Abstract
Purpose
To evaluate the effects of a hydroxypropyl guar (HPG) and hyaluronic acid (HA) ophthalmic solution in terms of post-cataract surgery dry-eye disease (DED) prevention.
Patients and Methods
In this retrospective study, the data of 419 patients not previously affected by DED, who had undergone unilateral cataract surgery in 17 Italian centers with different perioperative lubricating regimens, were retrospectively reviewed. Patients who had instilled HPG/HA solution 3 times/day in the preoperative week and for two postoperative months were included in group A; group B only instilled HPG/HA for two postoperative months; group C did not instill any perioperative artificial tears. All patients followed the same antibiotic and anti-inflammatory postoperative topical regimen. The scores of SPEED (Standard Patient Evaluation of Eye Dryness) questionnaire, tear break-up time (BUT) and corneal fluorescein staining (CFS, Oxford scale), performed at the preoperative visit and at one, four and eight postoperative weeks, were evaluated.
Results
In groups A and B, the SPEED scores were significantly lower than group C in the whole postoperative period. In group A, the SPEED scores were significantly lower than group B 1 and 4 weeks after surgery (p<0.001 and p=0.021). In group C, 25% of patients reported symptom scores corresponding to mild-moderate dry eye 4 and 8 weeks after surgery. The fluorescein tear BUT in groups A and B was significantly higher than group C in the whole postoperative period (p<0.001). In group A, BUT was significantly higher than group B 4 weeks after surgery (p=0.016). More patients showed no corneal fluorescein staining (CFS grade=0) in groups A and B than group C at all the postoperative visits.
Conclusion
The hydroxypropyl guar and hyaluronic acid ophthalmic solution was effective at reducing post-cataract surgery ocular discomfort and tear instability, particularly if also administered in the preoperative period.
Keywords: dry eye, cataract surgery, artificial tears, hydroxypropyl guar, hyaluronic acid, ocular surface
Introduction
Cataract surgery is one of the main causes of iatrogenic dry-eye disease (DED):1 it can both induce dry-eye symptoms in approximately one-third of patients2 and exacerbate preexisting dry eye. Moreover, symptoms may be combined with a reduced tear film stability and an increase in ocular surface staining,3 and these alterations may persist up to 3 months after surgery.4 However, the real incidence of dry-eye disease after cataract surgery is not known, due to discrepancy between symptoms and signs, difficulties in formulating the diagnosis, and possible pre-existing dry eye.1,3–7
Several factors may contribute to DED after cataract surgery, such as topical anesthesia, benzalkonium chloride–containing eyedrops, anterior segment tissue exposure to ultrasound and exposure to intense light from the operating microscope.8
Since DED symptoms can reduce patients’ quality of life, affecting their ability to perform daily activities,9 timely and effective diagnosis and treatment are necessary for cataract patients.10 Moreover, preoperative identification of patients with DED is crucial in order to prevent its exacerbation;4–7 nevertheless, optimizing their ocular surface before surgery may be useful for all phacoemulsification candidates.11
Artificial tears are the first-line treatment for DED.10 The two polymers hydroxypropyl guar (HPG) and hyaluronic acid (HA) are important constituents of many commercially available lubricating eyedrops. Together they are the main components of Systane Idra (Alcon Laboratories, Fort Worth, TX, USA), an ophthalmic formulation which contains HPG 0.175%, HA 0.15%, the demulcents propylene glycol (PG) and polyethylene glycol (PEG), sorbitol, aminomethylpropanol, boric acid, sodium borate, ethylenediaminetetraacetic acid (EDTA), sodium citrate, potassium chloride, sodium chloride, 0.001% Polyquad (polidronium chloride), and which is approved for the treatment of DED.12
Hydroxypropyl guar (HPG) is a mucomimetic gellable agent, with rheological properties very similar to those of tears.13 The efficacy of HPG ophthalmic solution in the treatment of DED after cataract surgery has been proved by clinical studies.14,15 Hyaluronic acid (HA), a mucopolysaccharide, is the key component of the extracellular matrix, plays a critical role in cell proliferation, anti-inflammation and wound repair, and has viscoelastic and hygroscopic properties.16,17 The efficacy of the HPG/HA combination in the treatment of dry eye has already been reported by a randomized double-masked clinical study.12
The purpose of our study was to retrospectively evaluate the effects of a hydroxypropyl guar and hyaluronic acid ophthalmic solution on post-cataract surgery dry-eye disease when instilled for 7 days before surgery and for eight postoperative weeks.
