Abstract
Among modern drug formulations, stimuli-responsive hydrogels also called “smart hydrogels” deserve a special attention. The basic feature of this system is the ability to change their mechanical properties, swelling ability, hydrophilicity, bioactive molecules permeability, etc., influenced by various stimuli, such as temperature, pH, electromagnetic radiation, magnetic field and biological factors. Therefore, stimuli-responsive matrices can be potentially used in tissue engineering, cell cultures and technology of innovative drug delivery systems (DDSs), releasing the active substances under the control of internal or external stimuli. Moreover, smart hydrogels can be used as injectable DDSs, due to gel–sol transition connected with in situ cross-linking process. Innovative smart hydrogel DDSs can be utilized as matrices for targeted therapy, which enhances the effectiveness of tumor chemotherapy and subsequently limits systemic toxicity. External stimulus sensitivity allows remote control over the drug release profile and gel formation. On the other hand, internal factors provide drg accumulation in tumor tissue and reduce the concentration of active drug form in healthy tissue. In this report, we summarise the basic knowledge and chemical strategies for the synthetic smart hydrogel DDSs applied in antitumor therapy.
Keywords: smart hydrogels, stimuli-responsive hydrogels, controlled release, drug delivery systems, anticancer drug delivery systems
Introduction
The first mention of hydrogels appeared in the literature at the beginning of the 19th century; they have been described as colloidal gels derived from inorganic salts. In the 1960s, Wichterle and Lim obtained a synthetic hydrogel system for biomedical applications.1–5 Currently, hydrogels are defined as three-dimensional, cross-linked polymer systems capable of absorbing significant amounts of water or body fluids while maintaining integrity.4,6 In an aqueous environment, they form three-dimensional, cross-linked structures due to the intermolecular interactions or chemical bonds between the chains of the hydrogel-forming polymer. An important role is played by hydrophilic functional groups, such as hydroxyl, amino or carboxyl, capable of ionizing in an aqueous environment. Hydrogel systems usually have a soft and elastic consistency. They possess the ability to absorb large amounts of water, small-size hydrophilic molecules and nutrients or metabolites, which makes them useful carriers in tissue engineering and DDSs.4,7–9
Hydrogels sensitive to environmental stimuli are also called “smart hydrogels”. The first system of this type was based on poly(acrylic acid) (PAAc), which altered its structural properties with pH changes.4 The use of stimuli-responsive hydrogels brings many possibilities, that is why they have been extensively studied in recent years. Among other advantages, it is possible to develop injectable hydrogels, where the sol–gel transition occurs at the site of administration. The use of injectable hydrogel-based DDSs shortens the duration of treatment, reduces the risk of infection of the implanted site, prevents scarring and raising the patient’s compliance. In addition, work is ongoing to obtain innovative hydrogel matrices that release biologically active substances such as anticancer drugs, genes or insulin in a controlled manner.5 The most interesting are undoubtedly hydrogels characterized by biocompatibility, biodegradability and sensitivity to environmental stimuli, such as temperature, pH, magnetic field, light or biological factors.9,10
The main problem that occurs in chemotherapy is the high toxicity of antitumor drugs. Typical methods of treatment with an anticancer drug solution administered intravenously are connected with severe side effects, which often prevents the use of appropriately high doses that could provide effective therapy. Antitumor drugs are especially toxic to cells that rapidly divide or take part in the metabolism and excretion of drugs, which results in their high toxicity to the liver, kidneys or hematopoietic system.11 Adverse effects associated with anticancer therapy can be significantly reduced when the drug distribution is limited to cancerous tissue. Targeted pharmacotherapy would allow high concentrations of the active drug only in this tissue, increasing the effectiveness of treatment and reducing systemic side effects to a minimum.
In recent years, many scientists have been researching new forms of drug delivery that would enable targeted therapy. One of the interesting solutions is undoubtedly the use of polymers, both in the design of prodrugs based on currently available cytostatics,12,13 and the development of therapeutic systems with a favorable release profile, controlled by external stimuli, such as smart hydrogels.
Although natural polymers, such as chitosan, dextran, cellulose and their chemical modification products are useful in smart hydrogels technology, they have some disadvantages. These systems are problematic when it comes to mechanical properties, maintaining the consistency is also difficult. Additionally, they need considerable chemical modification to overcome these problems and they are hard to process.14 The alternative to natural or chemically modified natural hydrogels seems to be biodegradable synthetic polymers, such as polyesters and poly(organophosphazenes) (POPs), which combine preferable properties and biosafety. Synthetic biodegradable polyesters are considered the most commercially competitive polymers for medical applications. They are also biocompatible and are used for the manufacturing of different medical devices, such as bone fixation devices, controlled drug delivery vehicles, implants, screws, stent, sutures, plate and materials for tissue engineering.
In this paper, the challenges and future research scope in the field of smart hydrogel DDSs are presented. The basic knowledge and chemical strategies for synthetic, smart hydrogel DDSs in antitumor therapy are also described. We trust that this review will be widely useful for the researchers and technologists who have been seeking for new directions with this kind of biomaterials.
Classification of Hydrogels
Hydrogels can be classified by various criteria. According to the used substrates and kind of polymerization processes, it is possible to distinguish homo- and copolymer or multipolymer systems. Homopolymer hydrogels are formed from one type of monomer, copolymeric systems are based on two types of monomers, while in the case of three or more different monomers, multipolymers are mentioned. Depending on the crystallographic properties, hydrogel systems may be crystalline, semi-crystalline or amorphous; their spatial structure may be based on hydrogen bonds, hydrocolloid aggregates or super-particles.6,15–19
Considering the charge of hydrogel-forming macromolecules; anionic, cationic, ampholytic or neutral systems are distinguished. Cationic hydrogels contain in their structure positively ionized moieties, anionic systems comprise negatively charged groups, and ampholytic groups – both types of the moieties. Depending on the mechanical properties, hydrogels may be affine or phantom.6
Hydrogel systems can also be divided depending on the particle size; here stands out macroscopic systems, micro- and nanogels. In contrast to classic hydrogels, micro- and nanogels are characterized by a size of particles much smaller than the internal diameter of injection needles, which is beneficial in the case of systems administered by injection.6 In addition, the smaller size means a larger area available for interactions with biological agents and improving the permeability from the hydrogel. Microgels of size less than 5 μm can be used for oral or inhaled administration, while nanogels can be injected intravenously. Nanogels are characterized by biocompatibility and good ability to absorb various types of molecules, which makes them a very interesting solution in the field of nanomedicine.6,20–22
One of the important stages in the synthesis of hydrogels is crosslinking, creating connections between macromolecules included in the final product. At this stage, hydrogels are classified into chemical and physical hydrogels. The first group contains macromolecules which are binded by chemical covalent bonds. On the other hand, in physically crosslinked systems the key role is played by hydrogen bonds or intermolecular interactions.4–6 The crosslinking process is responsible for creating a three-dimensional structure of the hydrogel, which determines its properties, including high water absorption capacity.
The mechanism of physical crosslinking is based on hydrophobic and/or ionic or crystallization interactions. In this case, the sol–gel transition is a reversible process, there are no chemical reactions that could lead to degradation of active substance incorporated in DDSs or cells in tissue engineering. In addition, the preparation of physically cross-linked hydrogels does not require a crosslinking agent, which is important in the context of the toxicity of the system.8,9 Up to date, many methods of physical crosslinking have been described, such as heating or cooling a solution of polymers, addition of electrolytes, formation of hydrogen bonds, formation of coacervates or alternating freezing and thawing of the solution.6
Chemical hydrogels have slightly different properties – they are physiologically resistant and have better mechanical properties. Generally, chemical crosslinking takes place by forming covalent bonds between individual polymer chains or low-molecular-weight monomers. In the latter example, crosslinking agent is added to monomer mixture and acts as a trigger for polymerization. The chemical crosslinking takes place under the influence of radical polymerization, chemical reaction, UV radiation or enzymatic activity, mainly transglutaminases or horseradish peroxidase.8,9
Physical and chemical crosslinking mechanisms are summarized in Table 1.
Table 1.
Physical and Chemical Crosslinking Mechanisms
| Physical Crosslinking | Chemical Crosslinking | ||||
|---|---|---|---|---|---|
| Mechanism | Ref. | Mechanism | Ref. | ||
| Heating or cooling polymer solution | [23] | Photocrosslinking | Photocrosslinker activated by photoirradiation forms free radicals able to create covalent bonds between polymer chains | [24] | |
| Ionic interaction | [25] | Michael-type addition | Reaction between nucleophilic groups (amino or thio) with aldehydes or ketones | [26] | |
| Complex coacervation | [27] | Thiol exchange/disulfide crosslinking | Creating disulfides by reaction between thiol groups | [28] | |
| Hydrogen bonding | [29] | Schiff-base crosslinking | Reaction between amine and aldehyde groups | [30] | |
| Maturation and aggregation | [31] | Click chemistry reactions | Alkyne-azide reactions | Addition reaction between azide and alkyne | [32] |
| Freeze-thaw cycles | [33] | Diels-Alder reaction | Cycloaddition of substituted alkene to conjugated diene | [34] | |
| Thiol-ene addition | Addition of thiol to substituted alkene | [35] | |||
It turns out that the production of hydrogels sensitive to external stimuli is possible both through the use of physical and chemical crosslinking,2,4,36,37 although, due to the reversibility of processes and the more dynamic nature of physical hydrogels, they are more widely studied in the context of these systems.4 In comparison with classical hydrogels, systems reacting to the stimuli of the environment are characterized by better properties in terms of their use in the innovative therapeutic systems or tissue engineering.
Stimuli-Responsive Hydrogels
Concerning standard hydrogel therapeutic systems, the release of the active substance occurs through shrinking or swelling of the material, diffusion and matrix decomposition.38 If the matrix has the ability to change its properties under the influence of environmental factors, such as temperature, pH or magnetic field, it is referred to as a stimuli-responsive hydrogel. Under the influence of these factors, such parameters as the ability to absorb water or physiological liquid, the shape of the matrix or solubility change, the sol/gel phase transition may also occur. The active control of the release kinetics of the pharmacological substance or gel formation at the site of administration is enabled by manipulation of the environmental factors or conditions prevailing at the site of administration.5
Thermoresponsive Hydrogels
Among the hydrogel systems sensitive to stimuli, thermoresponsive hydrogels are the most extensively studied.4 Polymer matrices forming such systems contain in their structure both, hydrophilic and hydrophobic fragments; their properties mostly depend on the hydrophobic/hydrophilic ratio. In response to temperature changes, the matrix changes its volume due to swelling or shrinking; there may be changes in the solubility of the polymer, conformation and phase transition.4,6
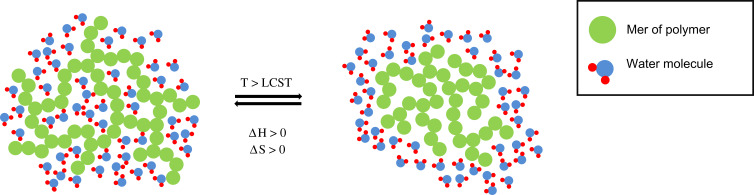
Depending on the properties, thermosensitive matrices are divided into systems showing lower critical solution temperature (LCST) or upper critical solution temperature (UCST). In the case of LCST hydrogels, the dependence of the solubility of polymers on the temperature is non-linear and decreases with temperature increase. At LCST, the solubility drops dramatically and the system changes from sol into a gel. The mechanism of the aforementioned transition is connected with the change of interactions between individual polymer chains and between the polymer and the surrounding molecules. When the temperature is lower than the LCST, the hydrophilic moieties of the polymer chains form hydrogen bonds with hydrophilic molecules located in their environment, and the hydrogel has a high solubility. As the temperature increases, the hydrogen bonds weaken, while the interactions between the hydrophobic elements of the polymer chains become stronger. As a result of this phenomenon, the solubility of the polymer decreases dramatically, and shrinkage of the matrix or phase transition may occur.4,6 This process has been presented in Figure 1.
Figure 1.
Phase transition mechanism of thermoresponsive hydrogels.
Phase transition at LCST in thermodynamic terms is associated with the predominant proportion of the entropy contribution, compared to the enthalpy contribution. The sol–gel transition process is characterized by the increase in enthalpy (ΔH > 0), associated with the endothermic cleavage of polymer-water molecule hydrogen bonds and the increase in the entropy of the system (ΔS > 0). When the polymer is solvated, its structure limits the mobility of water molecules, which reduces the entropy of the system, so desolvation leads to entropy increase. When T < LCST, then ΔH > TΔS, which results in a positive Gibbs function value (ΔG > 0), according to the equation ΔG = ΔH - TΔS. When the temperature increase above LCST, then TΔS > ΔH, ΔG for sol–gel transition becomes negative, and the unsolvated form is thermodynamically preferred in these conditions.39,40
Besides to LCST hydrogels, UCST systems are also described in the literature. In contrast to LCST, the solubility of the matrix increases with increasing of the temperature, the system shows gel–sol transition. Unfortunately, most solutions of this type have a phase transition temperature below 25°C, which makes them practically useless in the field of biomedical applications.4
Although LCST can assume values from a wide range, the most important hydrogels for biomedical applications are the systems with LCST close to the physiological temperature, ie, 30°C - 37°C. This allowed to develop an injectable in situ gel-forming systems. The example of phase transition in the appropriate temperature range is presented in Figure 2.
Figure 2.
The example thermoresponsive hydrogels obtained from poly(ethylene glycol) (PEG) and cyclic esters.
The suitability of LCST and UCST for medical purposes depends mainly on the molecular weight of the polymers used, as well as on the ability of the polymer chains to form hydrogen bonds.4,41–43
In addition, there are also hybrid systems exhibiting both LCST and UCST; they are characterized by a certain temperature range in which the matrix remains in the form of a gel. Examples of such hydrogels are systems based on poly(ethylene glycol) (PEG).4,44,45
The gelation mechanism of amphiphilic macromolecules can be explained in the context of micelle formation. These molecules are able to organize their structure into micelles spontaneously in aqueous media. When the temperature of the polymer solution is below LCST, the micelles are relatively small and their structure is well defined; hydrophobic blocks form the core of the micelle and hydrophilic moieties form the shell. As the temperature rises, the size of the micelles slightly increases, but the solution can flow freely. In some systems, the hydrophobic blocks from the micelles cores can interact with each other and form intermicellar bridges. About LCST, the size of micelles increases significantly, the hydrophobic interactions go stronger and phase transition occurs. Generally, the hydrophobic interactions are the main force responsible for gelation process. When the system temperature achieves UCST, the micellar structure disorganizes and the matrix start to flow.23,46
Thermoresponsive smart hydrogels are based on synthetic or natural macromolecules. In this paper, we decided to focus on the most common synthetic polymers used in smart hydrogels technology, such as poly(N-isopropylacrylamide) (PNIPAAm), Pluronics® (poloxamers), polyesters/PEG copolymers and POPs.47,48 These matrices show gel–sol transition at temperatures close to physiological and they are widely investigated as potential DDSs. Examples of polymer backbones of the mentioned hydrogels are presented in Table 2.
Table 2.
Synthetic Polymers for Thermoresponsivee Hydrogel Drug Delivery Systems
| Material | Abbreviation | Structure | Ref. |
|---|---|---|---|
| Poly(N-isopropyloacrylamide) | PNIPAAm | 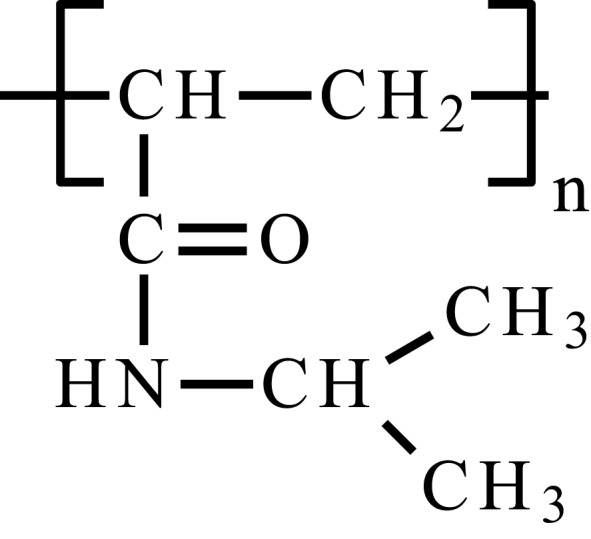 |
[49] |
| Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) | PEO-PPO-PEO, poloxamer | 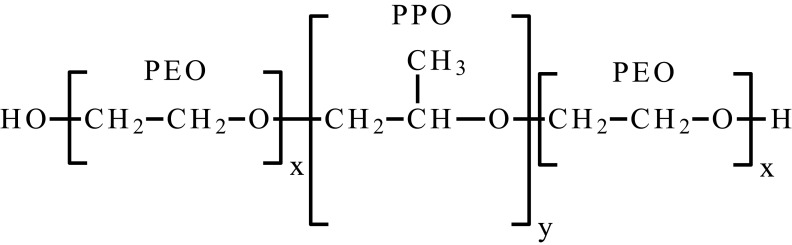 |
[50] |
| Poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) | PCL-PEG-PCL, PCEC | 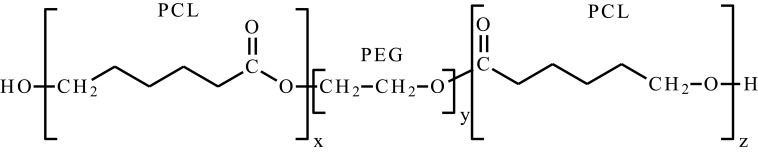 |
[23] |
| Poly(ethylene glycol) methyl ether-poly(ε-caprolactone)-poly(ethylene glycol) methyl ether | mPEG-PCL-mPEG, PECE | 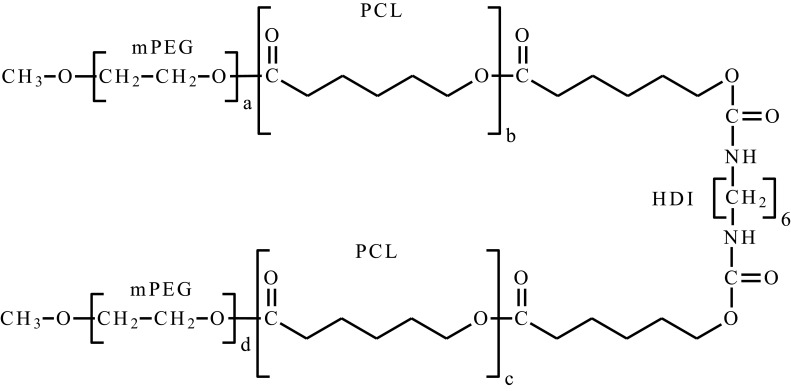 |
[23] |
| Poly(ethylene glycol) methyl ether-poly(lactic acid-co-glycolic acid)-poly(ethylene glycol) methyl ether | mPEG-PLGA-mPEG | 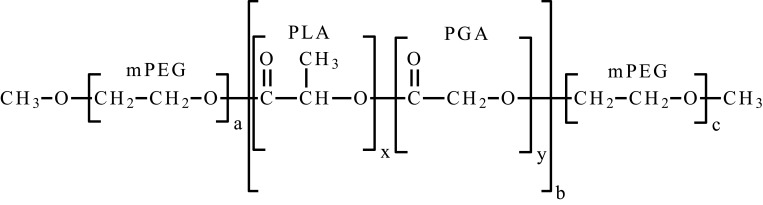 |
[51] |
| Poly(organofosfazene) bearing α-amino-ω-methoxy-PEG and L-isoleucine ethyl ester | [NP(AMPEG350)(IleOEt)]n | 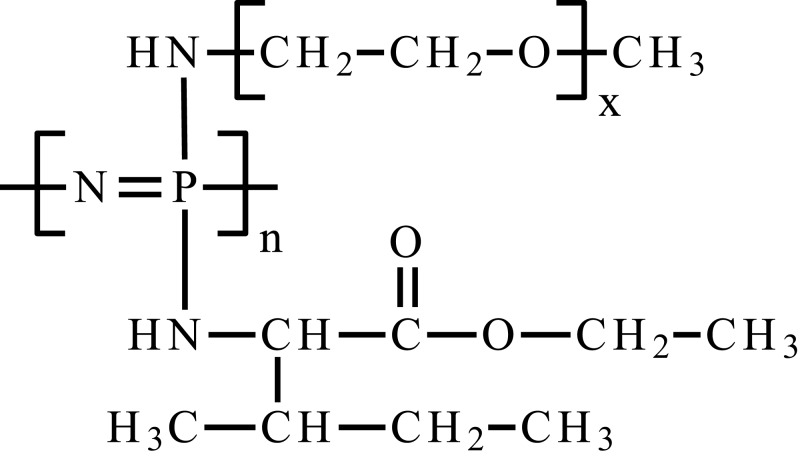 |
[52] |
PNIPAAm is a non-biodegradable synthetic polymer showing LCST phase transition at about 32 °C in pure water, which is important in biomedical applications. It has a soft and flexible coil structure below LCST, that changes when the critical temperature is achieved. The material becomes more hydrophobic, solubility of the material instantly decreases and aggregates are formed. The copolymerization of PNIPAAm with hydrophilic or hydrophobic substrates allows raising or lowering LCST value, respectively. This parameter is also influenced by environmental pH and the presence of salts.47,48,53
One of the strategies of tumor treatment is embolization. The method involves closing the lumen of a blood vessel supplying blood to the tumor, which leads to starvation. Chen et al54 developed a thermosensitive polymer system based on PNIPAAm to be used as a embolization factor for transcatheter arterial embolization (TAE) in hepatocellular carcinoma (HCC) therapy. The authors used cellulose nanowhiskers obtained from Acetobacter xylinum cellulose grafted by PNIPAAm polymers. The prepared system had appropriate mechanical properties and LCST similar to the temperature of the human body (about 34.6°C). Data obtained from transmission electron microscope (TEM) showed that nanowhiskers were a backbone of the system, surrounded by PNIPAAm aggregates responsible for the phase transition process. Nanowhiskers were subjected to the toxicity tests, and the system was considered to be non-toxic. The developed system seemed to be very promising, but researchers did not perform efficacy tests on an animal model.
