Abstract
The advent of interferon therapy for the treatment of multiple sclerosis (MS) was a massive advancement in the field and changed the course of the disease. While the exact mechanism of interferon therapy in MS is unknown, disease control is likely mediated by reducing Th1 and Th17 cells while increasing regulatory T cells and altering the cytokine profile. Interferon therapy not only gave physicians and patients an evidence-based treatment option to treat MS by decreasing relapses and the accrual of disability but it also provided valuable insight into disease pathophysiology that allowed for the development of further treatments. Currently, there are 18 disease-modifying therapies available for the treatment of MS with varying efficacies, routes of administration, and mechanisms. As treatment options in the field have evolved, interferon therapy is less commonly prescribed as first-line therapy, because the newer therapies are more effective and better tolerated. That being said, interferons still have a place in the field in both clinical practice and clinical trial research. In this review, we will summarize the safety and efficacy of interferon therapy and discuss its current place in MS care.
Keywords: multiple sclerosis, interferon-beta therapy, disease-modifying therapy
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system that leads to neuronal damage and irreversible disability, thought to be mediated by a T-cell autoimmune process. This theory of pathogenic T-cell involvement has become the target of many of the disease-modifying therapies (DMTs). Interferon (IFN) γ-secreting helper T cells (Th1), interleukin-17 secreting Th17 cells, and regulatory T cells (Tregs) are the most studied types of T cells in the pathogenesis and modulation of MS. With the more recent success of anti-B cell monoclonal antibody therapies in the treatment of MS, the role of B cells appears to be important in the pathogenesis of MS as well. It has been shown that the number of B cells, not the amount of antibody, correlates with relapse rates.1 This led to the theory that it is B cell-T cell interactions such as antigen presenting and modulation of cytokine secretion that are important drivers of the disease.
Interferons are a family of cytokines that are involved in the regulation of innate and adapted immunity, and therefore became an attractive target for immunomodulation therapy in MS. Interferons were initially studied for the treatment of multiple sclerosis on the basis of three rationales: 1) reports that intrathecal injections of natural interferon beta (IFNβ) significantly reduced exacerbations, 2) that intercurrent viral infections trigger new attacks, and 3) that interferons had immunomodulatory functions including inhibition of IFNγ synthesis, augment defective suppressor activity, and inhibit class II major histocompatibility complex antigen expression.2 Since these initial theories were proposed, it has been demonstrated that IFNβ has pleiotropic effects on the peripheral immune system including reducing pathogenic Th1 and Th17 cells and increasing Tregs that produce IL-10 via the JAK-STAT signaling pathway.3–5 Additionally, IFNβ has been shown to reduce CD27+ memory B cells and increase IL-10 producing transitional B cells, which is thought to be beneficial on disease activity.3 Finally, IFNβ may downregulate adhesion molecules suppressing the ability of pro-inflammatory cells to enter the CNS.5
There are currently multiple formulations of IFNβ that are approved for use in clinically isolated syndrome, relapsing remitting MS (RRMS), and secondary progressive MS (SPMS) with relapses. These include IFNβ-1b (Betaseron® and Extavia®) that are administered subcutaneously every other day at a dose of 250μg, IFNβ-1a that is administered intramuscularly once a week (Avonex®) at a dose of 30μg, IFNβ-1a (Rebif®) that is administered subcutaneously three times weekly at a dose of 22 or 44μg, and pegylated IFNβ-1a (Plegridy®) that is administered subcutaneously every 2 weeks at a dose of 125μg.5
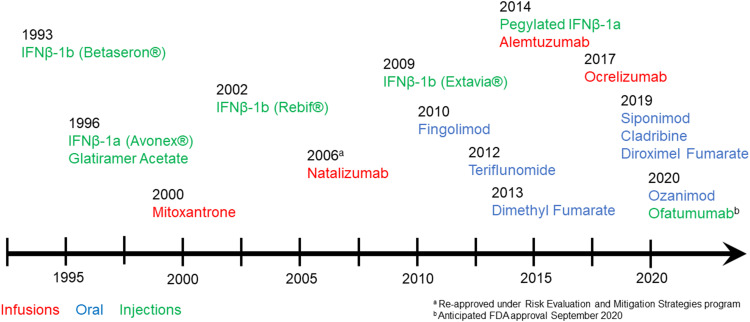
Interferons effect on disease activity in MS is multi-factorial and not fully understood. Since the pivotal approval of IFNβ, the therapeutic landscape has rapidly and continuously expanded with 18 FDA-approved DMTs for MS to date (Figure 1).6 The efficacy of interferons is now considered modest as newer therapies have demonstrated more potent disease control. There is also an increased adaptation of the use of highly effective therapy earlier in the disease course and a subsequent change in sequencing medications. In this increasingly complex treatment landscape, we will review the safety and efficacy of interferons and discuss their current role in the treatment of MS.
