Supplemental Digital Content is available in the text.
Keywords: brain, dose-response, nasal continuous positive airway pressure, nebulization, respiratory distress syndrome, surfactant
Objectives:
The current clinical treatment of neonates with respiratory distress syndrome includes endotracheal intubation and intratracheal instillation of exogenous surfactant. Nebulization of surfactant offers an attractive alternative. The aims of this study were to test nebulization as a noninvasive method of administering surfactant and determine the optimal dose for the treatment of respiratory distress syndrome–associated pathophysiology of the neonatal lungs.
Design:
Prospective, randomized, animal model study.
Setting:
An experimental laboratory.
Subjects:
Thirty-six newborn piglets.
Interventions:
Different doses (100, 200, 400, and 600 mg/kg) of poractant alfa were administered via a vibrating membrane nebulizer (eFlow-Neos; Pari Pharma GmbH, Starnberg, Germany) or a bolus administration using the intubation–surfactant–extubation (Insure) technique (200 mg/kg) to spontaneously breathing newborn piglets (n = 6/group) with bronchoalveolar lavage–induced respiratory distress syndrome during nasal continuous positive airway pressure (180 min).
Measurements and Main Results:
Pulmonary, hemodynamic, and cerebral effects were assessed. Histologic analysis of lung and brain tissue was also performed. After repeated bronchoalveolar lavage, newborn piglets developed severe respiratory distress syndrome. Rapid improvement in pulmonary status was observed in the Insure group, whereas a dose-response effect was observed in nebulized surfactant groups. Nebulized poractant alfa was more effective at doses higher than 100 mg/kg and was associated with similar pulmonary, hemodynamic, and cerebral behavior to that in the Insure group, but improved lung injury scores.
Conclusions:
In newborn piglets with severe bronchoalveolar lavage–induced respiratory distress syndrome, our results demonstrate that the administration of nebulized poractant alfa using an investigational customized eFlow-Neos nebulizer is an effective and safe noninvasive surfactant administration technique.
In neonatal ICUs (NICUs), the techniques for surfactant administration have changed greatly in recent years. Less invasive modifications of the intubation–surfactant–extubation (Insure) method for delivering surfactant to avoid even brief intubation and mechanical ventilation have been evaluated seeking to reduce intubation-related complications and improve the success of nasal continuous positive airway pressure (nCPAP) after surfactant administration (1). These modifications include the less invasive surfactant administration (LISA) method using an intratracheal surfactant instillation with the help of a thin catheter (e.g., nasogastric tube or vascular catheter), nebulized administration, pharyngeal administration, or laryngeal mask airway-guided administration (2–5). Among them, nebulized surfactant seems to be the least invasive means of administering surfactant, avoiding the risks associated with bolus fluid instillation into the trachea (transient airway obstruction, surfactant administration to a single lung, hemodynamic imbalance, etc) when the Insure or LISA approaches are applied (3, 4). Although preclinical and clinical studies using nebulized surfactant have been inconclusive, this is attributable to the use of different types of nebulizer and surfactant (with differing physical characteristics), as well as varying dosages, among other factors, and overall, the treatment appears to be safe and well tolerated (6–17).
A new device based on vibrating membrane technology (eFlow-Neos Nebulizer; Pari Pharma GmbH, Starnberg, Germany) has been miniaturized for use in premature and term neonates and is able to deliver considerably high doses of surfactant to the lungs (17–19). Furthermore, although it seems that the ultrastructure of poractant alfa changed immediately after nebulization, this alteration was transient and initial biophysical and ultrastructural properties were reinstated soon after surfactant nebulization (20), and in line with this its clinical effects were maintained (17, 19). Nonetheless, no studies have evaluated the optimal dose using eFlow-Neos nebulizer in a neonatal model, or explored the effects that this new surfactant administration technique may produce in the neonatal brain.
We hypothesized that the combination of nCPAP and nebulized surfactant, using an investigational customized eFlow-Neos nebulizer, would improve the clinical response in a dose-dependent way. The aims of this study were to test nebulization with this device as a noninvasive method of administering surfactant and to determine the optimal dose for the treatment of neonatal respiratory distress syndrome (RDS). To this end, we assessed the impact of different doses of nebulized surfactant on gas exchange, hemodynamic parameters, oxygen metabolism, and lung and brain injury scores in spontaneously breathing newborn piglets, with bronchoalveolar lavage (BAL)-induced RDS.
MATERIAL AND METHODS
Animal Preparation
The experimental protocol meets Spanish and European regulations for the protection of experimental animals (UE2010/63 and RD53/2013) and was approved by the Ethics Committee for Animal Welfare of Biocruces Bizkaia Health Research Institute.
First, 2- to 4-day-old newborn piglets were sedated with an IM injection containing ketamine (15 mg/kg), diazepam (2 mg/kg), and atropine (0.05 mg/kg) and anesthetized with sevoflurane (2–3%). A cuffed endotracheal tube (ET) (3.0 mm inner diameter [ID]; Well Lead Medical, Panyu, Guangzhou, China) was inserted and connected to a positive pressure ventilator (Carescape R860; Datex-Ohmeda, Madison, WI) with the following initial settings: Fio2 = 0.21–0.28, respiratory frequency (fR) = 28 breaths/min, positive end-expiratory pressure (PEEP) = 3 cm H2O, and positive inspiratory pressure (PIP) = 9–11 cm H2O to obtain a tidal volume (VT) = 8–10 mL/kg.
An arterial catheter was inserted into the femoral artery to monitor mean arterial blood pressure (MABP) and heart rate (HR) and to obtain blood samples for gas analysis. In addition, a 5F dual-lumen catheter was inserted into the jugular vein for administering fluids to maintain hydration (5 mL/kg/hr) and for obtaining venous blood samples. Blood flow in the right common carotid was measured by an ultrasonic flow probe (Transonic Systems, Ithaca, NY), as a proxy for cerebral blood flow. Rectal temperature was maintained between 38°C and 39°C with heating lamps.
