Summary
Background
The COVID-19 pandemic has placed unprecedented strain on health-care systems. Frailty is being used in clinical decision making for patients with COVID-19, yet the prevalence and effect of frailty in people with COVID-19 is not known. In the COVID-19 in Older PEople (COPE) study we aimed to establish the prevalence of frailty in patients with COVID-19 who were admitted to hospital and investigate its association with mortality and duration of hospital stay.
Methods
This was an observational cohort study conducted at ten hospitals in the UK and one in Italy. All adults (≥18 years) admitted to participating hospitals with COVID-19 were included. Patients with incomplete hospital records were excluded. The study analysed routinely generated hospital data for patients with COVID-19. Frailty was assessed by specialist COVID-19 teams using the clinical frailty scale (CFS) and patients were grouped according to their score (1–2=fit; 3–4=vulnerable, but not frail; 5–6=initial signs of frailty but with some degree of independence; and 7–9=severe or very severe frailty). The primary outcome was in-hospital mortality (time from hospital admission to mortality and day-7 mortality).
Findings
Between Feb 27, and April 28, 2020, we enrolled 1564 patients with COVID-19. The median age was 74 years (IQR 61–83); 903 (57·7%) were men and 661 (42·3%) were women; 425 (27·2%) had died at data cutoff (April 28, 2020). 772 (49·4%) were classed as frail (CFS 5–8) and 27 (1·7%) were classed as terminally ill (CFS 9). Compared with CFS 1–2, the adjusted hazard ratios for time from hospital admission to death were 1·55 (95% CI 1·00–2·41) for CFS 3–4, 1·83 (1·15–2·91) for CFS 5–6, and 2·39 (1·50–3·81) for CFS 7–9, and adjusted odds ratios for day-7 mortality were 1·22 (95% CI 0·63–2·38) for CFS 3–4, 1·62 (0·81–3·26) for CFS 5–6, and 3·12 (1·56–6·24) for CFS 7–9.
Interpretation
In a large population of patients admitted to hospital with COVID-19, disease outcomes were better predicted by frailty than either age or comorbidity. Our results support the use of CFS to inform decision making about medical care in adult patients admitted to hospital with COVID-19.
Funding
None.
Introduction
The ongoing COVID-19 pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first documented in China in late 2019.1 An increasing number of case series are being published that describe the clinical features and predictors of mortality in people with COVID-19.2, 3, 4 In these studies, older age has consistently been shown to be associated with poor outcomes, with increasing mortality linked to increasing age.5
Age is an attractive prognostic tool because it is easy to measure. However, we previously showed that on the individual level, age alone has little prognostic use.6, 7 A simple prognostic factor is needed to inform acute decisions on COVID-19 care pathways and one potential marker is frailty.8
In the UK, the National Institute for Clinical Excellence (NICE) released a rapid COVID-19 guideline recommending the use of the clinical frailty score (CFS)9 in discussions with patients regarding admission to intensive care units.8 The guideline was based on patient group recommendations; however, it came under criticism by the National Health Service Specialist Clinical Frailty Network who recommended that CFS should not be used in isolation but that the clinical discussions must be taken in conjunction with patient and carer wishes, and that the guidance might not apply to younger people or those with disabilities, in whom CFS has not been validated. There remains an important research gap with regards to supporting the use of CFS in the acute management of the ongoing pandemic.
Frailty is defined as “a medical syndrome with multiple causes and contributors that is characterised by diminished strength, endurance, and reduced physiologic function that increases an individual's vulnerability for developing increased dependency and/or death”.10 The prevalence of frailty in middle-aged and older patients varies according to the method of identifying frailty and the specific population but is estimated to be about 40%.11, 12 The likelihood of being frail increases with age, but can occur in younger adults.13 In addition, there is substantial evidence that frailty equates to worse patient outcomes in those admitted to hospital, including medical and surgical admissions as well as patients requiring intensive care.14 As a result it is becoming widely used as a trigger for specialist resource allocation, pathway decision aid, and shared decision making.15, 16
Research in context.
Evidence before this study
We searched PubMed, medRxiv, CINAHL, and Web of Science on April 30, 2020, using the search terms ”frailty” and “coronavirus2”. We searched for primary research and reviews published up to April 30, 2020, with no language restrictions. Despite the importance of frailty in UK national COVID-19 policy, our initial scoping literature review identified no published data at scale regarding frailty and outcomes in COVID-19.
Added value of this study
These data provide the first prevalence estimate of frailty in an adult patient population admitted to hospital with COVID-19. Using an established frailty scale (the clinical frailty scale [CFS]), we showed that patients classed as frail by the CFS are more likely to die from COVID-19 and are discharged from hospital less quickly than those who are not frail. These findings were independent of age and comorbidities.
Implications of all the available evidence
Clinical decisions are being made regarding the management of people with COVID-19 on the basis of their frailty status, using the clinical frailty scale. Our findings provide evidence to support those decisions.
