Abstract
Untreated progressive familial intrahepatic cholestasis (PFIC) type 2, or bile salt exporter protein deficiency, frequently leads to severe pruritus, impaired growth and progressive liver fibrosis with risk of organ failure. We describe a 15-month-old male patient with severe pruritus diagnosed with PFIC type 2 enrolled in an open-label phase 2 study who received 4 weeks of treatment with odevixibat, an ileal bile acid transporter inhibitor under development for cholestatic liver disease treatment. The patient experienced reductions in serum bile acids and improvement in itching and sleep scores, and odevixibat was well tolerated. After the odevixibat study, symptoms returned and the patient underwent partial external biliary diversion (PEBD). Odevixibat treatment and PEBD produced similar normalisation of serum bile acid levels and improvements in pruritus and sleep disruptions. Thus, odevixibat appeared to be as effective as invasive PEBD in treating serum bile acids and cholestatic pruritus in this patient.
Keywords: liver disease, paediatrics, congenital disorders, paediatric surgery, gastrointestinal system
Background
Progressive familial intrahepatic cholestasis (PFIC) type 2, or bile salt exporter protein (BSEP) deficiency, describes a rare liver disorder with onset in childhood characterised by severe cholestasis and low or normal gamma-glutamyltransferase (GGT) activity. The accumulation of hepatic bile acids leads to inflammation of the liver, and ultimately to liver fibrosis and failure. Furthermore, the retained bile acids can spill over into systemic circulation, resulting in abnormally high serum bile acid levels. PFIC diseases are believed to be responsible for 10%%–15% of paediatric cholestasis cases and of paediatric liver transplantations.1–4 The most frequent early symptoms include jaundice as well as severe pruritus and associated sleep disruption, leading to substantial reductions in health-related quality of life.1 3 4
Partial external biliary diversion (PEBD) is a surgical option for PFIC treatment that involves creation of a jejunal conduit between the gallbladder and an external abdominal stoma; PEBD has been shown to improve pruritus, liver function, liver histology and growth in patients with PFIC.5–8 However, risks associated with PEBD include post-surgical dehydration, electrolyte imbalances, surgical complications and presence of a lifelong stoma substantially affecting the patient’s quality of life.9 Patients not willing to receive an ostomy can be offered a partial internal biliary diversion (PIBD) which is often complicated by intermittent diarrhoea due to high concentration of bile salts in the colon.10
In general, surgical procedures like PEBD and PIBD were developed to interrupt the enterohepatic circulation and divert bile acids away from the liver.11 12 This reduction in bile acid load appears to reduce PFIC symptom severity and at least partially restore hepatic function,5 and recent reports indicate that reduced serum bile acids following surgical biliary diversion correlate with improved outcomes. For example, using retrospective data, the NAtural course and Prognosis of PFIC and Effect of biliary Diversion consortium found that for patients with BSEP-deficient PFIC who underwent surgical biliary diversion (n=61, which was most commonly PEBD in 47), pruritus was common prior to surgery (occurring in 97%) and occurred in fewer patients (46%) after surgery. This change corresponded with a reduction in mean serum bile acids (from 363 μmol/L to 48 μmol/L, respectively).13 Overall, a greater proportion of patients with lower serum bile acids after surgery survived with their native liver intact for up to 15 years versus those who had elevated serum bile acids post-surgery.13 In addition, a systematic review and meta-analysis evaluating studies with pre-PEBD and post-PEBD liver biochemistry values found that patients with PFIC with reduced serum bile acids post-PEBD were more likely to have favourable clinical responses such as improved pruritus and decreased need for liver transplant.14 Thus, the reduction in bile acids and improvement in clinical outcomes observed with PEBD supports the idea that disrupting the enterohepatic circulation is a workable option for treating patients with cholestatic liver disease. Ileal bile acid transporter (IBAT) inhibition is a targeted pharmacologic approach based on the same premise: inhibition of IBAT blocks intestinal resorption of bile acids, which interrupts the enterohepatic circulation and redirects bile acids away from the liver.15
Odevixibat (A4250) is a highly potent, orally administered, selective, reversible IBAT inhibitor currently under development for PFIC treatment.15 In an exploratory, open-label phase 2 study in children with PFIC, odevixibat treatment was well tolerated and associated with reduced pruritus, improved sleep and reduced serum bile acids.16 In this case report, we compare the effects of IBAT inhibition with PEBD surgery in a single patient with PFIC who received odevixibat as a participant in that phase 2 study followed by PEBD after the study ended and odevixibat was no longer available.
