Abstract
The dramatic increase in suspected COVID-19 cases in Africa has placed an enormous burden on public and private clinical facilities. To date, the most commonly used method for identifying and confirming the virus is the laboratory-based reverse transcription-polymerase chain reaction (RT-PCR) test. Unfortunately, testing capacities have been limited in many parts of Africa because of inadequate test kits, which have restricted scaling up beyond the few public health laboratories at designated locations. In this mini-review, we present Africa's preparedness and readiness for testing, why testing is crucial, the need to immediately strengthen existing facilities, and what it involves as part of combined approaches for managing the COVID-19 crisis. The review highlights the urgent need for speedy expansion and distribution of several laboratory testing platforms, including real-time PCR and serological assays to both public health facilities and fully accredited private clinical laboratories.
Keywords: RT-PCR, Serological, SARS-CoV-2, Laboratory, Test kits, Africa, Assay
Introduction
As the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2) or COVID-19 situation exacerbates worldwide, Africa has had its share of the virus outbreak. Since February 14, 2020, when the first case was confirmed on the continent (in Egypt), all 54 countries in Africa had confirmed cases as of May 13, 2020, with Lesotho being the latest country to record the virus (African News 2020). As of June 18, 2020, Africa had recorded 267,818 cases, with 7,219 deaths and 123,054 recoveries (Africa CDC, June 16, 2020). Significant variation in prevalence exists across the continent, ranging from four cases in Lesotho to 80,412 cases in South Africa (Shaban, 2020).
With the current outbreak characteristics in Africa, public health concerns could become critical if needed steps are not taken. Between and within-country statistics show sporadic and faster COVID-19 community transmission; countries may experience this scenario at the sub-national level (WHO, 2020c). Available data show that COVID-19 is gradually spreading across the African population (Salathé et al., 2020). Amidst this, some infected people may be unnoticed because of testing-related challenges, including persons with COVID-19 compatible clinical disease(s) (Salathé et al., 2020). WHO strongly encourages countries to prepare even before recording their first case (WHO, 2019a, WHO, 2019b). Preparation includes diverse individual protocols such as regular handwashing with soap and water or with an alcohol-based hand rub and social distancing (i.e., maintaining at least one meter distance between persons). Similarly, covering one's mouth and nose when coughing or sneezing and avoiding face self-touching constitute some of the individual protocols (WHO, 2019a, WHO, 2019b). Other protocols include staying at home if one feels unwell, refraining from smoking and all activities that weaken the lungs, avoiding unnecessary travel and staying away from crowds (WHO, 2020a).
Apart from these individual measures, one step towards identifying and treating persons with the virus is testing. WHO considers diagnostic testing for COVID-19, critical. It is essential to track the virus and understand its epidemiology to inform case management and suppress transmission (WHO, 2020b). Therefore, in this mini-review, we provide a discussion of Africa's preparedness towards testing, why testing is crucial, the need for and how to immediately strengthen existing facilities to manage the COVID-19 crisis.
Africa's preparedness towards testing
At present, testing-related competencies are restricted in many parts of Africa due to a limited number of test kits, preventing scaling up of testing beyond a few public health laboratories. It is evident from various country reports that the increasing number of suspected cases far outweighs the capacity of most health facilities in Africa, leaving many individuals untested (The New Humanitarian, 2020, Wild, 2020). According to del Rio and Malani, 2020a, del Rio and Malani, 2020b, developing vigorous testing capacities is an unmet need in dealing with the current outbreak, crucial for verifying persons with the virus as well as discovering those who are asymptomatic. Therefore, Africa's preparedness must focus on establishing COVID-19 testing capacities in each country. Countries with minimal testing capacity should be quickly equipped to refer samples of suspected cases to a WHO reference laboratory for COVID-19 testing through inter-laboratory collaboration. Countries with testing capacity at the national level should scale-up by decentralizing testing capacity through identified regional laboratories under the strict supervision of the COVID-19 national reference laboratory. Additionally, testing facilities can be located outside of hospitals to avoid overburdening already minimal hospital resources and the potential risk of facility-based (i.e., nosocomial) transmission to other patients and healthcare personnel (Salathé et al., 2020). For instance, private laboratory services and/or academic institutions with standardized laboratories could be additional options worth exploring, especially in geographical locations where difficulty in testing exists due to limited facilities (WHO, 2020c). Although testing alone will not prevent the spread of COVID-19, it is part of the mitigation strategies to keep the virus's current spread under control by preventing local transmission. This method should be readily available, and setbacks to it should be as few as practicable (Salathé et al., 2020).