Lung Injury and Study Design
Surfactant-deficient lung injury was achieved by repetitive saline lavage (30 mL/kg; 37°C with Fio2, 1) (21, 22). Taking into account that lung injury by saline lavage reduces the surfactant lipid concentration in alveolar lining fluids, decrease lung compliance, impair gas exchange, and facilitates alveolar collapse, after the first BAL procedure, the PEEP was increased to 5 cm H2O in order to keeping the lungs open, enabling all areas of the lungs to undergo gas exchange. At the end of the BAL procedure, positive pressure ventilation settings were Fio2 equals to 1.0, PEEP equals to 5 cm H2O, and fR and PIP were adjusted to a maximum of 42 breath/min and 25 cm H2O, to maintain VT equals to 8–10 mL/kg, to avoid barotrauma. Lavage procedures were repeated at 5-minute intervals until Pao2 less than 100 mm Hg was obtained. If animals continued to show severe respiratory failure (Pao2 < 100 mm Hg) after 30 minutes of stabilization on positive pressure ventilation, all newborn piglets received an IV bolus dose of 20 mg/kg of caffeine citrate (Peyona 20 mg/mL; Chiesi Farmaceutici, Parma, Italy) to stimulate spontaneous breathing. Tightly fitting short binasal prongs (made by cutting and joining two pieces of ET, with an ID of 3–5 mm and length of 4 cm, matched to the size of our piglets’ nasal orifice) were placed in all animals. Once spontaneous breathing was established, in a supine position, newborn piglets with surfactant-deficient lung injury were randomly assigned using a sealed envelope system to one of the following groups:
- nCPAP group (n = 6): once spontaneous breathing was established, the ET was removed and the piglets were maintained on nCPAP for 180 minutes, without surfactant treatment.
- nCPAP-Insure (Insure) group (n = 6): once spontaneous breathing was established, piglets (which were not extubated) received 200 mg/kg of poractant alfa (Curosurf; Chiesi Farmaceutici, Parma, Italy) although the ET. Immediately, after that, the ET was removed and the piglets were maintained on nCPAP for 180 minutes.
- nCPAP-nebulized surfactant (NS) groups (n = 6 per group): once spontaneous breathing was established, the ET was removed and the piglets received different doses nebulized of poractant alfa: 100 mg/kg (NS100), 200 mg/kg (NS200), 400 mg/kg (NS400), or 600 mg/kg (NS600) using an investigational customized vibrating membrane nebulizer (eFlow-Neos; Pari Pharma, Munich, Germany) which was placed between the prongs and the connection to the nCPAP circuit. After the end of surfactant nebulization, the nebulizer was immediately removed, and animals were maintained on nCPAP for 180 minutes.
The level of nCPAP was set at 5 cm H2O with a flow of 3 L/min in all animals during the experimental period. The Fio2 value was adjusted taking into account the pulmonary status (monitoring Pao2) of each animal at each moment of the study, seeking to maintain Pao2 values between 80 and 100 mm Hg.
Physiologic Measurements
In all randomized newborn piglets with BAL-induced RDS, all physiologic measurements were obtained immediately after the induction of anesthesia, after surgery, upon intubation at baseline (basal values), immediately after inducing RDS by BAL, and to confirm the respiratory failure, after the stabilization period following the insult (30 min of stabilization on conventional mechanical ventilation). All physiologic parameters were also measured immediately after extubation, during nCPAP 15 and 30 minutes after the start of nCPAP, and then every 30 minutes until the end of the experiment, at 180 minutes. Specifically, the following parameters were measured or calculated (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PCC/B268; legend, Supplemental Digital Content 4, http://links.lww.com/PCC/B271) in all randomized animals:
- Arterial pH, Pao2, Paco2 and base excess, lactic acid, and glucose (GemPremier4000; Instrumentation Laboratory, Lexington, MA);
- Pao2/Fio2 ratio;
- Hemodynamic parameters: HR, MABP, and carotid blood flow;
- Oxygen metabolism and intrapulmonary shunt: oxygen delivery (OD), oxygen consumption (Vo2), and intrapulmonary shunt (Qs/Qt) (IntelliVue Monitor; Philips Medical System, Eindhoven, The Netherlands). The Qs/Qt values were calculated as follows:
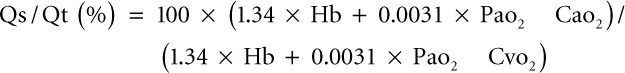 , where Pao2 = Fio2 × (Patm-47 × Paco2; Hb: hemoglobin (g/dL); Cao2: arterial oxygen content; and Cvo2: mixed venous oxygen content.
, where Pao2 = Fio2 × (Patm-47 × Paco2; Hb: hemoglobin (g/dL); Cao2: arterial oxygen content; and Cvo2: mixed venous oxygen content.- Airway flow, mean airway pressure, and VT monitored with a flow sensor connected to the ET, whereas values of lung mechanics were measured with a computerized system (M1014A; Philips Medical System) in the time intervals in which animals were intubated. The analyzer reported values for dynamic compliance (Cdyn), VT, and airway resistance at baseline, after the BAL-induced surfactant depletion, and after 30-minute stabilization of BAL-induced RDS. After 30 minutes of stabilization, all animals were extubated. During nCPAP treatment, lung mechanics cannot be measured. So that only with the objective to assess the effect of surfactant treatment on lung mechanics at the end of the follow-up period (at the end of the 3-hr experimental period), all studied animals were reintubated, connected to mechanical ventilation (using the same settings as at baseline), and lung mechanics were measured (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PCC/B268; legend, Supplemental Digital Content 4, http://links.lww.com/PCC/B271).
Lung Tissue Analysis
Postmortem, the lungs were removed and perfused with saline. The left lung was isolated, occluded, placed into liquid nitrogen, and stored at –80°C until use in biochemical analysis. Two different samples (ventral and dorsal) from the cranial, middle, and caudal lobes of the right lung were fixed in 4% formalin at 15 cm H2O for histologic analysis by a person blinded to the study.
Frozen lung samples were used for measurements of interleukin-8, interleukin-1B, and tumor necrosis factor (TNF)-α concentration using specific enzyme-linked immunosorbent assay kits for porcine interleukins (Abnova, Tapei City, Taiwan). Furthermore, enzyme activities were measured with enzyme assays, specifically catalase assays measuring the reduction of oxygen peroxide (H2O2) to H2O (23), where one unit of catalase is defined as the amount of enzyme needed to react with 1 µmol of H2O2/min and superoxide dismutase assays (Cayman Chemical, Ann Arbor, MI), whereas protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA) (24). On the other hand, slides from each 5-µm section cut from the formalin-fixed tissue were stained with hematoxylin-eosin and analyzed with light microscopy. Lung injury was scored by a pathologist blinded to treatment group using a semi-quantitative scoring system. Pathologic signs of lung injury (atelectasis, alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, edema, and necrosis) were each scored on a 0- to 4-point scale: 0 corresponding to no injury; 1, 2, and 3 to injury to 25%, 50%, and 75% of the field, respectively; and 4 to injury across the field (22, 25).