Figure 1.
Timeline of FDA approval for currently available disease-modifying therapies. Brand names only used for IFNβ formulations for clarity. Infusion therapies are in red text, oral therapies are in blue text and injectable therapies are in green text.
Pivotal Phase III Trials of RRMS
Prior to 1993 when IFNβ-1b became the first FDA-approved treatment for RRMS, there was nothing available that significantly impacted relapse rates, lesion accumulation, or disability accrual in MS. The first IFNβ trial that was published in 1993 represented a landmark in the history of MS treatment and led to a sea-change in the field. Further studies were subsequently performed looking at different formulations of IFNβ. Overall, the initial phase III trials of the first three interferon products showed a reduction in relapse rate by 18–34% in patients with relapsing-remitting MS.7 The results of these pivotal trials are summarized below and in Table 1.
Table 1.
Summaries of Pivotal Trials of Interferons in RRMS
| Trial | Primary Outcome | Secondary Outcome | Intervention | Significant Results | Adverse Events |
|---|---|---|---|---|---|
| IFNB MS Study Group,2 1993 (2 years) |
● Differences in ARR● Proportion of patients exacerbation-free | ● Time to exacerbation● MRI lesion load● Severity of exacerbations● Change in EDSS | 1.6MIU IFNB 1b or 8.0 MIU IFNB-1b SC every other day vs placebo |
● ARR lower in both treatment groups (placebo: 1.27; 1.6MIU 1.17; 8.0MIU 0.84)● More patients in 8.0MIU group exacerbation-free (did not remain significant at 3 years)● Prolongation of time to first and second exacerbations in 8.0MIU group● Benefit in MRI lesion burden (placebo with 20% increase, 1.6MIU with 10.5% increase, 8.0MIU with 0.1% decrease)● Less moderate and severe exacerbations in 8MIU group● No significant change in EDSS in any group | ● Mild, intermittent lymphopenia, neutropenia anemia, thrombocytopenia● Abnormal liver enzymes● Injection site pain● Flu-like illness● Muscle aches● Fever● Chills● Neutralizing antibodies: 11% in placebo, 47% in 1.6MIU, 45% in 8.0MIU |
| MSCRG,8 1996 (2 years) |
● Time to sustained disability progression | ● Exacerbations● MRI | 6.0 MIU IFNB-1a IM weekly vs placebo |
● Delay in time to sustained EDSS progression (34.9% in placebo vs 21.9% with progression at 104 weeks)● Decreased number of exacerbations and annual relapse rates (0.90 in placebo and 0.61 in IFN)● Decreased number and volume gadolinium enhancement | ● Mild anemia● Flu-like illness● Muscle aches● Chills● Fever● Asthenia● Neutralizing antibodies: 4% in placebo, 22% in IFN |
| PRISMS,9 1998 (2 years) |
● Annualized relapse rate | ● Times to first and second relapse● Proportion of relapse-free patients● Progression in disability● MRI activity | IFNB-1a 22µg or 44 µg SC three times weekly vs placebo | ● Decreased relapse rates in both treatment groups (2.56 in placebo, 1.82 in 22µg and 1.73 in 44 µg)● Median time to first relapse delayed by 3 and 5 months in 22µg and 44µg, respectively● Longer time to sustained progression● Decreased T2 and gadolinium-enhancing lesions in both treatment groups | ● Leukopenia● Elevated liver enzymes● Depression● Injection-site reactions● Flu-like illness● Neutralizing antibodies: 23.8% in 22µg and 12.5% in 44µg |
| ADVANCE,10 2014 (48 weeks) | ● Annualized relapse rates | ● Number of new or enlarging T2 lesions● Proportion of patients who relapsed● Proportion of patients with disability progression | Peginterferon beta-1a 125µg every 2 weeks or every 4 weeks, vs placebo | ● Decreased annualized relapse rates (0.397 in placebo, 0.256 in every 2-week group, 0.288 in every 4-week group)● Decreased proportion of patients who relapsed (0.291 in placebo, 0.187 in every 2-week group, and 0.222 in every 4-week group)● Decreased sustained disability (0.105 in placebo and 0.068 in both treatment groups)● Decreased number and volume of T2 lesions | ● Injection site reactions● Flu-like illness● Fever● Headache● |