Patients and Methods
In this retrospective observational multicenter cohort study, the records of 419 patients not previously affected by dry eye, who had undergone unilateral cataract surgery (phacoemulsification and intraocular lens implantation) in 17 ophthalmic centers in Italy, were evaluated. The study was performed in compliance with good clinical practice and was consistent with the tenets outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants. The study was approved by the Ethics committee of the University of Florence. The data of a maximum of 30 patients were collected in each single center, and then they were sent in anonymous form to the coordinating center (Eye clinic, University of Florence, Italy) and analyzed. The coordinating center received the data of 499 patients, of which 80 were excluded because either the inclusion criteria were not met, or exclusion criteria were present.
The inclusion criteria were: patients affected by uncomplicated cataract, patients older than 50 years who underwent uncomplicated standard phacoemulsification, with clear corneal incision and IOL implantation in the capsular bag, between September 2017 and March 2018; postoperative topical antiflammatory and antibiotic treatment which consisted in dexamethasone + tobramycin 4 times a day for 10 days, nepafenac 0.1% eyedrops 3 times a day for 1 month; the administration of HPG/HA ophthalmic solution 3 times/day in the preoperative week and for 2 postoperative months (group A), or HPG/HA for only two postoperative months (group B), or no perioperative lubricant eyedrops (group C); the availability of clinical records of the preoperative visit (between 30 and 7 days before surgery) and at least the 1-week (7 ± 2 days after surgery), 4-week (28 ± 3 days) and 8-week (56 ± 3 days) postoperative follow-ups, including dry-eye diagnostic tests (Schirmer test I without anesthesia at least at the preoperative visit, Standard Patient Evaluation of Eye Dryness, SPEED questionnaire, break-up time with fluorescein, BUT, corneal fluorescein staining, CFS, evaluated using the Oxford scale). The exclusion criteria were dry-eye disease diagnosed before cataract surgery (defined as a score of Schirmer test without anesthesia lower than 7.0 mm in 5 minutes, BUT lower than 10 seconds and SPEED questionnaire score >4, corneal fluorescein staining score >1 at the preoperative visit); concurrent ocular diseases that could affect the study results (eg anatomic or functional eyelid abnormalities, lagophthalmos, floppy eyelid syndrome); instillation of lubricant eyedrops in the month prior to the preoperative visit; administration in the perioperative period of eyedrops not mentioned in the inclusion criteria; use of systemic drugs that may contribute to dry-eye disease (antihistamine, decongestant, spasmolytic and antidepressant drugs); diabetes; modification of topical and systemic therapy during the course of the study; complicated cataract surgery and/or use of sutures during surgery, corneal limbal relaxing incisions, femtosecond laser-assisted cataract surgery; anterior corneal dystrophy; corneal leucoma; contact lens use; previous ocular surgery in the study eye.
Tear BUT was performed in all centers after fluorescein instillation (invasive BUT).
Corneal fluorescein staining (CFS) was evaluated using the Oxford grading scale which divides corneal staining into 6 grades, according to the severity, from 0 (absent) to 5 (severe).18
The SPEED questionnaire19 is composed of 8 items and evaluates the frequency and severity of 4 symptoms (dryness, grittiness or scratchiness; soreness or irritation; burning or watering; eye fatigue), using a 0–3 scale for frequency (0= never, 1=sometimes, 2=often, 3=constant) and a 0–4 scale for severity (0= no problems, 1= tolerable, 2=uncomfortable, 3=bothersome, 4=intolerable). A score ≤4 was considered as not associated with dry-eye disease, 4–9 mild dry eye, ≥10 moderate or severe dry eye, according to previous studies.20,21
The demographic characteristics, the results of Schirmer test I performed at the preoperative visit, and the scores of the SPEED questionnaire, BUT and CFS, performed at each visit in the study eye, were reviewed and analyzed for this retrospective study.