Case presentation
A male patient initially presented at age 7 months with therapy-resistant pruritus, cholestasis, steatorrhoea and low GGT activity; he was subsequently diagnosed with PFIC type 2 resulting from a variant in ABCB11, the gene encoding BSEP,17 with two heterozygotic exogenous changes compared to reference sequence NM_003742.2 (BSEP; in exon 23 c.3149C>T(p.R1050C) and in exon 23 c.3084A>G(p.A1028A; rs49769)) and a homozygotic exogenous change (in exon 12 c.1331T>C(p.V444A, rs2287622)). Because expression of BSEP was detected immunohistochemically, the gene variant most likely results in translation of a functionally deficient BSEP protein. The patient had failed conventional therapies (including ursodeoxycholic acid, colestyramine, rifampicin, naltrexone and phenobarbital), and at age 15 months was screened for, enrolled in and completed an open-label phase 2 study of odevixibat (ClinicalTrials.gov identifier: NCT02630875).
Treatment
At enrolment, the patient was 75 cm tall and weighed 9.0 kg. During the study, the patient received a daily dose of odevixibat 200 µg/kg for 4 weeks, along with concurrent rifampicin 40 mg two times per day, ursodeoxycholic acid 100 mg two times per day and naltrexone 2 mg two times per day (during the single-dose period of the study only). Although the patient responded well during the study, severe pruritus returned after the odevixibat trial ended, and the patient underwent PEBD about 3 months after the final odevixibat dose.
Outcome and follow-up
Patient response to odevixibat and PEBD
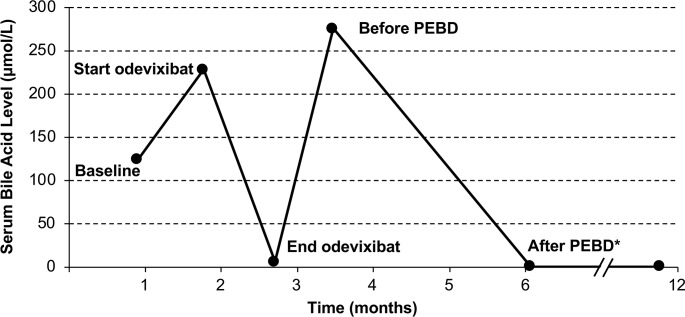
The sequential use of medical and surgical interventions for treatment of PFIC in the same patient permitted the comparative assessment of response to each treatment modality. At baseline of the phase 2 study, the patient’s total serum bile acid level was 124.3 µmol/mL which fell by 95% to 6.5 µmol/mL after 4 weeks of odevixibat treatment. Immediately preceding PEBD, the total serum bile acid level was 276 µmol/mL which fell to <1 µmol/mL following PEBD (figure 1).
Figure 1.
Bile acid levels over time in a patient with progressive familial intrahepatic cholestasis (bile salt exporter protein deficiency) who received treatment with the ileal bile acid transporter inhibitor odevixibat and subsequently underwent partial external biliary diversion (PEBD) surgery. *Values after PEBD surgery were <1.0 μmol/L.
Pruritus and sleep disruptions demonstrated similar patterns. During the odevixibat study, patient diary data showed reductions in visual analogue scale itch severity (VAS-Itch; 0–10 scale)18 scores of 5 points with odevixibat treatment (from 8 to 3 points). Pruritus improvements were also documented during the clinical study on the 4-point Whitington-itch scale5 (reduction from 2 to 1) and in Partial Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD19; 0‒10 scale) itch score (reduction from 8 to 3). A similar improvement (from 8 to 3) was observed in PO-SCORAD sleep disturbance score during odevixibat treatment. Following PEBD, VAS-Itch scores were reduced by six points (from eight points before PEBD to two points after surgery). In addition, sleep improvements were observed after PEBD that were qualitatively similar to those observed with odevixibat treatment.
Following PEBD surgery, the patient now continues on ursodeoxycholic acid 200 mg/day, supplemented with vitamin D 500 IU/day; recurrent infection-triggered pruritus has also required transient treatment with colestyramine.
Odevixibat safety
Throughout the odevixibat clinical study, the patient demonstrated elevations in eosinophils and neutrophils; however, only the values at screening (8.9% and 12.1%, respectively) were considered clinically significant by study investigators. The patient experienced two adverse events of mild intensity (nasopharyngitis and vomiting); neither was considered related to odevixibat or to laboratory abnormalities. Stool consistency and frequency did not change from baseline during treatment with odevixibat or following PEBD. Liver function tests other than serum bile acids were normal before and after both odevixibat treatment and PEBD except for mild and transient elevations during the post-operative phase and during viral upper airway infections.