The IFNβ-1b multiple sclerosis study group published the results of their multicenter, randomized, double-blind, placebo-controlled trial in 1993, based on two identical trials from 1988 to 1990. In this trial, placebo was compared to two different doses of IFNβ-1b (1.6 million international units (MIU) and 8.0 MIU), both administered by subcutaneous injections every other day. The primary endpoints of this trial were differences in the rates of exacerbations and the proportion of patients remaining exacerbation free. Results showed dose-dependent outcomes in significantly reducing the annual rate of exacerbation for patients receiving intermediate-dose and high-dose IFNβ-1b compared to placebo at 2 years (1.14 vs 0.84 vs 1.27, respectively). Additionally, there were significantly more patients who were relapse-free at 2 years in the high-dose IFNβ-1b groups compared to placebo (36 vs 18, respectively), though this was no longer significant at 3 years. Secondary outcomes demonstrated a significant prolongation of time to exacerbation in the group receiving high-dose IFNβ-1b compared to placebo, and significantly fewer hospitalizations and hospital days in the IFNβ-1b groups.2 The MRI data was also favorable in the IFNβ-1b groups compared to placebo in terms of T2 lesion load: at the end of the first year, the lesion load compared to baseline increased by 12.25% in the placebo group, increased by 4.1% in the intermediate dose group and decreased by 1.1% in the high-dose group.2 There was no significant change in the mean Expanded Disability Status Scale (EDSS) score in any of the arms at 3 years, though all three arms increased from baseline.
Adverse events included abnormal laboratory tests (elevated liver enzymes, neutropenia, anemia, thrombocytopenia), injection site reactions, and flu-like syndrome (fever, chills, myalgias, sweating). Mild, transient lymphopenia was the most common laboratory abnormality (65% of placebo patients, 76% in the intermediate dose group, and 80% in the high-dose group). Additionally, neutralizing antibodies were found in 47% of the intermediate dose and in 45% of the high-dose patients; however, there did not appear to be a relationship with exacerbation severity or time between exacerbations.
In 1996, the Multiple Sclerosis Collaborative Research Group published their study regarding the efficacy of intramuscular IFNβ-1a in decreasing disability progression. This study was a multi-center, double-blind, placebo-controlled trial, with 6.0MIU administered intramuscularly weekly.8 This trial’s primary endpoint evaluated time to sustained disability progression (at least 1.0 increase in baseline EDSS for at least 6 months) and showed a decrease in disability progression in the treated group compared to the placebo group (at 104 weeks 33.3% of placebo recipients had progression of disability vs 21.2% of IFNβ-1a recipients), and a significantly longer time to sustained disability progression in the treatment group. Additionally, the IFNβ-1a group had significantly fewer exacerbations (annual relapse rate 0.82 in the placebo group vs 0.67 in the treatment group), and a longer time to first exacerbation (47.3 weeks vs 36.1 weeks). Fewer gadolinium enhancing lesions were seen in the IFNβ-1a group and a larger within-person percent change in T2 lesions volume at 2 years (−6.5% in placebo group vs −13.2% in interferon group). They also found a significantly greater change in the EDSS scores in the placebo group compared to the treatment group.
Adverse events of the interferon in this trial were similar to the previous trial including flu-like symptoms, injection site reactions, and mild anemia. Depression was seen in both groups equally. Neutralizing antibodies were seen in 22% of the interferon recipients at week 104.
The PRISMS trial was published in 1998 to evaluate the efficacy of subcutaneous IFNβ-1a. This study looked at 2 different doses (22μg and 44μg) of IFNβ-1a injected three times weekly compared to placebo.9 This study showed a 27% and 33% risk reduction for the two doses of IFNβ-1a, respectively. It also showed a delayed median time to first relapse by 3 and 5 months, respectively. Additionally, the time to sustained progression was significantly longer in both IFNβ-1a groups compared to placebo, and there was a significant decrease in T2 MRI disease burden (increase of 10.9% in placebo group compared to decreases by 1.2% and 3.8% in the two treatment groups, respectively). Adverse events in the IFNβ-1a group included flu-like symptoms, injection site reactions, lymphopenia, and elevated liver enzymes. Depression was again seen in the placebo and treatment groups equally. Neutralizing antibodies were seen in 23.8% of the low-dose recipients and in 12.5% of the high-dose recipients and did not affect the mean relapse count. Overall, the reduction of relapse rates in this study were consistent with the first IFNβ-1b study and slightly favorable to the IFNβ-1a intramuscular study.