Statistical Analysis
Data were calculated as the mean ± standard deviation (SD). Between-group comparisons of age, Schirmer test, SPEED scores and BUT were performed using analysis of variance (one-way ANOVA) followed by Tukey post-hoc test. Within-group comparisons were made using paired t-tests. Correlations were analyzed using Spearman test. Between-group comparisons of CFS were performed using chi-square test. A p-value lower than 0.05 was considered statistically significant. The analyses were performed using RStudio and Stata 12 software (StataCorp, College Station, TX).
Results
In this study 419 eyes of 419 patients with a mean age of 72.5 ± 8.85 years (min 50, max 97), 70% females, 30% men, were included. Group A comprised 139 patients, group B and group C 140 patients each. Preoperative Schirmer test I, SPEED scores, BUT values and CFS score distributions were similar between groups (Table 1).
Table 1.
Preoperative Data of Enrolled Patients
| Group A | Group B | Group C | p-value | |
|---|---|---|---|---|
| Age (years) | 72.54 ± 8.56 | 71.58 ± 9.49 | 73.42 ± 8.39 | 0.228 |
| Schirmer test I (mm) | 12.79 ± 4.95 | 13.41 ± 5.50 | 12.60 ± 4.04 | 0.344 |
| SPEED score | 1.70 ± 1.44 | 1.68 ± 1.47 | 1.63 ± 1.58 | 0.907 |
| BUT (s) | 11.87 ± 2.09 | 12.12 ± 2.42 | 11.90 ± 2.41 | 0.613 |
| CFS score n(%) | ||||
| 0 | 90 (64.7%) | 98 (70%) | 106 (75.7%) | 0.135 |
| 1 | 49 (35.3%) | 42 (30%) | 34 (24.3%) |
Note: Age, Schirmer test I, SPEED score and BUT are reported as mean ± SD.
Abbreviations: SPEED, Standard Patient Evaluation of Eye Dryness; BUT, break-up time; CFS, corneal fluorescein staining; SD, standard deviation.
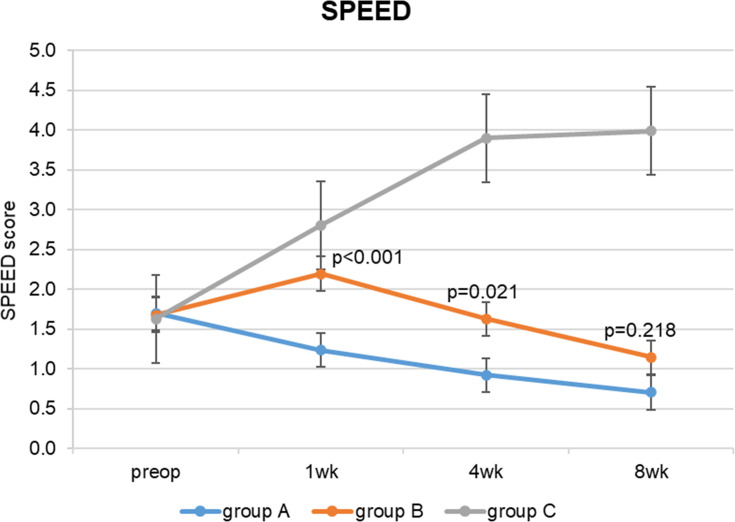
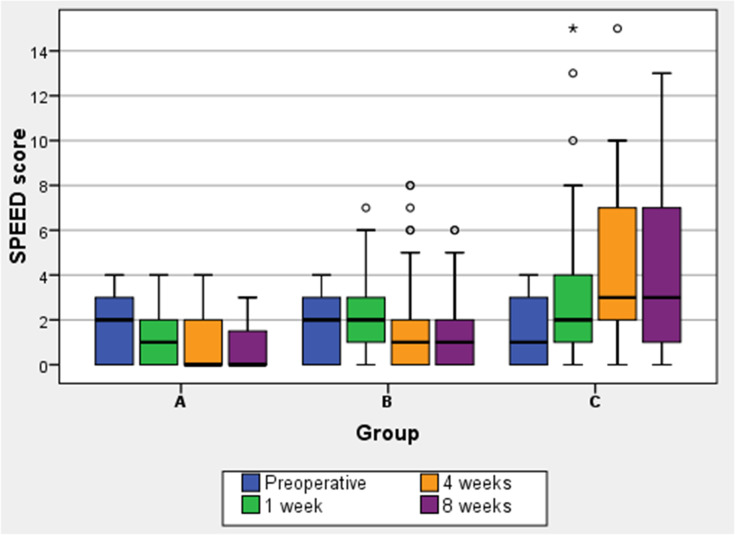
Regarding the mean SPEED questionnaire scores (Table 2), while in groups A and B they remained stable after cataract surgery, in group C (no perioperative lubricant eyedrops) they significantly increased, especially at 4 weeks (p<0.001). In groups A and B, the SPEED scores were significantly lower than group C in the whole postoperative period; at eight postoperative weeks the difference between groups C and A was 3.28 (95% confidence interval, CI, 2.66 to 3.90, p<0.001), the difference between groups C and B was 2.84 (95% CI 2.22 to 3.50, p<0.001). Group A (preoperative lubricant eyedrops) SPEED scores were significantly lower than group B 1 and 4 weeks after surgery: at 1 week the difference between groups B and A was 0.96 (95% confidence interval, CI, 0.41 to 1.51, p <0.001), at 4 weeks it was 0.71 (95% CI 0.09 to 1.32, p=0.021). Eight weeks after surgery group A and group B scores were similar, with a difference of 0.44 (95% CI −0.18 to 1.06, p=0.218). (Figure 1) Although mean SPEED scores were below or equal to 4 (no dry eye) even after surgery in all groups, the box and whisker plots (Figure 2) show that in group C 25% of patients had values between 7 and 10 after 4 weeks and between 7 and 13 (mild/moderate dry-eye symptoms) after 8 weeks.
Table 2.
SPEED Questionnaire and BUT Values
| Group A | Group B | Group C | p-value (ANOVA) | p-value (Tukey Post-Hoc Test) | |||
|---|---|---|---|---|---|---|---|
| A vs B | A vs C | B vs C | |||||
| SPEED score | |||||||
| Preop | 1.70 ± 1.44 | 1.68 ± 1.47 | 1.63 ± 1.58 | 0.907 | |||
| 1 wk | 1.24 ± 1.17 | 2.19 ± 1.82 | 2.81 ± 2.58 | 0.000 | 0.000 | 0.000 | 0.024 |
| 4 wk | 0.92 ± 1.05 | 1.63 ± 1.82 | 3.9 ± 3.16 | 0.000 | 0.021 | 0.000 | 0.000 |
| 8 wk | 0.71 ± 0.93 | 1.14 ± 1.44 | 3.98 ± 3.38 | 0.000 | 0.218 | 0.000 | 0.000 |
| BUT (s) | |||||||
| Preop | 11.87 ± 2.09 | 12.12 ± 2.42 | 11.90 ± 2.41 | 0.613 | |||
| 1 wk | 11.10 ± 2.51 | 10.76 ± 2.94 | 9.36 ± 2.67 | 0.000 | 0.552 | 0.000 | 0.000 |
| 4 wk | 11.94 ± 2.30 | 11.06 ± 2.96 | 11.06 ± 2.96 | 0.000 | 0.016 | 0.000 | 0.000 |
| 8 wk | 12.36 ± 2.40 | 11.84 ± 2.78 | 9.57 ± 2.83 | 0.000 | 0.232 | 0.000 | 0.000 |
Notes: Results are reported mean ± SD. P-values highlighted in bold are statistically significant.
Abbreviations: SPEED, Standard Patient Evaluation of Eye Dryness; BUT, break-up time; SD, standard deviation; preop, preoperative; wk, week; s, seconds; ANOVA, analysis of variance; vs, versus.
Figure 1.
SPEED questionnaire scores.
Note: Results are reported as mean ± standard error.
Abbreviations: SPEED, Standard Patient Evaluation of Eye Dryness; wk, week.
Figure 2.
Distributions of SPEED questionnaire scores, represented as box and whisker plots.
Notes: The bottom line of the box represents the first quartile value of SPEED scores, the top line of the box indicates the third quartile value. The middle line represents the median. Whiskers represent minimum and maximum values. Dots represent outliers, extreme values are marked with an asterisk (*).
Abbreviations: SPEED, Standard Patient Evaluation of Eye Dryness; wk, week.
