Abstract
Obesity represents an important public health concern because it substantially increases the risk of multiple chronic diseases and thereby contributing to a decline in both quality of life and life expectancy. Besides unhealthy diet, physical inactivity and genetic susceptibility, environmental pollutants also contribute to the rising prevalence of obesity epidemic. An environmental obesogen is defined as a chemical that can alter lipid homeostasis to promote adipogenesis and lipid accumulation whereas an endocrine disrupting chemical (EDC) is defined as a synthetic chemical that can interfere with the endocrine function and cause adverse health effects. Many obesogens are EDCs that interfere with normal endocrine regulation of metabolism, adipose tissue development and maintenance, appetite, weight and energy balance. An expanding body of scientific evidence from animal and epidemiological studies has begun to provide links between exposure to EDCs and obesity. Despite the significance of environmental obesogens in the pathogenesis of metabolic diseases, the contribution of synthetic chemical exposure to obesity epidemic remains largely unrecognised. Hence, the purpose of this review is to provide a current update on the evidences from animal and human studies on the role of fourteen environmental obesogens in obesity, a comprehensive view of the mechanisms of action of these obesogens and current green and sustainable chemistry strategies to overcome chemical exposure to prevent obesity. Designing of safer version of obesogens through green chemistry approaches requires a collaborative undertaking to evaluate the toxicity of endocrine disruptors using appropriate experimental methods, which will help in developing a new generation of inherently safer chemicals.
Keywords: Obesogens, Endocrine disrupting chemicals, Adipogenesis, Obesity, Green chemistry
Highlights
-
•
Many environmental obesogens are endocrine disrupting chemicals that interfere with normal endocrine regulation of metabolism.
-
•
Understanding the role of environmental obesogens in the epidemics of obesity is in an infant stage.
-
•
Green chemistry approach aims to design a safer version of these chemicals by understanding their hazardous effects.
-
•
Further studies are necessary to fully establish the hazardous effects of obesogens and their association to human obesity.
List of abbreviations
- EDC
Endocrine disrupting chemical
- NCDs
Non-communicable diseases
- BMI
Body mass index
- PPAR
Peroxisome proliferator-activated receptor
- RXR
Retinoid X receptor
- HCB
Hexachlorobenzene
- HCH
Hexachlorocyclohexane
- PCBs
Polychlorinated biphenyls
- DDT
Dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- BPA
Bisphenol A
- GLP-1
Glucagon-like peptide 1
- PVC
Polyvinyl chloride
- PET
Polyethylene terephthalate
- ATZ
Atrazine
- MSG
Monosodium glutamate
- ROS
Reactive oxygen species
- PBDE
Polybrominated diphenyl ethers
- PFOA
Perfluorooctanoic acid
- HFD
High fat diet
- CVD
Cardiovascular disease
1. Introduction
Obesity is an important global health concern, as it is one of the main predisposing factors for the emerging epidemic of non-communicable diseases (NCDs) and recognised by the UN Sustainable Development Goals [1]. Nearly 30% of the total population are obese (~2.1 billion) and the worldwide obesity rate has tripled since 1975 [2]. While caloric excess, sedentary lifestyle and genetic susceptibility are classically identified as the main drivers of obesity [3,4], these factors alone do not fully account for the genesis and pattern of the obesity epidemic. Since late 19th century, the environment to which humans are exposed has changed due to the increased production of synthetic chemicals, which are also a potential risk factor of obesity [5]. These chemicals can interfere with the action of hormones, which are involved in regulating metabolism and weight gain, and are referred to as “environmental obesogens” that are thought to promote obesity by interfering with metabolic homeostasis [6].
Although endocrine disruption has only recently received a great attention, the concept has been known about for a long time. Early indications of an endocrine-disrupting activity were reported in the 1920s using studies in pigs [7]. In 1960s, after exposure to industrial chemicals, endocrine disruption was reported widely in wildlife living on land, in water and in air. Today, humans are ubiquitously exposed to chemicals in daily life through their use in industrial and household products, pesticides, herbicides, plastics, detergents, flame retardants and personal care products. An expanding body of scientific evidence has begun to provide links between exposure to such chemicals and metabolic diseases such as obesity and diabetes [8,9]. Evidence from research studies has shown that a variety of environmental chemicals can influence adipogenesis and obesity. Today, there are more than 1000 chemicals reported to have endocrine effects [10].
Despite the significance of environmental obesogens in the pathogenesis of metabolic diseases, the contribution of synthetic chemical exposure to obesity remains largely unrecognised. Hence, the purpose of this review is to provide a current update on the evidences from animal and human studies on the role of environmental obesogens in obesity, a comprehensive view of the mechanisms of action of these obesogens and green and sustainable chemical strategies to overcome chemical exposure to prevent obesity [1].
2. Environmental obesogens hypothesis
In 2002, Baillie-Hamilton proposed a link between the increase in new industrial chemicals over the past four decades and the beginning of the obesity epidemic [11] suggesting that these so-called obesogens could have damaged many of the body’s natural weight-control mechanisms. This correlation along with experimental evidence led to the environmental obesogens hypothesis by Grun and Blumberg in 2006 [12]. The hypothesis suggests that prenatal or early-life exposure to synthetic chemicals may predispose exposed individuals to increased fat mass and excess weight. Studies in animal models have shown that certain environmental pollutants induce adipogenesis (i.e., formation of adipocytes (fat cells) from stem cells) and weight gain that is suggestive of the causative role of synthetic chemicals in the pathogenesis of obesity [13].
3. Endocrine disrupting chemicals (EDCs)
Endocrine Disrupting Chemicals (EDCs) are exogenous chemicals that interfere with the action of hormones. EDCs are used in everyday products from food packaging to fungicides and found abundant in our environment. Exposure to EDCs during early years of development have been shown to increase the risk of developing various chronic diseases including obesity and diabetes [14]. EDCs cause weight gain by altering lipid metabolism to promote adipogenesis and lipid accumulation [15]. This has been shown to occur through the following mechanisms [16,17]: (i). increasing the number and size of adipocytes and storage of fat per cell, (ii). altering endocrine pathways responsible for control of adipose tissue development, hormones that regulate appetite, satiety, and food preferences, basal metabolic rate, energy balance to favour storage of calories and insulin sensitivity and lipid metabolism in endocrine tissues such as pancreas, adipose tissue, liver, gastrointestinal tract, brain, and muscle.
The effect of EDCs on adipogenesis and obesity gained further attention after the postulation of the environmental obesogens hypothesis. These effects often begin during development, lead to obesity later in life [15]. Many environmental obesogens are EDCs that interfere with normal endocrine metabolic regulation, adipose tissue development and maintenance, appetite, weight and energy balance [18].
Studies have indicated that EDCs/obesogens are likely to increase the number and size of adipocytes by interfering with transcriptional regulators that control lipid flux, adipocyte proliferation, and adipocyte differentiation, particularly through the peroxisome proliferator-activated receptors (namely, PPAR-γ). Activation of the retinoid X receptor (RXR)–PPAR- γ heterodimer favours the differentiation of pre-adipocytes in adipose tissue and regulates lipid biosynthesis and storage [16]. Besides PPARs, steroid hormones have also shown to influence lipid storage and fat deposition. Another mechanism of EDC action may be through disrupting the energy balance between energy intake and energy expenditure [19], which has been shown to occur by either altering appetite, satiety, and food choices or through altering physical activity, resting metabolic rate, adaptive thermogenesis, and growth rates.
It has been shown that, during early stages of development, only low levels of EDCs are necessary to alter development as the protective mechanisms that exist in an adult such as the ability to repair DNA, a competent immune system, detoxifying enzymes, liver metabolism, the blood–brain barrier, and a normal metabolic rate may not yet be developed [9,20]. However, longer exposure to EDCs is required for the development of obesity in adulthood [20].
4. Epidemiological evidence of obesogens
The first publications that provided a proof of principle for the ability of chemicals to induce obesity, which were in line with obesogens hypothesis, showed that smoking during pregnancy can increase weight gain in children. This was further confirmed in 2008, where a meta-analysis of 14 epidemiological studies showed a strong association between maternal smoking during pregnancy and weight gain in the children [21]. By 2013, this finding was replicated in 30 different epidemiological studies [22]. Furthermore, these results were confirmed in human studies where nicotine administration in pregnant mothers resulted in increased body weight and fat disposition, adipocyte size and expression of genes involved in adipogenesis [23]. These studies led to the discovery of nicotine as an endocrine disruptor.
Similarly, the role of polychlorinated biphenyls (PCBs) as endocrine disruptors/obesogens was first shown by an epidemiological study [24], which showed that women exposed to PCBs during pregnancy gave birth to girls who were heavier than other girls. Following this study, several studies examined the role of PCBs in obesity. Several organochlorine pesticides (OCPs) have also been demonstrated to play a role in obesity as an endocrine disruptor. Prospective human studies have shown an association between elevated levels of dichlorodiphenyltrichloroethane (DDT) or its main metabolite dichlorodiphenyldichloroethylene (DDE) during pregnancy and development of obesity in offspring [25]. However, a few cohort studies that examined the effects of other OCPs such as hexachlorobenzene (HCB) and hexachlorocyclohexane (HCH) failed to provide an evidence of their obesogenic effect [26]. Even though animal studies have provided a link between EDCs and obesity [26], some of the epidemiological studies failed to confirm the link, which could be due to the differences in the sample size, measurement error and other environmental exposures.
5. Evidence of obesogens in animal models
Several chemicals have been shown to elicit biological effects that alter adipogenesis leading to weight gain. For instance, exposure of pregnant mice to tributyltin produced multipotent stromal cells that differentiated preferentially into adipocytes suggesting that tributyltin can act by altering adipocyte differentiation [27]. Studies have shown that such alterations during development of adipose tissues in early life can lead to the development of obesity in adulthood. Dietary soy phytoestrogens, such as genistein and daidzein, have been shown to modulate estrogen receptor signalling and reverse truncal fat accumulation in postmenopausal women and in ovariectomized rodent models [19]. Male offspring of rodents treated with phytoestrogens during pregnancy or lactation developed obesity at puberty. Neonatal exposure to diethylstilbestrol, a synthetic form of the female hormone estrogen, led to long-term weight gain in adulthood in female mice [28], which suggested that EDCs with estrogenic activity may act to mimic estrogen action on adipogenesis.
Bisphenol A (BPA) belongs to the list of compounds that have the obesogenic property, as rodent models have shown that exposure to BPA is associated with weight gain [29]. One of the mechanisms by which BPA increases body weight is through the activation of PI-3 kinase [30] where BPA can push the fibroblastic cells into the adipocyte differentiation pathway leading to increased accumulation of triglycerides and lipoprotein lipase.