Why testing is crucial
Africa requires an intense uprading of its testing capacities because of its limited capabilities in test centers and existing diagnostic facilities with both inadequate personnel and reagents. This is because providing laboratory testing for COVID-19 in suspected cases has clear benefits. Testing for COVID-19 would let infected persons be aware of their status (i.e., positive status). People who do not know their status may ignore essential preventive and management protocols (e.g., social distancing, self-isolation) and thus put others at risk of infection (Roser et al., 2020a). For example, a typical COVID-19 case starts with minor symptoms that on average manifest between 8 and 10 days after onset. Hence, testing will subsequently help to discover other cohorts, to contact trace, and to ensure preventive self-isolation of contacts. For African governments, this identification will facilitate follow-up measures to reduce the probability of infecting others. More extensive testing will provide a more precise estimate of the case-fatality ratio related to the time between disease onset and possible death, in the foreseeable future (Salathé et al., 2020).
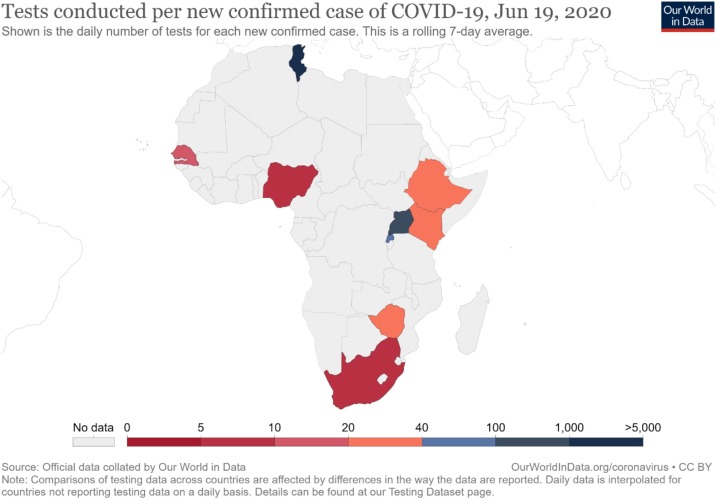
Through testing, proper evidence-based measures against the pandemic can be implemented, based on more accurate information about the number of cases. Such an estimation will facilitate the allocation of COVID-19 related hospital resources (e.g., ICU bed, nose/face mask, and other protective equipment requirements). Without clear data on the epidemiological situation on the ground, frontline health workers will face problems in their quest to manage the pandemic, in particular identifying what percentage of persons have mild, severe, and critical symptoms. The testing process will help understand the spread of the disease and thereby guide the implementation of appropriate interventions to minimize the infection's spread (Roser et al., 2020a, Salathé et al., 2020). By continuous monitoring of COVID-19 test results, a clear temporal picture on whether control measures have been effective or not will be established, thus providing needed information for alternate measures to be explored, at any specific time (Salathé et al., 2020). Unfortunately, many countries in Africa do not have adequate capacity for COVID-19 testing (see Figure 1 ). Consequently, this could be one area that can seriously undermine the control of the virus's possible spread on the continent (Agaba, 2020).
Figure 1.
Tests conducted per new confirmed cases of COVID-19 in Africa, June 19, 2020 (Roser et al., 2020b).
Bridging the testing gap
Drawing on the lessons of Ebola, the 2009 H1N1 influenza pandemic, and SARS-CoV, it will be critical to develop a country-specific framework guided by standardized WHO guidelines in response to an emerging viral outbreak like COVID-19. Immediate involvement of technical knowhow and the directives from reputable institutions (e.g., Centre for Disease Control [CDC]), local governments, public health departments, clinical laboratories, healthcare providers as well as other stakeholders should be on the frontline services (Binnicker, 2020). At the onset of the outbreak, it is essential that local institutions (e.g., CDC, Food and Drugs Authority [FDA]) and WHO, speedily develop new diagnostic tests based on their previous experience with infectious disease control. Test reagent manufacturers could be identified for the mass production of test reagents after developing an assay that has been verified, based on standard performance characteristics, by partnering public health agencies. After authorizing the usage of test kits, immediate local distribution by test manufacturers to qualified laboratories (i.e., state and local public health laboratories) should be the next step. The decision to include private clinical laboratories should be made after a thoroughly vetted process that could include:
-
1.
registering local clinical laboratories to meet approved equipment, safety set-up, and personnel to facilitate testing requirements,
-
2.
a follow-up site-visit by an expert from an already established CDC-qualified laboratory representative, and
-
3.
a double-blind certification panel directed by the CDC and/or test manufacturer to the clinical laboratory.