Typical application of thermosensitive hydrogels in the context of antitumor therapy is drug delivery. Qiao et al55 developed a system based on micelles made of a PNIPAAm-b-poly(L-alanine) block copolymer releasing doxorubicin (DOX). The system had the ability to self-assemble micelles due to the presence of PNIPAAm blocks forming a hydrophilic shell and poly(L-alanine) (P(L-Ala)) blocks forming a hydrophobic core to which lipophilic substances such as anticancer drugs could be incorporated. The system was obtained in the ring-opening polymerization (ROP) process, copolymerizing the amino-terminated PNIPAAm with L-alanine-N-carboxyanhydride (L-Ala-NCA), and then micelles were obtained by dialysis in a DMF/water. The average diameter of the micelles was about 200 nm. PNIPAAm-b-P(L-Ala) showed a phase transition in a narrow range of temperatures, with LCST about 30°C, lower than for PNIPAAm-NH2, which was related to hydrophobic P(L-Ala) blocks. The obtained system showed DOX loading capacity at the level of 9.6 wt%. The performed DOX kinetic studies revealed that at 25°C (below LCST), 25% DOX was released within 50 h. Therefore, at 37°C, the release of DOX was about 70%. It turned out that the system effectively releases the active substance for 20 h; the release was prolonged and controlled by temperature. The peptide bonds present in the macromolecule could be degraded in a physiological environment, which improved the degradability of the system. However, the presented system is not in situ gel-forming; the LCST of the system is too far from physiological temperature. Thermosensitivity between 25°C and 37°C has no practical application because the physiological temperature is always 37°C or could be locally elevated due to hyperthermia. The reasonable way to improve the applicability of the system is to elevate the LCST above physiological temperature and combine with hyperthermia device. Moreover, the problem is non-biodegradable PNIPAAm blocks as well as not conducted toxicity tests, which should be subjected to further studies.
Ghamkhari et al56 developed intravenous docetaxel (DTX) delivery system based on star-shaped amphiphilic copolymer HAPs-star-PCL-b-PNIPAAm (HAPs – hyperbranched aliphatic polyesters) nanomicelles. The authors obtained HAPs and added the PCL segments in the ROP process, yielded HAPs-star-PCL. In the next step, the RAFT-derivative (RAFT – reversible addition–fragmentation chain transfer) was obtained using (4-cyano-4-[(phenylcarbothioyl) sulfanyl] pentanoic acid) as the RAFT agent. HAPs-star-PCL-RAFT was then copolymerized with N-isopropylacrylamide producing the final product, HAPs-star-PCL-b-PNIPAAm. In the next step, nanomicelles containing DTX were prepared by the membrane dialysis method. They showed spherical shape and average diameter of 50 ± 10 nm. The obtained DDS showed LCST about 38 °C–40 °C. Loading content (LC) and encapsulation efficiency (EE) were 9.5% and 95.5%, respectively. Drug release kinetics experiments proved temperature and pH sensitivity of the system. Cumulative release after 222 h (about 9 days) in pH 5.4 and 7.4 was 58.16% and 41.38%, respectively. Interestingly, when the release temperature was elevated to 41 °C, the cumulative release was higher. The initial burst release of DTX was present, but it did not threaten the safety of the therapy. The release rate was prolonged with the kinetics similar to zero-order after initial burst release. Lower pH promoted release, which is beneficial in antitumor therapy, because the tumor is characterized by a reduced pH in relation to physiological values. Nanomicelles were found as biocompatible and showed efficient cellular uptake, so they are very promising as DDSs. Furthermore, due to thermosensitivity of the system, it could be used in combination with magnetic nanoparticles capable of inducing local hyperthermia, which would enhance the effectiveness by the synergy of actions.
Another group of thermoresponsive smart hydrogels are systems based on poloxamers–triblock copolymers of (PEO-b-PPO-b-PEO). They show LCST similar to physiological when the composition, concentration and molecular weight are appropriate; the LCST value may be controlled by these factors. The central block, PPO is hydrophobic, and the external PEO blocks are hydrophilic, the gelation mechanism is connected with micelles formation above critical micelle concentration (CMC) and critical micelle temperature (CMT). When the temperature is under CMT, all blocks of the copolymer are well hydrated, but when it raises, the solubility decreases and molecules tend to aggregate, which causes micelle formation. Poloxamers are bio-inert; however, they have limited integrity, low viscosity and poor mechanical properties, what is needed to be defeated in biomedical use.48,57
Shaker et al58 developed tamoxifen (TMC) niosomes embedded in poloxamer in situ gelling hydrogel system, suitable for conducting targeted therapy of breast cancer. Niosomes are spherical bilayer structures, where the hydrophobic layer is sandwiched by two hydrophilic layers.58 They are able to self-assembly, obtained with non-ionic surfactants, and/or membrane stabilizer. Niosomes as novel DDS, have essential advantages, namely proper cellular uptake and drug entrapment, prolonged drug release and good biocompatibility. The authors prepared TMC-loaded niosomes using Span 60 and cholesterol as surfactant and membrane stabilizer, respectively, in a molar ratio of 1:1, according to thin-lipid film hydration technique.58,59 The spherical-shape niosomes (diameter 212 nm) were incorporated into hydrogel obtained from poloxamer 188 (P188) and/or poloxamer 407 (P407). This system provided sustained release of TMC and high tumor concentration. In vitro drug release tests showed initial burst release of TMC in 24 h; in next 6 days the release kinetics was congenial to zero-order kinetics, 30–40% of TMC was released. Antitumor efficiency of the described system was evaluated in in vivo tests using Swiss Albino mice. Data obtained from the experiment showed improved antitumor efficacy when compared to TMC niosomes alone, free TMC and saline. The developed system is very promising; however, the authors suggest that further research is needed to increase the effectiveness of the system, eg by attaching some ligands to niosomes external layer.
One of the interesting methods used in tumor treatment is brachytherapy that employs radiopharmaceutical intratumoral injections. The main advantages of this method are high antitumor efficacies and relatively low side effects, because of limited local delivery of radionuclide. However, one of the problems is leakage of the substance out of the tumour, because of intratumoral pressure, also increased by injection.60–62 To solve this problem, Zhu et al60 developed an effective 90Y delivery system for unresectable HCC treatment, based on block copolymers of Pluronic® F127, poly(3-hydroxybutyrate) (PHB), and poly(propylene glycol) (PPG). Because of a high critical gelling concentration of poloxamers, connected with elevated viscosity in liquid state, what is undesirable in case of injectable hydrogels, the hydrophobic modificators were used. The addition of PHB and PPG decreased critical gelation concentration (CGC) providing optimal gelation conditions. The obtained poly(F-127/PHB/PPG urethane) copolymers were characterized by a molecular weight of about 22 kDa with a dispersity index (PDI) of 1.6–1.7. The hydrogels exhibited a gelling temperature close to the physiological temperature at low polymer concentration (about 5–7%) and had low viscosity in the liquid phase. The gelation test carried out in a 50% fetal bovine serum environment in a PBS-buffer solution at 37 °C, showed optimal properties and rapid gel formation. Furthermore, in vivo studies showed that some of the systems provided a long retention time of 90Y within the tumor, low viscosity and good injectability. The proposed solution is very promising, but lack of toxicity assay and gelation process inside the needle require further research and refinement.
One of the requirements for polymers used in biomedical applications is biocompatibility and biodegradability. A widely studied group among thermosensitive polymers are diblock, star-shaped and graft copolymers of PEG with cyclic esters. These systems are characterized by biocompatibility, are biodegradable or bioresorbable. To date, many papers have been published PEG/PCL,23,63,79 PEG/polylactide/PCL (PEG/PLA/PCL),80–83 PLA/PEG84–89 or PLA/polyglycolide/PCL (PLA/PGA/PCL)51,90–,97 copolymers to form smart hydrogels. In general, appropriately selecting hydrophilic/hydrophobic ratio, molecular structure, architecture, molecular weight and polymer concentration makes it possible to obtain injectable thermoresponsive smart hydrogel, gel-forming at the site of administration. These systems exhibit an amphiphilic character, in aqueous solutions they possess surfactant properties, which enables solubilization of lipophilic active substances.4,6 Typically, they are obtained by ROP characterized by lower Mn values (about 2000–6000 Da in general),23,63,65,68–86,88,92,93 when compared to PNIPAAm.55,56,98–100
Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone) is a natural substance with antioxidant, anti-inflammatory and anti-cancer properties. It demonstrates the ability to inhibit cell proliferation and has apoptotic properties towards human HCC.101 However, the applicability of embelin is limited due to its lipophilic character and poor solubility in water.101,102 To solve this problem, Peng et al101 developed a thermosensitive hydrogel embelin-delivery system, based on poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone)–PEG–poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone) (PECT).76,101 Embelin/PECT system was obtained by dissolution in tetrahydrofuran (THF) and, subsequently, dropwise addition to double-distilled water and 12 h mixing at room temperature. The system showed sol–gel transition in a body temperature. In vitro release study showed prolonged and zero-order release profile up to 3 weeks (57.08% cumulative release after 504 h), after initial burst release in the first 2 days. Biosafety and biocompatibility of PECT matrices and enhanced embelin/PECT hydrogels cytotoxicity (compared to control samples) have been confirmed in cell culture studies. Afterwards, the in vivo studies on BALB/c mice were performed. When subcutaneously injected, the system was stable for 4 weeks, allowing its relevance for prolonged therapy. In vivo studies confirmed increased antitumoral system efficacy and limited systemic toxicity, compared to a control group. It turned out that the proposed embelin/PECT system is very promising for a potential application in human HCC therapy.
One of the potent cytostatic agents is camptothecin (CPT), the topoisomerase I inhibitor. It has strong anticancer properties, but its applicability in therapy is limited due to high toxicity and low water solubility and bioavailability.103 Therefore, numerous attempts are made to use innovative DDSs, which would enable local distribution of drug and solve the problem of low drug bioavailability. Wu et al103 developed a system based on hollow mesoporous silica nanoparticles (HMSNs), filled with CPT and injected intratumor using a thermosensitive hydrogel, made of poly(D,L-lactide)-PEG-poly(D,L-lactide) triblock copolymer, to avoid the breast cancer recurrence. In the first stage of the experiment, HMSNs with core and shell diameters 80 nm and 20 nm, respectively, were obtained and filled with CPT. The obtained EE was found to be 66.04%. Safety, cell uptake and cytotoxicity studies proved that HMSNs-CPT carriers exhibited adequate properties and had undergone further work steps. Subsequently, CPT nanocarriers were mixed with thermosensitive hydrogel, which showed sol–gel transition about 38 °C. The release of CPT from the matrix has been extended; after 15 days, approximately 60% of cumulative release was observed, compared to 100% in control samples. In vitro and in vivo studies have shown the effectiveness of the system obtained, while reducing serious side effects.
Smart hydrogels based on POPs show interesting properties in DDS. First of all, they are biocompatible and biodegradable, have good mechanical properties, and their decomposition products are non-toxic. POPs are hybrid materials; containing organic and inorganic moieties in their structures. Structurally, they are poly(dichlorophosphazene) derivatives, whose chlorine atoms are substituted with alkoxy and/or amino groups. Depending on the substituents, POPs exhibit various properties and finding numerous applications in biomedicine.104 POPs are used to obtain thermosensitive smart hydrogels, and their properties depend on the type of substituents, their architecture and hydrophilic/hydrophobic balance.48,105 Appropriate adjustment of these parameters allows to obtain a gel-forming system at a temperature close to physiological, which is important for injectable drug delivery hydrogel matrices. Such systems are characterized by the ability to release both hydrophobic and hydrophilic molecules, ensuring prolonged delivery for 2 or 3 weeks, respectively.106,107 2-Methoxyestradiol (2-ME) is a methoxy-derivative of estradiol, a potential drug in anticancer therapy. Its mechanism of action is based primarily on the inhibition of angiogenesis, and its effectiveness is confirmed by in vitro and in vivo studies.108,109 However, 2-ME has several disadvantages, which limit its bioavailability, ie, poor solubility in water and a first-pass hepatic metabolism when administered per os.110 One of the methods to resolve this problem was proposed by Cho et al.109 They developed a thermosensitive 2-ME hydrogel release system based on POPs substituted by α-amino-ω-methoxypoly(ethylene glycol) (AMPEG), l-isoleucine ethyl ester (IleOEt) and glycyl lactate ethyl ester (GlyLacOEt) for potential use in breast cancer. The obtained POPs were characterized by an average molecular weight of about 35–38 kDa with PDI 2.38–2.73. The use of the polymer as a solubilizer significantly improved the solubility of 2-ME in an aqueous environment from 2.57 x 10−4 to 2.72 mg/mL. Moreover, the polymer exhibited a sol–gel transition at a temperature close to physiological. In vitro and in vivo experiments showed extended and controlled 2-ME release from the matrix up to 35 days, while the release kinetics were close to the zero-order kinetics.
The most studied groups of this type of biomaterials are thermosensitive smart hydrogels, which tend to be the nearest to wide-ranging clinical trials. Polyesters and POPs present the most promising and desirable features, as well as optimal mechanical and drug release properties due to their biodegradability. In our opinion, the researchers should focus on biodegradable, amphiphilic micelle-forming macromolecules, because of their ability to solubilize highly hydrophobic molecules, which usually anticancer drugs are. Furthermore, very promising solutions tend to be the systems composed of drug-loaded nanoparticles (eg, nanomicelles or HMSNs) immersed in thermoresponsive hydrogel, which comprises the advantages of both systems.
pH-Sensitive Hydrogels
Among the thermoresponsive hydrogels, there is a large group of polymers that change properties under the influence of pH. The macromolecule chains contain acidic or basic moieties that are able to donate or accept protons. Thus, pH-sensitive polymers are thus classified into two basic groups: polyanions and polycations, called polyacids and polybases, respectively.
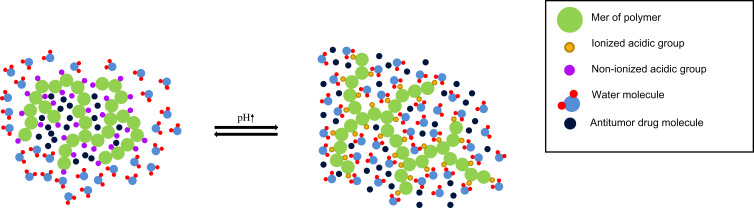
Polyanions in an alkaline environment (above pKa of polyacid) are deprotonated and the acidic groups are negatively charged. In the case of polycations, the situation is the opposite. Due to ionization, the conformation of the polymer molecule changes and solubility increases significantly. It turns out that ionization promotes swelling of the hydrogel; basic carriers at high pH will absorb small amounts of water.9,111 When the environmental pH increases, acidic moieties of polyanion become negatively charged and starts to repel each other. Moreover, the hydrophobic interactions between chains weaken and hydrogen bonds between ionized groups and water molecules are formed. Furthermore, dissociation leads to the formation of more ions, the osmotic pressure increases within the hydrogel structure; an osmotic gradient between inner and outer layers of hydrogel causes the inflow of water into the structure interior; the matrix is swelling. The mechanism of pH-sensitivity has been demonstrated in Figure 3.
Figure 3.
pH-responsive anionic smart hydrogels mechanism of action.
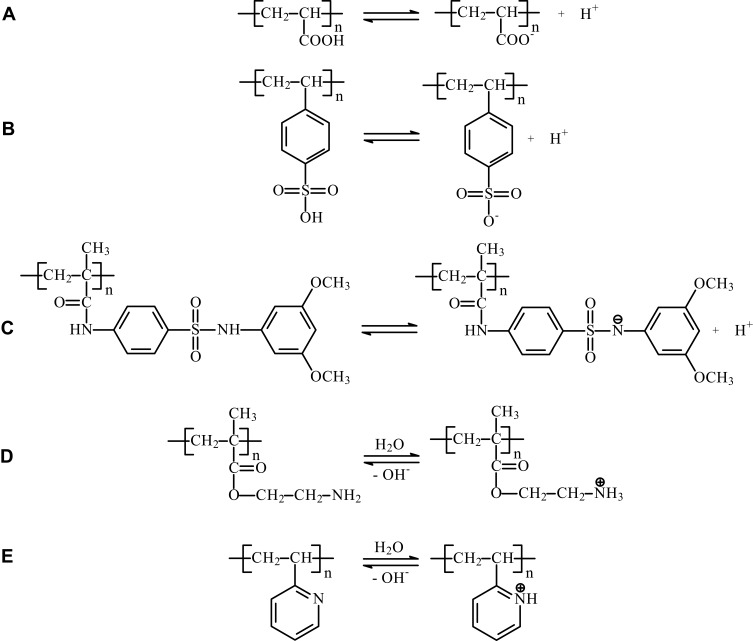
As the amount of absorbed water increases, the system becomes more permeable to drug molecules; thus, pH-responsive hydrogels can be used in oral therapeutic systems releasing a biologically active substance in the stomach or intestine.9 Analogously to LCST in thermosensitive polymers, pH-sensitive smart polymers show significant pH value, when their properties dramatically change. It is called critical or transition pH (pH*), dependent on pKa of polyelectrolyte.111–113 For biomedical applications, it is important to adjust the pH* of the polymer, so that the properties change or phase transition occurs in the appropriate, well-defined conditions. In the case of pH-sensitive smart hydrogels, two basic strategies for pH* value regulation are distinguished. The first is the copolymerization of a pH-sensitive polymer with other polyacids or polybases or incorporation of acidic or alkaline moieties into the polymer chains. The other way is to copolymerize the macromolecule with lipophilic structures, which changes the hydrophilic/hydrophobic balance and leads to pKa and pH* shifts. When the molecule is more hydrophobic, stronger electrostatic repulsion is needed to overcome the hydrophobic interactions and change the macromolecule conformation. Thus, copolymerization of monomers with hydrophobic moieties increases the pH* for polyanions and decreases it for polycations.111,112 Acidic and alkaline properties of different groups of pH-sensitive smart polymers are presented in Figure 4.
Figure 4.
Acidic and basic properties of pH-sensitive smart polymers. (A) Polyacrylates (PAAc), (B) sulfonic acid derivatives (styrene sulfonic acid), (C) sulfonamides, (D) polyamines (PMAA), (E) pyridine derivatives (P2VP).
According to pH-responsive hydrogels, polyacids (polyanions) are proton-donating polymers, able to change their properties, such as solubility and swelling due to environmental pH changes. pKa of polyacid differs from monomers pKa, it is influenced by molecular weight and polymer composition.111,114 The polymers used for pH-responsive hydrogels development are based on PAAc, poly(sulfonic acid) or sulfonamides derived vinyl monomers. The first two groups of polymers suffer from some limitations, they show wide-range pH sensitivity, the phase transition is not sharp and out-of-range sensitivity can be observed.111 Therefore, to overcome these disadvantages, matrices modified with sulfonamides were developed. Sulfonamide-based smart hydrogels show sharp and well-defined pH* which is very desired property in the context of biomedical applications.115
Polybases are a group of pH-sensitive smart polymers, containing alkaline moieties susceptible to protonation in acidic conditions. One of the most common polycations is primary, secondary and tertiary polymerized amines, such as poly(N,N’-dimethyl aminoethyl methacrylate) (PDMAEMA), poly(2-aminoethyl methacrylate) (PMAA) or poly(ethylene imine) (PEI). The free electron pair of the nitrogen atom forms a coordinate bond with H+, which determines the basic properties of the macromolecule.111 Another group of polycations is polymers comprising nitrogen-containing aromatic heterocycles. The nitrogen atom has a lone electron pair, able to proton binding. The most extensive studied polybasic smart polymers of this class are pyridine-based or imidazole-based macromolecular derivatives.111
Polyacids and polybases used in smart hydrogels technology are summarized in Tables 3 and 4.
