Summary
Background
An important feature of severe acute respiratory syndrome coronavirus 2 pathogenesis is COVID-19-associated coagulopathy, characterised by increased thrombotic and microvascular complications. Previous studies have suggested a role for endothelial cell injury in COVID-19-associated coagulopathy. To determine whether endotheliopathy is involved in COVID-19-associated coagulopathy pathogenesis, we assessed markers of endothelial cell and platelet activation in critically and non-critically ill patients admitted to the hospital with COVID-19.
Methods
In this single-centre cross-sectional study, hospitalised adult (≥18 years) patients with laboratory-confirmed COVID-19 were identified in the medical intensive care unit (ICU) or a specialised non-ICU COVID-19 floor in our hospital. Asymptomatic, non-hospitalised controls were recruited as a comparator group for biomarkers that did not have a reference range. We assessed markers of endothelial cell and platelet activation, including von Willebrand Factor (VWF) antigen, soluble thrombomodulin, soluble P-selectin, and soluble CD40 ligand, as well as coagulation factors, endogenous anticoagulants, and fibrinolytic enzymes. We compared the level of each marker in ICU patients, non-ICU patients, and controls, where applicable. We assessed correlations between these laboratory results with clinical outcomes, including hospital discharge and mortality. Kaplan–Meier analysis was used to further explore the association between biochemical markers and survival.
Findings
68 patients with COVID-19 were included in the study from April 13 to April 24, 2020, including 48 ICU and 20 non-ICU patients, as well as 13 non-hospitalised, asymptomatic controls. Markers of endothelial cell and platelet activation were significantly elevated in ICU patients compared with non-ICU patients, including VWF antigen (mean 565% [SD 199] in ICU patients vs 278% [133] in non-ICU patients; p<0·0001) and soluble P-selectin (15·9 ng/mL [4·8] vs 11·2 ng/mL [3·1]; p=0·0014). VWF antigen concentrations were also elevated above the normal range in 16 (80%) of 20 non-ICU patients. We found mortality to be significantly correlated with VWF antigen (r = 0·38; p=0·0022) and soluble thrombomodulin (r = 0·38; p=0·0078) among all patients. In all patients, soluble thrombomodulin concentrations greater than 3·26 ng/mL were associated with lower rates of hospital discharge (22 [88%] of 25 patients with low concentrations vs 13 [52%] of 25 patients with high concentrations; p=0·0050) and lower likelihood of survival on Kaplan–Meier analysis (hazard ratio 5·9, 95% CI 1·9–18·4; p=0·0087).
Interpretation
Our findings show that endotheliopathy is present in COVID-19 and is likely to be associated with critical illness and death. Early identification of endotheliopathy and strategies to mitigate its progression might improve outcomes in COVID-19.
Funding
This work was supported by a gift donation from Jack Levin to the Benign Hematology programme at Yale, and the National Institutes of Health.
Introduction
As of June 29, 2020, the global pandemic caused by severe acute respiratory syndrome coronavirus 2 has afflicted more than 10 million individuals worldwide, leading to more than 500 000 deaths.1 The disease consists of an early infectious phase in which the virus enters pulmonary epithelial cells via surface angiotensin-converting enzyme 2 (ACE2) receptors, causing viral pneumonia, followed by a systemic inflammatory phase characterised by respiratory failure and multiorgan dysfunction.2 Derangements in cytokine concentrations including interleukin-6 (IL-6) are believed to play a role in the disease, providing the rationale for the use of tocilizumab in patients with COVID-19.3
Among the more concerning features of COVID-19 infection is a coagulopathy characterised by high D-dimer and fibrinogen concentrations with minor changes in prothrombin time and platelet count.4, 5 This COVID-19-associated coagulopathy leads to a prothrombotic state, with venous thromboembolism prevalence of up to 69% in critically ill patients, irrespective of the use of pharmacological thromboprophylaxis, as well as reports of arterial thrombosis.6, 7 In autopsy series, microvascular thrombosis of the pulmonary vasculature has frequently been observed.8, 9
Research in context.