No information is available on the prevalence of frailty in patients admitted to hospital with COVID-19 or how it affects their outcomes. Such evidence will aid and support physicians in decision making with this complex group of patients. Additionally, it will provide an evidence base to support the NICE rapid COVID recommendation of CFS score being used to guide admission to critical care.
The aim of the COVID-19 in Older PEople (COPE) study was to establish the prevalence of frailty in patients with COVID-19 who were admitted to hospital and investigate its influence on mortality and duration of hospital stay.
Methods
Study design
The COPE study is a multicentre European observational cohort study conducted at 11 hospitals in the UK and Italy. Authority in the UK to conduct the study was granted by the Health Research Authority (20/HRA/1898), and in Italy by the ethics committee of University Hospital of Modena Policlinico (369/2020/OSS/AOUMO). Ethical approval was such that formal written consent from participants was not required. This manuscript follows the STROBE statement for reporting of cohort studies.
Participants
All patients aged 18 years or older admitted to the participating hospitals with a diagnosis of COVID-19 were included. Diagnostic criteria were laboratory-confirmed SARS-CoV-2-positive swabs or a clinical diagnosis made by the parent clinical team and based on signs, symptoms, or radiology consistent with COVID-19. Patients were excluded during data analysis if their hospital records were incomplete. No other exclusion criteria were applied. Clinical teams at each site screened inpatient admission lists for eligibility. Screening logs of eligible participants were retained at each site.
Data collection
To allow rapid data collection for this time-sensitive topic, this study used an existing network of clinical centres with experience in collecting frailty data using the CFS for academic and service assessment purposes, with the addition of one Italian site. Data were collected across ten centres in the UK (Ysbyty Ystrad Fawr [Caerphilly], Royal Gwent Hospital [Newport], Nevill Hall Hospital [Abergavenny], Southmead Hospital Bristol [Bristol], Aberdeen Royal Infirmary [Aberdeen], Royal Alexandra Hospital [Paisley], Royal Inverclyde Hospital [Inverclyde], Salford Royal Infirmary [Salford], Glasgow Royal Infirmary [Glasgow], and the University Hospital of Wales [Cardiff]) and one Italian hospital (University Hospital of Modena Policlinico [Modena]). All hospitals admit acutely unwell people with COVID-19 except two sites (Ysbyty Ystrad Fawr and Glasgow Royal Infirmary) that receive self-referred patients and patients triaged by paramedic staff. For all sites, assessment of frailty using the CFS was routinely collected data (as per NICE recommendations). In each site the assessment of CFS in patients with COVID-19 was overseen by specialist COVID-19 megateams—in the UK, a megateam consisted of a consultant geriatrician, an emergency physician, and an intensive care consultant. For all patients with COVID-19 admitted to hospital, CFS was documented in a dedicated section on each patient's admission booklet. CFS is a quick to use assessment tool and is most reliably performed by geriatricians who use it routinely.
The study analysed routinely generated hospital data for patients with COVID-19. A standardised case report format was used for recording data collected prospectively and supplemented by patient records and drug prescription charts. Before participating, all study personnel completed specific data collection training. It was also a prerequisite for study personnel to familiarise themselves with the process of frailty assessment through use of an open-access online resource.17 Training was supervised at a local level by the site's principal investigator. Local data protection policy was followed in order to record data securely at each site. Subsequently, each site transferred anonymised data to King's College London for statistical analysis.
Demographic data for age and sex were collected. Variables for analysis were selected from those used in recent COVID studies, which appear to be prognostic indicators.1, 2, 4, 18 These were clinical diagnosis of coronary artery disease, diabetes, and hypertension; smoking status (never, previous, or current); and blood biomarkers (C-reactive protein, with >40 mg/dL considered abnormal; albumin, with ≤34 g/L considered hypoalbuminaemia; and estimated glomerular filtration rate [eGFR], with <60 mL/min per 1·73 m2 considered moderate or worse renal function).
The CFS (appendix p 6) was used to assess frailty. It bases the frailty assessment on how a patient was 2 weeks before hospital admission. The CFS is an ordinal hierarchical scale that numerically ranks frailty from 1 to 9, with a score of 1 being very fit, 2 well, 3 managing well, 4 vulnerable, 5 mildly frail, 6 moderately frail, 7 severely frail, 8 very severely frail, and 9 terminally ill. We did not anticipate that there would be adequate number of events for each score so scores were grouped 1–2 (fit), 3–4 (becoming vulnerable, but not frail), 5–6 (initial signs of frailty but with some degree of independence), and 7–9 (severe or very severe frailty) for the purposes of the analyses. These groups were selected to fit with the clinical descriptions outlined in the CFS and we deemed them to be reasonable groupings of severity of frailty.
Outcomes
The primary outcome was mortality (time from hospital admission to mortality and day-7 mortality). The secondary outcome was time from hospital admission to discharge. For patients diagnosed with COVID-19 while an inpatient (hospital acquired or nosocomial infection), the date of diagnosis was used in lieu of the date of admission. Patients still in hospital at the latest follow-up point were censored for the time-to-mortality analysis. Patients that died were censored at the date of death for the time-to-discharge analysis. Patients still in hospital at latest follow-up with fewer than 7 days of follow-up data were excluded from the day-7 analysis.