Table 3.
Synthetic Polymers and Monomers Used in Polyanionic pH-Sensitive Hydrogels Technology
| Name | Chemical Formula | pKa | References | |
|---|---|---|---|---|
| Acrylic acid derivatives | ||||
| Poly(acrylic acid) | 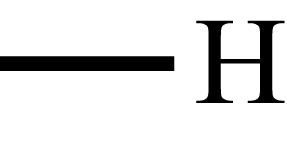 |
 |
4,28 | [111,116] |
| Poly(methacrylic acid) |  |
6 - 7 | [111,117] | |
| Poly(ethylacrylic acid) |  |
6,3 | [111,118] | |
| Poly(propyl acrylic acid) |  |
6,7 | [111,118] | |
| Poly(butyl acrylic acid) | 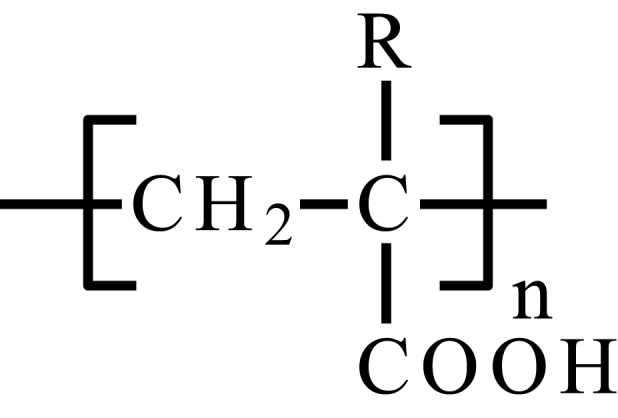 |
7,4 | [111,119] | |
| Sulfonic acid derivatives | ||||
| 2-acrylamido-2-methylpropylsulfonic acid | 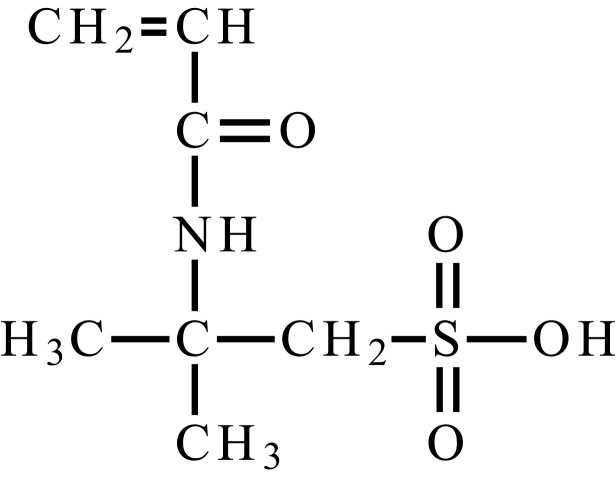 |
2-3 | [111] | |
| 2-methacryloxyethylsulfonic acid | 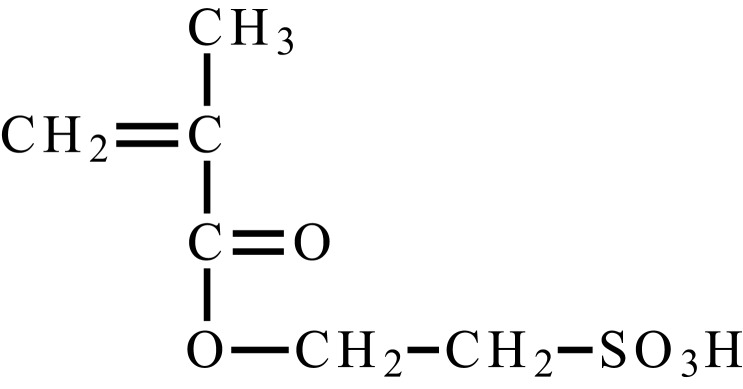 |
2-3 | [111] | |
| 3-methacryloxy-2-hydroxypropylsulfonic acid | 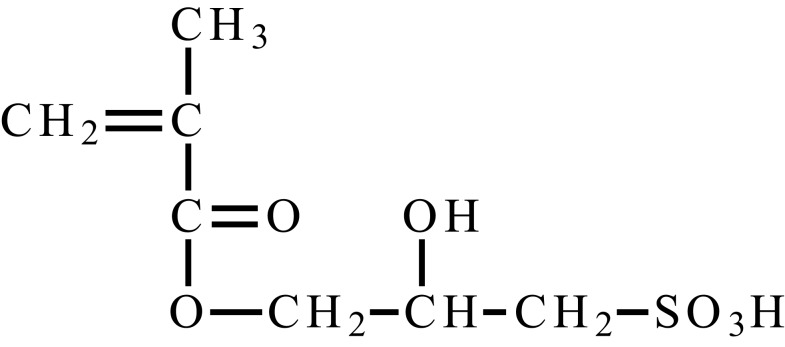 |
2-3 | [111] | |
| Ethylenesulfonic acid | 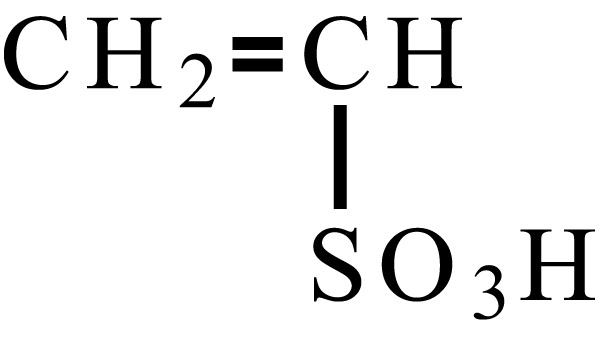 |
2-3 | [111] | |
| Styrenesulfonic acid | 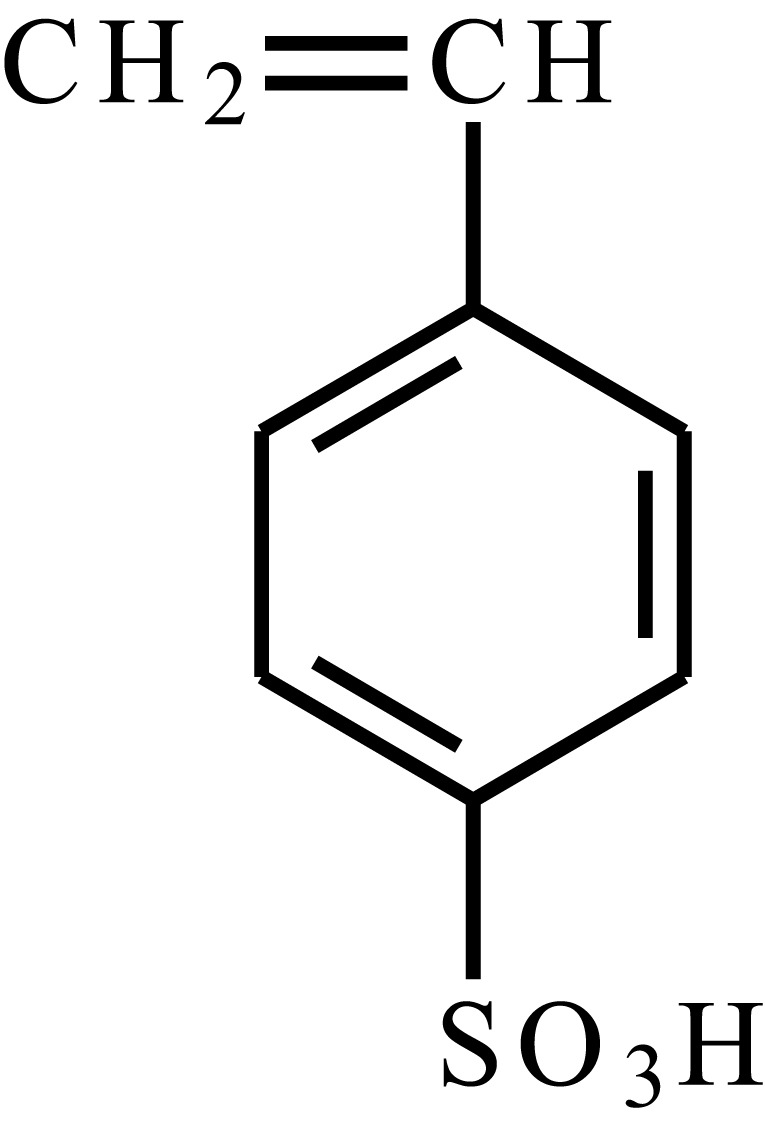 |
2-3 | [111] | |
| Sulfonamides | ||||
| Sulfapyridine | 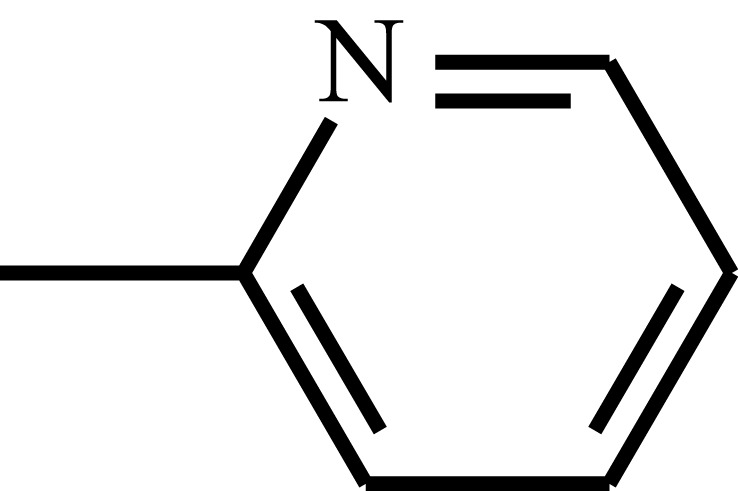 |
 |
8,43 | [111,120–122] |
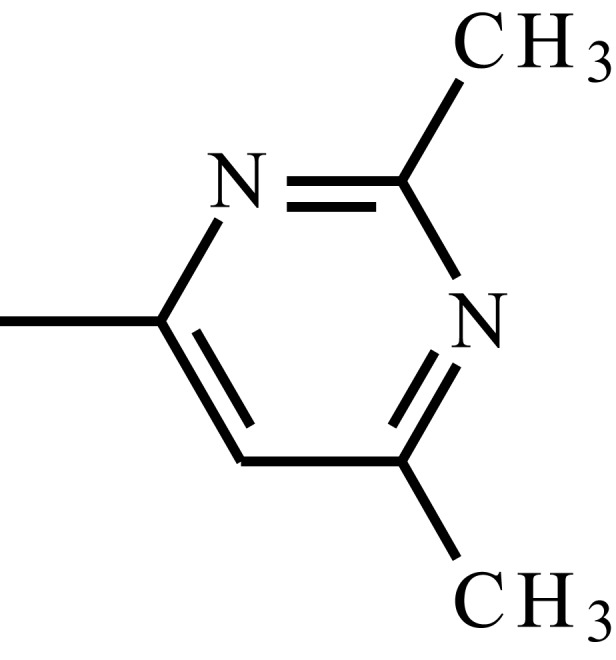
| Sulfisomidine | 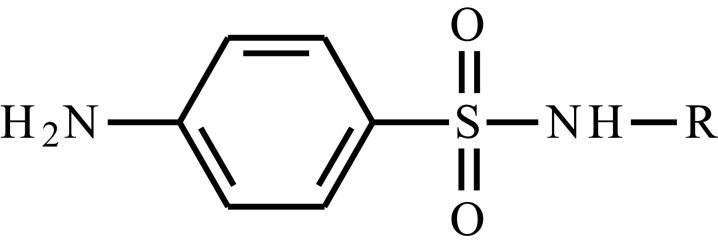 |
7,40 | [111,120,121] | |
| Sulfamethoxypyridazine | 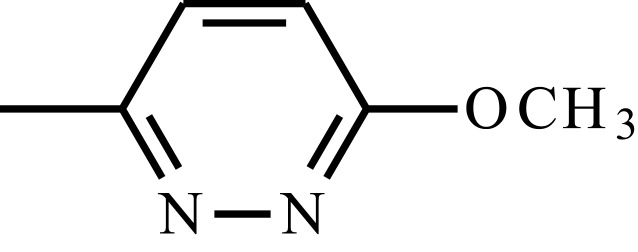 |
7,40 | [111,120,121] | |
| Sulfamethazine | 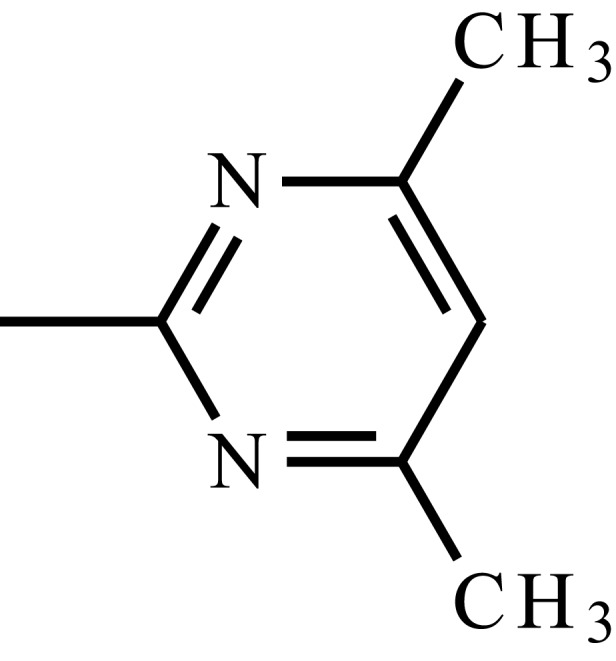 |
7,25 | [111,115,120,121] | |
| Sulfadimethoxine | 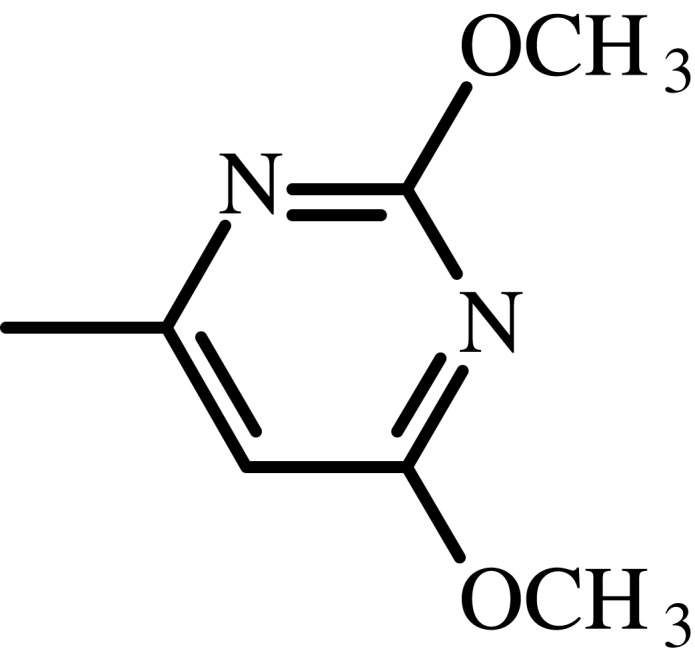 |
6,10 | [111,120,121,123] | |
Table 4.