6. Environmental obesogens
There are several environmental chemicals, which have been shown to act as EDCs. This section will focus on some of the commonly identified EDCs, their mechanisms of action as obesogens including epigenetic and transgenerational effects, in addition to the data connecting exposures to obesity in human populations (Table 1 ).
Table 1.
List of obesogens and their role in the environment and mechanism of action.
| Obesogen | Nature of the chemical | Chemical structurea | Role in the environment | Mechanism of action |
|---|---|---|---|---|
| Bisphenol A | 2,2-bis(4-hydroxyphenyl)propane | 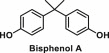 |
Used in the production of polycarbonate plastics and epoxy resins | An active agonist of estrogen-sensitive membrane receptor GPR30 and glucocorticoid receptors and it mimics the structure and function of the sex hormone estrogen [31,32]; Alters glucose metabolism, impairs adipogenesis and causes adipocyte dysfunction [33] |
| Phthalates | Diesters of 1,2-benzenedicarboxylic acid | 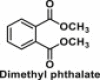 |
Used as plasticizers | A chemical with anti-androgenic and weakly estrogenic properties; influences obesity through mechanisms such as anti-thyroid hormone activities, and/or activation of peroxisome proliferator-activated receptors (PPAR), and epigenetic modulation [34]; maternal phthalate exposure is associated with alterations in methylation of critical placental genes [35] |
| Atrazine | 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine | 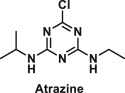 |
Used as herbicide | A chemical with androgenic inhibiting and weak estrogenic effects; binds to complex-I and III of the mitochondrial electron transport system and inhibits the oxidative phosphorylation of mitochondria [36]; Acts as an endocrine disrupter by Inhibiting cAMP-specific Phosphodiesterase-4 [37] |
| Organotins | Chemical compounds based on tin with hydrocarbon substituents | 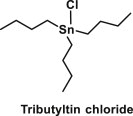 |
Used as polyvinyl chloride stabilizers, biocides, or antifouling paints | These chemicals interact with transcriptional regulators such as nuclear and steroid receptors and affect nuclear receptor signalling pathways (such as retinoid X receptor/PPARγ signalling pathway) leading to an alteration of glucose transporter, proinflammatory cytokines and lipid and carbohydrate metabolism [38] |
| Organophosphates | A class of organophosphorus compounds with the general structure O P(OR)3 | 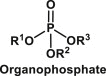 |
Used as insecticides, ophthalmic agents, herbicides and industrial chemicals | A chemical that disrupts the pathway synthesizing cyclic adenosine monophosphate controlled by adenylyl cyclase; increases lipid peroxidation [39] |
| Monosodium glutamate | Sodium salt of glutamic acid | 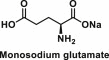 |
Used as a seasoning to make bland, nutritious foods taste good; also found naturally in algae, mushrooms, tomatoes, grapes and processed frozen foods, potato chips, salty snacks, sauces and sausages | It impairs the secretion of the gut hormone, glucagon-like peptide 1 (GLP-1), which is involved in satiety responses and insulin release [40]; induce neuronal necrosis in several brain regions including the hypothalamus [41] |
| Clozapine | Dibenzo-diazepine derivative | 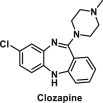 |
Used as a medicine in treating schizophrenia-related symptoms | It alters the function of key metabolic enzymes and affects electron transport chain during oxidative phosphorylation in the mitochondria; blocks muscarinic M1 and M2 receptors and inhibit GLP-1 secretion; enhances the production of cytokines that modulate immunological responses and promotes inflammation [42] |
| Polychlorinated biphenyls | Polyhalogenated aromatic hydrocarbons | 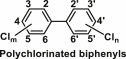 |
Used as plasticizers in paints, plastics and rubber products, in pigments, dyes and carbonless copy paper and in electrical, heat transfer and hydraulic equipments | These chemicals are hormonally active substances, mimicking the action of the thyroid hormone and estrogens [43]; disrupt the release of neurotransmitters that regulate neuroendocrine functions, cause alterations in intracellular calcium signalling and affect dopamine release [44] |
| Organobromines | 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane; Polybrominated diphenyl ethers | 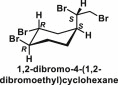 |
Used as a flame retardant | A chemical that affects androgen, estrogen, sex and thyroid hormone pathways (interference with thyroid function and testosterone metabolism); increases glycolysis and reduces glucose oxidation [45] |
| Perfluorooctanoic acid | A perfluorinated carboxylic acid |  |
Used in non-stick cookware, waterproof clothing and stain repellent on carpets, mattresses and microwaveable food items | Due to its structural resemblance to fatty acids, it has been found to alter energy metabolism and thyroid hormone homeostasis through the activation of PPARγ [46] |
| Genistein | A soy-derived isoflavone | 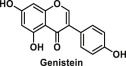 |
Used as an angiogenesis inhibitor and a phytoestrogen | It alters the expression of metabolic and adipogenic regulators, such as PPARγ [47]; disrupts the epigenetic regulation of Wnt10b, a key adipogenic gene; alters expression of lipid metabolism genes, disrupts lipolysis and lipogenesis and alters ATP synthesis [48] |
| Heavy metals | Arsenic | 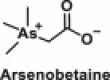 |
Used in plastics, mobile phone, solar panels, antiseptics, etc | The chemical has been shown to cause diabetes through the impairment of glucose-stimulated insulin secretion in pancreatic beta-cells, stimulation of pancreatic oxidative damage and insulin resistance in skeletal muscle, increment of gluconeogenesis in liver, and modulation of other hepatic insulin signalling pathways [49] |
| Dichloro-diphenyl-trichloroethane | Organochlorine | 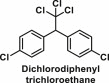 |
Used as insecticide | A chemical that impairs the function of visceral adipose tissue and decreases the response to energy surplus [50]; causes neuroendocrine disruption of the reproductive axis [51] |
| Nicotine | N-heterocyclic chemical compound | 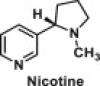 |
Found in the tobacco plant; used as medicine and stimulant | It modulates the actions of AMP-activated protein kinase, which integrates hormonal and nutritive signals in peripheral organs and hypothalamus, thereby playing a major role in regulation of energy balance; suppresses appetite by activating melanocortin-4 receptors expressed on hypothalamic pro-opiomelanocortin neurons [52] |
All the structures were drawn using ChemDraw.
6.1. Bisphenol A
Bisphenol A (BPA; 2,2-bis(4-hydroxyphenyl)propane) is primarily used in the production of polycarbonate plastics and epoxy resins. Polycarbonate plastics have numerous applications in consumer goods such as in food and drink packaging, water and infant bottles, compact discs, impact-resistant safety equipment, medical devices, sporting equipment etc. Epoxy resins are used as varnish to coat metal products such as food cans, bottle tops, and water supply pipes. BPA is also used as an additive in plastics such as polyvinyl chloride (PVC) and polyethylene terephthalate (PET) and is widely used in commercial products for many decades, but studies have shown that high levels of BPA may cause harm to both animals and humans [31,53,54].
Studies have shown BPA to be an active agonist of estrogen-sensitive membrane receptor GPR30 and glucocorticoid receptors [31,32] and it mimics the structure and function of the sex hormone estrogen and controls gene expressions to influence bodily processes, such as growth, cell repair and foetal development [53]. In addition, BPA may also interact and disrupt the functioning of thyroid gland [53] and affects the obesity-related biomarkers such as adiponectin, adipokine, leptin and ghrelin [31]. BPA has shown to increase the number and size of adipocytes by regulating the expression of genes such as fatty acid binding protein 4, (FABP4), cluster of differentiation 36 (CD36), and Proprotein convertase 1 (PCSK1) [55] and impair adipocyte metabolism [56]. Studies have shown that BPA exposure lowers the release of adiponectin which is a key player in lipid metabolism and fatty acid oxidation and decreased secretion of adiponectin deactivates fat combustion and hence leads to obesity-related metabolic syndrome [31]. BPA has also shown to promote adipogenesis by stimulating glucocorticoid receptors in combination with insulin in 3T3-L1 mouse fibroblasts cell lines [57].
The effects of BPA exposure have been noticed in all age groups including neonates, children, and adults (Table 2 ). It has been found that prolonged or high exposure to BPA during the early life causes more adverse effects related to obesity in adulthood [58]. Additionally, the World Health Organisation reported that BPA levels in breastfed babies were found to be nearly eight times lower than those fed on liquid formula using BPA-containing bottles [59]. Studies have also demonstrated a positive association between urinary BPA levels and obesity and diabetes in adults [60,61], children, and adolescents [62]; however, a few studies have questioned the strength of the association [63]. Given the amount of research that has been carried out in relation to BPA as an obesogen, future prevention efforts should now be employed to avoid BPA exposure, and more research is required to identify the duration, dose, and impact of long-term exposure of BPA to clarify its risk assessment.
Table 2.
Impact of the endocrine disruptors on obesity in different age groups.