The ADVANCE trial was published in 2014, looking at the safety and efficacy of pegylated IFNβ-1a (PEG IFNβ-1a) injected subcutaneously every 2 or 4 weeks compared to placebo. A polyethylene glycol (PEG) side chain was attached to the interferon molecule in order to prolong its half-life and increase systemic exposure without changing its biological properties.10 They found that adjusted annualized relapse rates at 48 weeks were significantly lower compared to placebo (0.397 in placebo vs 0.256 in the every 2-week group vs 0.288 in the every 4-week group). The trial also showed a significantly reduced risk of progression on disability compared to placebo, and fewer new or enlarging T2 lesions on MRI. Overall, every 2-week dosing was superior to every 4-week dosing, which was confirmed when the results of the 2-year study were published in 2015.11 Adverse events were similar to previous interferon studies including flu-like symptoms, injection site reactions and lab abnormalities included elevated liver enzymes. A 2-year extension study (ATTAIN) confirmed the long-term efficacy and safety of the medication.12 Less frequent injections with PEG IFNβ-1a was an important step in the advancement of DMT in terms of patient satisfaction and compliance.
Trials Looking at Time to Clinically Definite MS After First Event
Jacobs et al13 published the results of their CHAMPS trial in 2000 regarding the development of clinically definite MS after a first demyelinating event with MRI evidence of prior demyelination on weekly intramuscular IFNβ −1a therapy vs placebo. The results of this trial showed that there was a significantly lower probability of developing clinically definite MS in the interferon group during the 3-year study period (rate ratio of 0.49 when adjusted for age, type of event, and MRI characteristics). The interferon group also had favorable MRI outcomes.
Another study published in 2001 by the Early Treatment of Multiple Sclerosis study group looking at the effects of low-dose subcutaneous IFNβ −1a on the occurrence of relapses after a first neurologic event with a brain MRI suggestive of MS.14 The results showed that 34% of the participants in the interferon group vs 45% of the participants in the placebo group converted to clinically definite MS at 2 years and that the time to 30% of the patients converting to clinically definite MS was 569 days in the interferon group vs 252 days in the placebo group. Additionally, the annual relapse rate was lower, and the MRI characteristics were favorable in the interferon group. However, only 16% of the patients in the placebo group and 6% in the interferon group were free of accumulating new lesions on MRI in the 2-year period.
Pivotal Trials in Progressive MS
The European study group on IFNβ-1b in secondary progressive MS published their multi-center, double-blinded, randomized, placebo-controlled study in 1998, evaluating the role of subcutaneous IFNβ-1b every other day on disability progression in SPMS patients (EDSS 3.0–6.5).7 The primary outcome was progression of disability, measured by a 1.0 increase on the EDSS sustained for at least 3 months after all patients had been in the study for at least 2 years. Superimposed relapses were allowed in the study and were treated with IV steroids for 3 days. They found a significant difference in the time to confirmed progression of disability, in favor of the IFNβ-1b group, with progression delayed for 9–12 months during the study period, and a 30% reduction in annual relapse rate. Additionally, there was a 32.1% reduction in the proportion of patients becoming wheelchair-bound in the IFNβ-1b group during the study period. There were also significant effects on the MRI lesion volume in favor of the IFNβ-1b group. Adverse effects were similar to those seen in the previous interferon studies.
The secondary progressive efficacy clinical trial of recombinant IFNβ-1a in MS study group (SPECTRIMS) published the results of their multi-center, randomized, placebo-controlled study in 2001.15 They looked at two different doses of subcutaneous IFNβ-1a injected three times weekly (250μg dose and a body surface area adjusted dose of 160μg [5 MIU]/m2). Their findings showed no significant difference in time to sustained disability between the placebo and low-dose IFNβ-1a group, however, when broken down by sex, women showed a delay in progression at both doses while men did not. There was a significant benefit on the rate of exacerbations in both IFNβ-1a groups compared to placebo. Additionally, patients with pre-study relapses did better on IFNβ-1a compared to those without pre-study relapses. Side effects were similar to those seen in previous trials, including depression and suicide attempts in both groups.