Brain Tissue Analysis
To perform histologic analysis, the brain was fixed (4% formalin) and divided into cortex, inner regions (striatum, thalamus, and hippocampus), and cerebellum and brainstem. A total of 20 fields were analyzed with light microscopy. Pathologic features of brain injury (necrosis, inflammation, hemorrhage, edema, and infarction) were each scored on a 0- to 3-point scale: 0 corresponding to no injury; and 1, 2, and 3 to injury to mild, moderate, and severe injury across the field. The presence of more than five necrotic cells/field was considered to indicate neuronal necrosis (score range, 0–20) (26).
Statistical Analysis
Values are expressed as mean ± sem. Results were assessed using Levene test to confirm the homogeneity of variance between the different treatments and the Kolmogorov-Smirnoff test for normality (JMP8; Statistical Discovery, SAS, NC). Results related to gas exchange, hemodynamic parameters, oxygen metabolism, and lung mechanics were analyzed using one- and two-way analysis of variance as a function of group and time of repeated measures. Lung biochemical results and injury score and Brain Injury Score were analyzed using the nonparametric Wilcoxon test. A p value of less than 0.05 was considered significant.
RESULTS
The 36 newborn piglets from different litters randomized to one of the study groups were similar in age (4 ± 1 d) and size (2.0 ± 0.1 kg). Multiple BALs (range, 10–13) were needed to induce the targeted extent of severe lung injury (as indicated by Pao2 < 100 mm Hg; nCPAP: 68 ± 7 mm Hg; Insure: 68 ± 6 mm Hg; NS100: 60 ± 3 mm Hg; NS200: 64 ± 3 mm Hg; NS400: 58 ± 8 mm Hg; NS600: 62 ± 4 mm Hg), no significant differences being observed between groups in age, size, the number of BALs required, or in the amount of BAL recovered (being higher than 92% in all groups). The total number of piglets used in this study was 48, but 12 animals died before randomization, in the process of inducing severe BAL-induced RDS (Pao2 < 100 mm Hg). This indicates that our BAL-induced RDS model has a mortality rate of 25%.
The mean surfactant nebulization times per group were as follows: 13 ± 1, 22 ± 2, 52 ± 6, and 71 ± 3 minutes in NS100, NS200, NS400, and NS600 groups, respectively.
Pulmonary Outcomes
Gas Exchange and Lung Mechanics.
All animals had similar pH, Pao2/Fio2, Paco2, and Cdyn parameters at baseline, after induction of surfactant-deficient lung injury, and after 30 minutes of stabilization (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269; and Fig. 1), with no significant differences between groups. BAL produced (Fig. 1A), Cdyn (Fig. 1B), and pH (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269) along with a significant increase in Paco2 (Fig. 1C), indicating severe RDS.
Figure 1.
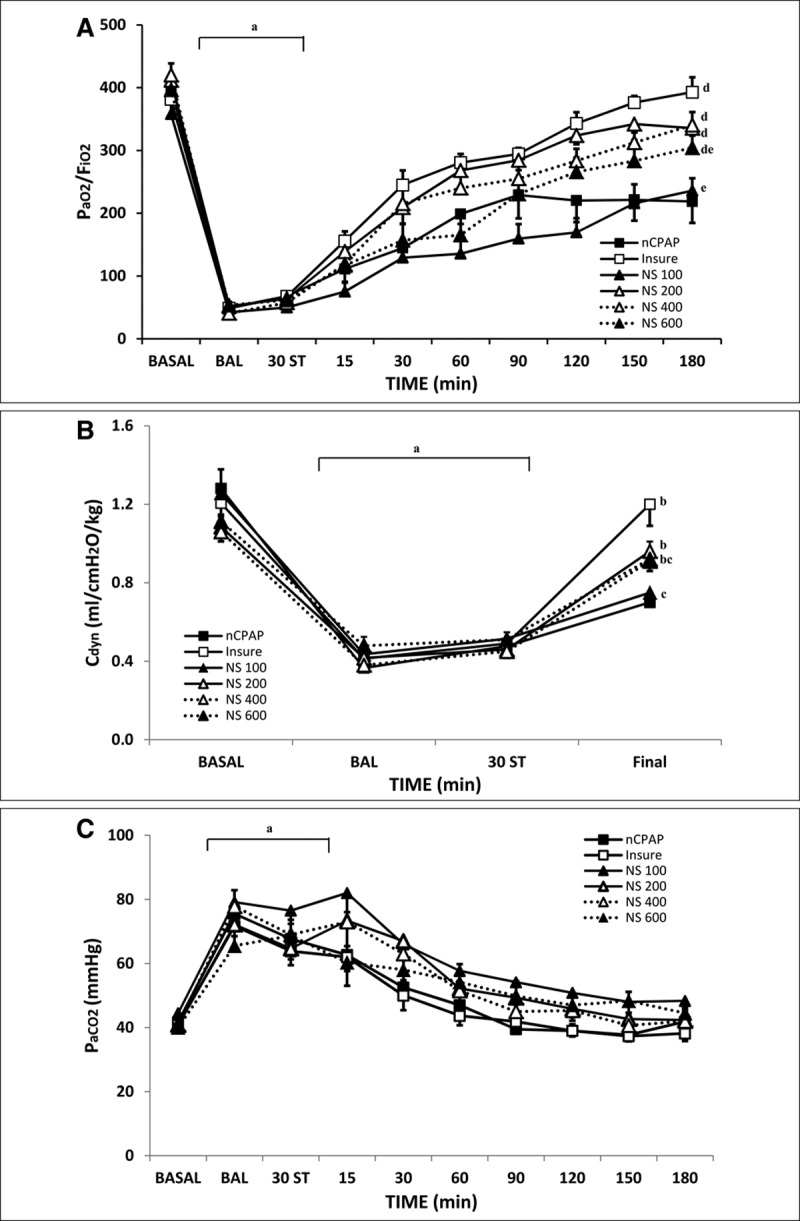
Changes in Pao2/Fio2 ratio, dynamic compliance (Cdyn), and Paco2 in bronchoalveolar lavage (BAL)-induced respiratory distress syndrome newborn piglets treated with nasal continuous positive airway pressure (nCPAP) without or with surfactant treatment, using the intubation–surfactant–extubation (Insure) method or different doses of nebulized surfactant (NS). Pao2/Fio2 (A), Cdyn (B), and Paco2 (C) values in the nCPAP (black square), Insure (white square), NS100 (black triangle, continuous line), NS200 (white triangle, continuous line), NS400 (white triangle, dotted line), and NS600 (black triangle, dotted line) groups. ap < 0.05 versus baseline, bp < 0.05 versus nCPAP group, and cp < 0.05 versus Insure group (one-way analysis of variance [ANOVA]); dp < 0.05 versus nCPAP group and ep < 0.05 versus Insure group (two-way ANOVA). Values are mean ± sem. BASAL = baseline point, ST = stabilization.