| Obesogens | Childhood obesity | Adolescent obesity | Adult onset obesity |
|---|---|---|---|
| Bisphenol A (BPA) | Urinary BPA (>5.4 ng/mL) was associated with childhood obesity (OR: 2.55 (95%CI: 1.65, 3.95) (p < 0.01) [144] | Urinary BPA (1.24 ng/mL) was associated with adolescent obesity OR: 1.10 (95% CI: 0.89–1.35) [145] | BPA (median concentration of 1.1 ng/mL) was associated with general obesity, OR: 1·78 (95% CI 1·10–2·89; p = 0·04) and abdominal obesity OR: 1·55 (1·04–2·32; p = 0·02) [146] |
| Phthalates | No clear trend was seen for the association between BMI and urinary monoethyl phthalate quartiles in boys and girls (p > 0.05) [68] | BMI (p-trend = 0.03) and WC (p-trend = 0.02) increased with urinary monoethyl phthalate quartile in adolescent girls [68] | BMI increased with urinary monoethyl phthalate quartile in 20–59-year-old women but the effect was less strong compared to adolescent girls (p-trend = 0.14) [68] |
| Atrazine | Odds of early menarche for girls with Diamino-chlorotriazine (atrazine analyte) exposures ≥ median was 1.86 (95% CI: 1.03, 3.38) [147] | NA | Farmers who were exposed to pesticides had higher urinary atrazine mercapturate compared with controls (P < 0.05); but no association was observed between atrazine mercapturate and oxidative stress markers (p > 0.05) [148] |
| Organotins | A trend towards higher weight gain was seen from birth to 3 months of age with increasing placenta tributyltin concentration (p = 0.024) [149] | NA | Mean measured levels of tributyltin in human serum samples reached concentrations (~27 nm) sufficient to activate high-affinity receptors such as RXR and PPARγ [150] |
| Organophosphates (OPs) | NA | NA | A significantly higher risk of arrhythmia was observed in the OPs poisoning cohort [subhazard ratio (SHR) = 1.25] compared with the non-OPs poisoning cohort, particularly in men (SHR = 1.33) and those under 49 years of age (SHR = 3.16) [94] |
| Monosodium glutamate (MSG) | NA | NA | For users in the highest tertile of MSG intake compared to non-users, the multivariable-adjusted odds ratios of overweight were 2.10 (95% CI, 1.13–3.90, P for trend = 0.03) and 2.75 (95% CI, 1.28–5.95, p = 0.04) [96] |
| Clozapine | Clozapine (mean dose 304.9 ± 121.9 mg/day) increased weight from 124.7 ± 25.6 lb to 134.2 ± 27.4 lb (p < 0.0001) and mean BMI from 21.4 kg/m2 to 22.9 kg/m2 (p < 0.001) [151] | Absolute and percentage average weight gains due to clozapine exposure (9.5 ± 10.4 kg; 14.8 ± 15.8%) [152] | Clozapine dose had no relation to weight change between 3 and 12 months of clozapine therapy in community-dwelling patient [153] |
| Polychlorinated biphenyls (PCBs) | Prenatal exposure to di-ortho PCBs was significantly associated with increased birth weight (β = 137; p = 0.02) [154] | NA | NA |
| Organobromines | A 10-fold increase in maternal serum 2,2′,4,4′,5,5′-Hexabromodiphenyl ether was associated with lower BMI z-score (β = −0.36, 95% CI, −0.60 to −0.13) at 2–8 years, smaller waist circumference (β = −1.81 cm; 95% CI, −3.13 to −0.50) at 4–8 years, and lower percent body fat (β = −2.37; 95% CI, −4.21 to −0.53) at 8 years [155] | NA | NA |
| Perfluorooctanoic acid (PFOA) | Exposure to PFOA in early life increased the z-score of childhood BMI (β = 0.10, 95% CI: 0.03, 0.17; I2 = 27.9%) [156] | NA | ORs (and 95% CI) for overweight risk by increasing PFOA exposure category for women were 1.0 (ref), 1.0 (0.8, 1.3), 1.0 (0.8, 1.2), 1.0 (0.8, 1.2), 0.9 (0.7, 1.1), and 0.9 (0.7, 1.1) and for men were 1.0 (ref), 0.9 (0.6, 1.1), 1.0 (0.7, 1.3), 1.0 (0.8, 1.4), 0.7 (0.5, 0.9), and 0.9 (0.7, 1.1). ORs for adult obesity risk were similar [157] |
| Genistein | NA | NA | Consumption of genistein for 2 months reduced basal insulin levels by 24% (p value = 0.05) and a reduced HOMA- IR index by 28% [158] |
| Heavy metals | Higher prenatal Cd levels were associated with higher obesity risk at 5 years of age where the effect of Cd (β = 3.18, se = 1.30, p = 0.014) was robust and corresponds to a ~25-fold increase in obesity for every one ng/g increase in blood weight of Cd [159] | The HOMA-IR value was significantly and positively related to the sum of the urinary inorganic and methylated arsenic concentrations and also the BMI Z score, with the regression coefficients (β) being 0.058 (p < 0.001) and 0.001 (p = 0.027), respectively [160] | Arsenic-related cancer ORs >20 were seen in those with elevated BMIs in both early adulthood and in later life [161] |
| Dichloro-diphenyl-trichloroethane (DDT) | There was no significant positive relation between in utero DDT exposure and obesity status of 7-year-old children [138] | Prenatal DDT exposure was associated with several adiposity measures in boys but not girls. Among boys, 10-fold increases in prenatal DDT were associated with increased BMI z-score (adj-β = 0.37, 95% CI: 0.08, 0.65) [162] | NA |
| Nicotine | Overweight and obese children with passive smoke exposure had greater overall and central adiposity than nonexposed overweight and obese children (p < 0.03) [163] | NA | Among smokers, the risk of obesity increased with the amount smoked and former heavy smokers were more likely to be obese than former light smokers (OR: 1.60, 95% 1.56–1.64, p < 0.001) [164] |
NA: Not applicable (literature not available).
OR: Odds ratio; CI: Confidence Interval.
6.2. Phthalates
Phthalates, for example, diesters of 1,2-benzenedicarboxylic acid, are frequently used as plasticizers [64]. Phthalates have been considered as EDCs with anti-androgenic and weakly estrogenic properties [65] and studies have suggested that phthalates are likely to influence obesity through mechanisms such as anti-thyroid hormone activities, and/or activation of peroxisome proliferator-activated receptors, and epigenetic modulation [34,66]. Epidemiological studies in adults and children (Table 2) have shown that higher concentrations of urinary phthalates were positively associated with obesity and cardiometabolic disease-related markers [67,68]. However, inconsistent associations have been shown between early-life exposure to phthalate metabolites and childhood growth and obesity [[69], [70], [71]]. While a study in 520 French boys found that prenatal phthalate exposure was significantly associated with increased body mass index (BMI) at age 5 [72], a study in 1239 from the US found that exposure to phthalates at ages 6–8 were associated with a predicted decrease in BMI from the ages of 7–13 [71]. Further large longitudinal studies in diverse study populations are required to confirm the relationship between phthalate exposure and obesity.
6.3. Atrazine
Atrazine [ATZ, 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine] is the second most extensively used herbicide in the Unites States [73], and Australian agriculture [74]. Given its role as a photosynthesis inhibitor [75], it is used extensively to control pre- and post-emergence broadleaf and grassy weeds in crops predominately in corn and sugarcane. ATZ functions by binding irreversibly to the plastoquinone binding protein of photosystem complex-II on thylakoid membranes in chloroplast and inhibiting the electron transfer and photosynthesis [76]. ATZ is also capable of binding to complex-I and III of the mitochondrial electron transport system and inhibiting the oxidative phosphorylation of mitochondria [36]. As per the testing of United States Department of Agriculture, 94% of US drinking water contains atrazine as a contaminant and approximately 7 million people were exposed to atrazine between 1998 and 2003 [77].
ATZ has been identified as a potent endocrine disruptor having androgenic inhibiting and weak estrogenic effects [78]. Animal studies have shown that long-term exposure to ATZ might contribute to the development of metabolic diseases outcomes such as insulin resistance and obesity, especially when exposure is linked with a high-fat diet [79]. In addition, the Agricultural Health Study in 11,273 pregnant mothers showed that women who reported agricultural exposure to herbicide, ATZ, during pregnancy had a risk of developing gestational diabetes [80]. Hence, environmental exposure to ATZ might be an important contributing factor to the obesity epidemic, as it results in damaging the mitochondrial function, affecting insulin signalling pathway, and inducing insulin resistance and obesity.
6.4. Organotins
Organotin compounds are those chemicals in which a tin atom is covalently bonded to the carbon atom of one or more organic substituents. They are represented by the general formula RSnX, where R is an alkyl group (methyl, ethyl, butyl, octyl) or aryl group and X is a halide (Cl, F) or other organic ligand (oxide, hydroxide, carboxylate or thiolate etc.) [81,82]. These compounds can be classified into four different classes: monoorganotins (RSnX3), diorganotins (R2SnX2), triorganotins (R3SnX) and tetraorganotins (R4Sn).
Organotins are industrially significant compounds which are widely used as polyvinyl chloride (PVC) stabilizers, biocides, or antifouling paints and have given rise to ubiquitous environmental contamination [83]. Organotin infamy is mainly due to trialkyltins (particularly tributyltin (TBT) and to lesser extent triphenyltin (TPT), which are active ingredients in antifouling paint formulations, especially for external marine applications. Thus, TBT is released into water and because of its low water solubility it gets deposited in sediments, resulting in the adverse and inexplicable effects on aquatic organisms [84]. Since, organotins are used in plastics, silicone and foams, therefore it results in their presence almost everywhere in clothes, wallpaper, medical devices, household piping, food containers and toys. Hence, humans are largely exposed to organotins not only through contaminated seafood but also through direct contact with these products and by ingestion and inhalation of dust. Organotins have been detected in human tissue samples and toxicity data has identified these compounds as carcinogens, endocrine disruptors, immunotoxicants and obesogens [12].
Organotins induce their metabolic- and endocrine-disrupting effects by interacting with transcriptional regulators such as nuclear and steroid receptors and thereby affecting different nuclear receptor signalling pathways and resulting in various morphophysiological effects [85]. These chemicals exert their obesogenic effect not only by stimulating adipogenesis by acting as agonist ligands for nuclear receptors PPARγ but also potentially affecting RXR/PPARγ signalling [86]. TBT has shown to increase the expression of adipocyte markers, lipid accumulation and glucose uptake in preadipocytes and induce the differentiation of pre-adipocytes to adipocytes by activating RXR and PPARγ [86]. Besides studies investigating the effects of TBT on the hypothalamic-pituitary-thyroid axis, there is a frame of facts indicating that TBT can also be considered a thyroid disruptor, thus ultimately contributing to the development of metabolic disorder and obesity [87,88].
6.5. Organophosphates
Organophosphates (also known as phosphate esters) are a class of organophosphorus compounds with the general structure O P(OR)3. They can be considered as esters of phosphoric acid. Examples of organophosphates include the following: insecticides (malathion, parathion, diazinon, fenthion, dichlorvos, chlorpyrifos and ethion), nerve gases (soman, sarin and tabun), ophthalmic agents (echothiophate and isoflurophate), antihelmintics (trichlorfon), herbicides (tribufos and merphos), industrial chemicals (tricresyl phosphate) [89]. Organophosphate toxicity can result from household or occupational exposure, military or terrorist action, or iatrogenic mishap. Exposure to organophosphates is also possible via intentional or unintentional contamination of food sources. The accidental inhalation or ingestion of these compounds in fish, dairy products, and other fatty foods that are contaminated, represent the most common way of human exposure [90]. Organophosphate pesticides can be absorbed by all routes, including inhalation, ingestion, and dermal absorption.
Previously organophosphate insecticides had been associated with neuropsychological conditions, but more recently and specifically, the concepts of obesogens is being investigated and researchers began to look at its effect on the endocrine system. A strict link has been reported between the early-life exposure to organophosphates (chlorpyrifos, diazinon or parathion in doses devoid of any acute signs of toxicity) in neonatal rats and the subsequent emergence of hyperinsulinemia and hyperlipidemia, depicting an overall pattern essentially resembling prediabetes, through the pathway synthesizing cyclic AMP controlled by adenylyl cyclase, the common site for disruption by organophosphates [91]. In addition, dietary chronic exposure to chlorpyrifos, has been shown to increase food intake and promote weight gain in mice with apolipoprotein E3 isoform unravelling relevant interactions between toxic exposure to chlorpyrifos and genetic predisposition [92]. A recent study in mice has shown that long-term exposure to chlorpyrifos alters the gut microbiome leading to weight gain and reduced insulin sensitivity [93]. Besides animal studies, a study in organophosphate-exposed cohort (N = 7561) and an age- and gender-matched control cohort (N = 30,244) showed that patients with acute poisoning from organophosphates had higher incidence rates of cardiovascular diseases compared with that of the non-organophosphatepoisoning cohort [94]. Given that obesity is a risk factor for Cardiovascular diseases (CVDs), the impact of organophosphates on CVDs could possibly be mediated through obesity.
