.022) and 0.94 (p = 0.491) thus showing that a substantial fraction of the apparent dependence on educational level is due to changes in BMI. Other studies (78) have also shown a consistent relation between blood pressure and BMI, although the causal relations between high blood pressure and different anthropometric measurements is still poorly understood (79). We can also see that the age dependence is much stronger than the dependence on educational level, with standardized linear regression coefficients of 0.330 and 0.254 vs. −0.099 and −0.083 for SBP and DBP, respectively.
Turning to the lipid profiles: we note that both total cholesterol and LDL have profile A, depending on age but not on BMI or educational level (Table 5). In contrast, the profiles for HDL are EBA (women) and EB (men), from which we conclude that, in this population, HDL is much more sensitive to lifestyle, as proxied by education, than total cholesterol and LDL. The relative independence of these metabolic factors from educational level using the standard threshold has some worrying implications. Considering educational level as a potential proxy for “healthy” lifestyle, this implies that there is no evidence that lifestyles differ with respect to consumption, generation and elimination of LDLs, or of total cholesterol. Moreover, in distinction to other risk factors the percentage of the population at risk of dyslipidemias is already high, even in the younger adult population. For example, in the 19-29 age group the incidence of total cholesterol > 200 mg/dL is 29.3% and LDL > 130 mg/dL is 18.8%. Even more worryingly, the proportion of HDL < 50 (women) and < 40 (men) are 52.2 and 42.3%, respectively, for age group 19–29, which are the same as the averages over all ages. In the case of LDL and HDL, which are well known risk factors for atherosclerosis (31), this implies that the risk factors are potentially present at a relatively constant above-threshold levels over the entire life of a subset of individuals. Although longitudinal data would have to be acquired to test this hypothesis, it suggests that metabolic screening, even in younger adults, may be a cost-effective way of preventing MS and its consequences in later life.
It is well-known that HDL is found in larger concentrations in women than in men (80), in concordance with our results. This has been linked to lower levels of cardiovascular risk in women before menopause (81, 82), a difference which tends to level off with age (80, 83). However, the fact that, in the present study, we observe a statistically significant linear increase in HDL as a function of educational level for both men and women is interesting, as other studies have shown contradictory findings. For instance, in Benetou et al. (84), as part of the EPIC study carried out in Greece, it was shown that HDL levels decreased (increased) for men (women) as a function of educational level. Whereas, in another part of the EPIC study in the UK (85), lower levels of HDL were found in the least educated group for both men and women, with the effect being stronger for women. What is clear is that educational level as a single proxy variable for lifestyle, which is a highly multi-factorial construct, reflects different results for some metabolic risk factors due to the adaptive nature of the response of individuals of distinct educational levels to an evolving obesogenic environment which, in its turn, depends on an array of socio-demographic, socio-economic, and socio-cultural factors. Thus, for instance, the relationship between socio-economic status/educational level and cholesterol levels has led to conclusions that abnormal values are positively linked to these variables (86–88), inversely linked (84, 89, 90), or not linked at all (91) thus showing that the relationship is not rigidly universal but, rather, a reflection of the complexity of the multi-factorial interaction that the proxy variable is representing. This is further complicated by the fact that this relationship may change in time. For instance, as discussed in Benetou et al. (84), the relation between educational level and lipid levels changed over a period of about 20 years among the young Greek population studied, changing from a positive relationship between total cholesterol and educational level to a negative one.
Although we find no significant link between total cholesterol and educational level, there is a strong age dependence, which is due to several important age-dependent mechanisms associated with the metabolism of cholesterol. For example, it is known that as a person becomes older, there is a decrease in the hepatic hydroxylase cholesterol-alpha-1 responsible for the synthesis of bile acids, a diminishing of the LDL receptors, a decrease in the number of Niemann Pick C1 transporters that are responsible for mediating the intestinal absorption of cholesterol and its biosynthesis and, finally, the decrease of bacterial populations, which play a predominant role in the enterohepatic circulation of bile acids (92). Some studies have observed that total cholesterol tends to decrease in the last years of life, and that in the group with a total cholesterol below 3.0 mmol/L, mortality increases by 40–50% compared to subjects who have total cholesterol between 4.5 and 5.4 mmol/L (93).
Triglycerides in the blood come from three sources: ingestion in the diet, endogenous synthesis (hepatic lipogenesis), and release from fatty deposits (adipocyte turnover). The first source depends directly on behavior, and is responsible for more than 60% of the daily intake of lipids (94). while the latter are insulin regulated. As also described here, it has also been noted that the average concentration of triglycerides tends to be higher in men than in women, although there are many associated variables, such as apolipoprotein AV, which are associated with postprandial triglyceride levels (95). However, general food intake, alcohol consumption (96) and hyperadrenergic states (97) will modify triglyceride levels. The profile for triglyceride is EBAS, indicating that it is dependent on all four risk factors. These relationships between hypertriglyceridemia and educational level, age and sex have been observed in several studies (85), as has the relation between BMI and triglyceride levels (98). In this case, the impact of educational level is particularly important, with a regression coefficient of −9.909 compared to 3.827 for BMI, indicating that the reduction in triglyceride level from a unit increment in educational level is 2.59 times greater than the increment due to a unit increase in BMI, thus indicating the importance of interventions to modify the daily intake of lipids (94). Moreover, as with LDL and HDL, the incidence of hypertriglyceridemia in the youngest adults 19–29 years old is very high at 31.5%, and this is a significant risk factor for CVD due to the correlation of hypertriglyceridemia with coronary heart disease (99, 100).