A study published in 2003 looked at weekly intramuscular IFNβ-1a at two doses compared to placebo in patients with primary progressive MS. This study did not show any significant effect on the primary endpoint of time to sustained progression in disability, but did show a lower rate of MRI lesion accumulation in the lower dose group compared to placebo.16
With the overall mixed results in these progressive trials, it could not be clearly stated that interferons were effective in SPMS, as they were in RRMS. The difference in results was suggested to be due to the shorter length of the European study, with patients that were younger, with a shorter disease duration, more contrast-enhancing lesions, and the higher rate of pre-study relapses in that trial suggesting a more inflammatory disease process.15,17 All of these studies demonstrated more of a benefit of treatment in the subgroup with more active disease, suggesting that patients with more inflammatory diseases were more likely to benefit from IFNβ therapy.17
Long-Term Experience with Interferon Therapy
As Bayas and Gold17 point out in their article summarizing 10 years of experience with interferon in 2003, the published data at that time demonstrated that IFNβ-1b was an effective treatment for patients with RRMS and SPMS with inflammatory components. The IFNβ treatment effect was dose and frequency-dependent and early treatment was beneficial. A recent systemic review by Melendez-Torres et al confirms the benefits of IFNβ therapy demonstrating comparative effectiveness on relapses and disability, and adherence rates when compared to glatiramer acetate.18 While interferon therapy was considered overall a safe treatment, patient support was needed to ensure compliance.17 Sabido-Espin and Munschauer evaluated discontinuation rates using Market Scan Commercial and Medicare Supplemental healthcare claims databases, which revealed that in a cohort of 5956 patients treated with IFNβ, 36.9% discontinued at 1 year, and 55.8% discontinued by 3 years.19 Mitigating adverse effects was considered especially important to foster patient compliance including thoroughly counseling patients on the benefit vs risk of the therapy, expected adverse effects, and that adverse effects tend to subside with longer duration of therapy. Additionally, the use of the “two-needle technique,” an automated injection device, helped to decrease injection site reactions.17 IFNβ therapy, along with glatiramer acetate, remained the mainstay of DMTs for over 15 years until the first oral DMTs were approved.
Changing Treatment Landscape
The Oral DMTs
In 2010 the first Phase 2 and Phase 3 studies of oral fingolimod were published, leading to the first FDA-approved oral DMT therapy for RRMS.20 While these trials were placebo-controlled, the TRANFORMS trial published in 2010 compared oral fingolimod to intramuscular IFNβ −1a.21 This study showed a significantly lower annualized relapse rate and improved MRI outcomes with fingolimod compared to IFNβ −1a. Shortly thereafter, two randomized, double-blind, placebo-controlled phase 3 studies, TOWER22 and TEMSO,23 led to the approval of teriflunomide in 2012, and DEFINE24 and CONFIRM25 looking at the safety and efficacy of dimethyl fumarate were published leading to its approval in 2013.24,26 Further, oral DMTs have since been approved including cladribine,27 siponimod,28 ozanimod,29 and diroximel fumarate.30 These trials and subsequent FDA approvals started the shift in clinical practice to using oral medications as first-line treatment. Patients had improved compliance with pills over injections, as well as superior efficacy, as demonstrated in real-world studies.31–34
Additionally, when there were no approved DMTs for pediatric use prior to 2018, many pediatric onset MS patients were started on IFNβ because of the safety history and extensive experience in adult patients. Since the PARADIGMS35 study was published showing fingolimod’s superior efficacy to intramuscular IFNβ −1a, and the medication’s subsequent FDA approval for use in pediatric patients, the practice has also been shifting away from starting IFNβ therapy in this younger group.
Early Highly Effective Therapy
There has been a recent shift in the MS field to use the highly effective, infusion-based monoclonal antibody treatments such as natalizumab, rituximab, and ocrelizumab as first-line treatments, and accordingly, fewer and fewer newly diagnosed patients are started on IFNβ therapy. This is especially true in patients that appear to have highly inflammatory disease. There is increasing evidence to support a critical time window where treatment has a greater impact on long-term disability which may be missed in a traditional escalation approach.36 Harding et al37 reported on a real-world study comparing outcomes on those who received early intensive treatment (EIT) with monoclonal antibody treatments (n=104) vs those who received injectables or oral therapies (escalation group, n=488). They found that even though patients who received EIT have more active disease, this group had better long-term outcomes compared to those in the escalation group. A recent real-world multi-center study of 741 pediatric patients also revealed better outcomes when using newer oral or monoclonal antibody treatment compared to injectables.38 There are two concurrent randomized, multi-centered trials (TREAT-MS (NCT03500328) and DELIVER-MS (NCT03535298)) that are actively enrolling patients to either escalation therapy vs early highly effective therapy looking at safety, efficacy, and cost-effectiveness.
Current Role for Interferon Use
Disease Stability
One of the main reasons for persistence use of IFNβ despite increased availability of treatment options is patients who had started IFNβ therapy before the approval of newer options and continue to do well in terms of clinical and MRI stability, and side effects profile. Patients should be continually monitored for disease activity, tolerability and compliance, and the topic of switching therapies should be considered if concerns arise. When both the patient and treating neurologist are comfortable with continuing IFNβ therapy then there is no compelling indication to switch or escalate therapy.