These procedures might be subject to modifications or special considerations depending on disease-specific characteristics (e.g., mode of transmission [blood-borne versus airborne] or recommended testing approach [molecular versus serological]) (Binnicker, 2020).
Because of the current pandemic's inherent continental testing challenges, there has been a faster regional response to the pandemic across Africa. The Africa Centres for Disease Control and Prevention, in partnership with WHO's African Region, have introduced an Africa Task Force for Novel Coronavirus that recently launched the “Partnership to Accelerate COVID-19 Testing (PACT): Test, Trace, Treat in Africa” on June 4, 2020 (Africa CDC, June 16, 2020). PACT is to provide the needed assistance for a joint continental strategy to help member states limit COVID-19 transmission. The Africa CDC recommends:
-
1.
ensuring an uninterrupted supply of test kits, reagents, and other essential supplies,
-
2.
pooling testing, reinforcing and expanding the existing workforce by providing training to additional laboratory technicians to run tests,
-
3.
decentralizing testing to other qualified laboratories within countries,
-
4.
considering rapid diagnostics where this can be validated and standardized,
-
5.
automating streamlining, and ensuring uninterrupted laboratory processes, and
-
6.
establishing a rapid and reliable specimen transport system (Africa CDC, 2020a).
The Africa CDC further suggests that to identify cases and their contacts, there is the need to:
-
1.
guarantee smart screening of at-risk populations,
-
2.
sensitize and activate local detection points like patent medicine vendors, traditional healers, and laboratories in order to identify cases,
-
3.
trace all case contacts,
-
4.
conduct active cases searching while investigating known cases,
-
5.
train and deploy community health workers to assist with contact tracing, community engagement, and education,
-
6.
facilitate and encourage care-seeking for symptomatic individuals, and
-
7.
engage and empower community members to self-report and seek care (Africa CDC, 2020a).
For treating symptomatic cases, the Africa CDC reiterates that these measures are essential:
-
1.
providing guidance on home-based care to individuals experiencing mild symptoms,
-
2.
implementing infection prevention and control practices,
-
3.
immediate triage and testing of symptomatic individuals at healthcare facilities,
-
4.
guaranteeing the availability of critical equipment and their functionality (e.g., oxygen and oxygen-delivering interfaces), and
-
5.
early identification and evidence-informed management of individuals with moderate to critical illness and medically vulnerable persons (e.g., older patients, individuals with comorbidities) (Africa CDC, 2020a).
Based on these recommendations, up to one million test kits have been distributed across the continent. Similarly, 625,000 Polymerase Chain Reaction (PCR) tests have been given to 51 member countries, and the extra support of 6600 GeneXpert cartridges have also been supplied to three member states (i.e., Comoros, Guinea, Sao Tome) that have limited or no capacity for PCR testing. Other pathogen genomics equipment and reagents have also been supplied to member countries except for Egypt (Africa CDC, June 16, 2020).
Laboratory testing capacity has increased from 2 to 44 African countries (https://apps.who.int/iris/bitstream/handle/10665/331763/SITREP_COVID-19_WHOAFRO_20200415-eng.pdf). Considerable efforts are now ongoing to increase diagnostic capacity across the continent. For instance, there are 44 hospitals with 32 specialized centers that are adequately resourced in response to the current outbreak in Morocco (https://www.moroccoworldnews.com/2020/03/296658/moroccoannounces-5-new-cases-of-covid-19-bringing-total-to-54/). As well, Algeria, Ghana, Nigeria, Senegal, South Africa, and a number of other African countries have established laboratories for within-country testing of COVID-19.
Conclusions
Countries in Africa have introduced different strategies to battle COVID-2019. However, these interventions are not without serious setbacks (e.g., limited or zero testing capacity, inadequately trained personnel). The World Health Organisation (WHO) and donor institutions could collaborate with local governments in the African region to create new health infrastructures (e.g., referral laboratories) and expand existing ones with relevant health logistics. Successful diagnosis of COVID-19 depends on proven scientific observations, laboratory findings, and epidemiological connections (Wang et al., 2020). Hence, data accuracy from the clinical testing will have a considerable impact on the other follow-up protocols to manage and prevent the virus spread (Stanfill et al., 2020). Therefore, a speedy expansion and geographical distribution of several laboratory testing platforms by public health facilities and fully accredited private clinical laboratories, including real-time PCR and serological assays, will be critical for a response to the pandemic (Sharfstein et al., 2020). Additionally, there should be interim guidance on the use of serological tests for COVID-19, especially in countries with limited and/ or no testing capacity. According to WHO (2020), although these procedures are fundamental, prioritizing testing should not impede other appropriate interventions (e.g., home isolation and quarantine) to further curb the virus's spread. Only then would Africa's COVID-19 expectations and needs be met at local levels on the continent.