For both glucose and Hb A1c the risk profile is EBA. The strong relation between BMI and glucose or Hb A1c has been noted in multiple studies. However, the dependence of fasting glucose level on educational level has been much less studied when compared to the relation with the incidence of DM2 (101). In one study, in a Korean population, incidence of fasting glucose levels > 100 mg/dL was found to be positively related to low educational level (102). Our results emphasize the importance of educational level as a protective factor as can be seen by comparing the regression coefficients for Hb A1c with respect to educational level and BMI, −0.137 and 0.030, respectively, indicating that a unit increase in educational level leads to a reduction in Hb A1c level that is 4.57 times larger than the corresponding increment due to a unit increase in BMI. The risk profile for basal insulin is B, indicating a dependence only on BMI. The fact that there is no significant dependence on age, neither in the case of the logistic regressions nor in linear regression, has been observed in other studies (103). In contrast to basal insulin, the risk profile of HOMA-IR is EBA, being a decreasing function of educational level and an increasing function of age and BMI, where a comparison of the regression functions and the odds ratios shows that BMI is the most important factor.
Uric acid comes from the final metabolism of purines, nitrogenous bases of DNA, and its concentration depends on both metabolism from food intake as well as its endogenous metabolism. The risk profiles for uric acid are EB for men and B for women. As noted here, it has been established that serum uric acid has higher average values in men than in women (104). Interestingly, uric acid increases in women but decreases in men as a function of educational level, though the statistical significance is weak in the case of women. Additionally, there is no significant dependence on age in either case but a statistically significant dependence on BMI (p < 0.001) as has been noted in other studies (105). The dependence of uric acid levels on age for women is potentially due to post-menopausal hormonal changes in uric acid levels (106). Increases in uric acid levels have been associated with insulin resistance (107), and as a risk factor for multiple diseases, such as DM2 (108–111), and MS (13, 112), as well as myocardial infarction, stroke, and congestive heart failure (113, 114).
Although we have taken educational level as a proxy for lifestyle, it is clearly not a direct causal factor. However, there are some obvious, and previously studied, variables that intermediate the relation between education and health, such as nutrition and exercise. Even if they have a direct causal connection with the metabolic factors we are considering, the relation between educational level and such lifestyle components is not clear. Do the better educated eat less? Eat healthier? Both? There is some evidence that the better educated both eat more healthily (115) and exercise more (116). Additionally, the relation between nutrition, exercise, and educational level will be affected by a host of socio-cultural factors, such that the relation between them is not necessarily generalizable across cultures.
There are many other factors that have been identified as variables that intermediate between education and health (117, 118). In particular, Mirowsky (119) concludes that “Education creates desirable outcomes because it trains individuals to acquire, evaluate and use information. It teaches individuals to tap the power of knowledge. As a result, education influences health in ways that are varied, present at all stages of adult life, cumulative, self-amplifying, and uniformly positive. Education develops the learned effectiveness that enables self-direction toward any and all values sought, including health.” We believe that this is fully in line with our results that education beyond the undergraduate level (effectively > 17 years of full time education) leads to very positive effects on metabolic health. This finding requires further research to identify what lifestyle characteristics, knowledge-base, or other factors, differentiate between subjects with an undergraduate and a postgraduate education. Obviously, one potentially important factor associated with our population is that it inhabits a very information rich environment—a university—but where there is a great deal of heterogeneity as to the degree of interaction with that information and, more importantly, how that information is incorporated into lifestyle and behavior. We would add some preliminary insights from our overall study that we believe to be relevant in potentially distinguishing between postgraduates and graduates: different eating habits, different degrees of health knowledge, more awareness of their physical/health state, greater disposition to change in the light of medical advice, more inclined to exercise, a more realistic perception of their health state, and they are taller—thus implying less excess consumption when compared to a smaller person. Some of these factors are physical in nature, but many point toward a difference in decision making.