Adverse Events or Intolerability on Other DMTs
Some patients may experience adverse events or are unable to tolerate non-interferon therapy. Common adverse events with many DMTs including oral and infusion therapies are laboratory abnormalities including elevated liver enzymes and leukopenia. While these abnormalities can be seen with IFNβ therapy, they tend to be asymptomatic, transient, and are rarely clinically significant.39,40
Though the field is transitioning towards using early, highly effective therapy, there are limitations to the use of these agents, and it is not clear for how long patients can remain on these therapies. The use of natalizumab is limited by JC virus status, and if a patient converts to a positive titer, natalizumab should be switched to an alternate DMT due to the risk of progressive multifocal leukoencephalopathy. Additionally, there may be limitations to the long-term use of B-cell therapy, such as decreased immunoglobulins and increased incidence of infections.41,42
IFNβ is an important option for patients who are very risk-averse, given the favorable safety profile and lengthy experience with this class of medication in the field.
De-Escalation
While the field is trending towards early highly effective therapy, it is not clear how long these patients need to remain on this type of therapy, and if or when the topic of treatment de-escalation should be considered. After a period of disease stability, it is not clear if the benefits of continuing these highly effective therapies outweigh the potential risks such as infections. This de-escalation concept is used in oncology and rheumatology where potent treatments are used initially, and then patients are maintained on safer therapies long term.43,44
A small study by Rieckmann et al45 looked at patients who had previously been on mitoxantrone therapy and had stable disease activity (relapse free for at least 6 months and no confirmed disability progression for at least 9 months prior to screening) and were subsequently put on either IFNβ-1a or no treatment. While the IFNβ-1a group had more patients who were relapse-free at 96 weeks, due to the small sample size there were no significant results.
Some evidence suggests that IFNβ therapy may be a superior option to teriflunomide when considering a lower-efficacy treatment. A recent study by Newsome et al46 performed a matching-adjusted comparison of patient data from the ADVANCE and ATTAIN trials evaluating PEG IFNβ-1a to the TEMSO and TOWER studies evaluating teriflunomide. The results showed that the proportion of patients with confirmed disability worsening at 108 weeks was significantly lower in the PEG IFNβ-1a group than the teriflunomide group, and that patients treated with PEG IFNβ-1a had lower annualized relapse rates at 108 weeks and 5 years compared to teriflunomide.46 While these outcomes have been supported by other meta-analyses, there have been no randomized, head-to-head trial proving this.
More studies evaluating de-escalation are needed to guide clinical practice; however, with their long-term safety record, interferons may be a good option for de-escalation once patients have transitioned from the highly inflammatory phase to the progressive stages of the disease where relapses are reduced in frequency, and the patient wishes to remain on therapy.
The Role of Interferons in Clinical Trials
One of the more common uses of interferons currently is as an active comparator in clinical trials for novel DMTs. While placebo-controlled trials are now considered unethical due to the plethora of effective medications available, interferons are commonly used as an active comparator under the umbrella of clinical equipoise. Trials including SUNBEAM29 and RADIANCE29 evaluating ozanimod, TENERE47 evaluating teriflunomide (no difference found in a primary composite endpoint), TRANSFORMS21 and PARADIGMS35 evaluating fingolimod in adults and children, respectively, OPERA I/II1 and OPERETTA (NCT01412333) evaluating ocrelizumab in adults and children, respectively, and CARE-MS I/II48,49 evaluating alemtuzumab have used or are using IFNβ therapy as a comparator for subsequent FDA approval.
Discussion
The advent of IFNβ therapy for the treatment of multiple sclerosis was revolutionary and changed the course of disease for a multitude of patients and their families. The study and development of IFNβ therapy has also been crucial to the further understanding of the pathogenesis of multiple sclerosis and the development of novel DMTs. The initial trials showed that early intervention is important for long-term outcomes and that groups with more active and inflammatory disease respond better to therapy. These points have been re-demonstrated in many subsequent clinical trials and have helped shape the use of DMTs in the field. The most recent 2017 revision to the McDonald Criteria allows for earlier diagnosis of MS and subsequently earlier treatment, because of these studies showing the importance of early treatment.50
Since the development of oral and infusion DMTs, fewer newly diagnosed patients are started on IFNβ due to increased efficacy, better patient satisfaction, and improved compliance with the newer DMTs. In the current treatment landscape with 18 choices for DMT, there is still a role for IFNβ in both clinical practice and in clinical research. There will likely always be a subset of patients who cannot tolerate oral or infusion DMTs due to adverse events, and IFNβ therapy will remain an option for this group. Additionally, patients who are highly risk-averse and want to proceed with the safest option will be comforted by the long history of favorable safety outcomes with IFNβ therapy. Another important role of IFNβ has been in the field of clinical trials. Because it is generally considered unethical to design a placebo-controlled trial for active MS, IFNβ has been critical in its role of an active comparator for novel DMTs to show efficacy for FDA approval.