6.6. Monosodium glutamate (MSG)
MSG is the sodium salt of glutamic acid [C5H8NO4Na], which is one of the most abundant naturally occurring non-essential amino acids. It is found naturally in algae, mushrooms, tomatoes and grapes. Today, MSG is artificially manufactured by the large-scale fermentation of starch for use in processed frozen foods, potato chips, salty snacks, sauces and sausages.
Lately, there has been a growing concern over MSGs being suspected as dietary obesogens which may be attributed to the altered regulatory mechanisms that hamper the fat metabolism [95]. A cross-sectional study involving 752 healthy Chinese, aged 40–59 years, has shown that the users in the highest tertile of MSG intake had 2.75 times increased risk of being overweight compared to non-users [96]. A prospective cohort study (China Health and Nutrition Survey) comprising 10,095 healthy Chinese adults, aged 18–65 years, also showed that the hazard ratio of overweight was 1.33 for participants in the highest quintile of MSG intake compared with those in the lowest quintile [97]. Furthermore, a study in 349 Thai individuals, aged 35–55 years, demonstrated that there was 1.16 times increased risk of being overweight for every 1 g increase in MSG intake [98].
Metabolic diseases such as obesity and diabetes may be influenced by endocrine disrupting interactions between consumed MSG and the hormones such as glucagon-like peptide 1 (GLP-1) which are involved in satiety responses and insulin release. A cell line study has shown that 72 h of exposure at dietary levels of MSG resulted in pre-lethal cytotoxicity and a significant decline in GLP-1 secretion, which highlights the possible role of MSG in inducing obesity by impairing GLP-1 secretion [40]. These evidences provide a starting point for further investigations of the role of MSG as an obesogen in disrupting the activities of other gut hormones.
6.7. Clozapine
Clozapine is a dibenzo-diazepine derivative, which shows strong antagonism towards several neurotransmitter receptors and is more effective in treating schizophrenia-related symptoms [99]. Prolonged use of clozapine has found to cause drug-induced metabolic syndrome in mammals that gave rise to adverse metabolic side effects such as obesity [100]. It has been shown that clozapine alters the function of key metabolic enzymes and affects electron transport chain during oxidative phosphorylation in the mitochondria [100]. Furthermore, clozapine treatment has been associated with increased production of reactive oxygen species (ROS) and antioxidant proteins in cells and tissues [101,102]. Studies have shown mitochondrial dysfunction, increased production of ROS and inflammation as mechanisms which are related to the development of obesity [103]. In addition, clozapine has been found to induce a preference for high-fat/high-sugar foods in both rats [104] and humans [105]. Animal studies have shown that clozapine blocks muscarinic M1 and M2 receptors and inhibits GLP-1 secretion [104] which influences the food preference and the secretion of high glucagon that affects the glucose homeostasis leading to impaired glucose tolerance and increase body weight.
6.8. Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are polyhalogenated aromatic hydrocarbons having up to 10 chlorine atoms attached to the biphenyl ring. Due to their non-flammability, high boiling points, chemical stability and insulating properties, PCBs are heavily used in various commercial applications such as plasticizers in paints, plastics and rubber products, in pigments, dyes and carbonless copy paper and in electrical, heat transfer and hydraulic equipment. Due to their high thermodynamic stability and persistent nature, they can be leached into the environment and bioaccumulate via entering the food chains. PCBs are mainly stored in the human adipose tissue and liver with elimination half-lives of around 10–15 years [43]. Experimental results also support their endocrine disrupting properties including disturbances in reproductive and metabolic physiology [106]. A study in 448 British mother-daughter dyads showed that prenatal exposure to PCBs was inversely associated with daughters’ birth weight [24].
Animal studies have shown that PCB-153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) [107], the most prevailed congener in human serum, significantly increased body weight in mice that were fed with high fat diet (HFD) suggesting that PCB-153 is a diet-dependent obesogen [108]. It was hypothesized that PCB-153 causes obesity by stimulating the production of abnormal adipocytokines and altering hepatic lipid metabolism, which might lead to the up-regulation of lipid biosynthesis gene expression and down-regulating beta-oxidation genes [108]. However, studies focussing on the precise mechanism by which PCB-153 cause obesity are highly warranted.
6.9. Organobromines
1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane (DBE-DBCH) is an exemplar organobromine compound, which is used as a flame retardant. Reports have shown that isomers of DBE-DBCH has multimodal endocrine disrupting potential as it affects androgen, estrogen, sex and thyroid hormone pathways [109]. Obesogenecity in birds could greatly affect their survival as increase in body weight may hamper the ability of flight and thereby their escape from predators. The mechanism by which DBE-DBCH may facilitate obesity is still not well understood but there are reports showing that these obesogens may act by disrupting sex steroids, which play a vital role in mobilizing stored lipids [110]. However, further mechanistic studies are in progress that are taking other possible pathways into consideration such as endocrine disruption of thyroid hormone pathway.
Polybrominated diphenyl ethers (PBDEs) are another class of organobromines that are utilized as synthetic flame retardants in many household and industrial products. Similar to PCBs, they also tend to accumulate in adipose tissue because of their persistent nature and lipophilicity [111]. PBDEs have been shown to disrupt endocrine homeostasis by reducing the thyroxine levels (T4) in the plasma of mice and rats and this has shown to disrupt lipid metabolism [112,113]. Animal studies have also shown that upon exposure to penta-BDE (Pentabromodiphenyl ether - a technical mixture of different PBDE congeners), an increase in lipolysis and reduction in glucose oxidation was observed; both of these characteristics are associated with obesity [45]. The present understanding of the environmental behaviour of PBDEs as an obesogen is far from complete and hence further research is necessary to more fully understand their role as an EDC.
6.10. Perfluorooctanoic acid
Perfluorooctanoic acid (PFOA) is a surfactant, which is used in non-stick cookware, waterproof clothing and stain repellent on carpets, mattresses and microwaveable food items [95]. PFOA has a tendency to accumulate in liver and kidney and has a mean serum half-life of four years. Due to its structural resemblance to fatty acids, it has been found to activate peroxisome proliferator-activated receptor (PPAR-α), which is a key transcription factor in lipid metabolism. Animal studies have shown that mice exposed prenatally to PFOA were more likely to become obese than controls when they reached adulthood [114]. Furthermore, PFOA exposed HFD-fed mice showed significant increase in body weight and peripheral adipose tissues. Exposure to PFOAs during development has also shown to increase insulin, leptin and body weight during mid-life [114]. However, it is still not clear whether PFOAs contribute to obesity in humans; hence, epidemiological studies in humans are required to establish a relationship between PFOAs and obesity.
6.11. Genistein
Genistein is the most active isoflavone (Table 1) found predominantly in legumes and it has attracted much attention of the scientific community as an angiogenesis inhibitor and a phytoestrogen [115]. Besides, it has also proven to be promising in the treatment of metabolic disorders owing to its antioxidant and anti-inflammatory activities [116]. Interestingly, at low doses, genistein was shown to be responsible for changes in the expression of metabolic and adipogenic regulators, such as PPAR-γ, thus promoting fat accumulation in adipose tissue, especially in male mice [47]. A study in rats has also shown that genistein can dysregulate the body composition, in a dose-dependent and gender-specific manner, thereby disrupting and reprogramming the signals dictating adipose tissue expansion, likely throughout the early-life epigenetic regulation of Wnt10b, one of the key adipogenic genes in adipose tissue [117]. Studies in humans have shown that genistein intake is associated with decreased BMI, weight, waist circumference, and total body fat mass in postmenopausal women [118]. However, the mechanisms by which genistein has its beneficial effect on obesity is still unclear.
6.12. Heavy metals
The term ‘heavy metals’ simply refers to metals having high densities, atomic numbers or weights, and are toxic at relatively low concentrations. Heavy metals belong to many aspects of modern human life (plastics, mobile phone, solar panels, antiseptics, and many more) and pose serious threat on human health [119,120] as they cannot be destroyed or degraded, and tend to bioaccumulate. However, heavy metals such as iron (Fe), cobalt (Co), and zinc (Zn) are considered as essential nutrients to humans.
Animal and human studies have shown that consuming As-contaminated water can significantly increase leptin levels in the serum of the offspring of pregnant rodents and women, placental tissue, and cord blood [[121], [122], [123]]. Epidemiological studies have shown that individuals living in those areas with high exposure to As had an increased risk of developing type 2 diabetes [49]. The mechanisms by which As is likely to increase the risk of diabetes in humans could be through the impairment of glucose-stimulated insulin secretion in pancreatic beta-cells, stimulation of pancreatic oxidative damage and insulin resistance in skeletal muscle, increment of gluconeogenesis in liver, and modulation of other hepatic insulin signalling pathways [[124], [125], [126], [127], [128]]. Furthermore, studies have shown that arsenobetaine (Table 1), an organoarsenic compound that is the main source of arsenic found in fish, can accumulate in human body or transform into toxic inorganic arsenic in the gastrointestinal tract by microorganisms [129]. A study in 369 individuals from the Korean Korea National Health and Nutrition Examination Survey demonstrated a significant association between urine arsenobetaine and pancreatic β-cell function (HOMA-β), which has been shown to be influenced by obesity [130].
Cd is a well-known human carcinogen ranked seventh on the list of toxicants of potential concerns as declared by the Agency for Toxic Substances and Disease Registry. Pb is another highly toxic and carcinogenic metals for humans with no safe blood levels as identified by the Centers for Disease Control and Prevention (CDC) [131]. Exposure to both Cd and Pb heavy metals, especially, during the prenatal period has been shown to be associated with lower birth weight and gestational age in humans [[132], [133], [134]]. Similar to those of human studies, animal studies of perinatal Pb exposure has also been identified to increase body weight, fat mass or food intake in adulthood [135,136]. Likewise, Cd exposure during early life has also been shown to increase fat mass in male mice. Studies have also shown that inflammation, oxidative stress, and insulin resistance [137] may also play vital roles in Cd and Pb-induced obesity.