As our results do not support the hypothesis that education is uniformly beneficial across all our considered metabolic variables, it must be determined why those most likely to live healthier lives fail in the case of certain variables, or is it that those variables are more difficult to improve, given a certain adjustment in lifestyle? If this is the case, public policy efforts should be focused on education-susceptible variables, prone to modification by exposure to information and intuitively incorporated into impactful life-style changes, while adopting a different approach for education-non-susceptible variables which are poor targets for educational campaigns. Although certain metabolic elements do not show significantly reduced risk for increasing educational level, overall metabolic health, as measured by the incidence of MS, is improved. Moreover, for those with one risk factor, the probability to have a second, or more risk factors, is significantly less. Thus, the more educated avoid a clustering of metabolic risk factors. Furthermore, as our population covers a large range of educational levels, we see that metabolic health keeps on improving beyond the undergraduate level, with masters/doctoral level participants being metabolically healthier than undergraduate level participants.
As well as a significant heterogeneity in metabolic risk profile as a function of educational level, we also see a significant heterogeneity in age, with the distribution of risk across the spectrum of factors for younger adults (19–29 years old) being quite different to that of older (> 40 years old) people. In particular, we saw that, in strong contrast to hyperglycemia, there were substantial fractions of young adults exhibiting dyslipidemias that were significant contributors to MS. This heterogeneity in age of metabolic risk implies that public health interventions also need to be specifically tailored to each age group.
5. Study Limitations
There are several limitations of the study. Firstly, as in the context of this paper, it is a cross-sectional study, we can make no inference as to any causal effects as a function of age. More specifically, the metabolic state of an individual is a result of their intrinsic physiology and their interaction with a changing environment over their lifetime. Although we do not know how that environment has changed, we can make the assumption that, for two individuals of the same age, the differences can be studied using educational level as a measure of the differences in the interaction. Additionally, even in a prospective study, it would be very difficult to track a set of environmental descriptors that proffered a sufficiently rich description of those aspects of the multi-factorial interaction between an individual and their environment that most affect metabolic state. Another limitation is that the population we consider is not representative of the wider Mexican population. However, we also believe that this is a strength. First of all, with respect to demographic characteristics it is highly heterogeneous, especially in terms of educational level, ranging from a primary only education up to postgraduate and therefore permits the explicit analysis of the impact of education beyond the undergraduate level as has been a chief objective of this paper. We may also observe differences due to educational level in a relatively uniform environment, where significant differences in the work place, such as in work-activity levels, or stress associated with job security, might be expected to be relatively small. Additionally, we believe that there are cross-cultural similarities between individuals at the higher levels of education that would indicate potentially similar results in analogous populations elsewhere. Another limitation is that educational level in this population is very much correlated with socio-economic status as measured by income. However, even if educational level was just a proxy for socio-economic status it would still be necessary to further investigate which specific lifestyle factors enabled postgraduates to be metabolically healthier than graduates. Finally, we are also assuming that educational level is a meaningful proxy for a large number of factors that constitute an individual's lifestyle.
6. Conclusion
In this paper we have shown that each metabolic biomarker and associated disorder has its own corresponding risk profile in terms of educational level, BMI, age, and sex and that these profiles can be used to classify metabolic disorders. Taking BMI as a control variable, educational level and age represent two complementary measures of exposure to metabolic insults, with age representing cumulative exposure and educational level, as a lifestyle proxy, representing the degree of exposure. Metabolic disorders can then be classified according to their dependence/independence on educational level and/or age taken as measures of degree and duration of metabolic insult exposure. We determined that in women WC, SBP, glucose, Hb A1c, HOMA-IR, triglycerides, and HDL depend on both degree and duration; whereas in men WC, DBP, total cholesterol, and LDL depend only on duration; uric acid and HDL only on degree, while in women basal insulin and uric acid depend on neither. Interestingly, all components of MS depend on both degree and duration except in the case of HDL.
The distinct risk profiles potentially indicate different aetiologies for the different disorders and this is also indicated by their quite different prevalence as a function of age. Moreover, we conclude that the distinct profiles indicate that both clinical and public health interventions for a given metabolic disorder need to be tailored to age and education (lifestyle) specific groups. The fact that educational level correlates with better health in many, but not all, of the metabolic variables and, in particular, in the components of MS, requires much more study to determine those characteristics that differentiate the lifestyle decisions of one educational group vs. another. In particular, given the better metabolic health of postgraduates vs. graduates, what are the differentiating factors and/or behaviors that lead to such a result?
Data Availability Statement
The datasets for this study can be found in http://www.c3.unam.mx/health/.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Facultad de Medicina of the Universidad Nacional Autónoma de México under project FM/DI/023/2014. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors wrote, revised the manuscript, and approve its submission for publication, certifying that the work is original and their own. CS conceived and designed the investigation, questionnaires, and protocols. AR-C, AH-C, RM-T, AB-M, and LC performed the data acquisition. JL-R made database. JE, LC, JL-R, and AR analyzed the data. CS, JE, AR-C, AB-M, LC, RF, and AR results interpretation and discussion.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Maria Concepción García Aguirre for the logistic and administrative support for the development of the project, Romel Calero Ramos for helping with the database accessibility, and Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran for the laboratory analysis.