Discussion
IBAT inhibition represents a pharmacologic alternative to surgical bile acid reduction by interrupting the highly efficient recovery of bile acids (estimated at >95%) from the distal colon and return to the hepatobiliary system via the enterohepatic circulation (figure 2).15 20 Because IBAT is localised principally on the brush border of ileal cells (facing the lumen of the gastrointestinal tract),21 IBAT inhibition using odevixibat could potentially be effected with minimal systemic exposure.15 Because patients with PFIC may require lifelong treatment with an IBAT inhibitor to maintain reductions in bile acid levels, it is important to consider the compound’s long-term safety effects. The low systemic exposure of odevixibat, along with the overall safety profile of odevixibat in the phase 2 trial, suggest that odevixibat is a safe compound for the long-term treatment of cholestatic liver disease. However, additional long-term studies (ranging from 6 months to 2 years) are ongoing or enrolling participants to assess the long-term effects, including safety, of IBAT inhibitors in paediatric cholestatic disease (ClinicalTrials.gov identifiers: NCT03566238, NCT03659916, NCT03905330, NCT04185363 and NCT04168385). The current surgical alternative, PEBD, has similar restrictions (ie, follow-up required to monitor potential post-surgical complications) and lifelong consequences (ie, ostomy).22
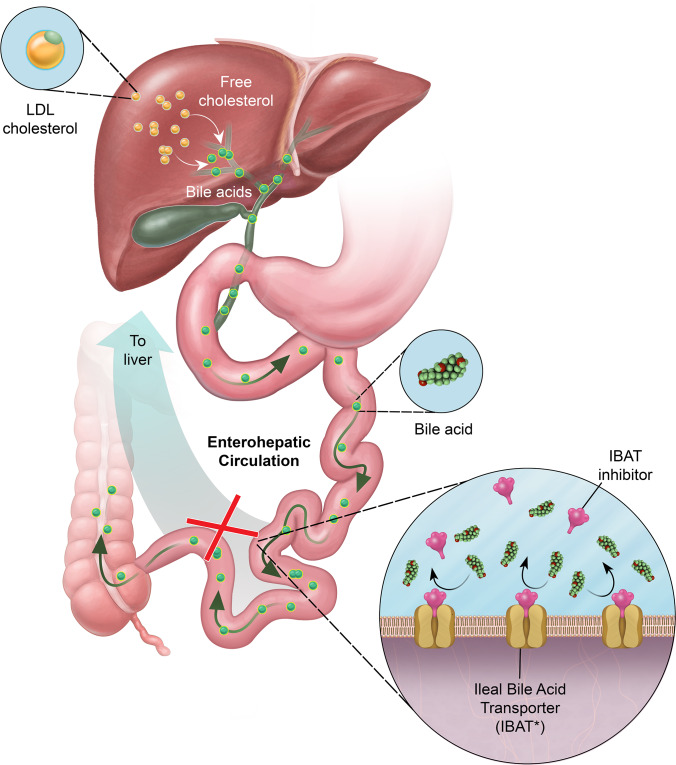
Figure 2.
Schematic illustration of the proposed mechanism of action of odevixibat (A4250). Bile acids are synthesised in the liver from LDL-derived cholesterol and secreted into the small intestine. Approximately 95% of secreted bile acids are transported into ileal cells by IBAT and recirculated back to the liver via the enterohepatic circulation. In cholestatic liver disease, bile acids accumulate in the liver, leading to clinically significant sequelae. Inhibition of IBAT by odevixibat is believed to lead to reduced circulation of bile acids back to the liver and thus might help to normalise the elevated bile acid levels observed in cholestatic liver disease. IBAT, ileal bile acid transporter; LDL, low-density lipoprotein. *Also known as apical sodium–dependent bile acid transporter. This illustration was professionally drawn for this case report and we hold the ownership (illustrator: Rob Flewell, CMI; anatomy by design, Inc.).