Other prespecified outcomes were long-term mortality (day-90 mortality and time from hospital admission to mortality); day-30 readmission; and the effect of drug classes, nosocomial infection, and deprivation on disease outcomes. These are not analysed here and will be reported in a future publication.
Statistical analysis
The original protocol planned to include a minimum of 500 patients. We estimated a minimum of 30% mortality in those that were frail, and 20% in those not frail (hazard ratio [HR] of 0·60). To detect this difference with 80% power and with a 5% significance, at least 500 patients were to be included. The sample size was increased to assess CFS categorised into four groups (rather than frail vs not frail).19
The first primary outcome measure (time to mortality), and the secondary outcome (time to discharge) were analysed with mixed-effects multivariable Cox proportional baseline hazards models. The analysis was fitted with a random effect20 to account for variation occurring at each hospital site, and adjusted for patient age group (<65 years, 65–79 years, and ≥80 years), sex (female or male), smoking status (never smoker, ex-smoker, or current smoker), C-reactive protein (>40 mg/dL or ≤40 mg/dL), diabetes (yes or no), hypertension (yes or no), coronary artery disease (yes or no), and eGFR (<60 mL/min per 1·73 m2 or ≥60 mL/min per 1·73 m2). Both a crude HR, and adjusted HR were estimated with associated 95% CIs. The baseline proportionality assumption was tested fitting log–log residuals. Each time-to-event analysis was reported with a Kaplan-Meier survival plot.
The primary outcome measure of day-7 mortality was analysed using a mixed-effects multivariable logistic regression, fitting each hospital as a random intercept effect, and adjusted with covariates consistent with the primary outcome. Both crude odds ratio (OR) and adjusted OR were presented with associated 95% CIs. Missing data were explored for patterns of missingness. We did exploratory subgroup analyses to explore the effect of frailty (CFS ≤4 or CFS ≥5) within each of the demographic and comorbidity subgroups of patients. Analyses were performed using Stata version 15. Kaplan-Meier survival plots were visualised in R.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Feb 27 and April 28, 2020, we screened 1707 participants from general medical, surgical, geriatric wards, respiratory wards. 143 participants were excluded. The reasons for exclusion were no clinical record found (n=66); no positive laboratory or clinical diagnosis found (n=61); and unable to access the patient record (n=16). The study included 1564 participants (table 1 ), of whom 1410 (90·2%) were recruited in the UK and 154 (9·8%) in Italy. 1500 (95·9%) patients were diagnosed via laboratory testing and 64 (4·1%) via clinical diagnosis only. Complete data were available for 1500 (95·9%) of included patients, and all outcomes were completed for all patients. 26 patients had missing smoking status and these were imputed as never smokers; 32 patients had missing C-reactive protein status and these were imputed as C-reactive protein less than 40 mg/dL; 14 patients had missing eGFR data and these were not imputed. A maximum of five patients had missing data for each of the remaining covariates. Given the minimal degree of missing data, a complete case population was used within each analysis. Data were collected between Feb 27 and April 28, 2020 (UK data collection began on March 6).
Table 1.
Demographics and frailty, by in-hospital mortality
| All patients (n=1564) | Dead (n=425) | Alive (n=1139) | |
|---|---|---|---|
| Sites | |||
| Hospital A | 115 (7·4%) | 15 (13·0%) | 100 (87·0%) |