Evidence before this study
We searched PubMed for articles published from inception up to May 23, 2020, using the keywords “coronavirus”, “COVID-19”, “von Willebrand factor”, “thrombomodulin”, and “endothelial cell”, with no language restrictions. The existing evidence at the time that we did our study was that patients with COVID-19 had an unusual coagulopathy characterised by increased venous and arterial thrombotic events, coagulation derangements, and microvascular thrombosis in the lungs, which some studies had speculated might be related to endothelial dysfunction.
Added value of this study
To the best of our knowledge, our study is the first to provide biochemical evidence that endotheliopathy is an important feature in the coagulopathy of COVID-19. Although other studies have shown elevated von Willebrand factor (VWF) in critically ill patients with COVID-19, this is the first to measure VWF in both critically ill and non-critically ill patients, along with several other previously unreported endothelial markers, including soluble P-selectin and soluble thrombomodulin. We show that endotheliopathy is widespread among hospitalised patients with COVID-19 and is more extensive in critically ill patients than in non-critically ill patients. We describe, for the first time, that soluble thrombomodulin concentrations might predict mortality and other clinical outcomes in patients with COVID-19. This finding requires further validation.
Implications of all the available evidence
Our study provides convincing biochemical evidence for endothelial involvement in COVID-19-associated coagulopathy and critical illness. Our preliminary findings identify a potential prognostic role for measurement of endothelial markers in patients with COVID-19 and suggest a need for future investigations of therapeutic strategies aimed at preserving endothelial function in COVID-19 and other related infectious processes.
A single-centre study from China found a strong association of disseminated intravascular coagulation and mortality in patients with COVID-19.4 However, other studies have suggested that the pathophysiology of COVID-19-associated coagulopathy is distinct from disseminated intravascular coagulation.5 The presence of endotheliitis and viral inclusions in endothelial cells, as reported in small autopsy series of patients with COVID-19, coupled with identification of the ACE2 receptor on vascular endothelial cells, has raised suspicion for endothelial cell injury or activation as a central feature of the pathophysiology of COVID-19, particularly during the inflammatory phase of the disease.9, 10 In an effort to better understand the pathological mechanisms underlying COVID-19-associated coagulopathy, we performed a biochemical study that, to our knowledge, for the first time assessed markers of endothelial cell and platelet activation in non-critically ill and critically ill patients with COVID-19. The primary objective of our study was to do exploratory analyses of haemostatic factors and markers of endothelial cell and platelet activation in patients with COVID-19 admitted to the intensive care unit (ICU) and those with COVID-19 not admitted to ICU. The secondary objective was to assess the relationship between these markers and clinical outcomes, including in-hospital mortality and discharges from the hospital.
Methods
Study design and participants
We did a cross-sectional study of adult (≥18 years) patients hospitalised in Yale-New Haven Hospital between April 13 and April 24, 2020, with a confirmed diagnosis of COVID-19, as measured using PCR assays on nasopharyngeal swab samples. The Institutional Review Board approved this study and waived the need for consent. We categorised patients according to whether they had been admitted to a medical ICU or a specialised non-ICU COVID-19 floor in our hospital. To ensure a maximum separation of severity of illness between the ICU and non-ICU cohort and to reduce bias where possible, we preferentially included ICU patients who were intubated and non-ICU patients with minimal oxygen support requirement, with patients selected via simple random sampling. Most ICU patients required ventilator support at the time laboratory measurements were taken, whereas all non-ICU patients were on no more than 3 L of supplemental oxygen. Patient characteristics including age, sex, ethnicity, major comorbidities, laboratory studies, and treatments were recorded. Clinical outcomes including hospital discharge and in-hospital death were assessed on May 23, 2020. We included an additional 13 asymptomatic, non-hospitalised individuals who volunteered to serve as controls for a subset of plasma biomarker measurements; these individuals represented staff, faculty, and spouses who signed consent for a separate protocol approved by the Institutional Review Board authorising their blood to be used for additional studies.