Glossary
Abbreviations
- DM2
type 2 diabetes mellitus
- CVD
cardiovascular disease
- MS
metabolic syndrome
- BMI
body mass index
- WC
waist circumference
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- Hb A1c
glycosylated hemoglobin
- HOMA-IR
homeostatic model assessment of insulin resistance
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- PP
systemic pulse pressure.
Footnotes
Funding. This work was supported by CONACyT through the network Complejidad, Ciencia y Sociedad, from Fronteras grants FC-2015-2/1093 and FC-2016-1/2277, and the Universidad Nacional Autónoma de México through DGAPA Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) [grant numbers: IV100116 and IN113619]. We also acknowledge support from SECTEI CDMX grant SECIT/093/2018 and a donation from Academic Relations, Microsoft Corporation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00180/full#supplementary-material
References
- 1.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. (2007) 30:8–13. 10.2337/dc06-1414 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Sattar N. The metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. (2008) 31:1898–904. 10.2337/dc08-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narayan KV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of body mass index on lifetime risk for diabetes mellitus in the United States. Diabetes Care. (2007) 30:1562–6. 10.2337/dc06-2544 [DOI] [PubMed] [Google Scholar]
- 4.Easton JF, Stephens CR, Angelova M. Risk factors and prediction of very short term versus short/intermediate term post-stroke mortality: a data mining approach. Comput Biol Med. (2014) 54:199–210. 10.1016/j.compbiomed.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Groop L. Genetics of the metabolic syndrome. Br J Nutr. (2000) 83:S39–48. 10.1017/S0007114500000945 [DOI] [PubMed] [Google Scholar]
- 6.Desai M, Jellyman J, Ross M. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes. (2015) 39:633–41. 10.1038/ijo.2015.13 [DOI] [PubMed] [Google Scholar]
- 7.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci. (2016) 53:52–67. 10.3109/10408363.2015.1084990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. (2009) 6:e1000058. 10.1371/journal.pmed.1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. (2008) 31:369–71. 10.2337/dc07-1795 [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. (1993) 153:2093–101. 10.1001/archinte.153.18.2093 [DOI] [PubMed] [Google Scholar]
- 11.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. (2011) 378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 12.Yousefzadeh G, Sheikhvatan M. Age and gender differences in the clustering of metabolic syndrome combinations: a prospective cohort research from the Kerman Coronary Artery Disease Risk Study (KERCADRS). Diabetes Metab Syndr. (2015) 9:337–42. 10.1016/j.dsx.2013.02.023 [DOI] [PubMed] [Google Scholar]
- 13.Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex-and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. (2017) 120:34–42. 10.1016/j.phrs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 14.Moore JX, Chaudhary N, Akinyemiju T. Peer Reviewed: Metabolic Syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prevent Chronic Dis. (2017) 14:E24 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leventhal AM, Huh J, Dunton GF. Clustering of modifiable biobehavioral risk factors for chronic disease in US adults: a latent class analysis. Perspect Public Health. (2014) 134:331–8. 10.1177/1757913913495780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leatherdale ST. An examination of the co-occurrence of modifiable risk factors associated with chronic disease among youth in the COMPASS study. Cancer Causes Control. (2015) 26:519–28. 10.1007/s10552-015-0529-0 [DOI] [PubMed] [Google Scholar]
- 17.Di Daniele N, Noce A, Vidiri MF, Moriconi E, Marrone G, Annicchiarico-Petruzzelli M, et al. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget. (2017) 8:8947–79. 10.18632/oncotarget.13553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutie PM, Giordano GN, Franks PW. Lifestyle precision medicine: the next generation in type 2 diabetes prevention? BMC Med. (2017) 15:171. 10.1186/s12916-017-0938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatzianagnostou K, Del Turco S, Pingitore A, Sabatino L, Vassalle C. The mediterranean lifestyle as a non-pharmacological and natural antioxidant for healthy aging. Antioxidants. (2015) 4:719–36. 10.3390/antiox4040719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Publ Health. (2011) 32:381–98. 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman EB, Woolf SH, Haley A. Understanding the relationship between education and health: a review of the evidence and an examination of community perspectives. Popul Health. (2015) 10:347–84. [Google Scholar]