IFNβ has played an important role in the treatment of multiple sclerosis since their development, and their role will likely remain important and will continually evolve as the field evolves.
Disclosure
CG has no conflicts of interest to disclose regarding this work. LHH has received speaking and consulting fees from Biogen, Genzyme, Genentech, Novartis, Bristol Myers Squibb, and EMD Serono.
References
- 1.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 2.IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB multiple sclerosis study group. Neurology. 1993;43(4):655–661. doi: 10.1212/WNL.43.4.655 [DOI] [PubMed] [Google Scholar]
- 3.Maimaitijiang G, Watanabe M, Shinoda K, et al. Long-term use of interferon-beta in multiple sclerosis increases Vdelta1(-)Vdelta2(-)Vgamma9(-) gammadelta T cells that are associated with a better outcome. J Neuroinflammation. 2019;16(1):179. doi: 10.1186/s12974-019-1574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayatollahi SA, Ghafouri-Fard S, Taheri M, Noroozi R. The efficacy of interferon-beta therapy in multiple sclerosis patients: investigation of the RORA gene as a predictive biomarker. Pharmacogenomics J. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Furber KL, Van Agten M, Evans C, Haddadi A, Doucette JR, Nazarali AJ. Advances in the treatment of relapsing-remitting multiple sclerosis: the role of pegylated interferon beta-1a. Degener Neurol Neuromuscul Dis. 2017;7:47–60. doi: 10.2147/DNND.S71986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disease Modifying Therapies for MS. Society TNMS. ed. nationalmssociety.org; 2020: 5–12. [Google Scholar]
- 7.Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491–1497. doi: 10.1016/S0140-6736(98)10039-9 [DOI] [PubMed] [Google Scholar]
- 8.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–294. doi: 10.1002/ana.410390304 [DOI] [PubMed] [Google Scholar]
- 9.Ebers GC. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. doi: 10.1016/S0140-6736(98)03334-0 [DOI] [PubMed] [Google Scholar]
- 10.Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13(7):657–665. doi: 10.1016/S1474-4422(14)70068-7 [DOI] [PubMed] [Google Scholar]
- 11.Kieseier BC, Arnold DL, Balcer LJ, et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler. 2015;21(8):1025–1035. doi: 10.1177/1352458514557986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newsome SD, Scott TF, Arnold DL, et al. Long-term outcomes of peginterferon beta-1a in multiple sclerosis: results from the ADVANCE extension study, ATTAIN. Ther Adv Neurol Disord. 2018;11:1756286418791143. doi: 10.1177/1756286418791143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS study group. N Engl J Med. 2000;343(13):898–904. doi: 10.1056/NEJM200009283431301 [DOI] [PubMed] [Google Scholar]
- 14.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357(9268):1576–1582. doi: 10.1016/S0140-6736(00)04725-5 [DOI] [PubMed] [Google Scholar]
- 15.Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MSSG. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56(11):1496–1504. doi: 10.1212/WNL.56.11.1496 [DOI] [PubMed] [Google Scholar]
- 16.Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology. 2003;60(1):44–51. doi: 10.1212/WNL.60.1.44 [DOI] [PubMed] [Google Scholar]
- 17.Bayas A, Gold R. Lessons from 10 years of interferon beta-1b (betaferon/betaseron) treatment. J Neurol. 2003;250(Suppl 4):IV3–8. doi: 10.1007/s00415-003-1402-8 [DOI] [PubMed] [Google Scholar]
- 18.Melendez-Torres GJ, Armoiry X, Court R, et al. Comparative effectiveness of beta-interferons and glatiramer acetate for relapsing-remitting multiple sclerosis: systematic review and network meta-analysis of trials including recommended dosages. BMC Neurol. 2018;18(1):162. doi: 10.1186/s12883-018-1162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabido-Espin M, Munschauer R. Reasons for discontinuation of subcutaneous interferon beta-1a three times a week among patients with multiple sclerosis: a real-world cohort study. BMC Neurol. 2017;17(1):57. doi: 10.1186/s12883-017-0831-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 21.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–256. doi: 10.1016/S1474-4422(13)70308-9 [DOI] [PubMed] [Google Scholar]
- 23.O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303. doi: 10.1056/NEJMoa1014656 [DOI] [PubMed] [Google Scholar]
- 24.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–1107. doi: 10.1056/NEJMoa1114287 [DOI] [PubMed] [Google Scholar]
- 25.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–1097. doi: 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 26.Havrdova E, Hutchinson M, Kurukulasuriya NC, et al. Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of: gold R, Kappos L, Arnold D, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367:1098-107;and Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97. Expert Opin Pharmacother. 2013;14(15):2145–2156. doi: 10.1517/14656566.2013.826190 [DOI] [PubMed] [Google Scholar]