Pao2/Fio2 ratio, Paco2, pH, and Fio2 values improved more rapidly in the Insure group than in the nCPAP group (Fig. 1; Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269). At the end of the experimental period, RDS resolved in the Insure group (being Pao2/Fio2 ratio > 350 mm Hg), whereas animals in the nCPAP group continued to show mild to moderate RDS (being Pao2/Fio2 ratio < 250 mm Hg). The administration of 200 or 400 mg/kg of nebulized surfactant produced a significant improvement in the values of these parameters, in line with the changes in the Insure group, whereas the administration of 100 or 600 mg/kg of nebulized surfactant did not achieve the same degree of improvement in Pao2/Fio2 ratio, pH, and Fio2 values as in the Insure group. At least, two animals in each nebulized group had a Paco2 of 55 mm Hg or more during the nebulization period.
Regarding Cdyn, in almost all of the surfactant-treated groups, it recovered to or close to baseline (100%, 89%, 87%, and 82% in the Insure, NS200, NS400, and NS600 groups, respectively), being Cdyn recoveries significantly compared with nCPAP group. However, only around 60% of Cdyn recovery was observed with nCPAP alone and 65% in the NS100 group (Fig. 1B), no significant differences observed between groups. No significant differences were observed between groups in VT or resistance parameters (data not shown).
FR remained high during the first 30–60 minutes of surfactant treatment in all surfactant-treated groups (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269). Furthermore, in the nCPAP group, changes in fR were observed from time to time due to transient episodes of apnea (which did not require stimulation and were not associated with important changes in pH, Pao2, or Paco2).
Lung Inflammatory Markers.
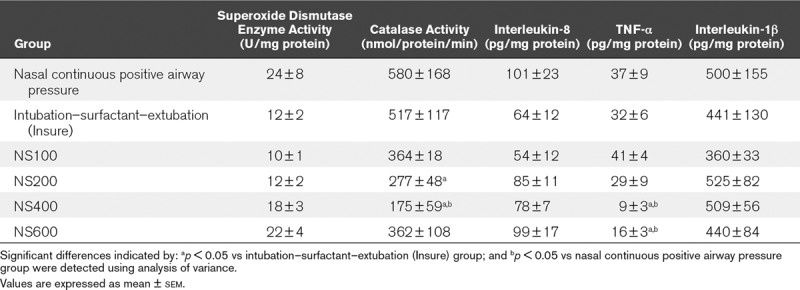
Table 1 shows the antioxidant enzyme activity and the concentrations of the acute phase cytokines interleukin-8, interleukin-1β, and TNF-α measured in lung homogenate. After 3 hours of ventilation with nCPAP, all surfactant-treated groups showed no significant reductions in lung antioxidant enzyme activity and cytokine levels. Furthermore, all but the lowest doses of surfactant nebulization (i.e., doses of 200, 400, and 600 mg/kg) were associated with a significant reduction in lung catalase activity and/or TNF-α level, but only the 400 mg/kg dose was associated with significant reductions in both lung markers.
TABLE 1.
Lung Biochemical Analysis in Newborn Piglets With Bronchoalveolar Lavage–Induced Respiratory Distress Syndrome Treated With Nasal Continuous Positive Airway Pressure Without or With Surfactant Treatment, Using the Intubation–Surfactant–Extubation (Insure) Method or Different Doses (100, 200, 400, or 600 mg/kg) of Nebulized Surfactant
Lung Injury.
All surfactant-treated groups obtained significant lower total lung injury scores than the nCPAP group. Furthermore, there was significantly less edema and alveolar hemorrhage in all nebulized surfactant groups than in the nCPAP group. Overall, the highest doses of surfactant nebulization (400 and 600 mg/kg) were associated with significant lower total lung injury scores than the Insure treatment (Table 2 and Fig. 2).
TABLE 2.
Total Lung Injury Scores in Newborn Piglets With Bronchoalveolar Lavage–Induced Respiratory Distress Syndrome Treated With Nasal Continuous Positive Airway Pressure Without or With Surfactant Treatment, Using the Intubation–Surfactant–Extubation (Insure) Method or Different Doses (100, 200, 400, or 600 mg/kg) of Nebulized Surfactant
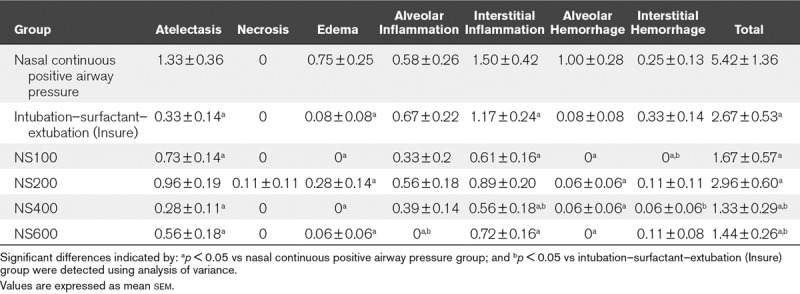
Figure 2.
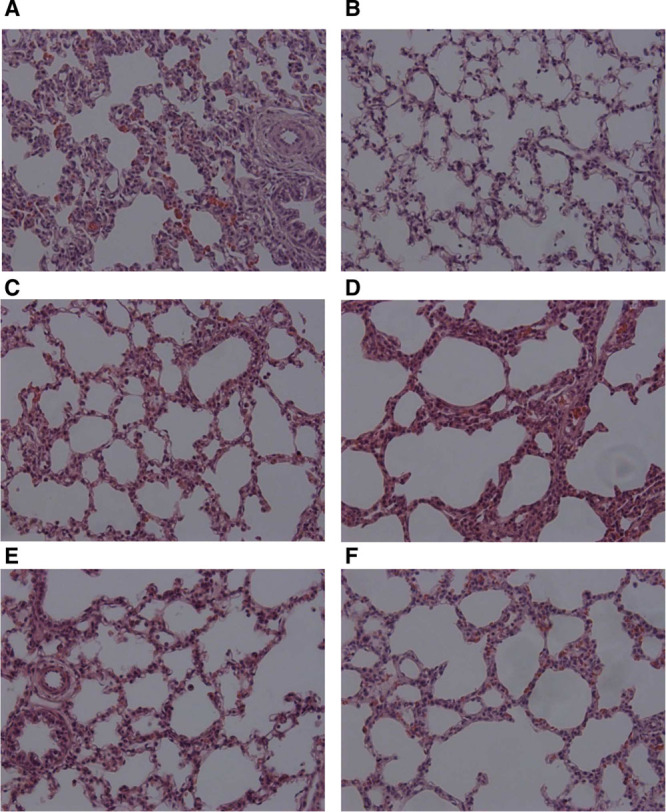
Photomicrographs (×200 magnification) of representative lung sections from nasal continuous positive airway pressure (A), intubation–surfactant–extubation (Insure) (B), nebulized surfactant (NS) 100 (C), NS200 (D), NS400 (E), and NS600 (F) groups. Panels were obtained from the middle region of the lung.