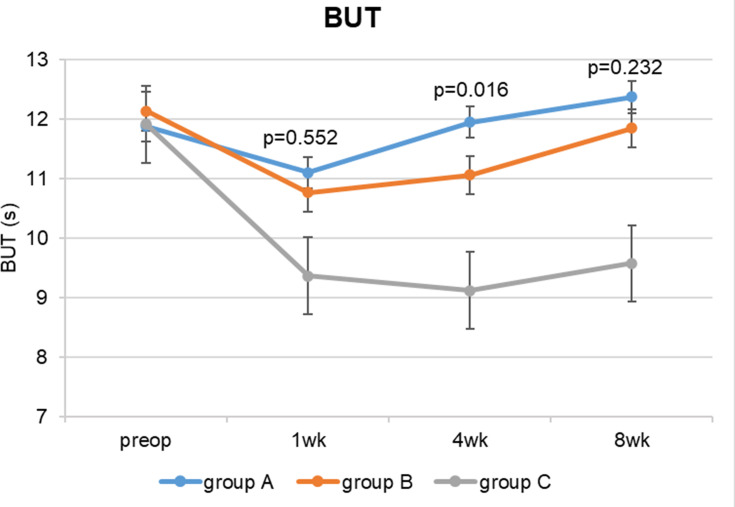
The fluorescein tear BUT (Table 2) was significantly higher in groups A and B than in group C (no lubricant eyedrops) in the whole postoperative period (p<0.001) (Figure 3). In group A (perioperative artificial tears), it was statistically significantly higher than group B 4 weeks after surgery, even though the difference was small and not clinically significant (0.88, 95% CI 0.13, 1.64, p=0.016). In all the 3 groups there was a statistically significant reduction in BUT scores after 1 week (p<0.001), but the differences between preoperative and 1-week values were not clinically significant (lower than 2 s); only in group C BUT dropped below 10. BUT returned similar to preoperative values at 4 weeks in group A, at 8 weeks in group B, and remained significantly lower in group C during the whole postoperative period (p<0.001). (Table 2 and Figure 3)
Figure 3.
Fluorescein tear break-up time at each study visit.
Abbreviations: BUT, break-up time; s, seconds; wk, week.
At eight postoperative weeks, the SPEED scores were inversely correlated with the BUT scores with a linear relationship (r=−0.5179, p<0.001).
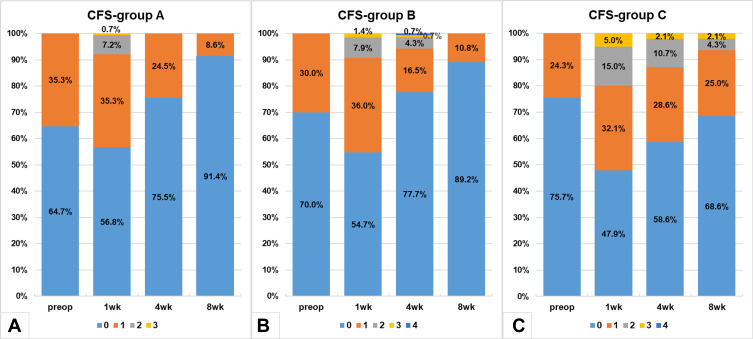
The distributions of CFS grades were significantly different in groups A and B compared to group C in the postoperative periods; more patients showed no corneal fluorescein staining (CFS grade=0) in groups A and B than group C at all the postoperative visits (Figure 4). The distributions of CFS scores in groups A and B were similar (p>0.05), except for week 4 (p=0.013), when the percentage of CFS>1 in group B was higher.
Figure 4.
Corneal fluorescein staining (Oxford scale) distributions in group A (A), group B (B) and group C (C) at each study visit.
Abbreviations: CFS, corneal fluorescein staining; wk, week.