- 27.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 28.Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. doi: 10.1016/S0140-6736(18)30475-6 [DOI] [PubMed] [Google Scholar]
- 29.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–1020. doi: 10.1016/S1474-4422(19)30239-X [DOI] [PubMed] [Google Scholar]
- 30.Naismith RT, Wolinsky JS, Wundes A, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2019;1352458519881761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergvall N, Makin C, Lahoz R, et al. Comparative effectiveness of fingolimod versus interferons or glatiramer acetate for relapse rates in multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin. 2013;29(12):1647–1656. doi: 10.1185/03007995.2013.847411 [DOI] [PubMed] [Google Scholar]
- 32.Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther. 2017;6(1):91–102. doi: 10.1007/s40120-017-0064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braune S, Grimm S, van Hovell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol. 2018;265(12):2980–2992. doi: 10.1007/s00415-018-9083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320–327. doi: 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379(11):1017–1027. doi: 10.1056/NEJMoa1800149 [DOI] [PubMed] [Google Scholar]
- 36.Ontaneda D, Tallantyre E, Kalincik T, Planchon SM, Evangelou N. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol. 2019;18(10):973–980. doi: 10.1016/S1474-4422(19)30151-6 [DOI] [PubMed] [Google Scholar]
- 37.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536–541. doi: 10.1001/jamaneurol.2018.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krysko KM, Graves JS, Rensel M, et al. Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020. doi: 10.1002/ana.25737 [DOI] [PubMed] [Google Scholar]
- 39.Francis GS, Grumser Y, Alteri E, et al. Hepatic reactions during treatment of multiple sclerosis with interferon-beta-1a: incidence and clinical significance. Drug Saf. 2003;26(11):815–827. doi: 10.2165/00002018-200326110-00006 [DOI] [PubMed] [Google Scholar]
- 40.Gold R, Rieckmann P, Chang P, Abdalla J, Group PS. The long-term safety and tolerability of high-dose interferon beta-1a in relapsing-remitting multiple sclerosis: 4-year data from the PRISMS study. Eur J Neurol. 2005;12(8):649–656. doi: 10.1111/j.1468-1331.2005.01083.x [DOI] [PubMed] [Google Scholar]
- 41.Marcinno A, Marnetto F, Valentino P, et al. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e498. doi: 10.1212/NXI.0000000000000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tallantyre EC, Whittam DH, Jolles S, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. 2018;265(5):1115–1122. doi: 10.1007/s00415-018-8812-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glinatsi D, Heiberg MS, Rudin A, et al. Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, Phase 4 study. Trials. 2017;18(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel RR, Ludmir EB, Augustyn A, et al. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: a systematic review of prospective trials. Oral Oncol. 2020;103:104608. doi: 10.1016/j.oraloncology.2020.104608 [DOI] [PubMed] [Google Scholar]
- 45.Rieckmann P, Heidenreich F, Sailer M, et al. Treatment de-escalation after mitoxantrone therapy: results of a Phase IV, multicentre, open-label, randomized study of subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. Ther Adv Neurol Disord. 2012;5(1):3–12. doi: 10.1177/1756285611428503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newsome SD, Mokliatchouk O, Castrillo-Viguera C, Naylor ML. Matching-adjusted comparisons demonstrate better clinical outcomes in patients with relapsing multiple sclerosis treated with peginterferon beta-1a than with teriflunomide. Mult Scler Relat Disord. 2020;40:101954. doi: 10.1016/j.msard.2020.101954 [DOI] [PubMed] [Google Scholar]
- 47.Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20(6):705–716. doi: 10.1177/1352458513507821 [DOI] [PubMed] [Google Scholar]
- 48.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. doi: 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 49.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828. doi: 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 50.Gaetani L, Prosperini L, Mancini A, et al. 2017 revisions of McDonald criteria shorten the time to diagnosis of multiple sclerosis in clinically isolated syndromes. J Neurol. 2018;265(11):2684–2687. doi: 10.1007/s00415-018-9048-8 [DOI] [PubMed] [Google Scholar]