Synthetic Polymers Used for Cationic pH-Sensitive Hydrogels
| Name | Abbreviation | Chemical Structure | pKa | Ref. | |
|---|---|---|---|---|---|
| Polyamines | |||||
| Poly(N,N’-dimethyl aminoethyl methacrylate) | PDMAEMA (PDMA) | 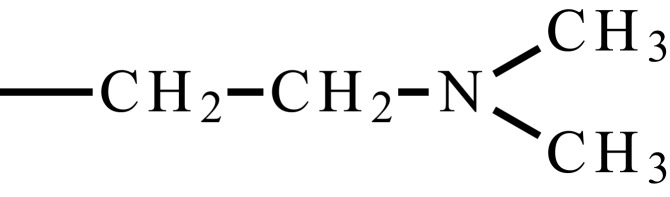 |
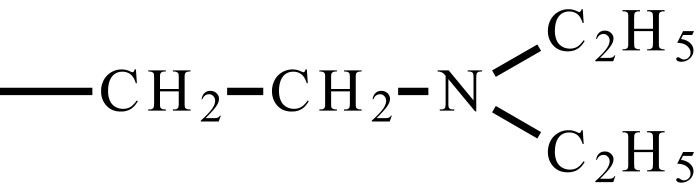 |
≈8 | [111,124] |
| Poly(N,N’-diethyl aminoethyl methacrylate) | PDEAEMA (PDEA) | 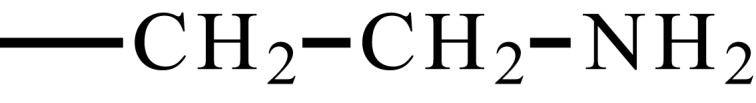 |
≈7,3 | [111,124] | |
| Poly(2-aminoethyl methacrylate) | PMAA |  |
≈7,6 | [111,120] | |
| Poly(ethylene imine) | PEI | 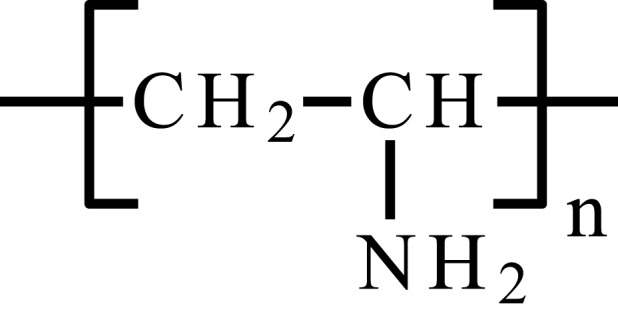 |
≈4,5 ≈6,7a | [111,120] | |
| Poly(vinylamine) | PVAm | 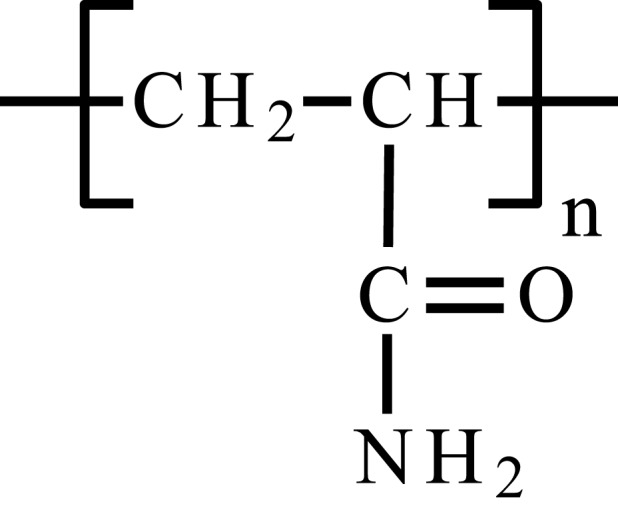 |
≈10,7b | [111,125] | |
| Poly(acrylamide) | PAAm | 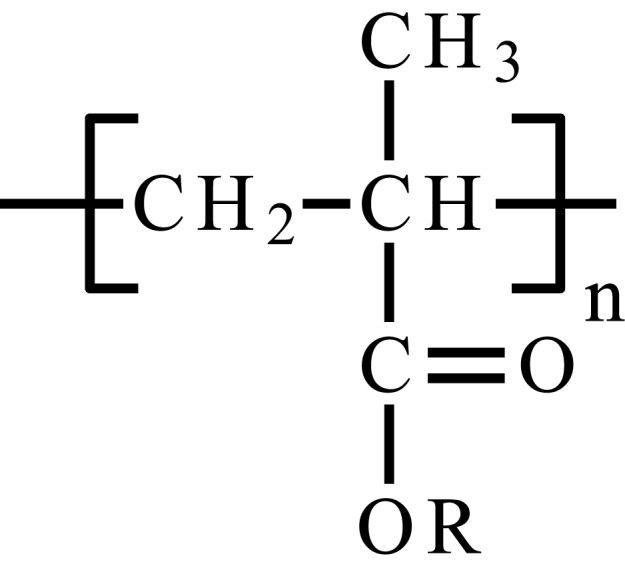 |
[111] | ||
| Pyridine and imidazole derivatives | |||||
| Poly(2-vinyl pyridine) | P2VP | 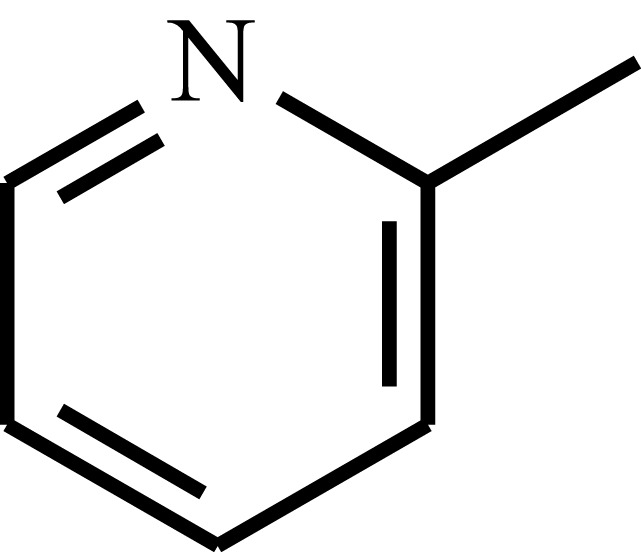 |
 |
≈5,9 | [111,126] |
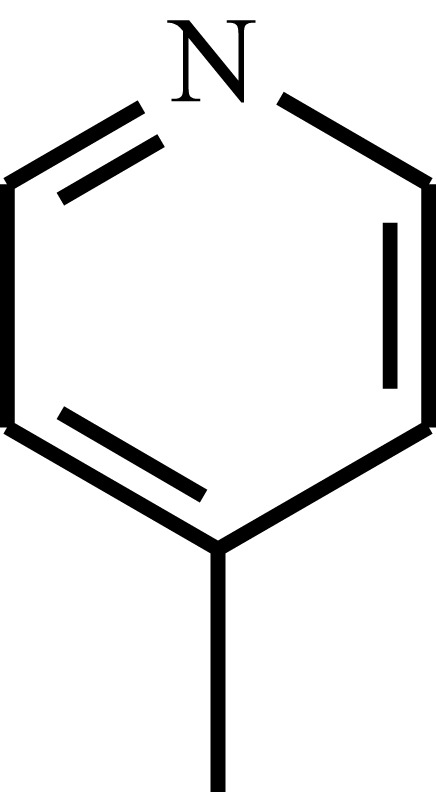
| Poly(4-vinyl pyridine) | P4VP | 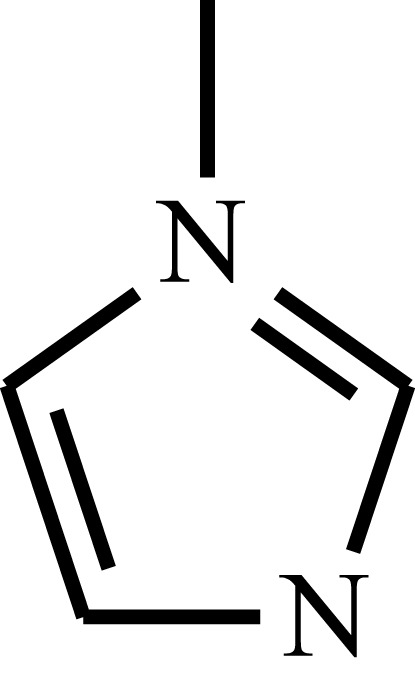 |
≈5,39 | [111,127] | |
| Poly(N-vinyl imidazole) | PVI | 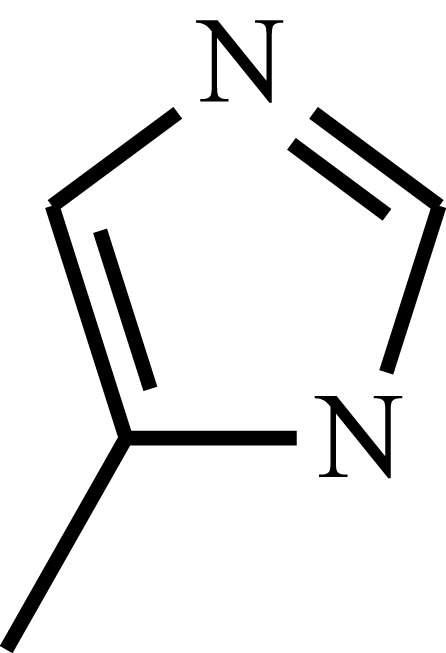 |
 |
≈6 | [111,128] |
| Poly(4-vinyl imidazole) | P4V | 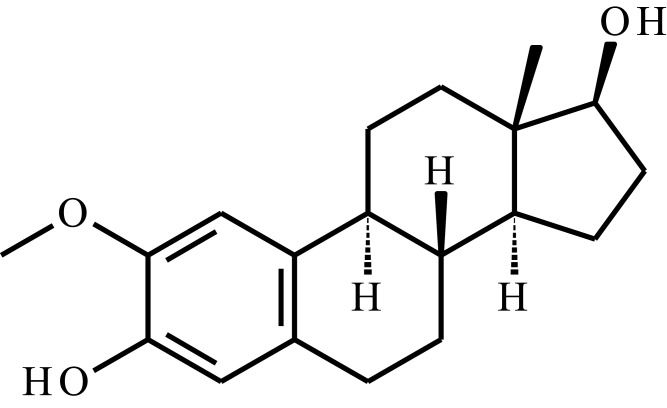 |
≈6 | [111,129] | |
Notes: a4,5 for primary and 6,7 for secondary amine groups. bpKa for ethylamine, analogue for PVAm. The PVAm behavior cannot be described by pKa.
One of the most explored field in pH-responsive smart hydrogels antitumor applications is oral DDSs. Since the vast majority of anticancer drugs are hydrophobic, oral DDSs could meet several basic requirements. First of all, the system should release the active substance in absorptive small intestine, it should have mucoadhesive properties to ensure a prolonged presence in the intestine lumen so that the drug molecules can effectively absorb into the bloodstream. In addition, the desired property is the ability to inhibit P-gp efflux pumps that pump out xenobiotics back to the gastrointestinal tract. pH-responsive hydrogels, which meet these criteria are very interesting as oral DDS, because they protect the active substance from the acidic environment of the stomach. Polyanionic smart hydrogels in low gastric pH are non-ionized and stay in a collapsed form, which protects the active substance from acidic conditions. When the hydrogel gets into the small intestine, it starts to swell, water permeability increases and provides drug release.25,112,130–132 Puranik et al25,133 developed an oral nanogel DOX delivery system based on poly(methacrylic acid) (PMAAc) backbone copolymerized with more hydrophobic monomers, such as n-butyl methacrylate (nBMA), tert-butyl methacrylate (t-BMA) methyl methacrylate (MMA) or n-butyl acrylate (nBA) to adjust suitable pH* and increase hydrophobic drug incorporation. Subsequently, obtained polymer was grafted with PEG to provide better integrity in the collapsed state of the hydrogel, because of hydrogen bonding with PEG chains. Moreover, PEG fragments inhibited P-gp efflux pumps in the small intestine,134 promoted mucoadhesion and played a role in biocompatibility.135 Thus, some chain fragments formed the hydrophobic core able to effective drug entrapment, and other chain fragments formed hydrophilic shell of the obtained nanoparticles. Among other formulations, poly(MAA-g-PEG-co-MMA) was described as the matrix with the most desirable properties. It exhibited the best drug loading capacity, appropriate physical properties and cytocompatibility. The average particle size was pH-dependent and increase from 277.3 nm at pH 4.5 to 472.3 nm at pH 7.5. Zeta-potential measurements show the significant potential decrease as pH becomes more alkaline. For poly(MAA-g-PEG-co-MMA), it varies from −3 to −20 mV, for pH 4.5 and 7.5, respectively. The release kinetics in vitro studies showed cumulative release of DOX at around 75% in 6 hours, with similar to zero-order kinetics, without significant “burst release”. Obtained oral DOX delivery system is very promising in tumor therapy, because of preferable administration route, but the lack of in vivo assay makes more testing required to determine the efficacy and safety of the therapy.
An interesting solution in the field of innovative oral DDSs was proposed by Wang et al.136 The authors encapsulated DTX in the micelles with amphiphilic properties. The resulting copolymer PCEC had the ability to form micelles with the hydrophilic shell (PEG fragments) and a hydrophobic core (PCL fragments), wherein DTX has been enclosed. Developed micelles were used to increase permeability and solubility of DTX. In the next step, drug-loaded micelles were incorporated into the smart pH-sensitive hydrogel, based on PEG-PCL-acryloyl chloride (AC) (PECA) modified by itaconic acid (IA) and PEG-methyl ether methacrylate (MPEGMA). The poly(ECA-IA-MEG) was obtained, which provided suitable conditions for oral DDS, especially side-specific intestinal drug release, important for chemotherapy. The DTX-micelles with an average diameter of 20 nm showed proper drug loading efficiency, EE and DL parameters for micelles were 97.02% and 7.76%, respectively. The antitumor activity of DTX-PCEC micelles was also enhanced, compared to free DTX. In vitro drug release assay showed that DTX-micelle-hydrogel released DTX within 336 h in a controlled and pH-dependent manner. There were significant differences between 1.2 and 6.8 pH release profiles, the cumulative release was higher in the less acidic environment. Moreover, when the release medium was simulated gastric and intestinal fluid, the difference was much greater, and cumulative release after 336 h was about 72% (intestinal fluid) and 23% (gastric fluid). Orally administered DTX-micelle-hydrogel in vivo antitumor efficiency tests showed similar tumor growth inhibition compared to free DTX (Taxotere® administered intravenous), but side effects were significantly limited. The presented solution is very interesting, but further experiments are needed to enhance the effectiveness above the classic intravenous DTX therapy.
One of the features of tumor cells is significantly increased metabolism in comparison to healthy cells, which is associated with intense cellular divisions and disturbed homeostasis, especially. Intensive metabolism causes a greater demand for oxygen and nutrients. To meet the problem, tumor tissues have the ability to surround themselves by a bundle of blood vessels in the angiogenesis process. However, due to the rapid multiplication of cells, the structure of the resulting vessels is disordered and unable to supply the tissue to oxygen sufficiently, which leads to chronic hypoxia. Under these conditions, cells start anaerobic respiration, which leads to the formation of lactic acid and acidification within the tumor tissue at a pH level of less than 6.5 (compared to physiological pH 7.5). Due to local acidification, it seems interesting to use pH-sensitive hydrogels, especially polybases, as antitumor DDSs.111,137,138
Lym et al139 have developed interesting pH-sensitive smart hydrogel DDS for human HCC treatment. The obtained solution has a dual mechanism; embolization of blood vessels nourishing tumor and DOX controlled release. The main assumption was the development of a smart hydrogel showing sol–gel transition under the influence of decreased pH of the tumor tissue. The flowing sol could be delivered via a microcatheter to hepatic artery and solidified in cancerous tissue, characterized by lower pH, and form a DOX depot. In the first step, the block copolymer containing CL, PEG and sulfamethazine (PCL-PEG-SM) was synthesized. Sulfamethazine (SM) is an anionic compound able to deprotonate in an alkaline environment, providing pH-sensitiveness of a hydrogel. The synthesized PCL-PEG-SM copolymer showed average molecular weight about 25,800 Da and 1.45 PDI, the pKa was about 6.80. Subsequently, the sol–gel transition assay was carried out. The system was liquid, flowable sol at pH 8.0, and when the pH decreased to 7.4 or 6.8, sol–gel transition was observed and the hydrogel formed solid and non-flowable structure. DOX in vitro release studies showed 65% cumulative release after 4 weeks, in a prolonged and controlled manner. Lipiodol®, radiopaque substance used as an X-ray contrast agent, was added to the developed system (10 wt%), which allowed easy observation of gelation processes in vivo. In previous tests, the insignificance of Lipiodol® to gelation processes was proven. To examine in vivo delivery of the hydrogel, the system in a sol state was administered by intraarterial or intratumor injection to VX2 rabbits. The white spots visible on computer tomography (CT) photographs showed the radiopaque solid gel location and its persistence in the site of administration for at least 5 hours, what proves the intratumoral gelation and usefulness of the hydrogel. Moreover, after tumor resection, the solid gel was observed in the vasculature (intraarterial administration). Summing up, the obtained smart hydrogel seems to be appropriate for intraarterial administration via a microcatheter to embolize the blood vessels and form a DOX depot. However, the lack of in vivo effectiveness assay is needed to verify its potential.
Another pH-sensitive chemoembolization hydrogel system was presented by Nguyen et al.140 In this study, the novel hydrogel system comprised of poly(CL-co-LA)-PEG-poly(CL-co-LA) (PCLA-PEG-PCLA) and SM, abbreviated (PCLA-PUSSM). The synthesis of PCLA-PUSSM was performed in three steps. Firstly, the triblock copolymer PCLA-PEG-PCLA was obtained via ROP process using Sn(Oct)2 as a catalyst. Subsequently, sulfamethazine acrylamide (SM-A) was coupled with α-thioglycerol in Michael addition, sulfide sulfamethazine monomer (SSM) was obtained. In the next step, HDI was used to polymerize SSM and connect it to free hydroxyl groups of triblock copolymer, PCLA-PUSSM was obtained (Mn 17470 Da, PDI 1.47). The gel-forming properties of the system were evaluated using tube inverting method and rheometric analysis in various conditions of pH and temperature. Briefly, in the injection conditions (25 °C, pH 8.5) 30 wt% PCLA-PUUSM flows freely, and forms stable gel in tumor conditions (37 °C, pH 6.5–7.2) and physiological conditions (37 °C, pH 7.4); the pH* value was estimated as 8.2. The in vitro drug release study showed prolonged and sustained DOX release profile; cumulative release after 2 weeks was estimated as about 25%. Moreover, MTT cytotoxicity assay showed only mild toxicity of the matrix; the hydrogel can be used as a biomedical device. Usefulness of the hydrogel as embolization agent was examined in vivo. Fifty minutes after subcutaneous injection into Sprague-Dawley rats the gel was observed. Moreover, in vivo degradation tests showed that the degradation rate was about 50% after 4 weeks. When the hydrogel was injected intratumorally, 5 h after injection the gel was clearly observed at the tumor site. The antitumor activity of DOX-loaded chemoembolization system was estimated in vivo by tumor growth inhibition tests. One of the prepared formulations (25 wt% PCLA-PUSSM, 20% Iohexol®, 10 mg/mL DOX), Formulation 3 showed promising properties. After 1 week postinjection, the percentage of tumor growth was 6% and 48% after 2 weeks (when compared to initial size). For comparison, the values observed for Formulation 1 (probably washed away from the side of administration) was 189% and 753%, respectively. The obtained data show the great potential of hydrogels as chemoembolization agents and will be an important branch of development for smart hydrogels.
Polyacids play a key role in the field of pH-sensitive hydrogels that are potentially useful in antitumor therapy because tumor tissue shows pH values below healthy tissues. Moreover, protection against gastric fluid is usually provided by polyacids that are shrunk in an acidic environment. Oral DDSs appear to be very significant, because this route of administration is associated with major patient comfort improvements. In our view, the future belongs to systems that allow therapy using different methods, combining drug delivery, embolization, and hyperthermia for example. We assume the synergistic impact from these combinations may significantly increase the therapy’s effectiveness.
Photosensitive Hydrogels
Among the photosensitive smart hydrogels, three basic groups are distinguished: hydrogels sensitive to near-infrared radiation (NIR), visible light and UV.4,6
One of the methods for obtaining light-responsive systems is the incorporation of specific chemical moieties, called chromophores, into the system. They have the ability to absorb and emit electromagnetic radiation, and as a result, the temperature of the system increases. This kind of matrices has hydrophobic and hydrophilic fragments in a polymeric chain. As a result of the rising temperature, hydrogen bonds between the chains and the surrounding water molecules are weakened, analogously to the thermosensitive hydrogels. In consequence, the hydrogel volume and water absorption capacity changes and phase transition can occur.6,141 Interestingly, in a field of antitumor drug delivery, the elevated temperature in the side of administration is very beneficial because of lower tolerance of tumor cells to overheating, when compared to healthy tissues.142,143 Therefore, one of the strategies of cancer treatment is photodynamic therapy connected with simultaneous chemotherapy, called chemo-photothermal therapy.144–146
Another solution is the use of ionizable functional groups under the influence of radiation. Ionization caused by radiation leads to increasing the osmotic pressure inside the matrix and the hydrogel swell, similarly as in the case of systems sensitive to pH changes.6,147
In addition, it is also possible to incorporate the chromophore groups into the matrix that change their physical and chemical properties under the influence of exposure to light; the resulting differences change the structure of the hydrogel.6
Among the smart hydrogels sensitive to UV radiation, there are two basic mechanisms associated with the creation or disintegration of the polymer chains. The synthesis of photo-cleavage systems requires the use of photosensitive cross-linkers, which under the influence of UV or visible radiation, change their chemical composition. The photoisomerization or photooxidation of the cross-linkers causes changes in a structure and properties, that may be a trigger for drug release.4 On the other hand, photopolymerization can lead to in situ cross-linking and gel formation at the site of administration in drug delivery. This solution needs a UV or visible light-sensitive initiators, that form free radicals by photoirradiation and starts the photopolymerization process.4,148,149
Kawano et al150 have developed an intravenous, injectable system containing gold nanorods coated with a PNIPAAm thermosensitive hydrogel capable of changing its properties under the NIR influence. Due to the photothermal effect, NIR radiation leads to an increase in the temperature of the hydrogel. As a consequence, hydrophilicity and water solubility of the polymers decrease and phase-transition occurs. The mechanism of gel formation is analogous to thermosensitive hydrogels. The PNIPAAm-coated nanorods exhibited 300 nm diameter spherical shape, and this system’s LCST was 34 °C. Due to the slow phase transition of the nanogel, the temperature is lower than physiological; however, it flows freely in the vasculature for 10 minutes. In vivo assay on mice showed significant gold accumulation in a kidney irradiated with NIR after 30 minutes of injection, when compared to a non-irradiated organ. As a result of radiation exposure, there was a phase transition associated with a decrease in the solubility of the system and “immobilization” of the matrix within the target tissue.150,151 Such form of approach appears to be very effective in the sense of targeted therapy, which enables a higher concentration of an active substance in the target tissue, at the same time reducing systemic dissemination, which is particularly important in the case of highly toxic therapy such as anti-cancer drugs. The authors proposed optimizing the method for rapid phase transition at higher temperatures (38–42 °C).
Pinto et al152 proposed a photosensitive hydrogel matrix, releasing carbon monoxide (CO). The evidence shows the antiproliferative and proapoptotic properties of CO,152–155 as well as increasing tumor cells sensitivity to chemotherapeutics.152,156 Neoplastic tissue is usually characterized by elevated levels of reactive oxygen species (ROS) and antioxidants, and the oxidative/antioxidative ratio seems to be very brittle. It is believed that CO may be a factor able to disturb the equilibrium by increasing mitochondrial ROS production, leading to cell apoptosis.152,157–160 One of the delivery strategies for bioactive CO to tumor cells is the use of CO releasing molecules (CORMs). They are usually complex compounds containing a metal atom (eg, Mn, Ru), surrounded by ligands, among which there are CO groups. A special group of CORMs are photosensitive compounds, abbreviated as photoCORMs. They allow spatio-temporal controlled CO release, triggered by light irradiation.152,161–165
The scientists developed a CO releasing hydrogel, containing [Mn(CO)3(qbt)(4-vpy)](CF3SO3) (qbt – 2-(quinolyl)benzothiazole) photoCORM, covalently bonded through 4-vinylpyridyne (4-vpy) to a 2-hydroxyethyl methacrylate polymer chain (HEMA). According to evidence, the use of the mentioned photoCORM system enables quick release of CO from the matrix at low light power. Additionally, this photoCORM discolorates from deep orange to colorless, due to CO release, which makes it easier to observe the process.152,166 The photosensitivity tests showed effective CO release under broad spectrum visible light exposure. The release profile was similar to zero-order kinetics, under strict control of light intensity. In the experiment described, light was provided by a developed CO-catheter, made of light-transmitting fiber end-capped with a photoCORM-hydrogel system. Human colorectal adenocarcinoma cancer cells (HT-29) and human embryonic kidney cells (HEK293) were used for in vitro apoptotic properties of the system assay. The data obtained from the experiment showed significant, light-power-dependent apoptotic effect against cancerous cell culture, and no significant effect against healthy cells. These results suggest the possibility of effective and non-toxic anticancer therapy, precisely controlled by an external light stimulus.