| Hospital B | 50 (3·2%) | 14 (28·0%) | 36 (72·0%) |
| Hospital C | 153 (9·8%) | 34 (22·2%) | 119 (77·8%) |
| Hospital D | 43 (2·7%) | 10 (23·3%) | 33 (76·7%) |
| Hospital E | 123 (7·9%) | 15 (12·2%) | 108 (87·8%) |
| Hospital F | 154 (9·8%) | 23 (14·9%) | 131 (85·1%) |
| Hospital G | 112 (7·2%) | 36 (32·1%) | 76 (67·9%) |
| Hospital H | 246 (15·7%) | 108 (43·9%) | 138 (56·1%) |
| Hospital I | 380 (24·3%) | 126 (33·2%) | 254 (66·8%) |
| Hospital J | 179 (11·5%) | 43 (24·0%) | 136 (76·0%) |
| Hospital K | 9 (0·6%) | 1 (11·1%) | 8 (88·9%) |
| Age, years | |||
| <65 | 488 (31·2%) | 55 (11·3%) | 433 (88·7%) |
| 65–79 | 535 (34·2%) | 168 (31·4%) | 367 (68·6%) |
| ≥80 | 541 (34·6%) | 202 (37·3%) | 339 (62·7%) |
| Sex | |||
| Female | 661 (42·3%) | 170 (25·7%) | 491 (74·3%) |
| Male | 903 (57·7%) | 255 (28·2%) | 648 (71·8%) |
| Smoking status | |||
| Never smokers | 814 (52·0%) | 205 (25·2%) | 609 (74·8%) |
| Ex-smokers | 603 (38·6%) | 185 (30·7%) | 418 (69·3%) |
| Current smokers | 121 (7·7%) | 26 (21·5%) | 95 (78·5%) |
| Missing | 26 (1·7%) | 9 (34·6%) | 17 (65·4%) |
| Diabetes | |||
| No | 1144 (73·1%) | 295 (25·8%) | 849 (74·2%) |
| Yes | 415 (26·5%) | 128 (30·8%) | 287 (69·2%) |
| Missing | 5 (0·3%) | 2 (40·0%) | 3 (60·0%) |
| Hypertension | |||
| No | 755 (48·3%) | 184 (24·4%) | 571 (75·6%) |
| Yes | 804 (51·4%) | 238 (29·6%) | 566 (70·4%) |
| Missing | 5 (0·3%) | 3 (60·0%) | 2 (40·0%) |
| Coronary artery disease | |||
| No | 1214 (77·6%) | 290 (23·9%) | 924 (76·1%) |
| Yes | 345 (22·1%) | 132 (38·3%) | 213 (61·7%) |
| Missing | 5 (0·3%) | 3 (60·0%) | 2 (40·0%) |
| Increased C-reactive protein (>40 mg/dL) | |||
| No | 439 (28·1%) | 66 (15·0%) | 373 (85·0%) |
| Yes | 1125 (71·9%) | 359 (31·9%) | 766 (68·1%) |
| Missing | 32 (2·0%) | 12 (37·5%) | 20 (62·5%) |
| Impaired renal function (eGFR <60 mL/min per 1·73 m2) | |||
| No | 980 (63·7%) | 202 (20·6%) | 778 (79·4%) |
| Yes | 570 (36·4%) | 217 (38·1%) | 353 (61·9%) |
| Missing | 14 (0·9%) | 6 (42·9%) | 8 (57·1%) |
| Clinical frailty score | |||
| 1: very fit | 91 (5·8%) | 7 (7·7%) | 84 (92·3%) |
| 2: fit | 197 (12·6%) | 22 (11·2%) | 175 (88·8%) |
| 3: managing well | 287 (18·4%) | 55 (19·2%) | 232 (80·8%) |
| 4: vulnerable | 185 (11·8%) | 52 (28·1%) | 133 (71·9%) |
| 5: mildly frail | 182 (11·6%) | 50 (27·5%) | 132 (72·5%) |
| 6: moderately frail | 251 (16·0%) | 84 (33·5%) | 167 (66·5%) |
| 7: severely frail | 260 (16·6%) | 96 (36·9%) | 164 (63·1%) |
| 8: very severely frail | 79 (5·1%) | 44 (55·7%) | 35 (44·3%) |
| 9: terminally ill | 27 (1·7%) | 12 (44·4%) | 15 (55·6%) |
| Missing | 5 (0·3%) | 3 (60·0%) | 2 (40·0%) |
Percentages for the dead and alive columns use the total for each row (from the all patients column) as the denominator. eGFR=estimated glomerular filtration rate.
The study population median age was 74 years (IQR 61–83; range 20–101); 903 (57·7%) of 1564 were men and 661 (42·3%) were women (table 1). 772 (49·4%) were classed as frail (CFS 5–8) and 27 (1·7%) were classed as terminally ill (CFS 9). At data cutoff (April 28, 2020), 425 (27·2%) of patients had died, with rates of 11·1–43·9% across the 11 hospitals. Median survival time for those that died was 7 days (IQR 4–11) after admission. 727 patients were discharged. The median duration of hospital stay for those discharged was 9 days (IQR 5–15). The distribution of cases and mortality across age groups and CFS is shown in the appendix (p 1); increased frailty was associated with mortality at all ages.
Frailty was associated with both mortality and time to discharge from hospital after adjustment for age, sex, smoking status, and other comorbidities, exhibiting worsening clinical outcome with increasing frailty (Table 2, Table 3 ).
Table 2.