Discussion
Cataract is one of the leading causes of visual impairment in the world,22 and the risk of experiencing dry-eye disease after phacoemulsification is significant, especially if other risk factors, such as age, female sex, concurrent use of systemic or topical drugs, diabetes, preoperative ocular surface disease, contact lens use, are present.1,4-7 Nevertheless, post-cataract surgery dry eye may also occur in the absence of preoperative significant ocular surface impairment or risk factors. It is often a transient condition, but it may become chronic in 10% of cases, leading to a decrease in quality of life.1,3
In our retrospective study, we have analyzed the records of patients not previously affected by DED, who underwent phacoemulsification for senile cataract, and who were prescribed different perioperative lubricating treatment regimens. In particular, our purpose was to evaluate the effects of an HPG/HA ophthalmic solution on ocular signs and symptoms of DED after cataract surgery, if administered from 7 days before surgery to 8 weeks postoperatively, compared to the postoperative administration only or no perioperative administration.
In our study, similarly to previous reports,1,3,4,10,11 we found an increase of DED symptoms and an impairment of tear film stability, evaluated using BUT, especially in the group which did not receive the HPG/HA solution.
Regarding the ocular symptoms of DED, we found differences between groups: while in groups treated with artificial tears the SPEED score remained stable after cataract surgery compared to preoperative values, in group C (no perioperative lubricant eyedrops) it significantly increased (meaning more ocular symptoms) at one postoperative week. Moreover, in the two treated groups, the SPEED scores were significantly lower than the non-treated group, and patients who administered HPG/HA solution from seven preoperative days reported fewer symptoms in the first four postoperative weeks rather than those treated only postoperatively. Interestingly, considering the SPEED score distribution, 25% of patients not treated with artificial tears reported mild-moderate dry-eye symptoms 4 and 8 weeks after surgery.
Regarding tear fluorescein BUT, an indicator of tear film stability,1 in all the three groups, it statistically significantly decreased compared to preoperative values, but not clinically significantly in the two groups treated with HPG/HA solution (the difference was lower than 2 seconds); moreover, in the not-treated group alone BUT dropped below 10. The return to the preoperative value was faster in the group treated preoperatively than the group treated only after surgery, and BUT remained significantly lower in the not-treated patients at 8 weeks.
In our patients, BUT was inversely correlated with the SPEED questionnaire scores, indicating a good correspondence between signs and symptoms.
The postoperative corneal fluorescein staining was significantly better in the groups treated with the HPG/HA solution, indicating less corneal damage and inflammation in those patients compared to those who did not administer artificial tears.
The changes in ocular signs and symptoms of DED during the study period were generally lower than in other reports,1,3,4,11 in the group not treated with artificial tears as well: a possible cause is the inclusion of patients without pre-existing dry eye or risk factors.
The main limitations of our study are the retrospective and multicentric design, which do not assure a complete standardization of the surgical technique (eg position of the main incision, number of mydriatic and anesthetic drops, duration of surgery, ultrasound exposure); however, in order to have homogeneous groups, strict inclusion and exclusion criteria were chosen, and the multicentric design permitted the enrollment of a large number of patients.
In our study, the HPG/HA solution, especially if administered preoperatively, showed a protective effect from iatrogenic DED: this highlights the importance of improving the homeostasis of the ocular surface not only in patients with established dry eye but also in patients without other evident risk factors for DED or without significant ocular surface impairment. The optimization of the ocular surface before cataract surgery can influence the postoperative outcome, reducing the risk of ocular symptoms and visual disturbances, or even biometric errors, important especially if premium intraocular lenses are implanted. Moreover, the combination of hydroxypropyl guar and hyaluronic acid in a single lubricant formulation can be advantageous, as it provides the properties of both polymers, potentially enhancing their beneficial effects; this may result in greater ocular surface hydration and protection, and longer retention time.12,23
Conclusion
The hydroxypropyl guar and hyaluronic acid ophthalmic solution was effective in protecting the ocular surface, reducing post-cataract surgery dry-eye signs and symptoms, particularly if administered in the preoperative period.
Acknowledgments
The authors thank Claudia Leo, statistician, Local care agency of Tuscany, North-West Area, for the statistical assistance. The authors also thank the other study investigators (all from Italy) for their important work: Dr. Elena Antoniazzi, Pavia; Dr. Piero Barboni, Bologna; Prof. Carlo Cagini, Perugia; Dr. Luigi Caretti, Rovigo; Dr. Antonella Franch, Venezia; Dr. Maria Concetta Nanni, Dr. Paolo Fantaguzzi, Forlì; Dr. Ugo Murialdo, Rapallo; Dr. Pietro Napoli, Cagliari; Dr. Francesco Paolercio, Vico Equense; Dr. Franco Passani, Massa e Carrara; Dr. Marta Sacchetti, Prof. Giorgio Lo Foco, Roma; Prof. Daniele Tognetto, Trieste; Prof. Claudio Traversi, Siena; Dr. Sandro Vergani, Milano; Dr. Silvia Visentin, San Donà di Piave; Prof. Luigi Zompatori, Tivoli.