The use of ultraviolet light as a trigger in drug release is connected with some problems. First of all, UV is a high-energy electromagnetic wave that is poorly permeable to tissues, which makes it difficult to treat deeper organs. In addition, prolonged UV exposure may have a cancerogenic effect.4,167–169 One of the strategies to overcome these obstacles is the use of up-converting nanoparticles (UCNPs). Under the influence of NIR radiation, UCNPs absorb longer-wave photons and then emit photons of UV light. In order to this, it is possible to significantly reduce the negative impact of UV on tissues, due to in situ local UV emission, and the use of better permeable NIR light.4,146,167,170,171
Yan et al171 introduced an interesting hydrogel system containing UCNPs for photo-controlled large biomacromolecules delivery. The obtained system was based on NaYF4:TmYb nanoparticles, capable of absorbing radiation from the NIR range and converting it to a UV-Vis radiation, which is a trigger for the photo-cleavable hydrogel structure. Nanoparticles had hexagonal prism shapes (length 36 nm and width 32 nm) with a narrow dispersity. The developed hydrogel consisted of polyacrylamide and PEG copolymer chains, cross-linked by photocleavable cross-linkers containing o-nitrobenzyl moieties, susceptible to UV-Vis radiation. The UCNPs and model biomacromolecules were incorporated into the hydrogel matrix. Under the NIR (980 nm) illumination, UCNPs emitted radiation with several different wavelengths from UV-Vis spectrum. It was shown that 250–400 nm is the most suitable for photocleavage because light from that range was efficiently absorbed by a hydrogel; the decomposition of the matrix was observed and the biomacromolecule was released. The release study was carried out using fluorescein isothiocyanate bovine serum albumin (FITC-BSA). After 50 minutes of 980 nm NIR radiation of the protein-loaded hydrogel, the cumulative release was about 67% and 38% for 5 W and 3.1 W laser power, respectively. When there was no radiation, the matrix decomposition was insignificant. The data showed NIR-dependent, controlled release of FITC-BSA. The described system seems to be appropriate to use as antitumor DDS because the activity of a highly cytotoxic drug is off when the light stimulus is absent. Moreover, it is possible to precisely regulate the drug release by adjusting the laser power and exposition time. Notwithstanding, there is no cytotoxicity and antitumor effectiveness evaluation, so further research is needed to consider this solution as anticancer DDS.
Photosensitive hydrogels are very promising as DDSs, especially since they can provide precise control over drug release. This strategy enables using vastly toxic drugs targeted directly to tumor tissue. In addition, there is a potential to combine targeted chemotherapy with a photothermal effect that can be synergistic in practice. The problems associated with photosensitive hydrogels are the possible toxicity of photo-sensitizing molecules and difficult access to deeper tissues by light, which requires the use of fibre-optic implant systems. For this purpose, scientists will look of biocompatible gel-forming materials as well as non-toxic photo-sensitizers. In addition, due to limited tissue penetration properties of UV radiation, consideration should be given to NIR-responsive systems or UCNPs.
Hydrogels Sensitive to Magnetic Field
Among the many substances incorporated in hydrogel matrices, systems containing magnetic-field-sensitive nanoparticles deserve special attention. In recent years, reports on magnetic-field-sensitive hydrogels have appeared in the literature. These systems are obtained by combining the hydrogel, most often sensitive to temperature stimuli, with iron oxide nanoparticles possessing paramagnetic properties (called superparamagnetic iron oxide nanoparticles, SPIONs). Under the influence of high-frequency magnetic field (HFMF), nanoparticles are vibrated, which leads to temperature increase and “activation” of the temperature-sensitive hydrogel, which changes its properties. Such a strategy enables, among other things, the release of the active substance under the control of an external stimulus, which is an alternating magnetic field (AMF). The advantages of the described solution are primarily low invasiveness and very good permeability to tissues, which enables the therapy of deeper organs.4
The ability of hydrogel-SPIONs matrices to side-specific elevation of temperature under the influence of AMF in the context of cancer treatment finds several interesting applications. It is known that cancerous tissues are more susceptible to elevated temperature when compared to healthy tissues.142 There are two methods of using elevated temperature in anticancer therapy: thermal ablation and apoptotic hyperthermia.172,173
Thermal ablation involves heating cancer cells to high temperatures in excess of 50 °C, which leads to necrosis.173–175 The use of SPIONs, which tends to accumulate in the tumor tissue, allows the precise side-specific elevation of the temperature to suitably high values using AMF, which leads to tissue burnout. However, it turned out that the attempts made did not give good enough results,173,176 and danger of damaging healthy cells occurred.177
Other potential methods of cancer treatment is apoptotic hyperthermia. Temperature in the range of 42 °C–45 °C is able to start the apoptosis process in the tumor cells. Raising the temperature to the desired values can be done using magnetic nanoparticles located in a variable magnetic field.173 In addition, it is possible to obtain a hydrogel system containing magnetic nanoparticles and releasing an anticancer substance. Such a system enables simultaneous hyperthermia and chemotherapy, which improves the effects of the therapy.
Meenach et al178 proposed a SPIONs containing hydrogel system susceptible to magnetic field stimulus. They synthesized a few polymers with PEGMMA backbone and cross-linked by and poly(ethylene glycol) dimethacrylate (PEGDMA) or tetra(ethylene glycol) dimethacrylate (TEGDMA) with different PEGMMA/PEGDMA ratios and PEGMMA molecular masses. The obtained hydrogels showed thermosensitivity, their swelling capacity reduced upon heating over LCST temperature. The hydrogel and SPIONs were subjected to biocompatibility assay and were classified as biocompatible and potentially useful for biomedical applications. SPIONs loaded hydrogel (iron oxide content about 5.5 wt%) matrices were exposed to AMF (297 kHz, 25 kA m−1) in order to estimate their ability to increase temperature increment under the influence of the magnetic field. The highest temperature that has been achieved was about 80 °C after 180 seconds of exposure. It was assessed that the temperature generated can be effectively controlled by manipulating the intensity of the magnetic field, the time of exposure and the content of magnetic nanoparticles in the matrix. Appropriately setting these parameters enables both thermoablation, requiring temperatures above 50 °C, and apoptotic hyperthermia in the range of 42 °C–45 °C. Importantly, when a pure hydrogel without SPIONs was subjected to AMF, the significant temperature increase was not observed. It proves the unique role of magnetic nanoparticles in the described process. The effectiveness and suitability for anticancer treatment were assessed by thermoablation in vitro tests on glioblastoma M059K cell line. The cells were placed on a Petri dish disposed over the hydrogel matrix and AMF generator so that only a small portion of the cells were in the heat range. It turned out that cells in the immediate vicinity of the matrix were killed. Simultaneously, the outer cells were not affected. The proposed system seems to be suitable for hyperthermia and thermoablation of tumor tissues, but the group did not conduct the effectiveness of apoptosis as a result of hyperthermia in a lower temperature range (42 °C–45 °C). The developed solution needs further research, especially in vivo antitumor efficacy tests.
Magnetic field-sensitive hydrogels enable the conduction of chemotherapy and hyperthermia therapy. In our view, because of strong magnetic field permeability to deeper tissues and remote temperature adjustability, it is the most desirable path in hyperthermia induction. Nevertheless, the use of magnetic nanoparticles in DDS technology is still a novel solution and requires further study and understanding of its properties and effects on the organism, especially its toxicity.
Biological Factors
Bioresponsive hydrogels are systems which change their properties, eg, swelling/shrinking behavior, erosion susceptibility or decomposition, due to the presence, activity and concentration of specific biological factors.4
Depending on the activating factors, biological-stimuli hydrogels are generally distinguished by three main groups: glucose-responsive, specific enzyme or the presence of antibodies sensitive.4
One of the technological solutions used in the design of insulin-releasing hydrogels in response to the glucose level changes is the use of glucose oxidase (GOX) and pH-responsive polymers. When the blood glucose level is elevated, GOX attached to the polymeric chain converts it to gluconic acid and H2O2. As a result, the pH decreases within the structure of the hydrogel, ionization of specific cationic functional groups occurs, swelling of the hydrogel and penetration of insulin into the bloodstream.4,114,179,180
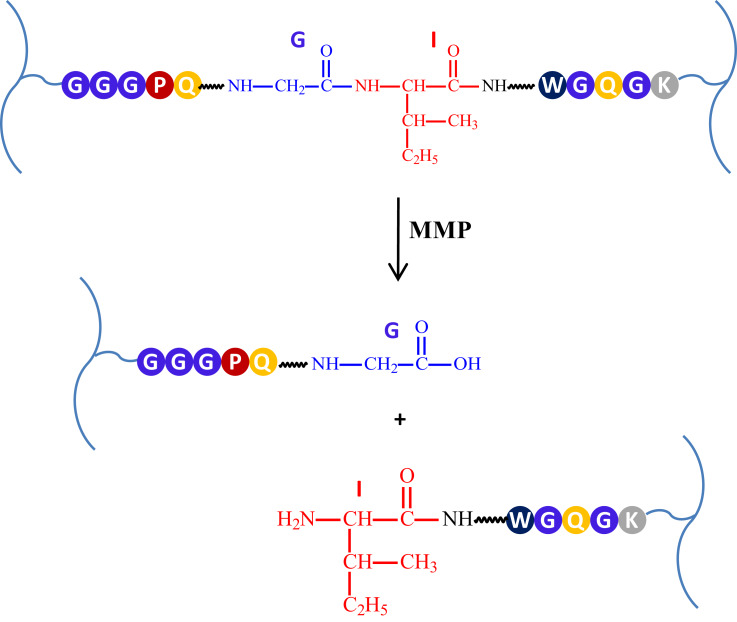
In the cellular and intracellular environment, the fundamental role in activation and regulation processes is played by enzymes. The presence of enzymes and their activity varies depending on the cell or organ compartment, and there are also differences in enzymes activity between the pathological and physiological conditions. Tumor cells do not stay in homeostasis, and their enzyme levels are dysregulated and differ from normal cells.181,182 Therefore, hydrogel systems sensitive to enzyme stimuli seem to be fascinating because they are selective and capable to be activated by specific factors. The first solution of this type in the context of tissue engineering was a hydrogel based on PEG, sensitive to the presence of metalloproteinase (MMP). For its synthesis, MMP-sensitive peptides were used as a cross-linking agent. This resulted in a system that was biodegradable under the influence of MMPs such as gelatinase and collagenase.4,183 The example of enzymatic cleavage is presented in Figure 5.
Figure 5.
The example of specific MMPs-dependent crosslinker cleavage.
There are three main criteria that enzyme-sensitive hydrogel systems must meet. Firstly, the polymer network must contain a chemical moiety being a substrate for the enzyme. These can be peptide fragments with a specific amino acid sequence for proteases or glycosidic bonds for glycosidases. Secondly, mentioned moieties need to be accessible to the enzymatic active center. Finally, enzymatic reaction in a susceptible polymer region must cause the change of biomaterial properties. The enzymatic cleavage of cross-linkers must lead to network decomposition and drug release, for instance.182
Matrices MMPs are a group of endopeptidases able to cleave peptide bonds. This group consist of 24 enzymes that play important roles in metabolism.184,185 They are divided into gelatinases, collagenases, stromelysins and membrane-type MMPs.185,186 MMPs and their specific inhibitors play a significant role in the cellular homeostasis. They are involved in proliferation and differentiation, survival, adhesion, migration and intercellular interactions. Importantly, unhealthy tissues, such as cancer tissue, show some disturbances of homeostasis, and MMPs enzymatic activity is significantly elevated. Moreover, tumor cells secrete MMPs and other proteolytical enzymes to extracellular matrix, decomposing it to make more space for tumor growth.187–189 Therefore, MMPs are promising biological triggers for enzyme-responsive antitumor DDSs. MMPs-sensitive hydrogels are generally formed by cross-linking the polymeric chains with specific, peptide-binded amino acid fragments, susceptible to MMPs activity. Due to the matrix decay, the enzymatic cleavage of these cross-linkers results in polymer network decomposition and drug release.185
Nazli et al189 have developed a MMPs-sensitive hydrogel system containing PEG-coated magnetic iron oxide nanoparticles (MIONPs), able to selective antitumor drug release. MMPs sensitivity was provided by incorporation the specific peptide fragment (GGGPQG↓IWGQGK; ↓marks the cleavage side) (PQ), vulnerable to enzymatic cleavage.190 The system was obtained by photoinitiator-coated MIONPs reaction with acrylate-PEG-PQ–PEG-acrylate conjugates, PEG-diacrylate and acrylate-PEG-RGDS. Acrylate-PEG-PQ–PEG-acrylate is derived from α1(I) collagen and determines the MMPs susceptibility. RGDS fragment is a specific ligand for αvβ3 integrin, enables nanocarriers tumor targeting and endocytosis. As a result of photopolymerization, the obtained nanomatrices have MIONP core and collagenase-degradable, cells targeting hydrogel shell. The average diameter of targeted nanocarriers was about 186.5 nm, their form was spherical and ellipsoidal. These nanocarriers were described as having a zeta potential of −3.37 mV. This ability protects macrophages from non-specific endocytosis, and also enables penetration into tumor cells. The conducted assay exhibited increased degradation of nanocarriers in collagenase-containing environment and DOX loading efficacy more than 97%. In vitro drug release study showed 60% and 36% cumulative release after 4 days, in a collagenase and non-collagenase release medium. Cytotoxicity of DOX-loaded targeted and non-targeted nanomatrices was evaluated by incubating with HeLa cells. Due to enhanced endocytosis, targeted nanocarriers have sufficient cytotoxicity. Moreover, the tests with healthy fibroblasts showed decreased cytotoxicity, because of lower collagenase levels and limited integrin expression. As a control, the cells were treated by DOX-free nanocarriers, and the significant toxicity of hydrogel matrices was not observed. The system proposed by Nazli and co-authors seems to be interesting as theranostic, capable of ensuring controlled drug release and imaging due to the efficient uptake of MIONP tumor cells, but more studies, especially in vivo assays, are needed to demonstrate the efficacy of these nanomatrices.
Among proteases mentioned above, there are enzymes able to cleave glycosidic bonds in carbohydrate structures. Hydrogels sensitive to the glycosidase activity in the context of anticancer therapy are used primarily as delivery systems of antineoplastic agents to the colon. The most widely studied are systems sensitive to β-mannanase, an enzyme present in the small intestine. The backbone of the hydrogel is based on natural macromolecules, such as glucomannan or guar gum. The release takes place by cleavage of glycosidic bonds and matrix decomposition.182
One of the strategies to obtain antigen-sensitive smart hydrogels is elaboration a system cross-linked by antigen–antibody interactions. Antigens and corresponding antibodies are covalently incorporated to polymer chains and due to non-covalent interactions between them, they form a reversible connection and the physical cross-linking process occurs. The resulting network is stable and shrinked because of polymer–polymer interactions. In the presence of free antigen, there are competitive interactions between the free antigen and the antigen associated with the hydrogel network. Antibodies grafted on polymers are blocked by free antigens, polymer–polymer interaction density decreases and hydrogel has better swelling and permeation properties. Such a system has been proposed by Miyata et al191 They used a chemically modified rabbit IgG and goat anti-rabbit IgG antibodies, grafted to acrylamide polymer chains. The hydrogel was selective and shows swelling behaviour only in rabbit IgG environment; with goat IgG addition the swelling was not observed. Rabbit IgG antigen-dependent permeation properties of the matrix were evaluated by hemoglobin release assay. The data showed effective protein release under rabbit IgG influence and hardly observable release without antigen presence. This strategy could be possibly utilized for antitumor therapy, but the evidence in this field is limited.
One of the futuristic ideas connected with hydrogel technology is intelligent contact lenses, able to act as biosensors. There is a correlation between specific biomarkers present in tear fluid and tumor disease. According to Ewans,192 the lacryglobin level in tears can be useful as a biomarker for cancer detection. Moreover, Tseng et al193 proposed various tear fluid biomarkers for cancer diagnosis and progression monitoring. Intelligent contact lenses are an interesting way to monitor online biomarker levels. The method is non-invasive and ensures high patient comfort. This method may cause tumors to be detected earlier, which significantly increases the patient’s survival prognosis.
Biological factors are known to be very specific in action, as enzymes and antibodies. Using this technique in smart hydrogel technology one can use these hydrogels to conduct guided therapy with precision. This technique is useful for accurate monitoring of drugs and for increasing therapy effectiveness. Future studies will concentrate on metalloproteinases and antibodies sensitive hydrogels, in particular nanogels, that can be internalized into tumor cells. This branch of smart hydrogel technology, however, is quite new and a lot of research has to be done to put this solution into clinical trials.
To the present several DDSs containing anti-cancer drugs have been obtained. Examples of smart hydrogel DDSs releasing various antitumoral agents are listed in Table 5.
Table 5.