The phase 2 study in which the patient in this case report was enrolled aimed to evaluate the safety and efficacy of odevixibat in patients diagnosed with pruritus associated with cholestatic liver disease. The phase 2 study also assessed pruritus and sleep as secondary efficacy endpoints, given the negative impact pruritus can have on patient sleep and quality of life.3 23 These pruritus and sleep outcomes were based on daily diaries recorded by caretakers or patients aged 11 years or older, who were instructed on the daily use of the diary. Each day, caregivers or age-appropriate patients evaluated the severity of itch (VAS Itch), characterised signs of visible scratching (Whitington Itch) and assessed problems with itch or sleep (PO-SCORAD Itch, PO-SCORAD Sleep). Because pruritus is a subjective symptom and may be difficult to assess objectively, reviews on chronic pruritus recommend using a combination of instruments to capture various aspects of pruritus (eg, severity and quality/course).24 25 The caregiver-reported or patient-reported scales used in the phase 2 study for measurement of pruritus and sleep are well known in specific fields (ie, dermatology) and/or are frequently used scales in clinical trials.19 24 25 One limitation of using these scales here is that they have not been specifically validated in children with cholestatic disease. However, at the time the phase 2 study was conducted, there were no alternative assessment tools. The lack of validated instruments in paediatric cholestasis has recently led to the development of new tools26 27 which were reported after the case report data were collected and now serve as assessments in phase 3 trials of IBAT inhibitors underway in paediatric patients with intrahepatic cholestasis (NCT03566238 and NCT03905330).
For the patient described here, IBAT inhibition using odevixibat led to reductions in serum bile acids. Moreover, the severity of cholestatic pruritus also improved with odevixibat, in parallel with the observed improvements following the subsequent PEBD. The efficacy of odevixibat, with respect to sustained reductions in serum bile acids and amelioration of cholestatic pruritus, remains to be determined. Furthermore, PFIC type 2 patients may require continued monitoring for hepatocellular carcinoma in spite of symptom control.28 However, the ability to effectively manage bile acid levels and reduce pruritus using IBAT inhibition to an extent comparable to that observed with PEBD may provide a novel approach in the treatment of PFIC.
Patient’s perspective.
Our son realised an equal improvement of symptoms with both odevixibat and surgical partial external biliary diversion. Considering the burden of surgery and continuous care of the stoma, we would have preferred medical therapy if available.
Learning points.
Progressive familial intrahepatic cholestasis (PFIC) type 2, or bile salt exporter protein (BSEP) deficiency, typically presents during infancy or early childhood with cholestasis, severe pruritus, and high bilirubin and serum bile acid levels but normal gamma-glutamyltransferase activity.
In patients with PFIC type 2, pruritus is frequently unrelenting, with severe quality of life impairment, and untreated PFIC leads to impaired growth and development and progressive liver fibrosis.
A 15-month-old patient with PFIC type 2 suffering from severe cholestatic pruritus was enrolled in a clinical trial of odevixibat, an ileal bile acid transporter inhibitor under development for treatment of cholestatic liver disease, and underwent partial external biliary diversion (PEBD) after the trial ended due to recurrence of symptoms.
Odevixibat appeared to be as effective as invasive PEBD in successfully treating cholestatic pruritus in this patient with PFIC type 2 as measured by symptom scores and serum bile acid levels over a 4-week treatment period.
Acknowledgments
This study was sponsored by Albireo AB. Editorial assistance was provided by Peloton Advantage, LLC, an OPEN Health company.
Footnotes
Contributors: CJS and ES designed and conducted the study. ES recruited the patient.CJS and ES collected the data. CJS analysed the data under supervision of ES. CJS and ES wrote the manuscript.
Funding: This study was funded by Albireo AB.