Disclosure
Eleonora Favuzza reports personal fees from Alcon Laboratoires and Johnson & Johnson Vision, outside the submitted work. Rita Mencucci reports personal fees from Laboratoires Thea, Alfa Intes, Alcon Laboratoires, Santen, and Johnson & Johnson Vision, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–538. [DOI] [PubMed] [Google Scholar]
- 2.Iglesias E, Sajnani R, Levitt RC, Sarantopoulos CD, Galor A. Epidemiology of persistent dry eye-like symptoms after cataract surgery: persistent postsurgical pain after cataract surgery. Cornea. 2018;37(7):893–898. doi: 10.1097/ICO.0000000000001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S, Wedrich A. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8(11):e78657. doi: 10.1371/journal.pone.0078657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutu C, Fukuoka H, Afshari NA. Mechanisms and management of dry eye in cataract surgery patients. Curr Opin Ophthalmol. 2016;27(1):24–30. doi: 10.1097/ICU.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 5.Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery. J Cataract Refract Surg. 2018;44:1090–1096. doi: 10.1016/j.jcrs.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 6.Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders: a report by the ASCRS cornea clinical committee. J Cataract Refract Surg. 2019;45(5):669–684. doi: 10.1016/j.jcrs.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 7.Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–1430. doi: 10.2147/OPTH.S120159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23(2):65–73. doi: 10.3341/kjo.2009.23.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15 [DOI] [PubMed] [Google Scholar]
- 10.Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699–1704. doi: 10.1016/j.jcrs.2014.12.056 [DOI] [PubMed] [Google Scholar]
- 11.Fogagnolo P, Favuzza E, Marchina D, et al. New therapeutic strategy and innovative lubricating ophthalmic solution in minimizing dry eye disease associated with cataract surgery: a randomized, prospective study. Adv Ther. 2020;37(4):1664–1674. doi: 10.1007/s12325-020-01288-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labetoulle M, Schmickler S, Galarreta D, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study. Clin Ophthalmol. 2018;12:2499–2508. doi: 10.2147/OPTH.S177176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benelli U. Systane lubricant eye drops in the management of ocular dryness. Clin Ophthalmol. 2011;5:783–790. doi: 10.2147/OPTH.S13773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez MA, Arriola-Villalobos P, Torralbo-Jiménez P, et al. The effect of preservative-free HP-guar on dry eye after phacoemulsification: a flow cytometric study. Eye. 2010;24(8):1331–1337. doi: 10.1038/eye.2010.24 [DOI] [PubMed] [Google Scholar]
- 15.Labiris G, Ntonti P, Sideroudi H, Kozobolis V. Impact of polyethylene glycol 400/propylene glycol/hydroxypropyl-guar and 0.1% sodium hyaluronate on postoperative discomfort following cataract extraction surgery: a comparative study. Eye Vis. 2017;4:13. doi: 10.1186/s40662-017-0079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88(6):821–825. doi: 10.1136/bjo.2003.027573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Hikida M, Nakano T, Ito S, Hamano T, Kinoshita S. Characterization of water retentive properties of hyaluronan. Cornea. 1993;12(5):433–436. doi: 10.1097/00003226-199309000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi: 10.1097/ICO.0b013e318294b0c0 [DOI] [PubMed] [Google Scholar]
- 20.Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular Surface Disease Index (OSDI) Versus the Standard Patient Evaluation of Eye Dryness (SPEED): a study of a nonclinical sample. Cornea. 2016;35(2):175–180. doi: 10.1097/ICO.0000000000000712 [DOI] [PubMed] [Google Scholar]
- 21.Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817–827. doi: 10.2147/OPTH.S130706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. doi:/S0042-96862004001100009 [PMC free article] [PubMed] [Google Scholar]
- 23.Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther. 2015;31(8):491–497. doi: 10.1089/jop.2014.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]