6.13. Dichlorodiphenyltrichloroethane (DDT)
DDT is a colourless, odourless and first modern synthetic organochlorine insecticide (Table 1) developed in 1940s. DDT and its metabolite product, dichlorodiphenyldichloroethylene (DDE), have been shown to be associated with adverse health problems which include diabetes and obesity in children [138]. Animal studies have shown that ancestral exposure to DDT can result in obesity transgenerationally suggesting that the etiology of obesity may in part be due to environmentally induced epigenetic transgenerational inheritance [139]. A study in rats have shown that exposure to DDE impairs the function of visceral adipose tissue and decreases the response to energy surplus, thereby contributing significantly to metabolic dysfunction and inflammation [140]. Cell line studies have shown that DDT can target PPARγ by increasing its expression or binding to it directly to activate downstream cascades that eventually leads to enhanced adipogenesis [50]. Besides affecting PPARγ, DDT has also shown to directly increase the expression of genes such as involved in lipid storage in adipocytes [50]. Given the role of DDT in endocrine diseases, a more careful risk consideration of the use of DDT is required.
6.14. Nicotine
Nicotine is found in the tobacco plant which is indigenous to the Americans and has been used as medicine and stimulant for at least 2000 years. Epidemiological studies showed that maternal smoking in pregnancy is a major risk factor for obesity even when exposure is limited only to early pregnancy stage [141,142]. Studies have shown that prenatal nicotinic overload prevents sympathetic responsiveness, which can lead to peripheral as well as central underactivity of noradrenergic systems; this in turn may increase appetite and decrease mobilization of fat from adipose tissue [143]. Additional studies are required to assess whether nicotine exposure in early pregnancy increases long-term risk of obesity and its related disease outcomes.
7. Role of obesogens on epigenetic modifications
Epigenetics is the study of heritable patterns of phenotype resulting from changes in a chromosome without alterations in the DNA sequence. In other words, epigenetic modifications refer to changes in the gene expression that are not caused by changes in the DNA sequences but are due to events such as DNA methylations and histone modifications [165]. Research in the field of EDCs has now focused on the effects of chemical exposures on the development of obesity through modulation of the epigenome during in utero and early postnatal stage. Exposure to a EDC appears to modify the epigenome, and available evidence demonstrates that chemical-induced epigenetic changes can be heritable [166]. Hence, exposure to EDCs may influence the metabolic status of an individual, with the potential for these effects to be transmitted to subsequent generations.
Even though there is no direct evidence to support an association of epigenetic mechanism for the actions of these environmental obesogens, studies in agouti mouse model offer a glimpse of this association [167]. Maternal dietary genistein supplementation in mice during gestation shifted the coat color of heterozygous viable yellow agouti offspring toward pseudoagouti. This phenotypic change was associated with increased methylation and the genistein-induced hypermethylation persisted into adulthood and protected the offspring from obesity [168]. Furthermore, exposure of pregnant rats to the fungicide, vinclozolin, led to transgenerational epigenetic modifications into at least the F4 generation [169]. EDCs can cause obesity and other related diseases by disrupting the epigenetic, structural, and functional mechanisms, which control energy homeostasis, lipid metabolism, appetite regulation, and adipogenesis.
8. Obesogens and COVID-19
At the time of writing this article, a novel human coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (formerly called HCoV-19), had emerged in Wuhan, China, in late 2019 and was identified as the causative agent for a cluster of pneumonia cases. SARS-CoV-2, which causes the COVID-19, had spread throughout China and globally causing unprecedented deaths. Interestingly, in 2003, a study in Chinese population had demonstrated a positive association between air pollution and severe acute respiratory syndrome (SARS) case fatality, where SARS patients from regions with high air pollution index (API) were twice as likely to die from SARS compared to those from regions with low APIs [170]. A study from the US had shown that long-term exposure to air pollution [each 10 μg/m3 elevation in particulate matters (PM10)] resulted in 6% increased risk of cardiopulmonary mortality [171]. Furthermore, exposure to PM10 was also linked to asthma and bronchitis. Given the data from these studies, it could be hypothesized that exposure to air pollutants might have an impact on the prognosis of SARS. Human studies have shown that nitrogen dioxide (NO2), which is one of the components of air pollution, was associated with higher fasting serum lipids among obese people suggesting obesity may exacerbate the effects of air pollution [172]. Animal studies have also shown that early-life exposure to air pollution particulates can lead to increased visceral obesity, insulin resistance, and inflammation suggesting the role of NO2 as an endocrine disruptor [173]. Given that COVID-19 is similar to SARS in causing respiratory illness, it is possible that exposure to NO2 could increase the mortality rate among COVID-19 patients; however, future studies are required to confirm this relationship.
9. Strategies for change and conclusions
To overcome the detrimental effects of obesogens on metabolic health, implementation of strategies such as expanded research programs and workshops, public health policies, and education efforts are highly warranted. Endocrine disruptor screening programs have been developed by the Environmental Protection Agency to assess the chemicals for their endocrine-disrupting effects [174] and this initiative should be expanded to identify obesogens and the molecular mechanisms by which they affect the metabolic health to specifically identify life-threatening chemicals. In addition, risk analysis should be developed by identifying groups who are exposed to high-risk obesogens and factors such as genetic variations that are linked to detoxification and metabolic pathways. Furthermore, instead of directly measuring the EDCs [175], development of clinical biomarkers will be useful in identifying individuals who are exposed to the obesogens and who can be prospectively monitored for the development of metabolic diseases such as obesity. Strategies to limit the production and the use of chemicals that affect the metabolic health and to ban the chemicals that are likely to induce transgenerational effects should be implemented.
One of the important goals of green chemistry is to avoid the hazardous exposure to these environmental obesogens/EDCs by designing a safer version of these synthetic chemicals [176]. This can be achieved by understanding the obesogen’s potential hazardous effect as early in the design process, which will enable the chemists to design new chemicals without these harardous effects. For instance, an endocrine disruption testing protocol has been developed for new chemical design by a team of 23 scientists and this protocol aims at measuring potential hormone-like or hormone-inhibiting effects of obesogens [177]. In addition, given the rapid advances in science, new assays are being incorporated into the protocol.
In conclusion, further experimental and epidemiological approaches are necessary to fully establish a magnitude of potentially hazardous effects of obesogens in humans, and its association to obesity and its related diseases. Research focussing on understanding the role of environmental obesogens in the epidemics of obesity is in an infant stage; hence, besides chemists, researchers from the field of genetics, molecular biology, epidemiology, physiology and clinical medicine are required to improve the understanding of the role of environment in obesity. For the design of safer version of obesogens through green chemistry approaches, a collaborative undertaking among the chemist, toxicologists and scientists is required; this will permit the evaluation of toxicity using appropriate experimental methods and will help lead to a new generation of inherently safer chemicals. These collaborative approaches will also educate the public and the lawmakers about the threat of endocrine disruptors and will limit the use of such obesogens through the implementation of public health policies. To overcome the challenges involved in studying the risk of obesity in relation to environmental obesogen exposures during early life, large sample size, prospective study designs, well characterised assessment of exposure and advanced statistical analysis are required to provide a strong evidence base for recommendations and strategies to prevent obesity and other related diseases.
Continued improvements in global legislation and adoption of practices such as, Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), in concert with regulatory bodies and non-governmental organisations will see a move to safer alternatives enabling better green and sustainable chemistry.
Funding
Newton Fund Researcher Links workshop grant, ID 2018-RLWK10-10480. The grant is funded by the UK Department for Business, Energy and Industrial Strategy and the Royal Society of Chemistry and delivered by the British Council.
CRediT authorship contribution statement
Radhika Gupta: Formal analysis, Resources, Data curation, Writing - review & editing, Software. Prashant Kumar: Formal analysis, Resources, Data curation, Writing - review & editing. Nighat Fahmi: Formal analysis, Resources, Data curation, Writing - review & editing. Bhaskar Garg: Formal analysis, Resources, Data curation, Writing - review & editing. Sriparna Dutta: Formal analysis, Resources, Data curation, Writing - review & editing. Shilpee Sachar: Formal analysis, Resources, Data curation, Writing - review & editing. Avtar S. Matharu: Writing - review & editing, Supervision. Karani S. Vimaleswaran: Conceptualization, Resources, Data curation, Writing - original draft, Writing - review & editing, Project administration, Formal analysis, Methodology, Supervision, Validation.
Declaration of Competing Interest
None.
Acknowledgements
The Authors would like to acknowledge that this work is the direct output of a Newton Fund Researcher Links Workshop grant, ID 2018-RLWK10-10480, between the United Kingdom and India, which allowing researchers to collaborate. The grant is funded by the UK Department for Business, Energy and Industrial Strategy and The Royal Society of Chemistry and delivered by the British Council. For further information, please visit www.newtonfund.ac.uk.