Time to mortality
| Crude HR (95% CI)* | p value | Adjusted HR†(95% CI)‡ | p value | ||
|---|---|---|---|---|---|
| Age, years | |||||
| <65 | 1 (ref) | .. | 1 (ref) | .. | |
| 65–79 | 3·30 (2·40–4·55) | <0·0001 | 2·58 (1·82–3·64) | <0·0001 | |
| ≥80 | 4·05 (2·95–5·57) | <0·0001 | 2·92 (2·02–4·22) | <0·0001 | |
| Sex | |||||
| Female | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 0·99 (0·81–1·21) | 0·93 | 1·07 (0·85–1·32) | 0·56 | |
| Smoking status | |||||
| Never | 1 (ref) | .. | 1 (ref) | .. | |
| Ex-smokers | 1·20 (0·98–1·47) | 0·079 | 0·95 (0·77–1·18) | 0·67 | |
| Current smokers | 0·84 (0·55–1·29) | 0·43 | 0·91 (0·59–1·42) | 0·69 | |
| Increased C-reactive protein (>40 mg/dL) | 2·22 (1·69–2·92) | <0·0001 | 2·61 (1·97–3·45) | <0·0001 | |
| Patients with diabetes | 1·12 (0·90–1·39) | 0·30 | 1·03 (0·82–1·29) | 0·83 | |
| Patients with coronary artery disease | 1·57 (1·26–1·95) | <0·0001 | 1·19 (0·94–1·49) | 0·83 | |
| Patients with hypertension | 1·24 (1·01–1·51) | 0·036 | 0·95 (0·77–1·18) | 0·66 | |
| Impaired renal function (eGFR <60 mL/min per 1·73 m2) | 1·93 (1·58–2·35) | <0·0001 | 1·43 (1·16–1·77) | 0·0007 | |
| Clinical frailty scale | |||||
| 1–2 | 1 (ref) | .. | 1 (ref) | .. | |
| 3–4 | 2·25 (1·47–3·45) | <0·0002 | 1·55 (1·00–2·41) | 0·052 | |
| 5–6 | 3·12 (2·05–4·76) | <0·0001 | 1·83 (1·15–2·91) | 0·011 | |
| 7–9 | 4·41 (2·90–6·71) | <0·0001 | 2·39 (1·50–3·81) | <0·0002 | |
eGFR=estimated glomerular filtration rate. HR=hazard ratio.
n=1520; 44 patients were not included in the analysis because of patient death or discharge on the day of admission.
The multivariable mixed-effects Cox regression was adjusted for age group, sex, smoking, C-reactive protein, diabetes, coronary artery disease, hypertension, renal function, and the clinical frailty scale.
n=1500; 20 further patients were not included in this analysis because of missing covariate data.
Table 3.
Time from hospital admission to discharge
| Crude HR (95% CI)* | p value | Adjusted HR†(95% CI)‡ | p value | ||
|---|---|---|---|---|---|
| Age, years | |||||
| <65 | 1 (ref) | .. | 1 (ref) | .. | |
| 65–79 | 0·74 (0·62–0·88) | 0·0006 | 0·82 (0·68–1·00) | 0·047 | |
| ≥80 | 0·56 (0·46–0·68) | <0·0001 | 0·62 (0·49–0·79) | <0·0001 | |
| Sex | |||||
| Female | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 1·03 (0·89–1·21) | 0·67 | 0·94 (0·80–1·10) | 0·42 | |
| Smoking status | |||||
| Never | 1 (ref) | .. | 1 (ref) | .. | |
| Ex-smokers | 0·90 (0·77–1·05) | 0·19 | 0·95 (0·81–1·12) | 0·54 | |
| Current smokers | 1·05 (0·78–1·43) | 0·74 | 0·97 (0·71–1·32) | 0·84 | |
| Increased C-reactive protein (>40 mg/dL) | 0·84 (0·71–0·98) | 0·028 | 0·73 (0·61–0·86) | <0·0002 | |
| Patients with diabetes | 0·89 (0·75–1·06) | 0·18 | 0·93 (0·77–1·12) | 0·43 | |
| Patients with coronary artery disease | 0·91 (0·74–1·10) | 0·33 | 1·10 (0·90–1·36) | 0·36 | |
| Patients with hypertension | 0·86 (0·75–1·00) | 0·056 | 0·93 (0·79–1·09) | 0·36 | |
| Impaired renal function (eGFR <60 mL/min per 1·73 m2) | 0·78 (0·66–0·92) | 0·0031 | 0·95 (0·79–1·14) | 0·58 | |
| Clinical frailty scale | |||||
| 1–2 | 1 (ref) | .. | 1 (ref) | .. | |
| 3–4 | 0·87 (0·71–1·05) | 0·15 | 0·94 (0·77–1·16) | 0·58 | |
| 5–6 | 0·61 (0·49–0·76) | <0·0001 | 0·70 (0·54–0·91) | 0·0084 | |
| 7–9 | 0·56 (0·44–0·72) | <0·0001 | 0·66 (0·50–0·87) | 0·0035 | |
eGFR=estimated glomerular filtration rate. HR=hazard ratio.
n=1520; 44 patients were not included in the analysis because of patient death or discharge on the day of admission.
The multivariable mixed-effects Cox regression was adjusted for age group, sex, smoking, C-reactive protein, diabetes, coronary artery disease, hypertension, renal function, and the clinical frailty scale.
n=1500; 20 further patients were not included in this analysis because of missing covariate data.