Examples of DDSs Releasing Various Antitumoral Agents
| Drug | Chemical Formula | Materials | Stimulus | Sustained-Release Time | Proposed Application and Administration Route | Ref. |
|---|---|---|---|---|---|---|
| 2-Methoxyestradiol (2-ME) | 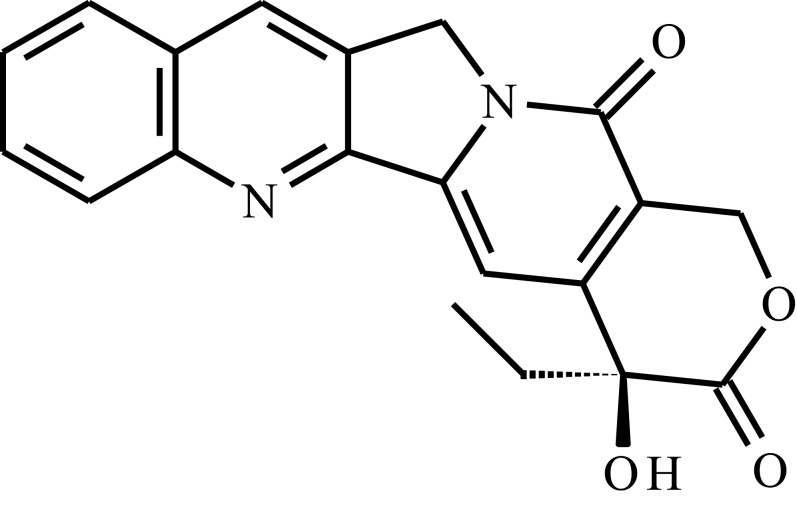 |
Poly(organophosphazenes) substituted by α-amino-ω-methoxypoly(ethylene glycol) (AMPEG), L-isoleucine ethyl ester (IleOEt) and glycyl lactate ethyl ester (GlyLacOEt) | Temp. | 35 days | Breast cancer, intratumoral injection | [109] |
| Camptothecin (CPT) | 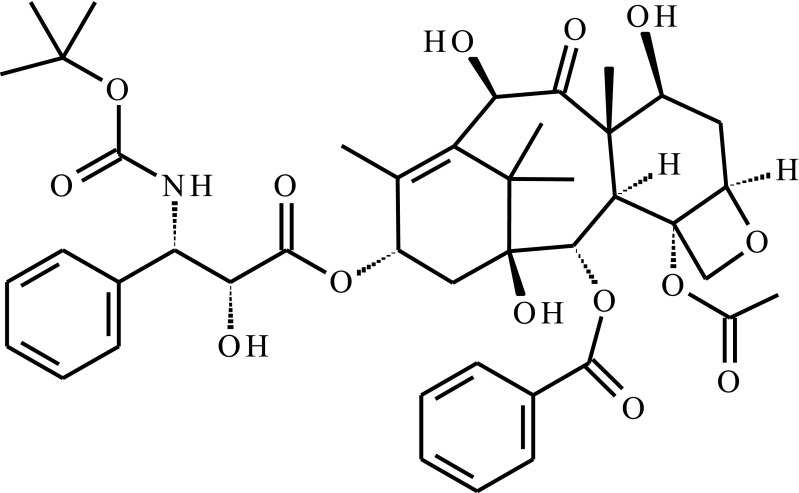 |
Poly(D,L-lactide)-poly(ethylene glycol)-poly(D,L-lactide) P(D,L-LA)-PEG-P(D,L-LA) triblock copolymer, hollow mesoporous silica nanoparticles (HMSNs) | Temp. | 15 days | Breast cancer, post-resection injection | [103] |
| Docetaxel (DTX) | 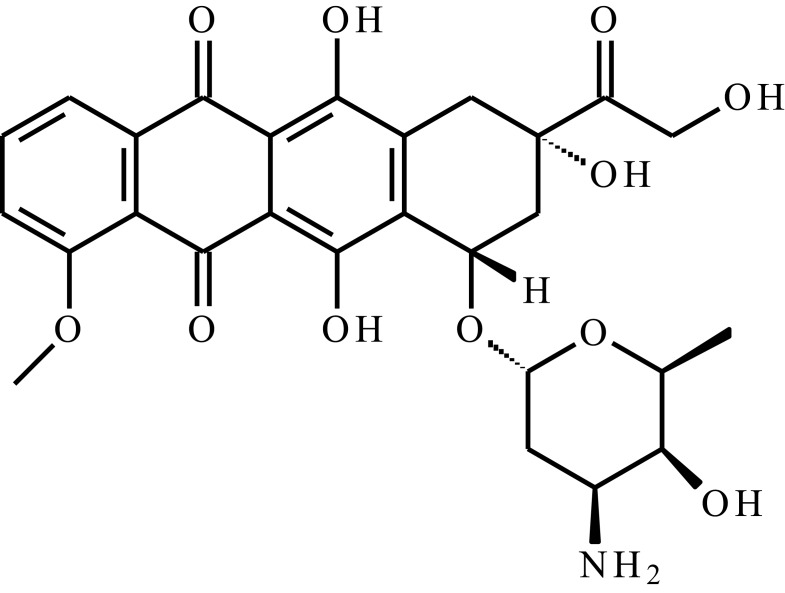 |
Star-liked block copolymer based on poly(ɛ-caprolactone)-b-poly(N-isopropylacrylamide) (HAPs-g-PCL-b-PNIPAAm) | Temp, pH | 222 h | Intravenous injection | [56] |
| Doxorubicin (DOX) | 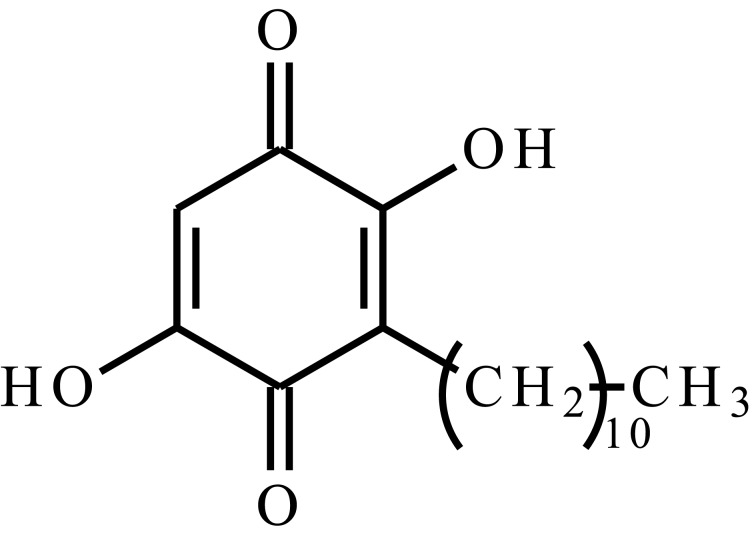 |
PNIPAAm-DOX hydrogel loaded into PEG-2,4,6-trimethoxybenzylidenepentaerythritol carbonate (PEG-PTMBPEC) polymersomes | Temp, pH | 144 h | Intravenous or intratumoral injection | [194] |
| PNIPAAm-b-poly(L-alanine) (PNIPAAm-b-PAla) | Temp. | >20h | Intravenous injection | [55] | ||
| PCL, PEG and sulfamethazine (PCL-PEG-SM) copolymer | pH | 4 weeks | HCC, transcatheter arterial chemoembolization agent | [139] | ||
| Acrylate-PEG-GGGPQG↓IWGQGK–PEG-acrylate conjugates, PEG-diacrylate conjugates and acrylate-PEG-RGDS conjugates-coated MIONPs | MMP | 4 days | Intravenous injection | [189] | ||
| Oligo(PEG) fumarate and sodium methacrylate | pH | 14 days | Intravenous injection | [195] | ||
| SPIONs coated with poly-(NIPAAm-stat-AAm)-block-PEI | AMF, Temp. | 96 ha | Intravenous injection | [196] | ||
| Embelin | 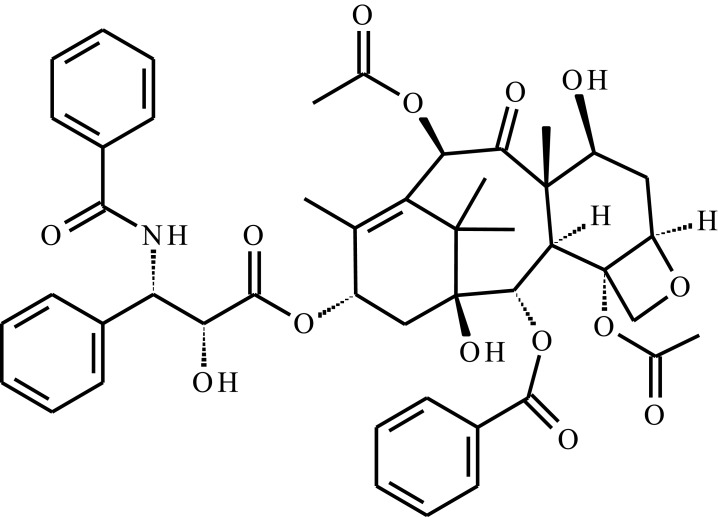 |
Triblock copolymer of poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone)–poly(ethylene glycol)–poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9- undecanone) (PECT) | Temp. | 21 days | HCC, peritumoral injection | [101] |
| Paclitaxel (PTX) |  |
Pluronic® P104 copolymerized with MMP-sensitive octapeptide | MMP, Temp. | 2–13 daysb | Intratumor injection | [197] |
| PCL-PEG-PCL | Temp, pH | 30 days | Intratumor injection | [198] | ||
| Paclitaxel and doxorubicin hydrochloride (PTX, DOX∙HCL) | PECT | Temp. | 30 days | Peritumoral injection | [199] | |
| 90Y (radiopharmaceutical) | Poly(Pluronic F-127/polyhydroxybutarate) diol/poly(propylene glycol) poly(F127/PHB/PPG urethane) | Temp. | HCC, intratumor injection | [60] | ||
| Tamoxifen citrate (TMC) |  |
Niosomes incorporated in poloxamer 407/poloxamer 188 | Temp. | 168 h | Breast cancer, intratumor injection | [58] |
Notes: a%cumulative release is strongly temperature-dependent. bDepending on the MMP concentration.
Summary and Future Remarks
In recent decades, attention in hydrogels in the context of DDSs has increased significantly. The main advantages of hydrogel systems are the low invasiveness associated with the application by injection, ease of formulation and the possibility obtaining a controlled, long-term drug release. Furthermore, therapeutic systems based on hydrogel show distribution that is strongly restricted to target tissues, allowing targeted therapy. This is particularly important for anticancer drugs that are associated with severe side effects when systemically administered.
A special group among hydrogel matrices are extensively studied the hydrogels sensitive to various stimuli. They primarily enable the development of injectable systems gelling at the application site, which greatly facilitates the therapy and increases the patient’s compliance. Furthermore, using this technology in DDSs, it is possible to achieve an adequate release profile, under the control of external stimulus, and spatiotemporal control over drug release. Moreover, internal factors sensitivity limits the release only to the target tissue, which reduces systemic toxicity. In addition to systems sensitive to a single stimulus, it is also possible to obtain matrices sensitive to many factors, depending on the type of monomers used or functional groups present in the polymer network structure.
In hydrogel DDSs technology, it is beneficial to develop matrices which meet certain requirements, such as biodegradability and biocompatibility, desirable mechanical properties, uniform distribution of a drug and reduced initial burst release. According to these requirements, the most widely applicable smart hydrogels are thermoresponsive matrices based on PEG/polyester copolymers and POPs, especially due to biodegradability and prolonged drug release profiles. The temperature stimulus seems to be the most versatile; thermosensitive smart hydrogel can be used as injectable DDS for various drugs. In antitumor therapy, the most promising are multi-stimuli smart hydrogels, able to combine chemotherapy with other methods of treatment, such as magnetic hyperthermia or photothermal therapy, which gives a synergistic effect with anticancer drugs. Moreover, drug release from DDSs sensitive to an external stimulus can be regulated by remote. Smart hydrogels sensitive to biological factors, as enzymes or antibodies, give the opportunity to conduct the therapy precisely targeted to tumor tissue, which allows the use of highly cytotoxic anticancer substances.
Smart hydrogels are able to release antitumor drug under control of external stimuli, such as photoirradiation or magnetic field. These factors can act as a trigger for drug release and enable real-time control over the release profile. These features make them promising when combined with relevant point-of-care (PoC) diagnostic devices, such as biosensors sensitive to tumor biomarkers, which closes the loop of care at the PoC.200 This opens the way for the medicine of the future, associated with an autonomous treatment system, in which the therapy is applied automatically in response to specific biomarkers of the disease.
It will be necessary for the researchers in the near future to elaborate the complex multi-stimulus responsive matrices capable of integrating several types of therapy. The main development direction, based on last year’s papers, is to modify known polymer structures of smart hydrogels to improve their properties and provide them with new, sophisticated features. For successful antitumor therapy, it may be necessary to combine multifunctional smart hydrogel system with sensitivity to biological stimuli which ensures precise targeting of the drug.
Given the intense development of modern pharmacy technology, there is still a lack of unambiguous legal regulations and standards that would require the use of stimulus-responsive hydrogels to effectively incorporate innovative technologies into the care. Although many innovative solutions have been developed and published, sadly very few of them are included in clinical trials. Smart hydrogel technology is still at the initial stage of development, but its significance in the context of modern drug forms is evident and it poses many possibilities for 21st-century medicine.
Acknowledgments
We would like to extend our sincere thanks to Koletta Kruk for her invaluable help with figures creation.
Abbreviations
DDDs, drug delivery systems; PAAc, poly(acrylic acid); POPs, poly(organophosphazenes); LCST, lower critical solution temperature; UCST, upper critical solution temperature; PEG, poly(ethylene glycol); PNIPAAm, poly(N-isopropylacrylamide); PEO, poly(ethylene oxide); PPO, poly(propylene oxide); PCL, poly(ε-caprolactone); PCEC, poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL); mPEG, poly(ethylene glycol) methyl ether; PECE, poly(ethylene glycol) methyl ether-poly(ε-caprolactone)-poly(ethylene glycol) methyl ether (mPEG-PCL-mPEG); PLGA, poly(lactic acid-co-glycolic acid); TAE, transcatheter arterial embolization; HCC, hepatolellular carcinoma; TEM, transmission electron microscope; DOX, doxorubicin; ROP, ring-opening polymerization; P(L-Ala), poly(L-alanine); (L-Ala-NCA), L-alanine-N-carboxyanhydride; DTX, docetaxel; HAPs, hyperbranched aliphatic polyesters; RAFT, reversible addition–fragmentation chain transfer; LC, loading content; EE, encapsulation efficiency; CMC, critical micelle concentration; CMT, critical micelle temperature; TMC, tamoxifen; P188, poloxamer 188; P407, poloxamer 407; PHB, poly(3-hydroxybutyrate); PPG, poly(propylene glycol); CGC, critical gelation concentration; PLA, polylactide; PGA, polyglycolide; F127, Pluronic F127; PDI, dispersity index; PBS, phosphate buffered saline; PECT, poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9undecanone)-poly(ethylene glycol)-poly(ε-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone); THF, tetrahydrofurane; CPT, camptothecin; HMSNs, hollow mesoporous silica nanoparticles; 2-ME, 2-methoxyestradiol; AMPEG, α-amino-ω-methoxypoly(ethylene glycol); IleOEt, L-isoleucine ethyl ester; GlyLacOEt, glycyl lactate ethyl ester; pH*critical pH; PDMAEMA, poly(N,N’-dimethyl aminoethyl methacrylate); PMAA, poly(2-aminoethyl methacrylate); PEI, poly(ethylene imine); PDEAEMA, poly(N,N’-diethylaminoethyl methacrylate); PVAm, poly(vinylamine); PAAm, poly(acrylamide); P2VP, poly(2-vinyl pyridine); P4VP, poly(4-vinyl pyridine); PVI, poly(N-vinyl imidazole); P4V, poly(4-vinyl imidazole); PMAAc, poly(methacrylic acid); nBMA, n-butyl methacrylate; t-BMA, tert-butyl methacrylate; MMA, methyl methacrylate; nBA, n-butyl acrylate; AC, acryloyl chloride; PECA, poly(ethylene glycol)-poly(ε-caprolactone)-acryloyl chloride; IA, itaconic acid; MPEGMA, poly(ethylene glycol)-methyl ether methacrylate; SM, sulfamethasine; CT, computer tomography; PCLA, poly(CL-co-LA)-PEG-poly(CL-co-LA); PCLA-PUSSM, poly(urethane sulfide SM) and PCLA multiblock copolymer; SM-A, SM acrylamide; SMM, sulfide SM monomer; HDI, hexamethylene 1,6-diisocyanate; NIR, near-infrared radiation; ROS, reactive oxygen species; CORMs, carbon monoxide releasing molecules; qbt, 2-(quinolyl)benzothiazole; 4-vpy, 4-vinylpyridine; HEMA, 2-hydroxyethyl methacrylate; UCNPs, up-converting nanoparticles; FITC-BSA, fluorescein isothiocyanate bovine serum albumin; SPIONs, superparamagnetic iron oxide nanoparticles; HFMF, high-frequency magnetic field; AMF, alternating magnetic field; PEGDMA, poly(ethylene glycol) dimethacrylate; TEGDMA, tetra(ethylene glycol) dimethacrylate; GOX, glucose oxidase; MMPs, metalloproteinases; MIONPs, magnetic iron oxide nanoparticles; PQ GGGPQG↓IWGQGK, peptide fragment; PoC, Point-of-Care.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Amin MCIM, Ahmad N, Pandey M, Abeer MM, Mohamad N. Recent advances in the role of supramolecular hydrogels in drug delivery. Expert Opin Drug Deliv. 2015;12(7):1149–1161. doi: 10.1517/17425247.2015.997707 [DOI] [PubMed] [Google Scholar]
- 2.Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release. 2014;190:254–273. doi: 10.1016/j.jconrel.2014.03.052 [DOI] [PubMed] [Google Scholar]
- 3.Wichterle O, Lím D. Hydrophilic gels for biological use. Nature. 1960;185(4706):117–118. doi: 10.1038/185117a0 [DOI] [Google Scholar]
- 4.Ferreira NN, Ferreira LMB, Cardoso VMO, Boni FI, Souza ALR, Gremião MPD. Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur Polym J. 2018;99:117–133. doi: 10.1016/j.eurpolymj.2017.12.004 [DOI] [Google Scholar]
- 5.Mathew AP, Uthaman S, Cho K-H, Cho C-S, Park I-K. Injectable hydrogels for delivering biotherapeutic molecules. Int J Biol Macromol. 2018;110:17–29. doi: 10.1016/j.ijbiomac.2017.11.113 [DOI] [PubMed] [Google Scholar]
- 6.Mahinroosta M, Jomeh Farsangi Z, Allahverdi A, Shakoori Z. Hydrogels as intelligent materials: a brief review of synthesis, properties and applications. Mater Today Chem. 2018;8:42–55. doi: 10.1016/j.mtchem.2018.02.004 [DOI] [Google Scholar]
- 7.Fu S, Ni P, Wang B, et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33(19):4801–4809. doi: 10.1016/j.biomaterials.2012.03.040 [DOI] [PubMed] [Google Scholar]
- 8.Moreira Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33(5):1281–1290. doi: 10.1016/j.biomaterials.2011.10.067 [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Gong C, Gou M, et al. Biodegradable poly(ɛ-caprolactone)–poly(ethylene glycol) copolymers as drug delivery system. Int J Pharm. 2009;381(1):1–18. doi: 10.1016/j.ijpharm.2009.07.033 [DOI] [PubMed] [Google Scholar]
- 10.Overstreet DJ, Dutta D, Stabenfeldt SE, Vernon BL. Injectable hydrogels. J Polym Sci Part B Polym Phys. 2012;50(13):881–903. doi: 10.1002/polb.23081 [DOI] [Google Scholar]
- 11.Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835–1849. doi: 10.1016/j.drudis.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Koukourakis GV, Kouloulias V, Koukourakis MJ, Zacharias GA, Zabatis H, Kouvaris J. Efficacy of the oral fluorouracil pro-drug capecitabine in cancer treatment: a review. Molecules. 2008;13(8):1897–1922. doi: 10.3390/molecules13081897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichifor M, Schacht EH, Seymour LW. Polymeric prodrugs of 5-fluorouracil. J Control Release. 1997;48(2–3):165–178. doi: 10.1016/S0168-3659(97)00048-5 [DOI] [Google Scholar]
- 14.Sgambato A, Cipolla L, Russo L. Bioresponsive hydrogels: chemical strategies and perspectives in tissue engineering. Gels. 2016;2(4):28. doi: 10.3390/gels2040028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppas N. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi: 10.1016/S0939-6411(00)00090-4 [DOI] [PubMed] [Google Scholar]
- 16.Bale S, Banks V, Haglestein S, Harding KG. A comparison of two amorphous hydrogels in the debridement of pressure sores. J Wound Care. 1998;7(2):65–68. doi: 10.12968/jowc.1998.7.2.65 [DOI] [PubMed] [Google Scholar]
- 17.Kurt B, Gulyuz U, Demir DD, Okay O. High-strength semi-crystalline hydrogels with self-healing and shape memory functions. Eur Polym J. 2016;81:12–23. doi: 10.1016/j.eurpolymj.2016.05.019 [DOI] [Google Scholar]
- 18.Li X, Wu W, Liu W. Synthesis and properties of thermo-responsive guar gum/poly(N-isopropylacrylamide) interpenetrating polymer network hydrogels. Carbohydr Polym. 2008;71(3):394–402. doi: 10.1016/j.carbpol.2007.06.005 [DOI] [Google Scholar]
- 19.Zhang X-Z, Jo Lewis P, Chu -C-C. Fabrication and characterization of a smart drug delivery system: microsphere in hydrogel. Biomaterials. 2005;26(16):3299–3309. doi: 10.1016/j.biomaterials.2004.08.024 [DOI] [PubMed] [Google Scholar]
- 20.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48(30):5418–5429. doi: 10.1002/anie.200900441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zha L, Banik B, Alexis F. Stimulus responsive nanogels for drug delivery. Soft Matter. 2011;7(13):5908. doi: 10.1039/c0sm01307b [DOI] [Google Scholar]
- 23.Gong C, Shi S, Wu L, et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel. Part 2: sol–gel–sol transition and drug delivery behavior. Acta Biomater. 2009;5(9):3358–3370. doi: 10.1016/j.actbio.2009.05.025 [DOI] [PubMed] [Google Scholar]
- 24.Sarvestani AS, Xu W, He X, Jabbari E. Gelation and degradation characteristics of in situ photo-crosslinked poly(l-lactide-co-ethylene oxide-co-fumarate) hydrogels. Polymer. 2007;48(24):7113–7120. doi: 10.1016/j.polymer.2007.10.007 [DOI] [Google Scholar]
- 25.Puranik AS, Pao LP, White VM, Peppas NA. Synthesis and characterization of pH-responsive nanoscale hydrogels for oral delivery of hydrophobic therapeutics. Eur J Pharm Biopharm. 2016;108:196–213. doi: 10.1016/j.ejpb.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 26.Jin R, Moreira Teixeira LS, Krouwels A, et al. Synthesis and characterization of hyaluronic acid–poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6(6):1968–1977. doi: 10.1016/j.actbio.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 27.Magnin D. Physicochemical and structural characterization of a polyionic matrix of interest in biotechnology, in the pharmaceutical and biomedical fields. Carbohydr Polym. 2004;55(4):437–453. doi: 10.1016/j.carbpol.2003.11.013 [DOI] [Google Scholar]
- 28.Choh S-Y, Cross D, Wang C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules. 2011;12(4):1126–1136. doi: 10.1021/bm101451k [DOI] [PubMed] [Google Scholar]
- 29.Nam HG, Nam MG, Yoo PJ, Kim J-H. Hydrogen bonding-based strongly adhesive coacervate hydrogels synthesized using poly(N -vinylpyrrolidone) and tannic acid. Soft Matter. 2019;15(4):785–791. doi: 10.1039/C8SM02144A [DOI] [PubMed] [Google Scholar]
- 30.Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26(18):3941–3951. doi: 10.1016/j.biomaterials.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Ren X, Zhang J, et al. Highly compressible lignin hydrogel electrolytes via double-crosslinked strategy for superior foldable supercapacitors. J Power Sources. 2020;449:227532. doi: 10.1016/j.jpowsour.2019.227532 [DOI] [Google Scholar]
- 32.Piluso S, Hiebl B, Gorb SN, Kovalev A, Lendlein A, Neffe AT. Hyaluronic acid-based hydrogels crosslinked by copper-catalyzed azide-alkyne cycloaddition with tailorable mechanical properties. Int J Artif Organs. 2011;34(2):192–197. doi: 10.5301/IJAO.2011.6394 [DOI] [PubMed] [Google Scholar]
- 33.Giannouli P, Morris ER. Cryogelation of xanthan. Food Hydrocoll. 2003;17(4):495–501. doi: 10.1016/S0268-005X(03)00019-5 [DOI] [Google Scholar]
- 34.Madl CM, Heilshorn SC. Rapid diels–alder cross-linking of cell encapsulating hydrogels. Chem Mater. 2019;31(19):8035–8043. doi: 10.1021/acs.chemmater.9b02485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y, Saeed AO, Hassan W, et al. “One-step” preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol Rapid Commun. 2012;33(2):120–126. doi: 10.1002/marc.201100534 [DOI] [PubMed] [Google Scholar]
- 36.Lehn J-M. Constitutional dynamic chemistry: bridge from supramolecular chemistry to adaptive chemistry In: Barboiu M, editor. Constitutional Dynamic Chemistry. Vol. 322 Berlin, Heidelberg: Springer Berlin Heidelberg; 2011: 1–32. doi: 10.1007/128_2011_256. [DOI] [PubMed] [Google Scholar]