Intrapulmonary Shunt and Oxygen Transport
BAL produced a significant increase in Qs/Qt values (Fig. 3A), with no significant changes in any of the systemic oxygen metabolism parameters (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269). Three hours of nCPAP ventilation without surfactant treatment was not associated with significant changes in OD or Vo2 (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269), whereas Qs/Qt gradually improved over time, although not reaching baseline (Fig. 3A). When surfactant was administered, Qs/Qt recovered to baseline values in Insure, NS200, and NS400 groups after 2 hours of treatment (Fig. 3A), but not in the NS100 and NS600 groups.
Figure 3.
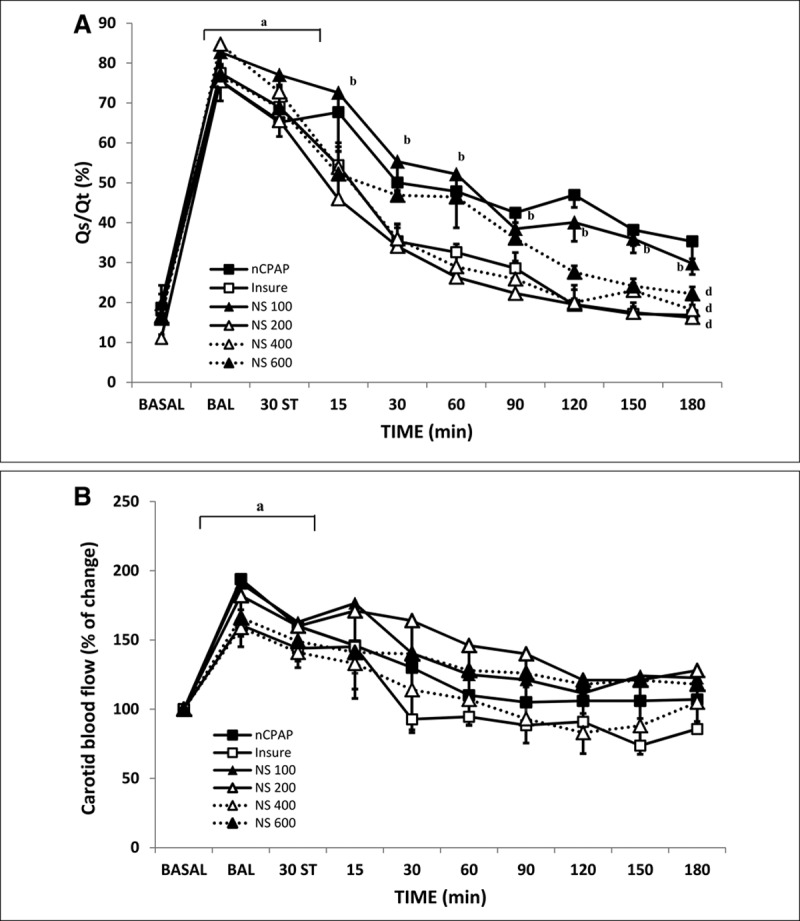
Changes in intrapulmonary shunt (Qs/Qt) and carotid blood flow (D) in bronchoalveolar lavage (BAL)-induced respiratory distress syndrome newborn piglets treated with nasal continuous positive airway pressure (nCPAP) without or with surfactant treatment, using the intubation–surfactant–extubation (Insure) method or different doses of nebulized surfactant (NS). Mean Qs/Qt (A) and mean carotid blood flow (B) values in the nCPAP (black square), Insure (white square), NS100 (black triangle, continuous line), NS200 (white triangle, continuous line), NS400 (white triangle, dotted line), and NS600 (black triangle, dotted line) groups. ap < 0.05 versus baseline; bp < 0.05 versus nCPAP group (one-way ANOVA); dp < 0.05 versus nCPAP group. Values are mean ± sem. BASAL = baseline point, ST = stabilization.
Furthermore, the combination of surfactant therapy (Insure and NS400) with nCPAP ventilation was associated with significant higher OD values than nCPAP alone (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/B269). The nebulization of 200 or 400 mg/kg of surfactant was associated with an increase in Vo2 values that lasted for less time than that observed in the Insure group.
Hemodynamic Assessment
At baseline, there were no differences between groups in the hemodynamic parameters studied. No significant changes in MABP were observed after BAL (Supplemental Fig. 2A, Supplemental Digital Content 3, http://links.lww.com/PCC/B270; legend, Supplemental Digital Content 4, http://links.lww.com/PCC/B271), whereas the HR rose significantly (Supplemental Fig. 2B, Supplemental Digital Content 3, http://links.lww.com/PCC/B270; legend, Supplemental Digital Content 4, http://links.lww.com/PCC/B271). During the study period, MABP and HR were significantly higher in animals in Insure and most NS (200, 400, or 600 mg/kg) groups than in those given nCPAP alone.
Cerebral Evaluation
As would be expected, as Pao2 values decreased, carotid blood flow increased significantly in all groups after the BAL procedure (Fig. 3B) in order to maintain cerebral oxygenation. The nCPAP strategy with or without surfactant administration gradually improved lung outcome and gas exchange, with a continuous decrease in carotid blood flow being observed over time in all studied groups, until it reached baseline values.
All groups studied obtained low brain injury scores, with similar hemorrhage, inflammation, and infarction scores in all regions studied (Table 3 and Fig. 4). Nevertheless, brain necrosis and edema scores were significantly lower in animals that received surfactant treatment than in those given nCPAP alone.
TABLE 3.
Total Brain Injury Scores in Newborn Piglets With Bronchoalveolar Lavage–Induced Respiratory Distress Syndrome Treated With Nasal Continuous Positive Airway Pressure Without or With Surfactant Treatment, Using the Intubation–Surfactant–Extubation (Insure) Method or Different Doses (100, 200, 400, or 600 mg/kg) of Nebulized Surfactant
Figure 4.