- 22.Pinillos-Franco S, García-Prieto C. The gender gap in self-rated health and education in Spain. A multilevel analysis. PLoS ONE. (2017) 12:e0187823. 10.1371/journal.pone.0187823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirowsky J. Education, Social Status, and Health. New York, NY: Routledge; (2017). 10.4324/9781351328081 [DOI] [Google Scholar]
- 24.Brunello G, Fort M, Schneeweis N, Winter-Ebmer R. The causal effect of education on health: what is the role of health behaviors? Health Econ. (2016) 25:314–36. 10.1002/hec.3141 [DOI] [PubMed] [Google Scholar]
- 25.Lynch JL, von Hippel PT. An education gradient in health, a health gradient in education, or a confounded gradient in both? Soc Sci Med. (2016) 154:18–27. 10.1016/j.socscimed.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 26.Bann D, Hamer M, Parsons S, Ploubidis GB, Sullivan A. Does an elite education benefit health? Findings from the 1970 British Cohort Study. Int J Epidemiol. (2016) 46:293–302. 10.1093/ije/dyw045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann S, Rohrmann S, Linseisen J, May AM, Kunst A, Besson H, et al. The association of education with body mass index and waist circumference in the EPIC-PANACEA study. BMC Public Health. (2011) 11:169. 10.1186/1471-2458-11-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roskam AJR, Kunst AE, Van Oyen H, Demarest S, Klumbiene J, Regidor E, et al. Comparative appraisal of educational inequalities in overweight and obesity among adults in 19 European countries. Int J Epidemiol. (2009) 39:392–404. 10.1093/ije/dyp329 [DOI] [PubMed] [Google Scholar]
- 29.Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Educational level, relative body weight, and changes in their association over 10 years: an international perspective from the WHO MONICA Project. Am J Public Health. (2000) 90:1260–8. 10.2105/AJPH.90.8.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. (2014) 2014:943162. 10.1155/2014/943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.GisteråA, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. (2017) 13:368–80. 10.1038/nrneph.2017.51 [DOI] [PubMed] [Google Scholar]
- 32.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. (2005) 28:1769–78. 10.2337/diacare.28.7.1769 [DOI] [PubMed] [Google Scholar]
- 33.Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. (2017) 2:775–81. 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. (2005) 48:1684–99. 10.1007/s00125-005-1876-2 [DOI] [PubMed] [Google Scholar]
- 35.Balkau B, Valensi P, Eschwége E, Slama G. A review of the metabolic syndrome. Diabetes Metab. (2007) 33:405–13. 10.1016/j.diabet.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 36.Ding EL, Smit LA, Hu FB. The metabolic syndrome as a cluster of risk factors: is the whole greater than the sum of its parts? Arch Intern Med. (2010) 170:484–5. 10.1001/archinternmed.2009.552 [DOI] [PubMed] [Google Scholar]
- 37.Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome-a new worldwide definition. Lancet. (2005) 366:1059–62. 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 38.Organization WH. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; (1999). [Google Scholar]
- 39.Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 40.Singh RG, Yoon HD, Poppitt SD, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its biomarkers: a systematic review and meta-analysis. Diabetes Metab Res Rev. (2017) 33:e2918. 10.1002/dmrr.2918 [DOI] [PubMed] [Google Scholar]
- 41.George P, Pearson E, Witham M. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. (2012) 29:e142–50. 10.1111/j.1464-5491.2012.03672.x [DOI] [PubMed] [Google Scholar]
- 42.Tucker LA, Erickson A, LeCheminant JD, Bailey BW. Dairy consumption and insulin resistance: the role of body fat, physical activity, and energy intake. J Diabetes Res. (2015) 2015:206959. 10.1155/2015/206959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 44.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. (2017) 127:1–4. 10.1172/JCI92035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. (2006) 444:840. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 46.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. (2008) 57:845–52. 10.1016/j.metabol.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassi N, Karagodin I, Wang S, Vassallo P, Priyanath A, Massaro E, et al. Lifestyle modification for metabolic syndrome: a systematic review. Am J Med. (2014) 127:1242.e1–e10. 10.1016/j.amjmed.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 48.Pronk NP, Anderson LH, Crain AL, Martinson BC, O'Connor PJ, Sherwood NE, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prevent Med. (2004) 27:25–33. 10.1016/j.amepre.2004.04.022 [DOI] [PubMed] [Google Scholar]
- 49.ECoPS W. Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee. Geneva: World Health Organization; (1995). [PubMed] [Google Scholar]