- 37.Davis AV, Yeh RM, Raymond KN. Supramolecular assembly dynamics. Proc Natl Acad Sci. 2002;99(8):4793–4796. doi: 10.1073/pnas.052018299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piotrowska U, Sobczak M. Enzymatic polymerization of cyclic monomers in ionic liquids as a prospective synthesis method for polyesters used in drug delivery systems. Molecules. 2014;20(1):1–23. doi: 10.3390/molecules20010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matanović MR, Kristl J, Grabnar PA. Thermoresponsive polymers: insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int J Pharm. 2014;472(1–2):262–275. doi: 10.1016/j.ijpharm.2014.06.029 [DOI] [PubMed] [Google Scholar]
- 40.Schild HG. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci. 1992;17(2):163–249. doi: 10.1016/0079-6700(92)90023-R [DOI] [Google Scholar]
- 41.Ruel-Gariépy E, Leroux J-C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58(2):409–426. doi: 10.1016/j.ejpb.2004.03.019 [DOI] [PubMed] [Google Scholar]
- 42.Prabaharan M, Mano JF. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol Biosci. 2006;6(12):991–1008. doi: 10.1002/mabi.200600164 [DOI] [PubMed] [Google Scholar]
- 43.de la Rosa VR, Hoogenboom R. Solution polymeric optical temperature sensors with long-term memory function powered by supramolecular chemistry. Chem Eur J. 2015;21(3):1302–1311. doi: 10.1002/chem.201405161 [DOI] [PubMed] [Google Scholar]
- 44.Seuring J, Agarwal S. Polymers with upper critical solution temperature in aqueous solution: unexpected properties from known building blocks. ACS Macro Lett. 2013;2(7):597–600. doi: 10.1021/mz400227y [DOI] [PubMed] [Google Scholar]
- 45.Seuring J, Agarwal S. Polymers with upper critical solution temperature in aqueous solution. Macromol Rapid Commun. 2012;33(22):1898–1920. doi: 10.1002/marc.201200433 [DOI] [PubMed] [Google Scholar]
- 46.Boffito M, Sirianni P, Di Rienzo AM, Chiono V. Thermosensitive block copolymer hydrogels based on poly(ɛ-caprolactone) and polyethylene glycol for biomedical applications: state of the art and future perspectives: thermosensitive PEG/PCL block copolymer hydrogels. J Biomed Mater Res A. 2015;103(3):1276–1290. doi: 10.1002/jbm.a.35253 [DOI] [PubMed] [Google Scholar]
- 47.Klouda L. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2015;97:338–349. doi: 10.1016/j.ejpb.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 48.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pei Y, Chen J, Yang L, et al. The effect of pH on the LCST of poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid). J Biomater Sci Polym Ed. 2004;15(5):585–594. doi: 10.1163/156856204323046852 [DOI] [PubMed] [Google Scholar]
- 50.Qiu Y, Hamilton SK, Temenoff J. Improving mechanical properties of injectable polymers and composites In: Injectable Biomaterials. Elsevier; 2011:61–91. doi: 10.1533/9780857091376.1.61 [DOI] [Google Scholar]
- 51.Jeong B, Bae YH, Kim SW. Thermoreversible gelation of PEG−PLGA−PEG triblock copolymer aqueous solutions. Macromolecules. 1999;32(21):7064–7069. doi: 10.1021/ma9908999 [DOI] [Google Scholar]
- 52.Lee BH, Lee YM, Sohn YS, Song S-C. A thermosensitive poly(organophosphazene) gel. Macromolecules. 2002;35(10):3876–3879. doi: 10.1021/ma012093q [DOI] [Google Scholar]
- 53.Chatterjee S, Hui P, Kan C. Thermoresponsive hydrogels and their biomedical applications: special insight into their applications in textile based transdermal therapy. Polymers. 2018;10(5):480. doi: 10.3390/polym10050480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Huang L, Sun H-J, Cheng SZD, Zhu M, Yang G. Stimuli-responsive nanocomposite: potential injectable embolization agent. Macromol Rapid Commun. 2014;35(5):579–584. doi: 10.1002/marc.201300720 [DOI] [PubMed] [Google Scholar]
- 55.Qiao P, Niu Q, Wang Z, Cao D. Synthesis of thermosensitive micelles based on poly(N-isopropylacrylamide) and poly(l-alanine) for controlled release of adriamycin. Chem Eng J. 2010;159(1–3):257–263. doi: 10.1016/j.cej.2010.02.035 [DOI] [Google Scholar]
- 56.Ghamkhari A, Sarvari R, Ghorbani M, Hamishehkar H. Novel thermoresponsive star-liked nanomicelles for targeting of anticancer agent. Eur Polym J. 2018;107:143–154. doi: 10.1016/j.eurpolymj.2018.08.008 [DOI] [Google Scholar]
- 57.Alexandridis P, Alan Hatton T. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids Surf Physicochem Eng Asp. 1995;96(1–2):1–46. doi: 10.1016/0927-7757(94)03028-X [DOI] [Google Scholar]
- 58.Shaker DS, Shaker MA, Klingner A, Hanafy MS. In situ thermosensitive Tamoxifen citrate loaded hydrogels: an effective tool in breast cancer loco-regional therapy. J Drug Deliv Sci Technol. 2016;35:155–164. doi: 10.1016/j.jddst.2016.05.007 [DOI] [Google Scholar]
- 59.Hood E, Gonzalez M, Plaas A, Strom J, VanAuker M. Immuno-targeting of nonionic surfactant vesicles to inflammation. Int J Pharm. 2007;339(1–2):222–230. doi: 10.1016/j.ijpharm.2006.12.048 [DOI] [PubMed] [Google Scholar]
- 60.Zhu J-L, Yu SW-K, Chow PK-H, Tong YW, Li J. Controlling injectability and in vivo stability of thermogelling copolymers for delivery of yttrium-90 through intra-tumoral injection for potential brachytherapy. Biomaterials. 2018;180:163–172. doi: 10.1016/j.biomaterials.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 61.Goh AS-W, Chung AY-F, Lo RH-G, et al. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorous32 (32P) brachytherapy delivery device—a first-in-man study. Int J Radiat Oncol. 2007;67(3):786–792. doi: 10.1016/j.ijrobp.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 62.Zhang K, Loong SLE, Connor S, et al. Complete tumor response following intratumoral 32P biosilicon on human hepatocellular and pancreatic carcinoma xenografts in nude mice. Clin Cancer Res. 2005;11(20):7532–7537. doi: 10.1158/1078-0432.CCR-05-0400 [DOI] [PubMed] [Google Scholar]
- 63.Bae SJ, Suh JM, Sohn YS, Bae YH, Kim SW, Jeong B. Thermogelling poly(caprolactone- b -ethylene glycol- b -caprolactone) aqueous solutions. Macromolecules. 2005;38(12):5260–5265. doi: 10.1021/ma050489m [DOI] [Google Scholar]
- 64.Zhao S-P, Zhang L-M, Ma D, Yang C, Yan L. Fabrication of novel supramolecular hydrogels with high mechanical strength and adjustable thermosensitivity. J Phys Chem B. 2006;110(33):16503–16507. doi: 10.1021/jp063005c [DOI] [PubMed] [Google Scholar]
- 65.Gong C, Qian Z, Liu C, et al. A thermosensitive hydrogel based on biodegradable amphiphilic poly(ethylene glycol)–polycaprolactone–poly(ethylene glycol) block copolymers. Smart Mater Struct. 2007;16(3):927–933. doi: 10.1088/0964-1726/16/3/043 [DOI] [Google Scholar]
- 66.Liu C, Gong C, Pan Y, et al. Synthesis and characterization of a thermosensitive hydrogel based on biodegradable amphiphilic PCL-Pluronic (L35)-PCL block copolymers. Colloids Surf Physicochem Eng Asp. 2007;302(1–3):430–438. doi: 10.1016/j.colsurfa.2007.03.006 [DOI] [Google Scholar]
- 67.Dayananda K, He C, Lee DS. In situ gelling aqueous solutions of pH- and temperature-sensitive poly(ester amino urethane)s. Polymer. 2008;49(21):4620–4625. doi: 10.1016/j.polymer.2008.08.010 [DOI] [Google Scholar]
- 68.Huynh DP, Nguyen MK, Pi BS, et al. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29(16):2527–2534. doi: 10.1016/j.biomaterials.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 69.Liu CB, Gong CY, Huang MJ, et al. Thermoreversible gel–sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J Biomed Mater Res B Appl Biomater. 2008;84B(1):165–175. doi: 10.1002/jbm.b.30858 [DOI] [PubMed] [Google Scholar]
- 70.Gong C, Shi S, Dong P, et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm. 2009;365(1–2):89–99. doi: 10.1016/j.ijpharm.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 71.Gong CY, Dong PW, Shi S, et al. Thermosensitive PEG–PCL–PEG hydrogel controlled drug delivery system: sol–gel–sol transition and in vitro drug release study. J Pharm Sci. 2009;98(10):3707–3717. doi: 10.1002/jps.21694 [DOI] [PubMed] [Google Scholar]
- 72.Gong CY, Shi S, Dong PW, et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel: part 1—synthesis, characterization, and acute toxicity evaluation. J Pharm Sci. 2009;98(12):4684–4694. doi: 10.1002/jps.21780 [DOI] [PubMed] [Google Scholar]
- 73.Huynh DP, Nguyen MK, Kim BS, Lee DS. Molecular design of novel pH/temperature-sensitive hydrogels. Polymer. 2009;50(12):2565–2571. doi: 10.1016/j.polymer.2009.03.060 [DOI] [Google Scholar]
- 74.Ma D, Zhang L-M, Xie X, Liu T, Xie M-Q. Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme. J Colloid Interface Sci. 2011;359(2):399–406. doi: 10.1016/j.jcis.2011.04.032 [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Chang L, Li X, et al. Controlled thermal gelation of poly(3-caprolactone)/poly(ethylene glycol) block copolymers by modifying cyclic ether pendant groups on poly(3- caprolactone). Soft matter. 2012;8:1575–1583. [Google Scholar]
- 76.Wang W, Deng L, Liu S, et al. Adjustable degradation and drug release of a thermosensitive hydrogel based on a pendant cyclic ether modified poly(ε-caprolactone) and poly(ethylene glycol)co-polymer. Acta Biomater. 2012;8(11):3963–3973. doi: 10.1016/j.actbio.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 77.Hyun H, Park S, Kwon D, Khang G, Lee H, Kim M. Thermo-responsive injectable MPEG-polyester diblock copolymers for sustained drug release. Polymers. 2014;6(10):2670–2683. doi: 10.3390/polym6102670 [DOI] [Google Scholar]
- 78.Lv F, Mao L, Liu T. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG–PCL copolymer: preparation, characterization and fluorescence imaging in vivo. Mater Sci Eng C. 2014;43:221–230. doi: 10.1016/j.msec.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 79.Buwalda SJ, Nottelet B, Coudane J. Robust & thermosensitive poly(ethylene glycol)-poly(ε-caprolactone) star block copolymer hydrogels. Polym Degrad Stab. 2017;137:173–183. doi: 10.1016/j.polymdegradstab.2017.01.015 [DOI] [Google Scholar]
- 80.Jiang Z, Deng X, Hao J. Thermogelling hydrogels of poly(ɛ-caprolactone-co-D,L-lactide)–poly(ethylene glycol)–poly(ɛ-caprolactone-co-D,L-lactide) and poly(ɛ-caprolactone-co-L-lactide)–poly(ethylene glycol)–poly(ɛ-caprolactone-co-L-lactide) aqueous solutions. J Polym Sci Part Polym Chem. 2007;45(17):4091–4099. doi: 10.1002/pola.22222 [DOI] [Google Scholar]
- 81.Kang YM, Lee SH, Lee JY, et al. A biodegradable, injectable, gel system based on MPEG-b-(PCL-ran-PLLA) diblock copolymers with an adjustable therapeutic window. Biomaterials. 2010;31(9):2453–2460. doi: 10.1016/j.biomaterials.2009.11.115 [DOI] [PubMed] [Google Scholar]
- 82.Sandker MJ, Petit A, Redout EM, et al. In situ forming acyl-capped PCLA–PEG–PCLA triblock copolymer based hydrogels. Biomaterials. 2013;34(32):8002–8011. doi: 10.1016/j.biomaterials.2013.07.046 [DOI] [PubMed] [Google Scholar]
- 83.Petit A, Redout EM, van de Lest CH, et al. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials. 2015;53:426–436. doi: 10.1016/j.biomaterials.2015.02.109 [DOI] [PubMed] [Google Scholar]
- 84.Velthoen IW, van Beek J, Dijkstra PJ, Feijen J. Thermo-responsive hydrogels based on highly branched poly(ethylene glycol)–poly(l-lactide) copolymers. React Funct Polym. 2011;71(3):245–253. doi: 10.1016/j.reactfunctpolym.2010.08.007 [DOI] [Google Scholar]
- 85.Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J. Biodegradable and thermoreversible PCLA–PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials. 2011;32(21):4725–4736. doi: 10.1016/j.biomaterials.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 86.Abebe DG, Fujiwara T. Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLA prepared via hybrid micelles of pre-mixed copolymers with different PEG lengths. Biomacromolecules. 2012;13(6):1828–1836. doi: 10.1021/bm300325v [DOI] [PubMed] [Google Scholar]
- 87.Buwalda SJ, Calucci L, Forte C, Dijkstra PJ, Feijen J. Stereocomplexed 8-armed poly(ethylene glycol)–poly(lactide) star block copolymer hydrogels: gelation mechanism, mechanical properties and degradation behavior. Polymer. 2012;53(14):2809–2817. doi: 10.1016/j.polymer.2012.05.006 [DOI] [Google Scholar]
- 88.Mao H, Pan P, Shan G, Bao Y. In situ formation and gelation mechanism of thermoresponsive stereocomplexed hydrogels upon mixing diblock and triblock poly(lactic acid)/poly(ethylene glycol) copolymers. J Phys Chem B. 2015;119(21):6471–6480. doi: 10.1021/acs.jpcb.5b03610 [DOI] [PubMed] [Google Scholar]
- 89.Mao H, Shan G, Bao Y, Wu ZL, Pan P. Thermoresponsive physical hydrogels of poly(lactic acid)/poly(ethylene glycol) stereoblock copolymers tuned by stereostructure and hydrophobic block sequence. Soft Matter. 2016;12(20):4628–4637. doi: 10.1039/C6SM00517A [DOI] [PubMed] [Google Scholar]
- 90.Jeong B, Lee DS, Shon J-I, Bae YH, Kim SW. Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers. J Polym Sci Part Polym Chem. 1999;37(6):751–760. doi: [DOI] [Google Scholar]
- 91.Lee DS, Shim MS, Kim SW, Lee H, Park I, Chang T. Novel thermoreversible gelation of biodegradable PLGA-block-PEO-block-PLGA triblock copolymers in aqueous solution. Macromol Rapid Commun. 2001;22(8):587–592. doi: [DOI] [Google Scholar]
- 92.Chung Y-M, Simmons KL, Gutowska A, Jeong B. Sol−gel transition temperature of PLGA- g -PEG aqueous solutions. Biomacromolecules. 2002;3(3):511–516. doi: 10.1021/bm0156431 [DOI] [PubMed] [Google Scholar]
- 93.Jackson JK, Hung T, Letchford K, Burt HM. The characterization of paclitaxel-loaded microspheres manufactured from blends of poly(lactic-co-glycolic acid) (PLGA) and low molecular weight diblock copolymers. Int J Pharm. 2007;342(1–2):6–17. doi: 10.1016/j.ijpharm.2007.04.022 [DOI] [PubMed] [Google Scholar]
- 94.Fraylich MR, Liu R, Richardson SM, et al. Thermally-triggered gelation of PLGA dispersions: towards an injectable colloidal cell delivery system. J Colloid Interface Sci. 2010;344(1):61–69. doi: 10.1016/j.jcis.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 95.Gao Y, Sun Y, Ren F, Gao S. PLGA–PEG–PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev Ind Pharm. 2010;36(10):1131–1138. doi: 10.3109/03639041003680826 [DOI] [PubMed] [Google Scholar]
- 96.Lin G, Cosimbescu L, Karin NJ, Tarasevich BJ. Injectable and thermosensitive PLGA-g-PEG hydrogels containing hydroxyapatite: preparation, characterization and in vitro release behavior. Biomed Mater. 2012;7(2):024107. doi: 10.1088/1748-6041/7/2/024107 [DOI] [PubMed] [Google Scholar]
- 97.Xie B, Jin L, Luo Z, et al. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin® to treat posterior segment disease. Int J Pharm. 2015;490(1–2):375–383. doi: 10.1016/j.ijpharm.2015.05.071 [DOI] [PubMed] [Google Scholar]
- 98.Dai X-H, Jin H, Cai M-H, et al. Fabrication of thermosensitive, star-shaped poly(L-lactide)-block-poly(N-isopropylacrylamide) copolymers with porphyrin core for photodynamic therapy. React Funct Polym. 2015;89:9–17. doi: 10.1016/j.reactfunctpolym.2015.02.002 [DOI] [Google Scholar]
- 99.Zhang J, Cui Z, Field R, Moloney MG, Rimmer S, Ye H. Thermo-responsive microcarriers based on poly(N-isopropylacrylamide). Eur Polym J. 2015;67:346–364. doi: 10.1016/j.eurpolymj.2015.04.013 [DOI] [Google Scholar]
- 100.Lee A-W, Hsu -C-C, Liu Y-Z, Wei P-L, Chen J-K. Supermolecules of poly(N -isopropylacrylamide) complexating Herring sperm DNA with bio-multiple hydrogen bonding. Colloids Surf B Biointerfaces. 2016;148:422–430. doi: 10.1016/j.colsurfb.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 101.Peng M, Xu S, Zhang Y, et al. Thermosensitive injectable hydrogel enhances the antitumor effect of embelin in mouse hepatocellular carcinoma. J Pharm Sci. 2014;103(3):965–973. doi: 10.1002/jps.23885 [DOI] [PubMed] [Google Scholar]
- 102.Huang Y, Lu J, Gao X, et al. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug Chem. 2012;23(7):1443–1451. doi: 10.1021/bc3000468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J, Qu Y, Shi K, et al. Camptothecin HMSNs/thermosensitive hydrogel composite for applications in preventing local breast cancer recurrence. Chin Chem Lett. 2018;29(12):1819–1823. doi: 10.1016/j.cclet.2018.10.004 [DOI] [Google Scholar]
- 104.Khan RU, Wang L, Yu H, et al. Recent progress in the synthesis of poly(organo)phosphazenes and their applications in tissue engineering and drug delivery. Russ Chem Rev. 2018;87(2):109–150. doi: 10.1070/RCR4757 [DOI] [Google Scholar]
- 105.Sohn YS, Kim JK, Song R, Jeong B. The relationship of thermosensitive properties with structure of organophosphazenes. Polymer. 2004;45(9):3081–3084. doi: 10.1016/j.polymer.2004.02.062 [DOI] [Google Scholar]
- 106.Kang G, Cheon S, Song S. Controlled release of doxorubicin from thermosensitive poly(organophosphazene) hydrogels. Int J Pharm. 2006;319(1–2):29–36. doi: 10.1016/j.ijpharm.2006.03.032 [DOI] [PubMed] [Google Scholar]
- 107.Kang GD, Cheon SH, Khang G, Song S-C. Thermosensitive poly(organophosphazene) hydrogels for a controlled drug delivery. Eur J Pharm Biopharm. 2006;63(3):340–346. doi: 10.1016/j.ejpb.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 108.Lippert C, Seeger H, Mueck AO, Lippert TH. The effects of A-ring and D-ring metabolites of estradiol on the proliferation of vascular endothelial cells. Life Sci. 2000;67(13):1653–1658. doi: 10.1016/S0024-3205(00)00747-5 [DOI] [PubMed] [Google Scholar]
- 109.Cho J-K, Hong K-Y, Park JW, Yang H-K, Song S-C. Injectable delivery system of 2-methoxyestradiol for breast cancer therapy using biodegradable thermosensitive poly(organophosphazene) hydrogel. J Drug Target. 2011;19(4):270–280. doi: 10.3109/1061186X.2010.499461 [DOI] [PubMed] [Google Scholar]
- 110.Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol nanocrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15(4):1460–1465. doi: 10.1158/1078-0432.CCR-08-1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bazban-Shotorbani S, Hasani-Sadrabadi MM, Karkhaneh A, et al. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J Control Release. 2017;253:46–63. doi: 10.1016/j.jconrel.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 112.Gil E, Hudson S. Stimuli-responsive polymers and their bioconjugates. Prog Polym Sci. 2004;29(12):1173–1222. doi: 10.1016/j.progpolymsci.2004.08.003 [DOI] [Google Scholar]