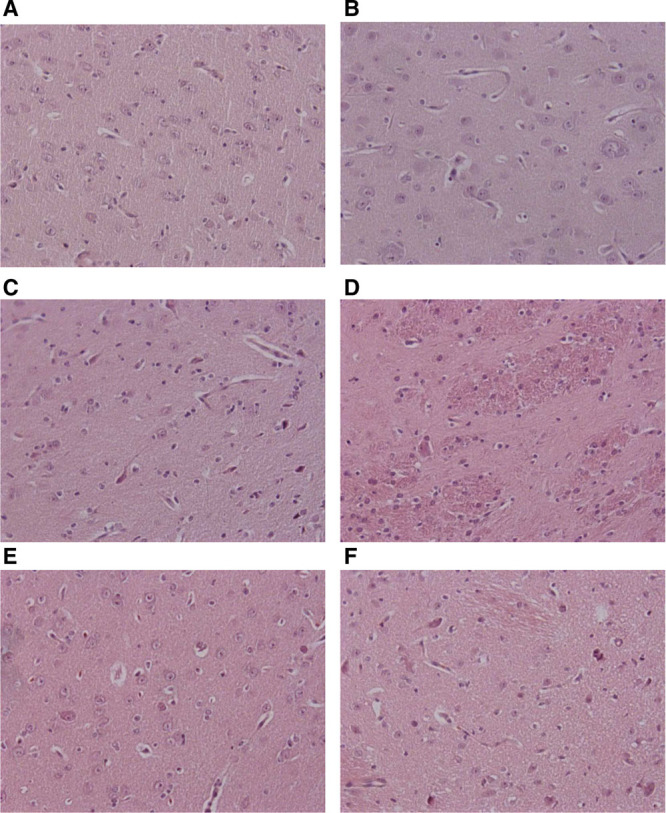
Photomicrographs (×200 magnification) of representative brain sections from nasal continuous positive airway pressure (A), intubation–surfactant–extubation (Insure) (B), nebulized surfactant (NS) 100 (C), NS200 (D), NS400 (E), and NS600 (F) groups. Panels were obtained from the striatum region of the brain.
DISCUSSION
We have been able to demonstrate, in our spontaneously breathing newborn piglet model of surfactant-deficient lung injury, that the investigational customized eFlow-Neos Nebulizer is safe and able to produce a clinically relevant improvement in physiologic parameters like oxygenation and lung function. Furthermore, a dose-response effect was observed in the nebulized groups, nebulized surfactant being more effective at doses higher than 100 mg/kg, these groups showing similar pulmonary, hemodynamic, and cerebral behavior to the Insure group, with lower lung injury scores.
In many hospitals, the procedure for surfactant administration for the treatment of neonatal RDS requires endotracheal intubation, bolus surfactant administration, and mechanical ventilation. Notably, this procedure is not without side effects (airway obstruction, hypoxia, bradycardia, and blood pressure fluctuation, among others), and current evidence suggests that mechanical ventilation of an immature surfactant-deficient lung is harmful and may exacerbate the development of bronchopulmonary dysplasia (27). In view of the comorbidities, in neonatology practice, there has been a notable trend toward greater use of noninvasive ventilation such as nCPAP, combined with LISA based on the Insure strategy or LISA techniques (using a nasogastric tube or vascular catheter) (1–4). Nonetheless, all of these approaches require instrumentation of the airway and still carry the risks associated with bolus fluid instillation into the trachea (transient airway obstruction, surfactant administration to a single lung, hemodynamic imbalance, etc) (3, 4). Furthermore, if due to the severity of the RDS, the neonate requires mechanical ventilation, it would be necessary to instrument the airways again to place the ET and set up mechanical ventilation.
Nebulization during nCPAP treatment represents the least invasive way of administering surfactant, and it has been explored in small pilot studies in premature infants (13–17), these indicating the safety and feasibility of the method. Nevertheless, only two of the studies showed positive effects: the earliest showed an immediate positive effect on OD (13) and the most recent reported a reduced need for intubation in the first 3 days of life (17). The mixed results concerning clinical response and surfactant distribution observed in clinical (13–17) and animal (6–12) studies are likely due to the use of nebulizers not specifically designed for the neonatal population or to the special physicochemical properties of a compound like surfactant affecting the nebulization.
The new aerosol technology under study, the vibrating membrane nebulizer, eFlow-Neos, has been designed to enhance the delivery of surfactant to the neonatal respiratory system taking into consideration the particular characteristics of surfactant (lipid-protein composition, high viscosity, etc). First, this vibrating membrane nebulizer is portable and simple to use, avoids surfactant aerosol dilution, and reduces the residual volume in the device (28). Second, it has been previously demonstrated that it is able to nebulize surfactant at an appropriate particle size (2.5–3.5 μm) (18–20) capable of penetrating deep into the distal airways (18, 19). Third, high distal airways delivery efficacies of greater than 14% have been observed in “in vitro” (29) and animal models (18, 19). Finally, although transient alterations in surfactant morphology and characteristics were observed after nebulization, presumably after rearranging on a surface, the initial biophysical and ultrastructural properties were reinstated (20). In addition to this, while in surfactant bolus administration, delivered phospholipids should be absorbed or taken up by type II alveolar epithelium cells, the surfactant delivered by different type nebulizers (6–20) should undergo a dynamic process to reach homeostasis over the time of nebulization. All the aforementioned studies suggest that eFlow-Neos nebulizer is a promising innovative approach for effective delivery of nebulized surfactant in neonates.
The present study was designed with the objective of identifying the optimal dose of nebulized surfactant for the treatment of neonatal RDS. Improvements in oxygenation, intrapulmonary shunt, and compliance in spontaneously breathing newborn piglets with surfactant-deficient lung injury treated with 200 or 400 mg/kg of nebulized poractant alfa were observed compared with outcomes in untreated controls (19). In contrast, nebulization of 100 or 600 mg/kg did not match the improvement observed in Insure-treated animals, as previously reported (19). Although other research has demonstrated improved ventilation and lung mechanics, even with minimal deposition in the lungs (as little as 2–3 mg/kg) (6), the administration of 100 mg/kg of nebulized surfactant (corresponding to 14 mg/kg, supposing a lung deposition of approximately 14% of dose) (18, 19, 29) does not seem to be enough to produce a positive clinical response. On the other hand, 600 mg/kg of nebulized surfactant only slightly improved oxygenation, intrapulmonary shunt, and compliance (19), probably due to the prolonged time that the nebulizer needs to be in place between the Y-piece of the ventilator’s breathing circuit and the nasal prongs (> 1 hr to nebulize the total dose) which may reduce or delay the clinical response. The continuous nebulization of 600 mg/kg of nebulized surfactant may produce surfactant accumulation in the airways, which would partly explain the impairment in Pao2 and Paco2 values during surfactant nebulization (19). Furthermore, after removal of the nebulizer, pulmonary outcomes started to improve over the course of the experimental period (19), and hence, it may be the case that with a longer follow-up, this higher dose would be seen to produce an equivalent response to that seen with 200 or 400 mg/kg of nebulized surfactant.