- 50.McPherson RA, Pincus MR. Henry's Clinical Diagnosis and Management by Laboratory Methods E-Book. St. Louis, MO: Elsevier Health Sciences; (2017). [Google Scholar]
- 51.Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's Principles of Internal Medicine. Vol. 2. New York, NY: Mcgraw-Hill; (2008). [Google Scholar]
- 52.Collaboration NRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. (2016) 387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dart AM, Kingwell BA. Pulse pressure-a review of mechanisms and clinical relevance. J Am Coll Cardiol. (2001) 37:975–84. 10.1016/S0735-1097(01)01108-1 [DOI] [PubMed] [Google Scholar]
- 54.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. (2000) 23:295–301. 10.2337/diacare.23.3.295 [DOI] [PubMed] [Google Scholar]
- 55.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. (2006) 23:469–80. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 56.Barquera S, Campos-Nonato I, Hernández-Barrera L, Flores M, Durazo-Arvizu R, Kanter R, et al. Obesity and central adiposity in Mexican adults: results from the Mexican National Health and Nutrition Survey 2006. Salud Pública de México. (2009) 51:S595–603. 10.1590/S0036-36342009001000014 [DOI] [PubMed] [Google Scholar]
- 57.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. (2003) 3:21. 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Chia K. Estimation of prevalence rate ratios for cross sectional data: an example in occupational epidemiology. Br J Indust Med. (1993) 50:861–2. 10.1136/oem.50.9.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wasserstein RL, Lazar NA. The ASA's statement on p-values: context, process, and purpose. Am Stat. (2016) 70:129–33. 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 60.Easton JF, Stephens CR, Sicilia HR. An analysis of real, self-perceived, and desired BMI: is there a need for regular screening to correct misperceptions and motivate weight reduction? Front Public Health. (2017) 5:12. 10.3389/fpubh.2017.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Easton JF, Stephens CR, Sicilia HR. The effect of a medical opinion on self-perceptions of weight for Mexican adults: perception of change and cognitive biases. BMC Obesity. (2017) 4:16. 10.1186/s40608-017-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz G, Correia MC. (eds). The Economics of Gender in Mexico: Work, Famil State, and Market. The World Bank (2001). p. 9–45. [Google Scholar]
- 63.Wilkinson RG, Marmot M. Social Determinants of Health: the Solid Facts. Copenhagen: World Health Organization; (2003). [Google Scholar]
- 64.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. (2003) 35:263–85. 10.1017/S0021932003002633 [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Marignac VL, Valladares A, Cameron E, Chan A, Perera A, Globus-Goldberg R, et al. Admixture in Mexico City: implications for admixture mapping of type 2 diabetes genetic risk factors. Hum Genet. (2007) 120:807–19. 10.1007/s00439-006-0273-3 [DOI] [PubMed] [Google Scholar]
- 66.Tyrrell J, Jones SE, Beaumont R, Astley CM, Lovell R, Yaghootkar H, et al. Height, body mass index, and socioeconomic status: Mendelian randomisation study in UK Biobank. BMJ. (2016) 352:i582. 10.1136/bmj.i582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boing AF, Subramanian S. The influence of area-level education on body mass index, waist circumference and obesity according to gender. Int J Public Health. (2015) 60:727–36. 10.1007/s00038-015-0721-8 [DOI] [PubMed] [Google Scholar]
- 68.Judge TA, Cable DM. When it comes to pay, do the thin win? The effect of weight on pay for men and women. J Appl Psychol. (2011) 96:95–112. 10.1037/a0020860 [DOI] [PubMed] [Google Scholar]
- 69.Dalton M, Cameron A, Zimmet P, Shaw J, Jolley D, Dunstan D, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. (2003) 254:555–63. 10.1111/j.1365-2796.2003.01229.x [DOI] [PubMed] [Google Scholar]
- 70.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. (2004) 79:379–84. 10.1093/ajcn/79.3.379 [DOI] [PubMed] [Google Scholar]
- 71.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. (2012) 13:275–86. 10.1111/j.1467-789X.2011.00952.x [DOI] [PubMed] [Google Scholar]
- 72.Bray GA. The Metabolic Syndrome and Obesity. Totowa NJ: Humana Press; (2007). 10.1007/978-1-59745-431-5 [DOI] [Google Scholar]
- 73.Svensson E, Reas DL, Sandanger I, Nygard JF. Urban?rural differences in BMI, overweight and obesity in Norway (1990 and 2001). Scand J Public Health. (2007) 35:555–8. 10.1080/14034940701349217 [DOI] [PubMed] [Google Scholar]
- 74.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res. (2011) 34:281–5. 10.1038/hr.2010.241 [DOI] [PubMed] [Google Scholar]
- 75.Kishore J, Gupta N, Kohli C, Kumar N. Prevalence of hypertension and determination of its risk factors in rural Delhi. Int J Hypertens. (2016) 2016:7962595. 10.1155/2016/7962595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Chen J, Wang K, Edwards C. Education as an important risk factor for the prevalence of hypertension and elevated blood pressure in Chinese men and women. J Hum Hypertens. (2006) 20:898–900. 10.1038/sj.jhh.1002086 [DOI] [PubMed] [Google Scholar]