References
- 1.Jaacks L.M., Vandevijvere S., Pan A., McGowan C.J., Wallace C., Imamura F., et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 3.Vimaleswaran K.S., Loos R.J. Progress in the genetics of common obesity and type 2 diabetes. Expet Rev. Mol. Med. 2010;12:e7. doi: 10.1017/S1462399410001389. [DOI] [PubMed] [Google Scholar]
- 4.Vimaleswaran K.S., Bodhini D., Lakshmipriya N., Ramya K., Anjana R.M., Sudha V., et al. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. 2016;13:39. doi: 10.1186/s12986-016-0098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neel B.A., Sargis R.M. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60(7):1838–1848. doi: 10.2337/db11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelishadi R., Poursafa P., Jamshidi F. Role of environmental chemicals in obesity: a systematic review on the current evidence. J Environ Public Health. 2013;2013:896789. doi: 10.1155/2013/896789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNutt S.H., Purwin P., Murray C. Vulvo-vaginitis in swine: preliminary report. J. Am. Vet. Med. Assoc. 1928;73:484. [Google Scholar]
- 8.Thayer K.A., Heindel J.J., Bucher J.R., Gallo M.A. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ. Health Perspect. 2012;120(6):779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heindel J.J., vom Saal F.S. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol. Cell. Endocrinol. 2009;304(1-2):90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Schug T.T., Johnson A.F., Birnbaum L.S., Colborn T., Guillette L.J., Jr., Crews D.P., et al. Minireview: endocrine disruptors: past lessons and future directions. Mol. Endocrinol. 2016;30(8):833–847. doi: 10.1210/me.2016-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baillie-Hamilton P.F. Chemical toxins: a hypothesis to explain the global obesity epidemic. J. Alternative Compl. Med. 2002;8(2):185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 12.Grun F., Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 13.Di Gregorio I., Busiello R.A., Burgos Aceves M.A., Lepretti M., Paolella G., Lionetti L. Environmental pollutants effect on Brown adipose tissue. Front. Physiol. 2018;9:1891. doi: 10.3389/fphys.2018.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Special Report on Environmental Endocrine Disruption: an Effects Assessment and Analysis. U.S. Environmental Protection Agency; Washington, D.C.: 1997. [Google Scholar]
- 15.Casals-Casas C., Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 16.Swedenborg E., Ruegg J., Makela S., Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009;43(1):1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- 17.Combarnous Y., Nguyen T.M.D. Comparative overview of the mechanisms of action of hormones and endocrine disruptor compounds. Toxics. 2019;7(1) doi: 10.3390/toxics7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grun F., Blumberg B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darbre P.D. Endocrine disruptors and obesity. Curr Obes Rep. 2017;6(1):18–27. doi: 10.1007/s13679-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken E., Levitan E.B., Gillman M.W. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int. J. Obes. 2008;32(2):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behl M., Rao D., Aagaard K., Davidson T.L., Levin E.D., Holloway A.C. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ. Health Perspect. 2013;121:170–180. doi: 10.1289/ehp.1205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr. Neuropharmacol. 2007;5(3):213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel J.F., Hartman T.J., Sjodin A., Northstone K., Taylor E.V. Prenatal exposure to polychlorinated biphenyls and fetal growth in British girls. Environ. Int. 2018;116:116–121. doi: 10.1016/j.envint.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kezios K.L., Liu X., Cirillo P.M., Cohn B.A., Kalantzi O.I., Wang Y., et al. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod. Toxicol. 2013;35:156–164. doi: 10.1016/j.reprotox.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Merrill M., Birnbaum L.S. Childhood obesity and environmental chemicals. Mt. Sinai J. Med. 2011;78(1):22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchner S., Kieu T., Chow C., Casey S., Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010;24(3):526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaludjerovic J., Ward W.E. Diethylstilbesterol has gender-specific effects on weight gain and bone development in mice. J. Toxicol. Environ. Health. 2008;71(15):1032–1042. doi: 10.1080/15287390801988947. [DOI] [PubMed] [Google Scholar]
- 29.Rubin B.S., Soto A.M. Bisphenol A: perinatal exposure and body weight. Mol. Cell. Endocrinol. 2009;304(1-2):55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuno H., Iwanami J., Kidani T., Sakayama K., Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol. Sci. 2005;84(2):319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 31.MacKay H., Patterson Z.R., Abizaid A. Perinatal exposure to low-dose bisphenol-A disrupts the structural and functional development of the hypothalamic feeding circuitry. Endocrinology. 2017;158(4):768–777. doi: 10.1210/en.2016-1718. [DOI] [PubMed] [Google Scholar]
- 32.Thomas P., Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102(1-5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Ariemma F., D’Esposito V., Liguoro D., Oriente F., Cabaro S., Liotti A., et al. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desvergne B., Feige J.N., Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol. Cell. Endocrinol. 2009;304(1-2):43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Grindler N.M., Vanderlinden L., Karthikraj R., Kannan K., Teal S., Polotsky A.J., et al. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci. Rep. 2018;8(1):6086. doi: 10.1038/s41598-018-24505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagarkar S., Gandhi D., Devi S.S., Sakharkar A., Kapley A. Atrazine exposure causes mitochondrial toxicity in liver and muscle cell lines. Indian J. Pharmacol. 2016;48(2):200–207. doi: 10.4103/0253-7613.178842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucka M., Pogrmic-Majkic K., Fa S., Stojilkovic S.S., Kovacevic R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol. Appl. Pharmacol. 2012;265(1):19–26. doi: 10.1016/j.taap.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tinkov A.A., Ajsuvakova O.P., Skalnaya M.G., Skalny A.V., Aschner M., Suliburska J., et al. Organotins in obesity and associated metabolic disturbances. J. Inorg. Biochem. 2019;191:49–59. doi: 10.1016/j.jinorgbio.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Oksay T., Naziroglu M., Ergun O., Dogan S., Ozatik O., Armagan A., et al. N-acetyl cysteine attenuates diazinon exposure-induced oxidative stress in rat testis. Andrologia. 2013;45(3):171–177. doi: 10.1111/j.1439-0272.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 40.Shannon M., Green B., Willars G., Wilson J., Matthews N., Lamb J., et al. The endocrine disrupting potential of monosodium glutamate (MSG) on secretion of the glucagon-like peptide-1 (GLP-1) gut hormone and GLP-1 receptor interaction. Toxicol. Lett. 2017;265:97–105. doi: 10.1016/j.toxlet.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Olney J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(3880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 42.Kluge M., Schuld A., Schacht A., Himmerich H., Dalal M.A., Wehmeier P.M., et al. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology. 2009;34(1):118–128. doi: 10.1016/j.psyneuen.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Dirinck E., Jorens P.G., Covaci A., Geens T., Roosens L., Neels H., et al. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19(4):709–714. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- 44.Bell M.R. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 2014;19:134–144. doi: 10.1016/j.coph.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoppe A.A., Carey G.B. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 2007;15(12):2942–2950. doi: 10.1038/oby.2007.351. [DOI] [PubMed] [Google Scholar]
- 46.Rosen M.B., Lee J.S., Ren H., Vallanat B., Liu J., Waalkes M.P., et al. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol. Sci. 2008;103(1):46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- 47.Penza M., Montani C., Romani A., Vignolini P., Pampaloni B., Tanini A., et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147(12):5740–5751. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- 48.Szkudelska K., Nogowski L. Genistein--a dietary compound inducing hormonal and metabolic changes. J. Steroid Biochem. Mol. Biol. 2007;105(1-5):37–45. doi: 10.1016/j.jsbmb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Navas-Acien A., Silbergeld E.K., Streeter R.A., Clark J.M., Burke T.A., Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ. Health Perspect. 2006;114(5):641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bateman M.E., Strong A.L., McLachlan J.A., Burow M.E., Bunnell B.A. The effects of endocrine disruptors on adipogenesis and osteogenesis in mesenchymal stem cells: a review. Front. Endocrinol. 2016;7:171. doi: 10.3389/fendo.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parent A.S., Naveau E., Gerard A., Bourguignon J.P., Westbrook G.L. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J. Toxicol. Environ. Health B Crit. Rev. 2011;14(5-7):328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tweed J.O., Hsia S.H., Lutfy K., Friedman T.C. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metabol. 2012;23(7):334–342. doi: 10.1016/j.tem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertoli S., Leone A., Battezzati A. Human bisphenol A exposure and the "diabesity phenotype". Dose Response. 2015;13(3) doi: 10.1177/1559325815599173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amin M.M., Ebrahim K., Hashemi M., Shoshtari-Yeganeh B., Rafiei N., Mansourian M., et al. Association of exposure to Bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int. J. Environ. Health Res. 2019;29(1):94–106. doi: 10.1080/09603123.2018.1515896. [DOI] [PubMed] [Google Scholar]
- 55.Vom Saal F.S., Nagel S.C., Coe B.L., Angle B.M., Taylor J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012;354(1-2):74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneyer A. Getting big on BPA: role for BPA in obesity? Endocrinology. 2011;152(9):3301–3303. doi: 10.1210/en.2011-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sargis R.M., Johnson D.N., Choudhury R.A., Brady M.J. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18(7):1283–1288. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim K.Y., Lee E., Kim Y. The association between bisphenol A exposure and obesity in children-A systematic review with meta-analysis. Int. J. Environ. Res. Publ. Health. 2019;16(14) doi: 10.3390/ijerph16142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toxicological and Health Aspects of Bisphenol A. Ottawa, Canada. 2010. [Google Scholar]
- 60.Carwile J.L., Michels K.B. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ. Res. 2011;111(6):825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T., Li M., Chen B., Xu M., Xu Y., Huang Y., et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012;97(2):E223–E227. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 62.Vafeiadi M., Roumeliotaki T., Myridakis A., Chalkiadaki G., Fthenou E., Dermitzaki E., et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ. Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Lakind J.S., Goodman M., Mattison D.R. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014;44(2):121–150. doi: 10.3109/10408444.2013.860075. [DOI] [PubMed] [Google Scholar]
- 64.Schettler T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 81-5. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi S., Iida M., Kobayashi S., Jin K., Matsuda T., Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology. 2005;210(2-3):223–233. doi: 10.1016/j.tox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Kim S.H., Park M.J. Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab. 2014;19(2):69–75. doi: 10.6065/apem.2014.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trasande L., Attina T.M., Sathyanarayana S., Spanier A.J., Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ. Health Perspect. 2013;121(4):501–506. doi: 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatch E.E., Nelson J.W., Qureshi M.M., Weinberg J., Moore L.L., Singer M., et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ. Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maresca M.M., Hoepner L.A., Hassoun A., Oberfield S.E., Mooney S.J., Calafat A.M., et al. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ. Health Perspect. 2016;124(4):514–520. doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harley K.G., Berger K., Rauch S., Kogut K., Claus Henn B., Calafat A.M., et al. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr. Res. 2017;82(3):405–415. doi: 10.1038/pr.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deierlein A.L., Wolff M.S., Pajak A., Pinney S.M., Windham G.C., Galvez M.P., et al. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016;27(4):492–499. doi: 10.1097/EDE.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Botton J., Philippat C., Calafat A.M., Carles S., Charles M.A., Slama R., et al. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ. Res. 2016;151:601–609. doi: 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiely T., Donaldson D., Grube A. U.S. Environmental Protection Agency; Washington, DC: 2004. Pesticide Industry Sales and Usage: 2000 and 2001 Market Estimates. [Google Scholar]