- 113.Zhu L, Smith PP, Boyes SG. pH-responsive polymers for imaging acidic biological environments in tumors. J Polym Sci Part B Polym Phys. 2013;51(14):1062–1067. doi: 10.1002/polb.23302 [DOI] [Google Scholar]
- 114.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64:49–60. doi: 10.1016/j.addr.2012.09.024 [DOI] [PubMed] [Google Scholar]
- 115.Park SY, Bae YH. Novel pH-sensitive polymers containing sulfonamide groups. Macromol Rapid Commun. 1999;20(5):269–273. doi: [DOI] [Google Scholar]
- 116.Kurkuri MD, Aminabhavi TM. Poly(vinyl alcohol) and poly(acrylic acid) sequential interpenetrating network pH-sensitive microspheres for the delivery of diclofenac sodium to the intestine. J Control Release. 2004;96(1):9–20. doi: 10.1016/j.jconrel.2003.12.025 [DOI] [PubMed] [Google Scholar]
- 117.Kharlampieva E, Sukhishvili SA. Polyelectrolyte multilayers of weak polyacid and cationic copolymer: competition of hydrogen-bonding and electrostatic interactions. Macromolecules. 2003;36(26):9950–9956. doi: 10.1021/ma0350821 [DOI] [Google Scholar]
- 118.Grainger SJ, El-Sayed MEH. Stimuli-sensitive particles for drug delivery In: Biologically-Responsive Hybrid Biomaterials. World Scientific; 2010:171–190. doi: 10.1142/9789814295680_0008 [DOI] [Google Scholar]
- 119.Hoffman AS, Stayton PS, Press O, et al. Bioinspired polymers that control intracellular drug delivery. Biotechnol Bioprocess Eng. 2001;6(4):205–212. doi: 10.1007/BF02931981 [DOI] [Google Scholar]
- 120.Huh KM, Kang HC, Lee YJ, Bae YH. pH-sensitive polymers for drug delivery. Macromol Res. 2012;20(3):224–233. doi: 10.1007/s13233-012-0059-5 [DOI] [Google Scholar]
- 121.Kang SI, Bae YH. pH-induced solubility transition of sulfonamide-based polymers. J Control Release. 2002;80(1–3):145–155. doi: 10.1016/S0168-3659(02)00021-4 [DOI] [PubMed] [Google Scholar]
- 122.Kang SI, Na K, Bae YH. Sulfonamide-containing polymers: a new class of pH-sensitive polymers and gels. Macromol Symp. 2001;172(1):149–156. doi: [DOI] [Google Scholar]
- 123.Kang SI, Bae YH. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J Control Release. 2003;86(1):115–121. doi: 10.1016/S0168-3659(02)00409-1 [DOI] [PubMed] [Google Scholar]
- 124.Schmalz A, Hanisch M, Schmalz H, Müller AHE. Double stimuli-responsive behavior of linear and star-shaped poly(N,N-diethylaminoethyl methacrylate) in aqueous solution. Polymer. 2010;51(6):1213–1217. doi: 10.1016/j.polymer.2009.11.023 [DOI] [Google Scholar]
- 125.Pelton R. Polyvinylamine: a tool for engineering interfaces. Langmuir. 2014;30(51):15373–15382. doi: 10.1021/la5017214 [DOI] [PubMed] [Google Scholar]
- 126.Tsitsilianis C, Stavrouli N, Bocharova V, et al. Stimuli responsive associative polyampholytes based on ABCBA pentablock terpolymer architecture. Polymer. 2008;49(13–14):2996–3006. doi: 10.1016/j.polymer.2008.04.058 [DOI] [Google Scholar]
- 127.Arizaga A, Ibarz G, Piñol R. Stimuli-responsive poly(4-vinyl pyridine) hydrogel nanoparticles: synthesis by nanoprecipitation and swelling behavior. J Colloid Interface Sci. 2010;348(2):668–672. doi: 10.1016/j.jcis.2010.05.051 [DOI] [PubMed] [Google Scholar]
- 128.Molina MJ, Gómez-Antón MR, Piérola IF. Determination of the parameters controlling swelling of chemically cross-linked pH-sensitive Poly(N -vinylimidazole) hydrogels. J Phys Chem B. 2007;111(42):12066–12074. doi: 10.1021/jp074385k [DOI] [PubMed] [Google Scholar]
- 129.Ihm J, Han K, Hwang C, et al. Poly (4-vinylimidazole) as nonviral gene carrier: in vitro and in vivo transfection. Acta Biomater. 2005;1(2):165–172. doi: 10.1016/j.actbio.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 130.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58(15):1655–1670. doi: 10.1016/j.addr.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 131.Sharpe LA, Daily AM, Horava SD, Peppas NA. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv. 2014;11(6):901–915. doi: 10.1517/17425247.2014.902047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- 133.Puranik AS, Pao LP, White VM, Peppas NA. In vitro evaluation of pH-responsive nanoscale hydrogels for the oral delivery of hydrophobic therapeutics. Ind Eng Chem Res. 2016;55(40):10576–10590. doi: 10.1021/acs.iecr.6b02565 [DOI] [Google Scholar]
- 134.Shen Q, Lin Y, Handa T, et al. Modulation of intestinal P-glycoprotein function by polyethylene glycols and their derivatives by in vitro transport and in situ absorption studies. Int J Pharm. 2006;313(1–2):49–56. doi: 10.1016/j.ijpharm.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 135.Plapied L, Duhem N, Des Rieux A, Préat V. Fate of polymeric nanocarriers for oral drug delivery. Curr Opin Colloid Interface Sci. 2011;16(3):228–237. doi: 10.1016/j.cocis.2010.12.005 [DOI] [Google Scholar]
- 136.Wang Y, Chen L, Tan L, et al. PEG–PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials. 2014;35(25):6972–6985. doi: 10.1016/j.biomaterials.2014.04.099 [DOI] [PubMed] [Google Scholar]
- 137.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 138.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343–366. doi: 10.1016/S0167-8140(84)80077-8 [DOI] [PubMed] [Google Scholar]
- 139.Lym JS, Nguyen QV, Ahn DW, et al. Sulfamethazine-based pH-sensitive hydrogels with potential application for transcatheter arterial chemoembolization therapy. Acta Biomater. 2016;41:253–263. doi: 10.1016/j.actbio.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 140.Nguyen QV, Lym JS, Huynh CT, et al. A novel sulfamethazine-based pH-sensitive copolymer for injectable radiopaque embolic hydrogels with potential application in hepatocellular carcinoma therapy. Polym Chem. 2016;7(37):5805–5818. doi: 10.1039/C6PY01141A [DOI] [Google Scholar]
- 141.Suzuki A. Light induced phase transition of poly(N-isopropylacrylamide-co-chlorophyllin) gels. J Intell Mater Syst Struct. 1994;5(1):112–116. doi: 10.1177/1045389X9400500113 [DOI] [Google Scholar]
- 142.Jha S, Sharma PK, Malviya R. Hyperthermia: role and risk factor for cancer treatment. Achiev Life Sci. 2016;10(2):161–167. doi: 10.1016/j.als.2016.11.004 [DOI] [Google Scholar]
- 143.van der Zee J, González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet. 2000;355(9210):1119–1125. doi: 10.1016/S0140-6736(00)02059-6 [DOI] [PubMed] [Google Scholar]
- 144.Raza A, Hayat U, Rasheed T, Bilal M, Iqbal HMN. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: a review. J Mater Res Technol. 2018. doi: 10.1016/j.jmrt.2018.03.007 [DOI] [Google Scholar]
- 145.Chauhan DS, Indulekha S, Gottipalli R, et al. NIR light-triggered shrinkable thermoresponsive PNVCL nanoshells for cancer theranostics. RSC Adv. 2017;7(70):44026–44034. doi: 10.1039/C7RA07485A [DOI] [Google Scholar]
- 146.Hu J, Chen Y, Li Y, Zhou Z, Cheng Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials. 2017;112:133–140. doi: 10.1016/j.biomaterials.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 147.Lugao AB, Malmonge SM. Use of radiation in the production of hydrogels. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At. 2001;185(1–4):37–42. doi: 10.1016/S0168-583X(01)00807-2 [DOI] [Google Scholar]
- 148.Coons LS, Rangarajan B, Godshall D, Scranton AB. Photopolymerizations of Vinyl Ester: glass fiber composites In: Scranton AB, Bowman CN, Peiffer RW, editors. Photopolymerization. Vol. 673 Washington, DC: American Chemical Society; 1997: 203–218. doi: 10.1021/bk-1997-0673.ch015. [DOI] [Google Scholar]
- 149.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–4314. doi: 10.1016/S0142-9612(02)00175-8 [DOI] [PubMed] [Google Scholar]
- 150.Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjug Chem. 2009;20(2):209–212. doi: 10.1021/bc800480k [DOI] [PubMed] [Google Scholar]
- 151.Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Adv Drug Deliv Rev. 2011;63(14–15):1257–1266. doi: 10.1016/j.addr.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 152.Pinto MN, Chakraborty I, Sandoval C, Mascharak PK. Eradication of HT-29 colorectal adenocarcinoma cells by controlled photorelease of CO from a CO-releasing polymer (photoCORP-1) triggered by visible light through an optical fiber-based device. J Control Release. 2017;264:192–202. doi: 10.1016/j.jconrel.2017.08.039 [DOI] [PubMed] [Google Scholar]
- 153.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9(9):728–743. doi: 10.1038/nrd3228 [DOI] [PubMed] [Google Scholar]
- 154.Chakraborty I, Carrington SJ, Roseman G, Mascharak PK. Synthesis, structures, and CO release capacity of a family of water-soluble PhotoCORMs: assessment of the biocompatibility and their phototoxicity toward human breast cancer cells. Inorg Chem. 2017;56(3):1534–1545. doi: 10.1021/acs.inorgchem.6b02623 [DOI] [PubMed] [Google Scholar]
- 155.Carrington SJ, Chakraborty I, Mascharak PK. Rapid CO release from a Mn(i) carbonyl complex derived from azopyridine upon exposure to visible light and its phototoxicity toward malignant cells. Chem Commun. 2013;49(96):11254. doi: 10.1039/c3cc46558f [DOI] [PubMed] [Google Scholar]
- 156.Wegiel B, Gallo D, Csizmadia E, et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73(23):7009–7021. doi: 10.1158/0008-5472.CAN-13-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li X, Fang P, Mai J, Choi ET, Wang H, Yang X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6(1):19. doi: 10.1186/1756-8722-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med. 2008;45(5):562–569. doi: 10.1016/j.freeradbiomed.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chin BY, Jiang G, Wegiel B, et al. Hypoxia-inducible factor 1 stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci. 2007;104(12):5109–5114. doi: 10.1073/pnas.0609611104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.García-Gallego S, Bernardes GJL. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew Chem Int Ed. 2014;53(37):9712–9721. doi: 10.1002/anie.201311225 [DOI] [PubMed] [Google Scholar]
- 162.Heinemann SH, Hoshi T, Westerhausen M, Schiller A. Carbon monoxide – physiology, detection and controlled release. Chem Commun. 2014;50(28):3644–3660. doi: 10.1039/C3CC49196J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rimmer RD, Pierri AE, Ford PC. Photochemically activated carbon monoxide release for biological targets. Toward developing air-stable photoCORMs labilized by visible light. Coord Chem Rev. 2012;256(15–16):1509–1519. doi: 10.1016/j.ccr.2011.12.009 [DOI] [Google Scholar]
- 164.Schatzschneider U. Novel lead structures and activation mechanisms for CO-releasing molecules (CORMs): CO-releasing molecules (CORMs). Br J Pharmacol. 2015;172(6):1638–1650. doi: 10.1111/bph.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schatzschneider U. PhotoCORMs: light-triggered release of carbon monoxide from the coordination sphere of transition metal complexes for biological applications. Inorganica Chim Acta. 2011;374(1):19–23. doi: 10.1016/j.ica.2011.02.068 [DOI] [Google Scholar]
- 166.Govender P, Pai S, Schatzschneider U, Smith GS. Next generation PhotoCORMs: polynuclear tricarbonylmanganese(I)-functionalized polypyridyl metallodendrimers. Inorg Chem. 2013;52(9):5470–5478. doi: 10.1021/ic400377k [DOI] [PubMed] [Google Scholar]
- 167.Jalani G, Naccache R, Rosenzweig DH, Haglund L, Vetrone F, Cerruti M. Photocleavable hydrogel-coated upconverting nanoparticles: a multifunctional theranostic platform for NIR imaging and on-demand macromolecular delivery. J Am Chem Soc. 2016;138(3):1078–1083. doi: 10.1021/jacs.5b12357 [DOI] [PubMed] [Google Scholar]
- 168.Wang X, Wang C, Zhang Q, Cheng Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem Commun. 2015;5. doi: 10.1039/C5CC08391E [DOI] [PubMed] [Google Scholar]
- 169.Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G. Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev. 2005;105(6):2647–2694. doi: 10.1021/cr030707e [DOI] [PubMed] [Google Scholar]
- 170.Haase M, Schäfer H. Upconverting nanoparticles. Angew Chem Int Ed. 2011;50(26):5808–5829. doi: 10.1002/anie.201005159 [DOI] [PubMed] [Google Scholar]
- 171.Yan B, Boyer J-C, Habault D, Branda NR, Zhao Y. Near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J Am Chem Soc. 2012;134(40):16558–16561. doi: 10.1021/ja308876j [DOI] [PubMed] [Google Scholar]
- 172.Ansari MO, Ahmad MF, Shadab GGHA, Siddique HR. Superparamagnetic iron oxide nanoparticles based cancer theranostics: a double edge sword to fight against cancer. J Drug Deliv Sci Technol. 2018;45:177–183. doi: 10.1016/j.jddst.2018.03.017 [DOI] [Google Scholar]
- 173.Nedyalkova M, Donkova B, Romanova J, Tzvetkov G, Madurga S, Simeonov V. Iron oxide nanoparticles – in vivo/in vitro biomedical applications and in silico studies. Adv Colloid Interface Sci. 2017;249:192–212. doi: 10.1016/j.cis.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 174.García-Jimeno S, Ortega-Palacios R, Cepeda-Rubio M, Vera A, Leija L, Estelrich J. Improved thermal ablation efficacy using magnetic nanoparticles: a study in tumor phantoms. Prog Electromagn Res. 2012;128:229–248. doi: 10.2528/PIER12020108 [DOI] [Google Scholar]
- 175.Jordan A, Scholz R, Wust P, Fa H. Magnetic #uid hyperthermia (MFH): cancer treatment with AC magnetic “eld induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater. 1999;7. [Google Scholar]
- 176.Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest Radiol. 2002;37(10):580–586. doi: 10.1097/00004424-200210000-00008 [DOI] [PubMed] [Google Scholar]
- 177.Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia. 2002;18(4):267–284. doi: 10.1080/02656730110108785 [DOI] [PubMed] [Google Scholar]
- 178.Meenach SA, Hilt JZ, Anderson KW. Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010;6(3):1039–1046. doi: 10.1016/j.actbio.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 179.Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1–49. doi: 10.1016/j.mser.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kawamura A, Miyata T. Biologically stimuli-responsive hydrogels In: Li Q, editor. Intelligent Stimuli-Responsive Materials. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2013:335–362. doi: 10.1002/9781118680469.ch10 [DOI] [Google Scholar]
- 181.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chandrawati R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp Biol Med. 2016;241(9):972–979. doi: 10.1177/1535370216647186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10(3–4):515–522. doi: 10.1089/107632704323061870 [DOI] [PubMed] [Google Scholar]
- 184.Vartak DG, Gemeinhart RA. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15(1):1–20. doi: 10.1080/10611860600968967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nultsch K, Germershaus O. Matrix metalloprotease triggered bioresponsive drug delivery systems – design, synthesis and application. Eur J Pharm Biopharm. 2018;131:189–202. doi: 10.1016/j.ejpb.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 186.Daniele A, Abbate I, Oakley C, et al. Clinical and prognostic role of matrix metalloproteinase-2, −9 and their inhibitors in breast cancer and liver diseases: a review. Int J Biochem Cell Biol. 2016;77:91–101. doi: 10.1016/j.biocel.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 187.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 188.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745 [DOI] [PubMed] [Google Scholar]
- 189.Nazli C, Demirer GS, Yar Y, Acar HY, Kizilel S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloids Surf B Biointerfaces. 2014;122:674–683. doi: 10.1016/j.colsurfb.2014.07.049 [DOI] [PubMed] [Google Scholar]
- 190.Zhang X, Xu B, Puperi DS, et al. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015;14:11–21. doi: 10.1016/j.actbio.2014.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miyata T, Asami N, Uragami T. A reversibly antigen-responsive hydrogel. Nature. 1999;399(6738):766–769. doi: 10.1038/21619 [DOI] [PubMed] [Google Scholar]
- 192.Evans V, Vockler C, Friedlander M, Walsh B, Willcox MD. Lacryglobin in human tears, a potential marker for cancer. Clin Experiment Ophthalmol. 2001;29(3):161–163. doi: 10.1046/j.1442-9071.2001.00408.x [DOI] [PubMed] [Google Scholar]
- 193.Tseng R, Chen -C-C, Hsu S-M, Chuang H-S. Contact-lens biosensors. Sensors. 2018;18(8):2651. doi: 10.3390/s18082651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oroojalian F, Babaei M, Taghdisi SM, Abnous K, Ramezani M, Alibolandi M. Encapsulation of thermo-responsive gel in pH-sensitive polymersomes as dual-responsive smart carriers for controlled release of doxorubicin. J Control Release. 2018;288:45–61. doi: 10.1016/j.jconrel.2018.08.039 [DOI] [PubMed] [Google Scholar]
- 195.Dadsetan M, Taylor KE, Yong C, Bajzer Ž, Lu L, Yaszemski MJ. Controlled release of doxorubicin from pH-responsive microgels. Acta Biomater. 2013;9(3):5438–5446. doi: 10.1016/j.actbio.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dani RK, Schumann C, Taratula O, Taratula O. Temperature-tunable iron oxide nanoparticles for remote-controlled drug release. AAPS PharmSciTech. 2014;15(4):963–972. doi: 10.1208/s12249-014-0131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garripelli VK, Kim J-K, Son S, Kim WJ, Repka MA, Jo S. Matrix metalloproteinase-sensitive thermogelling polymer for bioresponsive local drug delivery. Acta Biomater. 2011;7(5):1984–1992. doi: 10.1016/j.actbio.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin X, Deng L, Xu Y, Dong A. Thermosensitive in situ hydrogel of paclitaxel conjugated poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone). Soft Matter. 2012;8(12):3470. doi: 10.1039/c2sm07172j [DOI] [Google Scholar]
- 199.Xu S, Wang W, Li X, Liu J, Dong A, Deng L. Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOX-HCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency. Eur J Pharm Sci. 2014;62:267–273. doi: 10.1016/j.ejps.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 200.Tan EKW, Au YZ, Moghaddam GK, Occhipinti LG, Lowe CR. Towards closed-loop integration of point-of-care technologies. Trends Biotechnol. 2019;37(7):775–788. doi: 10.1016/j.tibtech.2018.12.004 [DOI] [PubMed] [Google Scholar]