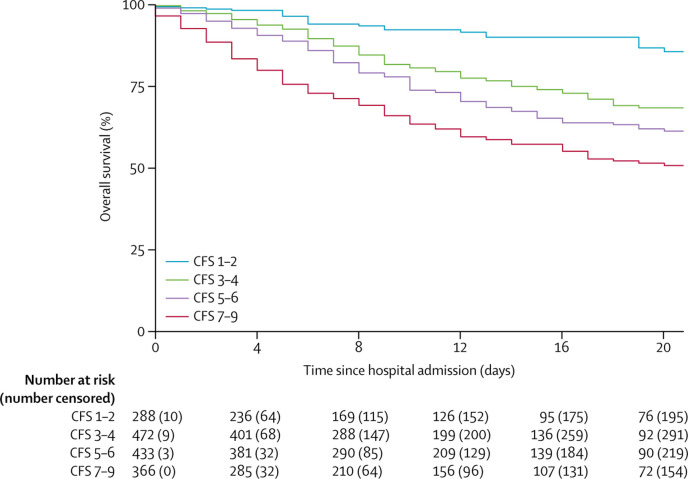
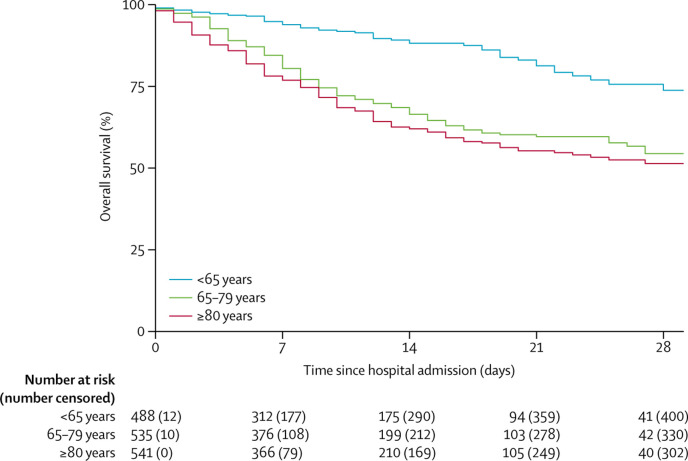
The crude HR for time from hospital admission to mortality was 2·25 (95% CI 1·47–3·45; p<0·0002) for CFS 3–4, 3·12 (2·05–4·76; p<0·0001) for CFS 5–6, and 4·41 (2·90–6·71; p<0·0001) for CFS 7–9, all compared with CFS 1–2 (table 2, figure 1 ). Compared with patients aged younger than 65 years, the crude HR was 3·30 (95% CI 2·40–4·55; p<0·0001) for those aged 65–79 years and 4·05 (2·95–5·57; p<0·0001) for those aged 80 years or older (table 2, figure 2 ). Coronary artery disease, reduced renal function, increased C-reactive protein, and hypertension were also associated with mortality. After adjusting for the other covariates, increasing frailty was generally associated with increased mortality. Compared with CFS 1–2, the adjusted HRs were, 1·55 (95% CI 1·00–2·41; p=0·052) for CFS 3–4, 1·83 (1·15–2·91; p=0·011) for CFS 5–6, and 2·39 (1·50–3·81; p<0·0002) for CFS 7–9. Older age, increased C-reactive protein, and impaired renal function were also associated with mortality in adjusted analyses (table 2). Residual log–log plots did not offer any indication of any breach of proportionality. Similar results were recorded for the coprimary outcome of day-7 mortality for CFS 7–9 (appendix p 2). The adjusted ORs for day-7 mortality were 1·22 (95% CI 0·63–2·38; p=0·56) for CFS 3–4, 1·62 (0·81–3·26; p=0·17) for CFS 5–6, and 3·12 (1·56–6·24; p<0·0012) for CFS 7–9, all compared with CFS 1–2.
Figure 1.
Overall survival by CFS category
CFS=clinical frailty score.
Figure 2.
Overall survival by age
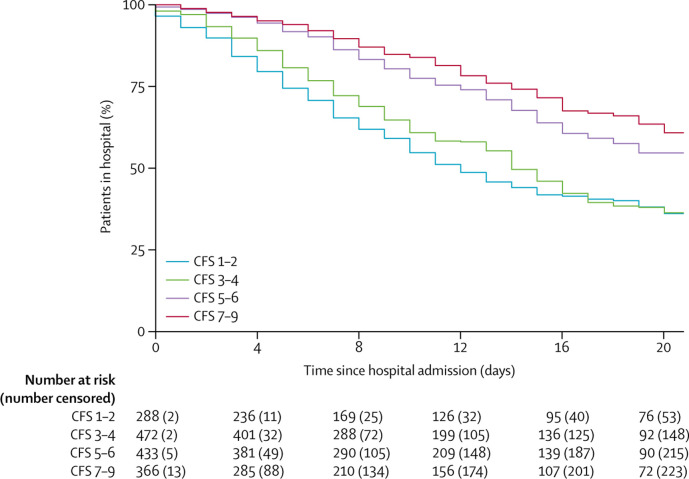
The time from hospital admission to discharge analysis showed that CFS 5–6 and 7–9 were associated with a longer duration of hospital stay than CFS 1–2. Compared with CFS 1–2, the adjusted HRs were 0·94 (95% CI 0·77–1·16; p=0·58) for CFS 3–4, 0·70 (0·54–0·91; p=0·0084) for CFS 5–6, and 0·66 (0·50–0·87, p=0·0035) for CFS 7–9 (figure 3 , table 3). In addition to frailty, age 65 years and older and increased C-reactive protein were associated with longer hospital stay.