- 77.Cundiff JM, Uchino BN, Smith TW, Birmingham W. Socioeconomic status and health: education and income are independent and joint predictors of ambulatory blood pressure. J Behav Med. (2015) 38:9–16. 10.1007/s10865-013-9515-8 [DOI] [PubMed] [Google Scholar]
- 78.Doll S, Paccaud F, Bovet Pa, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes. (2002) 26:48. 10.1038/sj.ijo.0801854 [DOI] [PubMed] [Google Scholar]
- 79.Bourgeois B, Watts K, Thomas DM, Carmichael O, Hu FB, Heo M, et al. Associations between height and blood pressure in the United States population. Medicine. (2017) 96:e9233. 10.1097/MD.0000000000009233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. (1999) 99:1165–72. 10.1161/01.CIR.99.9.1165 [DOI] [PubMed] [Google Scholar]
- 81.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. (2004) 109:726–32. 10.1161/01.CIR.0000115516.54550.B1 [DOI] [PubMed] [Google Scholar]
- 82.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. (2004) 50:1189–200. 10.1373/clinchem.2004.032763 [DOI] [PubMed] [Google Scholar]
- 83.Castelli WP. Epidemiology of coronary heart disease: The Framingham study. Am J Med. (1984) 76:4–12. 10.1016/0002-9343(84)90952-5 [DOI] [PubMed] [Google Scholar]
- 84.Benetou V, Chloptsios Y, Zavitsanos X, Karalis D, Naska A, Trichopoulou A. Total cholesterol and HDL-cholesterol in relation to socioeconomic status in a sample of 11,645 Greek adults: the EPIC study in Greece. Scand J Public Health. (2000) 28:260–5. 10.1177/14034948000280040801 [DOI] [PubMed] [Google Scholar]
- 85.Shohaimi S, Boekholdt MS, Luben R, Wareham NJ, Khaw KT. Distribution of lipid parameters according to different socio-economic indicators-the EPIC-Norfolk prospective population study. BMC Public Health. (2014) 14:782. 10.1186/1471-2458-14-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Commun Health. (1998) 52:399–405. 10.1136/jech.52.6.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garrison RJ, Gold RS, Wilson P, Kannel WB. Educational attainment and coronary heart disease risk: the Framingham Offspring Study. Prevent Med. (1993) 22:54–64. 10.1006/pmed.1993.1004 [DOI] [PubMed] [Google Scholar]
- 88.Heiss G, Haskell W, Mowery R, Criqui MH, Brockway M, Tyroler H. Plasma high-density lipoprotein cholesterol and socioeconomic status. The Lipid Research Clinics Program Prevalence Study. Circulation. (1980) 62:IV108–115. [PubMed] [Google Scholar]
- 89.Brunner E, Shipley MJ, Blane D, Smith GD, Marmot MG. When does cardiovascular risk start? Past and present socioeconomic circumstances and risk factors in adulthood. J Epidemiol Commun Health. (1999) 53:757–64. 10.1136/jech.53.12.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iribarren C, Luepker RV, McGovern PG, Arnett DK, Blackburn H. Twelve-year trends in cardiovascular disease risk factors in the Minnesota Heart Survey: are socioeconomic differences widening? Arch Intern Med. (1997) 157:873–81. 10.1001/archinte.157.8.873 [DOI] [PubMed] [Google Scholar]
- 91.Manhem K, Dotevall A, Wilhelmsen L, Rosengren A. Social gradients in cardiovascular risk factors and symptoms of Swedish men and women: the Göteborg MONICA study 1995. J Cardiovasc Risk. (2000) 7:359–68. 10.1177/204748730000700509 [DOI] [PubMed] [Google Scholar]
- 92.Morgan A, Mooney KM, Wilkinson SJ, Pickles N, Mc Auley MT. Cholesterol metabolism: a review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res Rev. (2016) 27:108–24. 10.1016/j.arr.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 93.Charlton J, Ravindrarajah R, Hamada S, Jackson SH, Gulliford MC, Newman A. Trajectory of total cholesterol in the last years of life over age 80 years: cohort study of 99,758 participants. J Gerontol A. (2017) 73:1083–9. 10.1093/gerona/glx184 [DOI] [PubMed] [Google Scholar]
- 94.Rhoades RA, Bell DR. Chapter 26: Gastrointestinal physiology. In: Medical Physiology: Principles for Clinical Medicine. 4th Edition Philadelphia, PA: Lippincott Williams & Wilkins; (2012). [Google Scholar]
- 95.Olano-Martin E, Abraham EC, Gill-Garrison R, Valdes AM, Grimaldi K, Tang F, et al. Influence of apoA-V gene variants on postprandial triglyceride metabolism: impact of gender. J Lipid Res. (2008) 49:945–53. 10.1194/jlr.M700112-JLR200 [DOI] [PubMed] [Google Scholar]
- 96.Tabara Y, Arai H, Hirao Y, Takahashi Y, Setoh K, Kawaguchi T, et al. The causal effects of alcohol on lipoprotein subfraction and triglyceride levels using a Mendelian randomization analysis: The Nagahama Study. Atherosclerosis. (2017) 257:22–8. 10.1016/j.atherosclerosis.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 97.Hoeke G, Nahon KJ, Bakker LE, Norkauer SS, Dinnes DL, Kockx M, et al. Short-term cooling increases serum triglycerides and small high-density lipoprotein levels in humans. J Clin Lipidol. (2017) 11:920–8. 10.1016/j.jacl.2017.04.117 [DOI] [PubMed] [Google Scholar]