Although our study was not designed to evaluate surfactant distribution in the lung, the lower total Lung Injury Scores and lung biochemical markers observed after natural surfactant administration using an investigational customized eFlow-Neos nebulizer may result in a better surfactant pool sizes and distribution pattern of surfactant (18, 19). In particular, more surfactant may be deposited in lower lung lobes (30), those which are less developed in animals and neonates with RDS (31), during nCPAP support (32). Overall, our study suggests that a nominal surfactant dose of 400 mg/kg may be the best of those tested because it provides a good short-term pulmonary response (19), with a reduction in lung markers and lung injury, and would help to increase the intrapulmonary surfactant pool (19). In line with this, a recent double-blind, parallel, stratified, randomized control trial has been reported (17), and these results showed that early postnatal surfactant nebulization of 200 mg/kg of poractant alfa may reduce the need for intubation in the first 3 days of life compared with nCPAP alone in premature infants with mild RDS, although no positive effect was observed in moderate RDS (17). This lack of response to nebulized surfactant in moderate RDS could be related to the dose deposited in the lung (17), and this problem could be addressed by nebulization of higher doses (> 200 mg/kg). One of the benefits of nebulized surfactant is the expansion of treatment to a wider range of infants which exhibit mild RDS symptoms (17). Those infant may not receive therapeutic surfactant treatments to avoid potential future complications associated with intubation, as bronchopulmonary dysplasia. Borderline babies, who might do well initially, often develop signs of RDS over the next couple of days and may have a more difficult course because of delayed surfactant treatment. So that those infants may benefit from nebulized surfactant treatment, increasing also the hospitalization cost savings (fewer days in mechanical ventilation, in the NICU) with minimal patient handling and disruption (28).
Nebulization was associated with a transient increase in Paco2 in a third of the piglets in each group (secondary to external dead space), but this resolved immediately following nebulizer removal (17). No other significant changes in MABP, HR, or systemic oxygen metabolism were observed during the nebulization period (17), independent of nebulization group. Over time, MABP, HR, and OD stayed higher in surfactant-treated groups than in the nCPAP group, but they remained within the physiologic range (22).
Another important consideration is the effect of new respiratory technology at birth on other organ systems, especially the brain. This is of particular importance given that preterm infants have a significantly higher risk of acute and chronic brain injury than term infants (33). There is growing evidence from animal studies that ventilation-induced lung injury leads to systemic and brain inflammation and injury, due to hemodynamic instability and a localized cerebral inflammatory response (34). In our study, neither of the surfactant therapies used (bolus or nebulizer) was observed to have any significant clinical effects on carotid blood flow or brain injury score, confirming the safety of this new way of administering surfactant.
Limitations of this study include the use of newborn piglets (2–4 d old) rather than premature animals. The intubated premature lamb has been the gold standard animal model to study the pathogenesis and the treatments of neonatal RDS for many decades; nevertheless, the use of noninvasive support such as nCPAP using premature lambs remains complicated, and the pulmonary outcomes after surfactant treatment in combination with nCPAP are difficult to interpret due to the high variability in response to nCPAP and surfactant (30). Furthermore, fetal lamb cerebral development is quite advanced compared with that in human fetuses (35). Surfactant washout lavage models have frequently been used in adult and juvenile animals to implement successful animal models of acute pulmonary failure in the context of RDS (21, 36, 37). Although, piglets’ nasal and pharyngeal anatomy differs from that of human infants (the distance from the nasal orifice to pharynx being longer and narrower), the newborn piglet model was chosen because the brain maturation, lung volume, and birth weights resemble those of newborn infants. The level of nCPAP used for the treatment of RDS in human neonates ranges between 5 and 8 cm H2O depending on the lung illness type and severity. In the present study, as in previous studies (19, 22, 37) using nCPAP, a pressure of 5 cm H2O was used; however, it is possible that the use of higher level of nCPAP could have obtained different physiologic and histologic results.
CONCLUSIONS
Nebulization delivery of poractant alfa at doses of at least 200 mg/kg using an investigational customized eFlow-Neos Nebulizer is a safe therapeutic approach for relieving surfactant deficiency in spontaneously breathing newborn piglets supported with nCPAP. Furthermore, there is histologic and biochemical evidence of less lung injury trend with poractant alfa nebulization treatment. Nominal surfactant dose of 200 or 400 mg/kg delivered by the eFlow-Neos nebulizer significantly improved pulmonary outcomes in a similar way to Insure surfactant administration. Nonetheless, long-term studies are required with the selected dose in order to assess the need for intubation, duration of ventilation, surfactant redosing, physiologic stability, and so on, comparing with the outcomes with the use of nCPAP alone.
ACKNOWLEDGMENTS
We thank Alfredo Alonso Digon for excellent technical assistance and for his valuable support in the experimental handling of the animals; and Redes Temáticas de Investigación Cooperativa en Salud funded by the Plan Nacional de Investigación, Desarrollo e Innovación (PN I+D+I) 2013-2016 (Spain), Instituto de Salud Carlos III-Sub-Directorate General for Research Assessment and Promotion, and the European Regional Development Fund, ref. RD16/0022.
Supplementary Material
Footnotes
*See also p. 701.