Figure 3.
Time to discharge from hospital by CFS
CFS=clinical frailty score.
The subgroup analyses showed a consistent finding with the main analyses, that frail patients (CFS 5–9) had both an increased risk of mortality and longer duration of hospital stay compared with patients who were not frail (appendix pp 3–5). Frailty did not appear to be associated with any specific subgroup (eg, those aged ≥80 years).
Discussion
The COPE study was designed to provide urgent and much needed evidence on the effect of frailty in adult patients admitted to hospital with COVID-19. Our data show that the prevalence of frailty (CFS 5–8) was 49·4% and that frailty was associated with both earlier death and longer time spent in hospital. These outcomes worsened with increasing frailty, with similar findings after adjustment for age and comorbidity.
These data show that the prevalence of frailty, using the clinical frailty scale, was slightly higher than expected for a European hospital population.11, 12 The first of these studies suggested prevalence of frailty to be 39·6% in a large (n=12 282) but single centre UK estimate,12 the second suggested frailty to be 42% in a much younger population drawn from the 500 000-person UK Biobank.11 However, the varying degree of frailty across the sites in our study appears consistent with the natural variation expected. Our findings suggest that contracting COVID-19 is probably more severe for frail people and adds empirical substance to the widely held belief that a poor outcome is associated with increased age or increased frailty.
Mortality in our population was 27·2%, which is in line with current mortality estimates for COVID-19 globally. In a large patient cohort in New York (NY, USA) 555 (21%) of 2634 people died;3 the participants had a median age of 63 years (estimated IQR 49–74) compared with a median age of 74 years (61–83) in our study.3 The mortality rate in our study is slightly lower than that reported in the original patient characterisation from Wuhan, China (28·3%),4 which was also a younger population, with a median age of 56 years (46–67, range 18–87). Additionally, Docherty and colleagues21 recently reported a mortality rate of 26% (6769 of 20 133 participants) in a large UK descriptive study. The median age of their population was 73 years (IQR 58–82). Their findings are similar to ours and probably the same impact of frailty would be observed within their larger cohort.
These data also show that frailty helps to predict the risk of death in patients with COVID-19, similar to in other diseases.6, 19 The primary aim of this study was to provide evidence to support clinicians in making decisions about appropriate allocation of health-care resources in the urgent setting of the COVID-19 pandemic. Notably, the decision support tool recommended for use in the UK National Health Service includes the CFS that is used in this study.8 These results support the use of the CFS in the decision making process and it could be considered for adoption in other countries.
Time from hospital admission to discharge was significantly longer in frail patients (CFS 5–6 and CFS 7–9) than in patients with low frailty scores. Again, findings are consistent with the non-COVID-19 literature, and further support the hypothesis that frailty is an appropriate tool for use in patients with COVID-19. Also, people with high frailty scores probably require longer to recover and rehabilitate from COVID-19 and require more complex discharge planning,22 but these were not assessed in this study. Notably, frailty should not be used alone for clinical decisions.23
These data were collected from representative hospitals situated across England, Scotland, and Wales. 10% of participants were from Italy, which adds to the wider European generalisability. However, incidence of COVID-19 tended to appear in hotspots around the country and caution should be used until the pandemic has been analysed across countries worldwide. The demographic findings, such as the increased mortality associated with increased C-reactive protein levels and prevalence of comorbidities (hypertension, diabetes, and coronary artery disease) are also in line with other estimates, suggesting that our data are comparable with other populations.2, 3 It is unfortunate that because of the constraints of the pandemic, we were unable to rapidly collect more clinical data, for example body-mass index. We deliberately focused only on core variables within the study and group to achieve rapid reporting of frailty data. Further, as data regarding the pandemic become more widely available, comorbidities are being shown to be of increasing importance, so these additional data would have added value to the manuscript but were not readily available at the time of data collection. The study data quality and completeness allowed all analyses to include at least of 97% of included patients.
It is possible that inaccuracies might have occurred during data collection; however, training in data collection was provided to all study personnel and the research team are experienced in collecting observational data in frail people from multiple UK sites.6, 19
The UK guidelines recommend frailty assessment using the CFS in the COVID-19 pandemic.8 The CFS is a simple, quick, and easy-to-use frailty measure, although not well validated in people younger than 65 years or those with learning disabilities.9 Other frailty measures are available for inpatient frailty assessment but are usually longer to complete or rely on routinely collected data to calculate the frailty score.14, 24 With scarce research evidence, we did not feel it appropriate to analyse other frailty assessments, despite their potential usefulness during this pandemic. This study also provides evidence regarding the validated use of the CFS in the frailty assessment of people with COVID-19.
Another limitation is that patients were only included if admitted to hospital. This criterion will have excluded patients that were discharged from or died in emergency departments, and excluded cases diagnosed in the community, who did not present to secondary care. It will also have excluded many frailer individuals living in residential and nursing facilities. Hence, these findings are only generalisable to an inpatient population.