Procedures
Venous blood was collected from hospitalised patients and non-hospitalised controls and processed according to standard laboratory techniques. Due to diurnal variations in plasminogen activator inhibitor-1 (PAI-1), for hospitalised patients, blood specimens were collected with the first scheduled morning draw, which in all but five patients occurred between 0300 h and 0700 h. Blood collection from non-hospitalised controls was not subject to the same time restrictions and occurred between 1100 h and 1400 h. For coagulation and endothelial cell and platelet marker measurements, blood was collected in 3·2% sodium citrate tubes and centrifuged at 4000 rpm for 20 min. The resulting plasma supernatant was used for further testing. Measurements of D-dimer and fibrinogen were done at our institution's clinical laboratory using a BCS XP System (Siemens; Malvern, PA, USA) with manufacturer reagents and controls per laboratory protocol. Measurements of antithrombin activity, protein C activity, protein S activity, α2-antiplasmin activity, von Willebrand factor (VWF) antigen, VWF activity, and factor VIII activity were taken at our institution's laboratory using the ACL TOP (Instrumentation Laboratory; Bedford, MA, USA) with manufacturer reagents and controls per laboratory protocol. VWF antigen and VWF activity were measured using a latex enhanced immunoassay; the VWF antigen assay used polystyrene particles coated with rabbit polyclonal antibody directed against VWF, while the VWF activity assay used a lyophilised suspension of polystyrene latex particles coated with anti-VWF mouse monoclonal antibody directed against the platelet-binding epitope of VWF. Factor VIII activity was measured using a one-stage partial thromboplastin time-based test in which patient plasma was diluted and added to reagent or manufacturer lyophilised, factor VIII-deficient plasma, with correction of the partial thromboplastin time being proportional to the factor VIII activity percentage, as derived from a calibration curve. For measurements of PAI-1 and thrombin-antithrombin complexes (TAT), plasma supernatant was frozen, then sent to national Clinical Laboratory Improvement Amendments-certified reference laboratories, where testing for these analytes was done by ELISA.
Soluble P-selectin, soluble CD40 ligand (sCD40L), and soluble thrombomodulin were measured using ELISA assays (soluble P-selectin: R&D Systems, DPSE00; soluble thrombomodulin: Abcam, ab46508; and sCD40L: R&D Systems, DCDL40). These assays were run on samples that had adequate volume remaining after the in-hospital testing described previously was completed. For soluble P-selectin and soluble thrombomodulin, samples were diluted in a 1:4 ratio before addition to ELISA plates. For sCD40L, samples were undiluted. Assays were done in duplicates according to the manufacturer's instructions.
Biomarkers are presented with their standard reference range (as per our single-centre laboratory). This includes VWF antigen and activity, which are reported as percentages compared with calibration curves using values obtained from the standardised reference population used for clinical laboratory testing throughout our hospital system.
Statistical analysis
As this was a descriptive study that sought to identify haematological abnormalities in a subset of patients with COVID-19 who were ill enough to be hospitalised, we examined the differences between coagulation studies and endothelial cell markers in ICU patients and non-ICU patients, and controls where applicable, using the unpaired two-sided t test if samples conformed to the normal distribution, with Welch correction for unequal variances. For samples that did not satisfy the normal distribution, we used the unpaired two-tailed Mann-Whitney U test. We used D'Agostino-Pearson and Anderson-Darling tests of normality. We compared endothelial cell parameters in ICU patients, non-ICU patients, and healthy controls using one-way ANOVA with post-hoc multiple comparisons of means for samples with the normal distribution and Kruskal-Wallis rank test with post-hoc multiple comparisons of mean ranks for samples that did not conform to the normal distribution. We evaluated the associations of endothelial cell markers with each other and with mortality using non-parametric Spearman correlation to account for non-normal distribution of all interrogated parameters except soluble P-selectin.