Dr. Rey-Santano and Ms. Mielgo contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Rey-Santano, Ms. Mielgo, Dr. Gomez-Solaetxe, and Dr. Loureiro’s institutions received funding from Chiesi Farmaceutici and Carlos III Health Institute (PI14/00024) (co-financed by the European Regional Development Fund [ERDF] “A way to make Europe”). Dr. Rey-Santano, Ms. Mielgo, Dr. Gomez-Solaetxe, Dr. Salomone, and Dr. Loureiro disclosed off-label product use of vibrating membrane nebulizer (eFlow-Neos). Ms. Ricci, Dr. Bianco, and Dr. Salomone disclosed that they are Chiesi employees. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 1994; 331:1051–1055 [DOI] [PubMed] [Google Scholar]
- 2.Aldana-Aguirre JC, Pinto M, Featherstone RM, et al. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017; 102:F17–F23 [DOI] [PubMed] [Google Scholar]
- 3.Göpel W, Kribs A, Ziegler A, et al. ; German Neonatal Network: Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): An open-label, randomised, controlled trial. Lancet 2011; 378:1627–1634 [DOI] [PubMed] [Google Scholar]
- 4.Kribs A, Roll C, Göpel W, et al. ; NINSAPP Trial Investigators: Nonintubated surfactant application vs conventional therapy in extremely preterm infants: A randomized clinical trial. JAMA Pediatr 2015; 169:723–730 [DOI] [PubMed] [Google Scholar]
- 5.Roberts KD, Brown R, Lampland AL, et al. Laryngeal mask airway for surfactant administration in neonates: A randomized, controlled trial. J Pediatr 2018; 193:40–46.e1 [DOI] [PubMed] [Google Scholar]
- 6.Lewis JF, Ikegami M, Jobe AH, et al. Aerosolized surfactant treatment of preterm lambs. J Appl Physiol (1985) 1991; 70:869–876 [DOI] [PubMed] [Google Scholar]
- 7.Henry MD, Rebello CM, Ikegami M, et al. Ultrasonic nebulized in comparison with instilled surfactant treatment of preterm lambs. Am J Respir Crit Care Med 1996; 154:366–375 [DOI] [PubMed] [Google Scholar]
- 8.Ellyett KM, Broadbent RS, Fawcett ER, et al. Surfactant aerosol treatment of respiratory distress syndrome in the spontaneously breathing premature rabbit. Pediatr Res 1996; 39:953–957 [DOI] [PubMed] [Google Scholar]
- 9.Dijk PH, Heikamp A, Bambang Oetomo S. Surfactant nebulisation prevents the adverse effects of surfactant therapy on blood pressure and cerebral blood flow in rabbits with severe respiratory failure. Intensive Care Med 1997; 23:1077–1081 [DOI] [PubMed] [Google Scholar]
- 10.Fok TF, Al-Essa M, Dolovich M, et al. Nebulisation of surfactants in an animal model of neonatal respiratory distress. Arch Dis Child Fetal Neonatal Ed 1998; 78:F3–F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampland AL, Wolfson MR, Mazela J, et al. Aerosolized KL4 surfactant improves short-term survival and gas exchange in spontaneously breathing newborn pigs with hydrochloric acid-induced acute lung injury. Pediatr Pulmonol 2014; 49:482–489 [DOI] [PubMed] [Google Scholar]
- 12.Walther FJ, Hernández-Juviel JM, Waring AJ. Aerosol delivery of synthetic lung surfactant. PeerJ 2014; 2:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorch G, Hartl H, Roth B, et al. Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr Pulmonol 1997; 24:222–224 [DOI] [PubMed] [Google Scholar]
- 14.Arroe M, Pedersen-Bjergaard L, Alberstsen P, et al. Inhalation of aerosolized surfactant (Exosurf) to neonates treated with nasal continuous positive airway pressure. J Matern Fetal Neonatal Med 1998; 3:346–352 [Google Scholar]
- 15.Berggren E, Liljedahl M, Winbladh B, et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr 2000; 89:460–464 [DOI] [PubMed] [Google Scholar]
- 16.Finer NN, Merritt TA, Bernstein G, et al. An open label, pilot study of Aerosurf® combined with nCPAP to prevent RDS in preterm neonates. J Aerosol Med Pulm Drug Deliv 2010; 23:303–309 [DOI] [PubMed] [Google Scholar]
- 17.Minocchieri S, Berry CA, Pillow JJ; CureNeb Study Team: Nebulised surfactant to reduce severity of respiratory distress: A blinded, parallel, randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2019; 104:F313–F319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linner R, Perez-de-Sa V, Cunha-Goncalves D. Lung deposition of nebulized surfactant in newborn piglets. Neonatology 2015; 107:277–282 [DOI] [PubMed] [Google Scholar]
- 19.Bianco F, Ricci F, Catozzi C, et al. From bench to bedside: In vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir Res 2019; 20:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minocchieri S, Knoch S, Schoel WM, et al. Nebulizing poractant alfa versus conventional instillation: Ultrastructural appearance and preservation of surface activity. Pediatr Pulmonol 2014; 49:348–356 [DOI] [PubMed] [Google Scholar]
- 21.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand 1980; 24:231–236 [DOI] [PubMed] [Google Scholar]
- 22.Rey-Santano C, Mielgo VE, Gomez-Solaetxe MA, et al. Non-invasive ventilation and surfactant treatment as the primary mode of respiratory support in surfactant-deficient newborn piglets. Pediatr Res 2018; 83:904–914 [DOI] [PubMed] [Google Scholar]
- 23.Holmes RS, Masters CJ. On the tissue and subcellular distribution of multiple forms of catalase in the rat. Biochim Biophys Acta 1969; 191:488–490 [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254 [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann AM, Roberts KD, Lampland AL, et al. Improved gas exchange and survival after KL-4 surfactant in newborn pigs with severe acute lung injury. Pediatr Pulmonol 2010; 45:782–788 [DOI] [PubMed] [Google Scholar]
- 26.Rey-Santano C, Mielgo VE, López-de-Heredia-y-Goya J, et al. Cerebral effect of intratracheal aerosolized surfactant versus bolus therapy in preterm lambs. Crit Care Med 2016; 44:e218–e226 [DOI] [PubMed] [Google Scholar]
- 27.Cowan F, Whitelaw A, Wertheim D, et al. Cerebral blood flow velocity changes after rapid administration of surfactant. Arch Dis Child 1991; 66:1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillow JJ, Minocchieri S. Innovation in surfactant therapy II: Surfactant administration by aerosolization. Neonatology 2012; 101:337–344 [DOI] [PubMed] [Google Scholar]
- 29.Minocchieri S, Burren JM, Bachmann MA, et al. Development of the premature infant nose throat-model (PrINT-Model): An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr Res 2008; 64:141–146 [DOI] [PubMed] [Google Scholar]
- 30.Hütten MC, Kuypers E, Ophelders DR, et al. Nebulization of poractant alfa via a vibrating membrane nebulizer in spontaneously breathing preterm lambs with binasal continuous positive pressure ventilation. Pediatr Res 2015; 78:664–669 [DOI] [PubMed] [Google Scholar]
- 31.Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol 1986; 29:502–513 [PubMed] [Google Scholar]
- 32.Niemarkt HJ, Kuypers E, Jellema R, et al. Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res 2014; 76:166–170 [DOI] [PubMed] [Google Scholar]
- 33.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ 2010; 88:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polglase GR, Miller SL, Barton SK, et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One 2012; 7:e39535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol 2009; 40:156–167 [DOI] [PubMed] [Google Scholar]
- 36.Lampland AL, Meyers PA, Worwa CT, et al. Gas exchange and lung inflammation using nasal intermittent positive-pressure ventilation versus synchronized intermittent mandatory ventilation in piglets with saline lavage-induced lung injury: An observational study. Crit Care Med 2008; 36:183–187 [DOI] [PubMed] [Google Scholar]
- 37.Ricci F, Catozzi C, Murgia X, et al. Physiological, biochemical, and biophysical characterization of the lung-lavaged spontaneously-breathing rabbit as a model for respiratory distress syndrome. PLoS One 2017; 12:e0169190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