Competing interests: ES is a consultant for Albireo AB and has received travel support from Albireo AB and Alexion.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jacquemin E. Progressive familial intrahepatic cholestasis. J Gastroenterol Hepatol 1999;14:594–9. 10.1046/j.1440-1746.1999.01921.x [DOI] [PubMed] [Google Scholar]
- 2.Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012;36 Suppl 1:S26–35. 10.1016/S2210-7401(12)70018-9 [DOI] [PubMed] [Google Scholar]
- 3.Mehl A, Bohorquez H, Serrano M-S, et al. Liver transplantation and the management of progressive familial intrahepatic cholestasis in children. World J Transplant 2016;6:278–90. 10.5500/wjt.v6.i2.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol 2014;4:25–36. 10.1016/j.jceh.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology 1988;95:130–6. 10.1016/0016-5085(88)90301-0 [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237–67. 10.1016/j.jhep.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Porte RJ, Verkade HJ, et al. Partial external biliary diversion in children with progressive familial intrahepatic cholestasis and Alagille disease. J Pediatr Gastroenterol Nutr 2009;49:216–21. 10.1097/MPG.0b013e31819a4e3d [DOI] [PubMed] [Google Scholar]
- 8.Schukfeh N, Metzelder ML, Petersen C, et al. Normalization of serum bile acids after partial external biliary diversion indicates an excellent long-term outcome in children with progressive familial intrahepatic cholestasis. J Pediatr Surg 2012;47:501–5. 10.1016/j.jpedsurg.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Stapelbroek JM, van Erpecum KJ, Klomp LWJ, et al. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol 2010;52:258–71. 10.1016/j.jhep.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 10.Bustorff-Silva J, Sbraggia Neto L, Olímpio H, et al. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis--a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg 2007;42:1337–40. 10.1016/j.jpedsurg.2007.03.029 [DOI] [PubMed] [Google Scholar]
- 11.van der Woerd WL, Houwen RH, van de Graaf SF. Current and future therapies for inherited cholestatic liver diseases. World J Gastroenterol 2017;23:763–75. 10.3748/wjg.v23.i5.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H-L, Wu S-H, Hsu S-H, et al. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci 2018;25:75. 10.1186/s12929-018-0475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wessel DBE, Thompson RJ, Gonzales E, et al. Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol 2020 10.1016/j.jhep.2020.02.007. [Epub ahead of print: 20 Feb 2020]. [DOI] [PubMed] [Google Scholar]
- 14.Verkade HJ, Thompson RJ, Arnell H, et al. Systematic review and meta-analysis: partial external biliary diversion in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 2020. 10.1097/MPG.0000000000002789. [Epub ahead of print: 15 May 2020]. [DOI] [PubMed] [Google Scholar]
- 15.Graffner H, Gillberg P-G, Rikner L, et al. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther 2016;43:303–10. 10.1111/apt.13457 [DOI] [PubMed] [Google Scholar]
- 16.Sturm E, Baumann U, Lacaille F, et al. The ileal bile acid transport inhibitor A4250 reduced pruritus and serum bile acid levels in children with cholestatic liver disease and pruritus: final results from a multiple-dose, open-label, multinational study [abstract 1200]. Hepatology 2017;66:646A–7.28295448 [Google Scholar]
- 17.Jansen PL, Strautnieks SS, Jacquemin E, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 1999;117:1370–9. 10.1016/S0016-5085(99)70287-8 [DOI] [PubMed] [Google Scholar]
- 18.Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012;92:502–7. 10.2340/00015555-1246 [DOI] [PubMed] [Google Scholar]
- 19.Stalder J-F, Barbarot S, Wollenberg A, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy 2011;66:1114–21. 10.1111/j.1398-9995.2011.02577.x [DOI] [PubMed] [Google Scholar]
- 20.Li T, Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol 2015;74:263–302. 10.1016/bs.apha.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson PA, Haywood J, Craddock AL, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 2003;278:33920–7. 10.1074/jbc.M306370200 [DOI] [PubMed] [Google Scholar]
- 22.Wang KS, Tiao G, Bass LM, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology 2017;65:1645–54. 10.1002/hep.29019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremer AE, Beuers U, Oude-Elferink RPJ, et al. Pathogenesis and treatment of pruritus in cholestasis. Drugs 2008;68:2163–82. 10.2165/00003495-200868150-00006 [DOI] [PubMed] [Google Scholar]
- 24.Pereira MP, Ständer S. Measurement tools for chronic pruritus: assessment of the symptom and the associated burden: a review. Itch 2019;4:e29. [Google Scholar]
- 25.Ständer S, Augustin M, Reich A, et al. Pruritus assessment in clinical trials: consensus recommendations from the International forum for the study of itch (IFSI) special interest group scoring itch in clinical trials. Acta Derm Venereol 2013;93:509–14. 10.2340/00015555-1620 [DOI] [PubMed] [Google Scholar]
- 26.Kamath BM, Abetz-Webb L, Kennedy C, et al. Development of a novel tool to assess the impact of Itching in pediatric cholestasis. Patient 2018;11:69–82. 10.1007/s40271-017-0266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson R, Gwaltney C, Paty J, et al. Development of patient- and observer-reported outcome measures for paediatric cholestatic liver diseases [abstract]. Annual Meeting of the European Association for the Study of Liver Diseases; April 10-14, Vienna, Austria, 2019. [Google Scholar]
- 28.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 2006;44:478–86. 10.1002/hep.21287 [DOI] [PubMed] [Google Scholar]