- 98.Shamai L, Lurix E, Shen M, Novaro GM, Szomstein S, Rosenthal R, et al. Association of body mass index and lipid profiles: evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg. (2011) 21:42–7. 10.1007/s11695-010-0170-7 [DOI] [PubMed] [Google Scholar]
- 99.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. (2007) 115:450–8. 10.1161/CIRCULATIONAHA.106.637793 [DOI] [PubMed] [Google Scholar]
- 100.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. (2005) 36:232–40. 10.1016/j.arcmed.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 101.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. (2011) 40:804–18. 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 102.Ko MJ, Kim MK. The relationship between high blood glucose and socio-economic position in childhood and adulthood in Korea: findings from the Korean National Health and Nutrition Examination, 2007-09. Int J Epidemiol. (2012) 41:733–42. 10.1093/ije/dys045 [DOI] [PubMed] [Google Scholar]
- 103.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E, et al. Independent influence of age on basal insulin secretion in nondiabetic humans. J Clin Endocrinol Metab. (1999) 84:863–8. 10.1210/jcem.84.3.5542 [DOI] [PubMed] [Google Scholar]
- 104.Meisinger C, Döring A, Stöckl D, Thorand B, Kowall B, Rathmann W. Uric acid is more strongly associated with impaired glucose regulation in women than in men from the general population: the KORA F4-Study. PLoS ONE. (2012) 7:e37180. 10.1371/journal.pone.0037180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Oliveira EP, Moreto F, de Arruda Silveira LV, Burini RC. Dietary, anthropometric, and biochemical determinants of uric acid in free-living adults. Nutr J. (2013) 12:11. 10.1186/1475-2891-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wingrove CS, Walton C, Stevenson JC. The effect of menopause on serum uric acid levels in non-obese healthy women. Metab Clin Exp. (1998) 47:435–8. 10.1016/S0026-0495(98)90056-7 [DOI] [PubMed] [Google Scholar]
- 107.Qiu Q, Gong Y, Liu X, Dou L, Wang Y, Wang B, et al. Serum uric acid and impaired glucose tolerance: the Cardiometabolic Risk in Chinese (CRC) Study. Cell Biochem Biophys. (2015) 73:155–62. 10.1007/s12013-015-0597-5 [DOI] [PubMed] [Google Scholar]
- 108.American Diabetes Association Standards of medical care in diabetes–2011. Diabetes Care. (2011) 34(Suppl. 1):S11–61. 10.2337/dc11-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sluijs I, Beulens JW, van der A DL, Spijkerman AM, Schulze MB, van der Schouw YT. Plasma Uric Acid is associated with increased risk of type 2 Diabetes independent of diet and metabolic risk factors. J Nutr. (2012) 143:80–5. 10.3945/jn.112.167221 [DOI] [PubMed] [Google Scholar]
- 110.Lin J, Chiou W, Weng H, Fang J, Liu T. Application of three-dimensional body scanner: observation of prevalence of metabolic syndrome. Clin Nutr. (2004) 23:1313–23. 10.1016/j.clnu.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 111.Chien KL, Chen MF, Hsu HC, Chang WT, Su TC, Lee YT, et al. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem. (2008) 54:310–6. 10.1373/clinchem.2007.095190 [DOI] [PubMed] [Google Scholar]
- 112.Chang JB, Chen YL, Hung YJ, Hsieh CH, Lee CH, Pei D, et al. The role of uric acid for predicting future metabolic syndrome and type 2 diabetes in older people. J Nutr Health Aging. (2017) 21:329–35. 10.1007/s12603-016-0749-3 [DOI] [PubMed] [Google Scholar]
- 113.Holme I, Aastveit A, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein Mortality RISk study (AMORIS). J Intern Med. (2009) 266:558–70. 10.1111/j.1365-2796.2009.02133.x [DOI] [PubMed] [Google Scholar]
- 114.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971-1992. JAMA. (2000) 283:2404–10. 10.1001/jama.283.18.2404 [DOI] [PubMed] [Google Scholar]
- 115.Centre for Diet U, Activity Research (CEDAR) Food, Income and Education: Who Eats More of What? (2014) Available online at: http://wwwcedariphcamacuk/resources/evidence/food-income-education-graphic/.
- 116.Pleis JR, Lucas JW, Ward BW. Summary health statistics for US adults: National Health Interview Survey, 2008. Vital Health Stat. (2009). 1–157. [PubMed] [Google Scholar]
- 117.Cutler DM, Lleras-Muney A. Education and Health: Evaluating Theories and Evidence. National Bureau of Economic Research, NBER Working Paper No. 1(2352) 2006 10.3386/w12352 [DOI] [Google Scholar]
- 118.Devaux M, Sassi F, Church J, Cecchini M, Borgonovi F. Exploring the relationship between education and obesity. OECD J. (2011) 5:121–59. 10.1787/eco_studies-2011-5kg5825v1k23 [DOI] [Google Scholar]
- 119.Mirowsky J, CE R. Education, Social Status, and Health. New York, NY: Aldine de Gruyter; (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study can be found in http://www.c3.unam.mx/health/.