- 74.Atrazine Chemical Review: Australian Pesticides and Veterinary Medicines Authority: Australian Pesticides and Veterinary Medicines Authority. 2017. https://apvma.gov.au/node/12371 Available from: [Google Scholar]
- 75.Knauert S., Escher B., Singer H., Hollender J., Knauer K. Mixture toxicity of three photosystem II inhibitors (atrazine, isoproturon, and diuron) toward photosynthesis of freshwater phytoplankton studied in outdoor mesocosms. Environ. Sci. Technol. 2008;42(17):6424–6430. doi: 10.1021/es072037q. [DOI] [PubMed] [Google Scholar]
- 76.Schottler S.P., Eisenreich S.J. Herbicides in the great lakes. Environ. Sci. Technol. 1994;28(12):2228–2232. doi: 10.1021/es00061a036. [DOI] [PubMed] [Google Scholar]
- 77.United States Geological Survey . 2017. Measured Atrazine in Streams.https://water.usgs.gov/nawqa/home_maps/atrazine_streams.html Available from: [Google Scholar]
- 78.Mnif W., Hassine A.I., Bouaziz A., Bartegi A., Thomas O., Roig B. Effect of endocrine disruptor pesticides: a review. Int. J. Environ. Res. Publ. Health. 2011;8(6):2265–2303. doi: 10.3390/ijerph8062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim S., Ahn S.Y., Song I.C., Chung M.H., Jang H.C., Park K.S., et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PloS One. 2009;4(4) doi: 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saldana T.M., Basso O., Hoppin J.A., Baird D.D., Knott C., Blair A., et al. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30(3):529–534. doi: 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Carvalho Oliveira R., Santelli R.E. Occurrence and chemical speciation analysis of organotin compounds in the environment: a review. Talanta. 2010;82(1):9–24. doi: 10.1016/j.talanta.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 82.Sekizawa J., Suter G., II, Birnbaum L. Integrated human and ecological risk assessment: a case study of tributyltin and triphenyltin compounds. Hum. Ecol. Risk Assess. 2010;9(1):325–342. [Google Scholar]
- 83.Blunden S.J., Cusack P.A., Hill R. Royal Society of Chemistry; London: 1985. The Industrial Use of Tin Chemicals. [Google Scholar]
- 84.Wang X.H., Wu Y.L., Cai Y.R., Xie W., Xu J. [Pollution history and sources of organotin compounds in aquaculture water of Tong’an Bay, Xiamen] Huanjing Kexue. 2011;32(7):1916–1923. [PubMed] [Google Scholar]
- 85.Nakanishi T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J. Toxicol. Sci. 2008;33(3):269–276. doi: 10.2131/jts.33.269. [DOI] [PubMed] [Google Scholar]
- 86.Yanik S.C., Baker A.H., Mann K.K., Schlezinger J.J. Organotins are potent activators of PPARgamma and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol. Sci. 2011;122(2):476–488. doi: 10.1093/toxsci/kfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Oliveira M., Rodrigues B.M., Olimpio R.M.C., Graceli J.B., Goncalves B.M., Costa S.M.B., et al. Disruptive effect of organotin on thyroid gland function might contribute to hypothyroidism. Internet J. Endocrinol. 2019;2019:7396716. doi: 10.1155/2019/7396716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santos-Silva A.P., Andrade M.N., Pereira-Rodrigues P., Paiva-Melo F.D., Soares P., Graceli J.B., et al. Frontiers in endocrine disruption: impacts of organotin on the hypothalamus-pituitary-thyroid axis. Mol. Cell. Endocrinol. 2018;460:246–257. doi: 10.1016/j.mce.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 89.Kamanyire R., Karalliedde L. Organophosphate toxicity and occupational exposure. Occup. Med. (Lond.) 2004;54(2):69–75. doi: 10.1093/occmed/kqh018. [DOI] [PubMed] [Google Scholar]
- 90.Munoz-Quezada M.T., Lucero B.A., Iglesias V.P., Munoz M.P., Cornejo C.A., Achu E., et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int. J. Occup. Environ. Health. 2016;22(1):68–79. doi: 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slotkin T.A. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod. Toxicol. 2011;31(3):297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peris-Sampedro F., Basaure P., Reverte I., Cabre M., Domingo J.L., Colomina M.T. Chronic exposure to chlorpyrifos triggered body weight increase and memory impairment depending on human apoE polymorphisms in a targeted replacement mouse model. Physiol. Behav. 2015;144:37–45. doi: 10.1016/j.physbeh.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Liang Y., Zhan J., Liu D., Luo M., Han J., Liu X., et al. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. 2019;7(1):19. doi: 10.1186/s40168-019-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung D.Z., Yang H.J., Li Y.F., Lin C.L., Chang S.Y., Sung F.C., et al. The long-term effects of organophosphates poisoning as a risk factor of CVDs: a nationwide population-based cohort study. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0137632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holtcamp W. Obesogens: an environmental link to obesity. Environ. Health Perspect. 2012;120(2):a62–a68. doi: 10.1289/ehp.120-a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He K., Zhao L., Daviglus M.L., Dyer A.R., Van Horn L., Garside D., et al. Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study. Obesity (Silver Spring) 2008;16(8):1875–1880. doi: 10.1038/oby.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He K., Du S., Xun P., Sharma S., Wang H., Zhai F., et al. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS) Am. J. Clin. Nutr. 2011;93(6):1328–1336. doi: 10.3945/ajcn.110.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Insawang T., Selmi C., Cha’on U., Pethlert S., Yongvanit P., Areejitranusorn P., et al. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 2012;9(1):50. doi: 10.1186/1743-7075-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimbron J., Khandaker G.M., Toschi C., Jones P.B., Fernandez-Egea E. A systematic review and meta-analysis of randomised controlled trials of treatments for clozapine-induced obesity and metabolic syndrome. Eur. Neuropsychopharmacol. 2016;26(9):1353–1365. doi: 10.1016/j.euroneuro.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 100.Contreras-Shannon V., Heart D.L., Paredes R.M., Navaira E., Catano G., Maffi S.K., et al. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0059012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heiser P., Sommer O., Schmidt A.J., Clement H.W., Hoinkes A., Hopt U.T., et al. Effects of antipsychotics and vitamin C on the formation of reactive oxygen species. J. Psychopharmacol. 2010;24(10):1499–1504. doi: 10.1177/0269881109102538. [DOI] [PubMed] [Google Scholar]
- 102.Polydoro M., Schroder N., Lima M.N., Caldana F., Laranja D.C., Bromberg E., et al. Haloperidol- and clozapine-induced oxidative stress in the rat brain. Pharmacol. Biochem. Behav. 2004;78(4):751–756. doi: 10.1016/j.pbb.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 103.Martinez J.A. Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J. Physiol. Biochem. 2006;62(4):303–306. doi: 10.1007/BF03165759. [DOI] [PubMed] [Google Scholar]
- 104.Smith G.C., Vickers M.H., Cognard E., Shepherd P.R. Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: implications for glucose metabolism and food choice behaviour. Schizophr. Res. 2009;115(1):30–40. doi: 10.1016/j.schres.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Henderson D.C., Sharma B., Fan X., Copeland P.M., Borba C.P., Freudenreich O., et al. Dietary saturated fat intake and glucose metabolism impairments in nondiabetic, nonobese patients with schizophrenia on clozapine or risperidone. Ann. Clin. Psychiatr. 2010;22(1):33–42. [PubMed] [Google Scholar]
- 106.Mennigen J.A., Thompson L.M., Bell M., Tellez Santos M., Gore A.C. Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats. Environ. Health. 2018;17(1):18. doi: 10.1186/s12940-018-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.National Toxicology P. NTP technical report on the toxicology and carcinogenesis studies of 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (Gavage studies) Natl. Toxicol. Progr. Tech. Rep. 2006;529:4–168. [PubMed] [Google Scholar]
- 108.Wahlang B., Falkner K.C., Gregory B., Ansert D., Young D., Conklin D.J., et al. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J. Nutr. Biochem. 2013;24(9):1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Darbre P.D. Overview of air pollution and endocrine disorders. Int. J. Gen. Med. 2018;11:191–207. doi: 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marteinson S.C., Fernie K.J. Is the current-use flame retardant, DBE-DBCH, a potential obesogen? Effects on body mass, fat content and associated behaviors in American kestrels. Ecotoxicol. Environ. Saf. 2019;169:770–777. doi: 10.1016/j.ecoenv.2018.11.104. [DOI] [PubMed] [Google Scholar]
- 111.Hamers T., Kamstra J.H., Sonneveld E., Murk A.J., Kester M.H., Andersson P.L., et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 112.Hallgren S., Darnerud P.O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177(2-3):227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 113.Hallgren S., Sinjari T., Hakansson H., Darnerud P.O. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch. Toxicol. 2001;75(4):200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- 114.Hines E.P., White S.S., Stanko J.P., Gibbs-Flournoy E.A., Lau C., Fenton S.E. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell. Endocrinol. 2009;304(1-2):97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 115.Polkowski K., Mazurek A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000;57(2):135–155. [PubMed] [Google Scholar]
- 116.Behloul N., Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013;698(1-3):31–38. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 117.Strakovsky R.S., Lezmi S., Flaws J.A., Schantz S.L., Pan Y.X., Helferich W.G. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol. Sci. 2014;138(1):161–174. doi: 10.1093/toxsci/kft331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goodman-Gruen D., Kritz-Silverstein D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause. 2003;10(5):427–432. doi: 10.1097/01.GME.0000058866.35869.B4. [DOI] [PubMed] [Google Scholar]
- 119.Wang S.L., Chang F.H., Liou S.H., Wang H.J., Li W.F., Hsieh D.P. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ. Int. 2007;33(6):805–811. doi: 10.1016/j.envint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Samarghandian S., Azimi-Nezhad M., Shabestari M.M., Azad F.J., Farkhondeh T., Bafandeh F. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscipl. Toxicol. 2015;8(3):151–154. doi: 10.1515/intox-2015-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodriguez K.F., Ungewitter E.K., Crespo-Mejias Y., Liu C., Nicol B., Kissling G.E., et al. Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ. Health Perspect. 2016;124(3):336–343. doi: 10.1289/ehp.1509703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gossai A., Lesseur C., Farzan S., Marsit C., Karagas M.R., Gilbert-Diamond D. Association between maternal urinary arsenic species and infant cord blood leptin levels in a New Hampshire Pregnancy Cohort. Environ. Res. 2015;136:180–186. doi: 10.1016/j.envres.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmed S., Mahabbat-e Khoda S., Rekha R.S., Gardner R.M., Ameer S.S., Moore S., et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ. Health Perspect. 2011;119(2):258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diaz-Villasenor A., Sanchez-Soto M.C., Cebrian M.E., Ostrosky-Wegman P., Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic beta-cells. Toxicol. Appl. Pharmacol. 2006;214(1):30–34. doi: 10.1016/j.taap.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 125.Diaz-Villasenor A., Burns A.L., Salazar A.M., Sordo M., Hiriart M., Cebrian M.E., et al. Arsenite reduces insulin secretion in rat pancreatic beta-cells by decreasing the calcium-dependent calpain-10 proteolysis of SNAP-25. Toxicol. Appl. Pharmacol. 2008;231(3):291–299. doi: 10.1016/j.taap.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 126.Padmaja Divya S., Pratheeshkumar P., Son Y.O., Vinod Roy R., Andrew Hitron J., Kim D., et al. Arsenic induces insulin resistance in mouse adipocytes and myotubes via oxidative stress-regulated mitochondrial sirt3-FOXO3a signaling pathway. Toxicol. Sci. 2015;146(2):290–300. doi: 10.1093/toxsci/kfv089. [DOI] [PubMed] [Google Scholar]