This study, which was designed to assess the effect of frailty on outcomes in people of all ages with COVID-19, showed that frailty increases risk of mortality, even after accounting for age and other known comorbidities linked to COVID-19. Overall, these findings support the use of frailty as a trigger for specialist resource allocation, pathway decision aid, and in shared decision making in people with COVID-19. The findings show the importance of frailty assessment, rather than age, in combination with other measures in the context of COVID-19.
Data sharing
All data sharing and collaboration requests should be directed to the corresponding author.
Contributors
BC, JHew, KMc, PKM, and SJM conceived the concept of the study. BC, JTC, and JHew managed the project. JHew, BC, AV-M, TJQ, PB, AV, LP, MS, AP, JTC, EB, AE, FR, EM, MH, JHes, FB-P, EC, SJM, and KMc did data entry. EM, JHes, and FR did the literature review. JHew, BC, PKM, SJM, and KMc did data analysis. BC and RS did statistical analysis. RS did graphics. JHew, and BC wrote the first draft of the manuscript. All authors contributed to writing of the manuscript.
COPE study collaborators
Charlotte Davey, Sheila Jones, Kiah Lunstone, Alice Cavenagh, Charlotte Silver, Thomas Telford, and Rebecca Simmons (Ysbyty Yystad Fawr); Tarik El Jichi Mutasem, Sandeep Singh, Dolcie Paxton, and Will Harris (North Bristol Trust); Norman Galbraith, Emma Bhatti, Jenny Edwards, and Siobhan Duffy (Royal Alexandra Hospital, Paisley); Carly Bisset and Ross Alexander (Inverclyde Royal Infirmary); Madeline Garcia, Shefali Sangani, Thomas Kneen, and Thomas Lee (Salford Royal Infirmary); Aine McGovern (Glasgow Royal Infirmary); and Prof Giovanni Guaraldi (University Hospital of Modena Policlinico, Italy).
Declaration of interests
We declare no competing interests.
Contributor Information
Kathryn McCarthy, Email: kathryn.mccarthy@nbt.nhs.uk.
COPE Study Collaborators:
Charlotte Davey, Sheila Jones, Kiah Lunstone, Alice Cavenagh, Charlotte Silver, Thomas Telford, Rebecca Simmons, Tarik El Jichi Mutasem, Sandeep Singh, Dolcie Paxton, Will Harris, Norman Galbraith, Emma Bhatti, Jenny Edwards, Siobhan Duffy, Carly Bisset, Ross Alexander, Madeline Garcia, Shefali Sangani, Thomas Kneen, Thomas Lee, Aine McGovern, and Giovanni Guaraldi
Supplementary Material
References
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewitt J, Carter B, McCarthy K. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. 2019;48:388–394. doi: 10.1093/ageing/afy217. [DOI] [PubMed] [Google Scholar]
- 7.New York State Task Force on Life and the Law Ventilator allocation guidelines. November, 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf
- 8.National Institute for Health and Care Excellence NICE updates rapid COVID-19 guideline on critical care. March 25, 2020. https://www.nice.org.uk/news/article/nice-updates-rapid-covid-19-guideline-on-critical-care [PubMed]
- 9.Rockwood K, Song X, MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley JE, Vellas B, van Kan GA. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med. 2016;35:24–34. doi: 10.1016/j.ejim.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Smart R, Carter B, McGovern J. Frailty exists in younger adults admitted as surgical emergency leading to adverse outcomes. J Frailty Aging. 2017;6:219–223. doi: 10.14283/jfa.2017.28. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert T, Neuburger J, Kraindler J. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesari M, Costa N, Hoogendijk EO, Vellas B, Canevelli M, Pérez-Zepeda MU. How the frailty index may support the allocation of health care resources: an example from the INCUR study. J Am Med Dir Assoc. 2016;17:448–450. doi: 10.1016/j.jamda.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Muscedere J, Waters B, Varambally A. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.East Midlands Emergency Medicine Educational Media Lightning learning: clinical frailty scale. April 21, 2017. https://static1.squarespace.com/static/546e1217e4b093626abfbae7/t/58fdd215be6594f266ea05d0/1493029401898/Clinical+Frailty+Scale+%28Lightning+Learning%29.pdf
- 18.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmar KL, Law J, Carter B. Frailty in older patients undergoing emergency laparotomy: results from the UK observational Emergency Laparotomy and Frailty (ELF) study. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003402. published online June 7. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez RG. Parametric frailty and shared frailty survival models. Stata J. 2002;2:22–44. [Google Scholar]
- 21.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis G, Whitehead MA, Robinson D, O'Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moug S, Carter B, Myint PK. Decision-making in COVID-19 and frailty. Geriatrics. 2020;5:E30. doi: 10.3390/geriatrics5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollinghurst J, Fry R, Akbari A. External validation of the electronic Frailty Index using the population of Wales within the secure anonymised information linkage databank. Age Ageing. 2019;48:922–926. doi: 10.1093/ageing/afz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sharing and collaboration requests should be directed to the corresponding author.