Funding
We sincerely thank the German Research Foundation through the Neurocognition and Action-Biomechanics Research Group, Bielefeld University, Germany for providing financial support for the publication of this research.
Ethical approval
Ethical approval for this study was not required per our institutions’ policies, since the work did not involve the use of human subjects or animal experiments.
Conflict of interest
The authors have no conflict of interest to declare.
References
- Africa CDC . 2020. Partnership to accelerate COVID-19 testing (PACT) in Africa. Retrieved on June 20, 2020. Available from: https://africacdc.org/download/partnership-to-accelerate-covid-19-testing-pact-in-africa-social-media-materials/ [Google Scholar]
- Agaba J. 2020. Experts call for accelerated testing in Africa as WHO predicts up to 190,000 COVID-19 deaths. Retrieved on June 19, 2020. Available from: https://allianceforscience.cornell.edu/blog/2020/06/experts-call-for-accelerated-testing-in-africa-as-who-predicts-up-to-190000-covid-19-deaths/ [Google Scholar]
- Binnicker M.J. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- del Rio C., Malani P.N. 2019 novel coronavirus—important information for clinicians. JAMA. 2020 doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- https://apps.who.int/iris/bitstream/handle/10665/331763/SITREP_COVID-19_WHOAFRO_20200415-eng.pdf [Accessed 13 June 2020]
- https://www.moroccoworldnews.com/2020/03/296658/moroccoannounces-5-new-cases-of-covid-19-bringing-total-to-54/
- Roser M., Ritchie H., Ortiz-Ospina E. Coronavirus disease (COVID-19)–statistics and research. Our World in Data. 2020 [Google Scholar]
- Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. 2020. Coronavirus Pandemic (COVID-19) Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/coronavirus [Online Resource] [Google Scholar]
- Salathé M., Althaus C.L., Neher R., Stringhini S., Hodcroft E., Fellay J. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150(1112) doi: 10.4414/smw.2020.20225. [DOI] [PubMed] [Google Scholar]
- Shaban A.R.A. 2020. News; African News. Available from: https://www.africanews.com/2020/06/18/coronavirus-in-africa-breakdown-of-infected-virus-free-countries/ [Accessed 19 June 2020] [Google Scholar]
- Sharfstein J.M., Becker S.J., Mello M.M. Diagnostic testing for the novel coronavirus. JAMA. 2020 doi: 10.1001/jama.2020.3864. [DOI] [PubMed] [Google Scholar]
- Stanfill M.H., Giannangelo K., Fenton S.H. 2020. Health information management best practices for quality health data during the COVID-19 global pandemic. Retrieved on June 19, 2020. Available from: https://perspectives.ahima.org/health-information-management-best-practices-for-quality-health-data-during-the-covid-19-global-pandemic/ [Google Scholar]
- The New Humanitarian . 2020. African countries struggle to find the coronavirus test kits they need. Retrieved on June 19, 2020. Available from: https://www.thenewhumanitarian.org/news/2020/05/18/Africa.coronavirus-test-kits. [Google Scholar]
- Wang Y., Kang H., Liu X., Tong Z. Combination of rt-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-COV-2 outbreak. J Med Virol. 2020 doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S. 2020. African countries are struggling to access Covid-19 test reagents on the open market. Retrieved on June 19, 2020. Available from: https://qz.com/africa/1854277/african-countries-struggle-to-get-covid-19-test-reagents/ [Google Scholar]
- World Health Organization . 2019. Novel coronavirus (2019-ncov) technical guidance: laboratory testing for 2019-ncov in humans. https://www.Who.Int/emergencies/diseases/novel-Coronavirus. [Google Scholar]
- World Health Organisation . 2020. Coronavirus disease (COVID-19) advice for the public. Retrieved on June 19, 2019. Available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. [Google Scholar]
- World Health Organization . World Health Organization; 2020. Laboratory testing strategy recommendations for COVID-19: interim guidance. March 22, 2020 (No. WHO/COVID-19/lab_testing/2020.1) [Google Scholar]
- World Health Organization . World Health Organization; 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance. March 2, 2020 (No. WHO/COVID-19/laboratory/2020.4) [Google Scholar]
- World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [Accessed 08 April 2020] [Google Scholar]