- 127.Liu S., Guo X., Wu B., Yu H., Zhang X., Li M. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci. Rep. 2014;4:6894. doi: 10.1038/srep06894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamann I., Petroll K., Hou X., Anwar-Mohamed A., El-Kadi A.O., Klotz L.O. Acute and long-term effects of arsenite in HepG2 cells: modulation of insulin signaling. Biometals. 2014;27(2):317–332. doi: 10.1007/s10534-014-9714-y. [DOI] [PubMed] [Google Scholar]
- 129.Newcombe C., Raab A., Williams P.N., Deacon C., Haris P.I., Meharg A.A., et al. Accumulation or production of arsenobetaine in humans? J. Environ. Monit. 2010;12(4):832–837. doi: 10.1039/b921588c. [DOI] [PubMed] [Google Scholar]
- 130.Baek K., Lee N., Chung I. Association of arsenobetaine with beta-cell function assessed by homeostasis model assessment (HOMA) in nondiabetic Koreans: data from the fourth Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2009. Ann Occup Environ Med. 2017;29:31. doi: 10.1186/s40557-017-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Betts K.S. CDC updates guidelines for children’s lead exposure. Environ. Health Perspect. 2012;120(7):a268. doi: 10.1289/ehp.120-a268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berkowitz Z., Price-Green P., Bove F.J., Kaye W.E. Lead exposure and birth outcomes in five communities in Shoshone County, Idaho. Int. J. Hyg Environ. Health. 2006;209(2):123–132. doi: 10.1016/j.ijheh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Sanin L.H., Gonzalez-Cossio T., Romieu I., Peterson K.E., Ruiz S., Palazuelos E., et al. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics. 2001;107(5):1016–1023. doi: 10.1542/peds.107.5.1016. [DOI] [PubMed] [Google Scholar]
- 134.Tian L.L., Zhao Y.C., Wang X.C., Gu J.L., Sun Z.J., Zhang Y.L., et al. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol. Trace Elem. Res. 2009;132(1-3):51–59. doi: 10.1007/s12011-009-8391-0. [DOI] [PubMed] [Google Scholar]
- 135.Wu J., Wen X.W., Faulk C., Boehnke K., Zhang H., Dolinoy D.C., et al. Perinatal lead exposure alters gut microbiota composition and results in sex-specific bodyweight increases in adult mice. Toxicol. Sci. 2016;151(2):324–333. doi: 10.1093/toxsci/kfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faulk C., Barks A., Liu K., Goodrich J.M., Dolinoy D.C. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics. 2013;5(5):487–500. doi: 10.2217/epi.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun H., Wang N., Nie X., Zhao L., Li Q., Cang Z., et al. Lead exposure induces weight gain in adult rats, accompanied by DNA hypermethylation. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0169958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Warner M., Aguilar Schall R., Harley K.G., Bradman A., Barr D., Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the CHAMACOS cohort. Environ. Health Perspect. 2013;121(5):631–636. doi: 10.1289/ehp.1205656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skinner M.K., Manikkam M., Tracey R., Guerrero-Bosagna C., Haque M., Nilsson E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11:228. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pestana D., Teixeira D., Meireles M., Marques C., Norberto S., Sa C., et al. Adipose tissue dysfunction as a central mechanism leading to dysmetabolic obesity triggered by chronic exposure to p,p’-DDE. Sci. Rep. 2017;7(1):2738. doi: 10.1038/s41598-017-02885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mendez M.A., Torrent M., Ferrer C., Ribas-Fito N., Sunyer J. Maternal smoking very early in pregnancy is related to child overweight at age 5-7 y. Am. J. Clin. Nutr. 2008;87(6):1906–1913. doi: 10.1093/ajcn/87.6.1906. [DOI] [PubMed] [Google Scholar]
- 142.Grzeskowiak L.E., Hodyl N.A., Stark M.J., Morrison J.L., Clifton V.L. Association of early and late maternal smoking during pregnancy with offspring body mass index at 4 to 5 years of age. J Dev Orig Health Dis. 2015;6(6):485–492. doi: 10.1017/S2040174415007151. [DOI] [PubMed] [Google Scholar]
- 143.Levin E.D., Slotkin T.A. In: Handbook of Developmental Neurotoxicology. Slikker W.J., Chang L.W., editors. Academic Press; San Diego: 1998. Developmental neurotoxicity of nicotine; pp. 587–615. [Google Scholar]
- 144.Bhandari R., Xiao J., Shankar A. Urinary bisphenol A and obesity in U.S. children. Am. J. Epidemiol. 2013;177(11):1263–1270. doi: 10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jacobson M.H., Woodward M., Bao W., Liu B., Trasande L. Urinary bisphenols and obesity prevalence among U.S. Children and adolescents. J Endocr Soc. 2019;3(9):1715–1726. doi: 10.1210/js.2019-00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu B., Lehmler H.J., Sun Y., Xu G., Liu Y., Zong G., et al. Bisphenol A substitutes and obesity in US adults: analysis of a population-based, cross-sectional study. Lancet Planet Health. 2017;1(3):e114–e122. doi: 10.1016/S2542-5196(17)30049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Namulanda G., Taylor E., Maisonet M., Boyd Barr D., Flanders W.D., Olson D., et al. In utero exposure to atrazine analytes and early menarche in the Avon Longitudinal Study of Parents and Children Cohort. Environ. Res. 2017;156:420–425. doi: 10.1016/j.envres.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lerro C.C., Beane Freeman L.E., Portengen L., Kang D., Lee K., Blair A., et al. A longitudinal study of atrazine and 2,4-D exposure and oxidative stress markers among Iowa corn farmers. Environ. Mol. Mutagen. 2017;58(1):30–38. doi: 10.1002/em.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rantakokko P., Main K.M., Wohlfart-Veje C., Kiviranta H., Airaksinen R., Vartiainen T., et al. Association of placenta organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: a cohort study. Environ. Health. 2014;13(1):45. doi: 10.1186/1476-069X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kannan K., Senthilkumar K., Giesy J. Occurrence of butyltin compounds in human blood. Environ. Sci. Technol. 1999;33:1776–1779. [Google Scholar]
- 151.Sporn A.L., Bobb A.J., Gogtay N., Stevens H., Greenstein D.K., Clasen L.S., et al. Hormonal correlates of clozapine-induced weight gain in psychotic children: an exploratory study. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(9):925–933. doi: 10.1097/01.chi.0000170552.15798.dd. [DOI] [PubMed] [Google Scholar]
- 152.Fleischhaker C., Heiser P., Hennighausen K., Herpertz-Dahlmann B., Holtkamp K., Mehler-Wex C., et al. Weight gain in children and adolescents during 45 weeks treatment with clozapine, olanzapine and risperidone. J. Neural. Transm. 2008;115(11):1599–1608. doi: 10.1007/s00702-008-0105-9. [DOI] [PubMed] [Google Scholar]
- 153.Lau S.L., Muir C., Assur Y., Beach R., Tran B., Bartrop R., et al. Predicting weight gain in patients treated with clozapine: the role of sex, body mass index, and smoking. J. Clin. Psychopharmacol. 2016;36(2):120–124. doi: 10.1097/JCP.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 154.Lignell S., Aune M., Darnerud P.O., Hanberg A., Larsson S.C., Glynn A. Prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may influence birth weight among infants in a Swedish cohort with background exposure: a cross-sectional study. Environ. Health. 2013;12:44. doi: 10.1186/1476-069X-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vuong A.M., Braun J.M., Sjodin A., Webster G.M., Yolton K., Lanphear B.P., et al. Prenatal polybrominated diphenyl ether exposure and body mass index in children up to 8 Years of age. Environ. Health Perspect. 2016;124(12):1891–1897. doi: 10.1289/EHP139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu P., Yang F., Wang Y., Yuan Z. Perfluorooctanoic acid (PFOA) exposure in early life increases risk of childhood adiposity: a meta-analysis of prospective cohort studies. Int. J. Environ. Res. Publ. Health. 2018;15(10) doi: 10.3390/ijerph15102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barry V., Darrow L.A., Klein M., Winquist A., Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ. Res. 2014;132:62–69. doi: 10.1016/j.envres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 158.Guevara-Cruz M., Godinez-Salas E.T., Sanchez-Tapia M., Torres-Villalobos G., Pichardo-Ontiveros E., Guizar-Heredia R., et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2019-000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Green A.J., Hoyo C., Mattingly C.J., Luo Y., Tzeng J.Y., Murphy S.K., et al. Cadmium exposure increases the risk of juvenile obesity: a human and zebrafish comparative study. Int. J. Obes. 2018;42(7):1285–1295. doi: 10.1038/s41366-018-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin H.C., Huang Y.K., Shiue H.S., Chen L.S., Choy C.S., Huang S.R., et al. Arsenic methylation capacity and obesity are associated with insulin resistance in obese children and adolescents. Food Chem. Toxicol. 2014;74:60–67. doi: 10.1016/j.fct.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 161.Steinmaus C., Castriota F., Ferreccio C., Smith A.H., Yuan Y., Liaw J., et al. Obesity and excess weight in early adulthood and high risks of arsenic-related cancer in later life. Environ. Res. 2015;142:594–601. doi: 10.1016/j.envres.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Warner M., Ye M., Harley K., Kogut K., Bradman A., Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: the CHAMACOS study. Environ. Res. 2017;159:606–612. doi: 10.1016/j.envres.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davis C.L., Tingen M.S., Jia J., Sherman F., Williams C.F., Bhavsar K., et al. Passive smoke exposure and its effects on cognition, sleep, and health outcomes in overweight and obese children. Child. Obes. 2016;12(2):119–125. doi: 10.1089/chi.2015.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dare S., Mackay D.F., Pell J.P. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Portela A., Esteller M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 166.Baccarelli A., Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21(2):243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dolinoy D.C. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008;66(Suppl 1):S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dolinoy D.C., Weidman J.R., Waterland R.A., Jirtle R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nilsson E.E., Anway M.D., Stanfield J., Skinner M.K. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135(5):713–721. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cui Y., Zhang Z.F., Froines J., Zhao J., Wang H., Yu S.Z., et al. Air pollution and case fatality of SARS in the People’s Republic of China: an ecologic study. Environ. Health. 2003;2(1):15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pope C.A., 3rd, Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K., et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim J.S., Chen Z., Alderete T.L., Toledo-Corral C., Lurmann F., Berhane K., et al. Associations of air pollution, obesity and cardiometabolic health in young adults: the Meta-AIR study. Environ. Int. 2019;133(Pt A):105180. doi: 10.1016/j.envint.2019.105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zheng Z., Xu X., Zhang X., Wang A., Zhang C., Huttemann M., et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. 2013;58(1):148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Browne P., Noyes P.D., Casey W.M., Dix D.J. Application of adverse outcome pathways to U.S. EPA’s endocrine disruptor screening program. Environ. Health Perspect. 2017;125(9) doi: 10.1289/EHP1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sosa-Ferrera Z., Mahugo-Santana C., Santana-Rodriguez J.J. Analytical methodologies for the determination of endocrine disrupting compounds in biological and environmental samples. BioMed Res. Int. 2013;2013:674838. doi: 10.1155/2013/674838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anastas N.D., Warner J.C. The incorporation of hazard reduction as a chemical design criterion in green chemistry. Chem. Health Saf. 2005;12(2):9–13. [Google Scholar]
- 177.Schug T.T., Abagyan R., Blumberg B., Collins T.J., Crews D., DeFur P.L., et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 2013;15(1):181–198. doi: 10.1039/C2GC35055F. [DOI] [PMC free article] [PubMed] [Google Scholar]