Given the specificity of soluble thrombomodulin to endothelial cells and findings on correlation analysis, we segregated patients into two groups according to their soluble thrombomodulin test results. We chose the median soluble thrombomodulin value for the entire cohort as a classification cutoff because there were no predefined upper and lower limits for the soluble thrombomodulin assay. We then did Kaplan–Meier analyses and compared in-hospital survival of patients with high or low soluble thrombomodulin values using the log-rank test for both full and ICU cohorts. Finally, we compared the rates of hospital discharges in patients with high and low soluble thrombomodulin using the χ2 test of proportions. All statistical analysis was done using GraphPad Prism (version 8.4.2; GraphPad Software, San Diego, CA, USA) and Stata (version 16; StataCorp, College Station, TX, USA). A p<0·05 was considered statistically significant.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. GG, ABP, MLM, HJC, and AIL had full access to all of the data and the final responsibility to submit for publication.
Results
68 adult patients (48 ICU, 20 non-ICU) were included in the final study cohort. In addition, 13 non-hospitalised, asymptomatic controls (five men and eight women, with a mean age of 48 years [SD 10; range 30–65]) were included as a comparator group for measurements of specific endothelial cell and platelet biomarkers that did not have standard reference ranges. Except where noted, all coagulation parameters were measured in all 68 patients, whereas endothelial cell and platelet biomarkers were measured in 50 patients (40 ICU and ten non-ICU) for whom blood remained after coagulation testing. Laboratory studies were done from April 13 to April 24, 2020. Obesity, hypertension, hyperlipidaemia, and diabetes were common, affecting up to a half of all patients (table 1 ). Three (4%) patients had active malignancy and three (4%) had cirrhosis, while one (1%) patient had a history of cirrhosis that resolved after liver transplant; all such patients were in the ICU cohort. 41 (60%) patients were on prophylactic-dose anticoagulation, 13 (19%) on intermediate-dose anticoagulation (enoxaparin 0·5 mg/kg twice daily), and 11 (16%) on therapeutic anticoagulation; three (4%) patients were not anticoagulated (appendix). 48 (71%) patients, including all but two ICU patients, had received tocilizumab before their coagulation factors were measured. Among all patient characteristics analysed, only sex showed a significant difference in distribution between ICU and non-ICU subgroups (table 1).
Table 1.
Patient characteristics
| All patients (n=68) | ICU (n=48) | Non-ICU (n=20) | p value | ||
|---|---|---|---|---|---|
| Age, years | 62 (16; 20–93) | 64 (16; 20–92) | 58 (15; 24–93) | 0·15 | |
| Sex | .. | .. | .. | 0·033 | |
| Female | 27 (40%) | 15 (31%) | 12 (60%) | .. | |
| Male | 41 (60%) | 33 (69%) | 8 (40%) | .. | |
| Ethnicity | .. | .. | .. | 0·088 | |
| Black | 16 (24%) | 14 (29%) | 2 (10%) | .. | |
| White | 35 (51%) | 20 (42%) | 15 (75%) | .. | |
| Hispanic | 16 (24%) | 13 (27%) | 3 (15%) | .. | |
| Asian | 1 (1%) | 1 (2%) | 0 | .. | |
| Comorbidities | |||||
| Obesity | 25 (37%) | 20 (42%) | 5 (25%) | 0·27 | |
| Congestive heart failure | 5 (7%) | 4 (8%) | 1 (5%) | 1·0 | |
| Hyperlipidaemia | 18 (26%) | 13 (27%) | 5 (25%) | 1·0 | |
| Hypertension | 38 (56%) | 28 (58%) | 10 (50%) | 0·60 | |
| Diabetes | 20 (29%) | 16 (33%) | 4 (20%) | 0·38 | |
| Coronary artery disease, myocardial infarction, or heart disease | 10 (15%) | 9 (19%) | 1 (5%) | 0·26 | |
| Atrial fibrillation | 4 (6%) | 3 (6%) | 1 (5%) | 1·0 | |
| Stroke or transient ischaemic attack | 7 (10%) | 3 (6%) | 4 (20%) | 0·18 | |
| Chronic kidney disease | 7 (10%) | 7 (15%) | 0 | 0·096 | |
| Active malignancy | 3 (4%) | 3 (6%) | 0 | 0·55 | |
Data are n (%) or mean (SD; range), unless specified otherwise. p values are for comparison between ICU and non-ICU patients. ICU=intensive care unit.
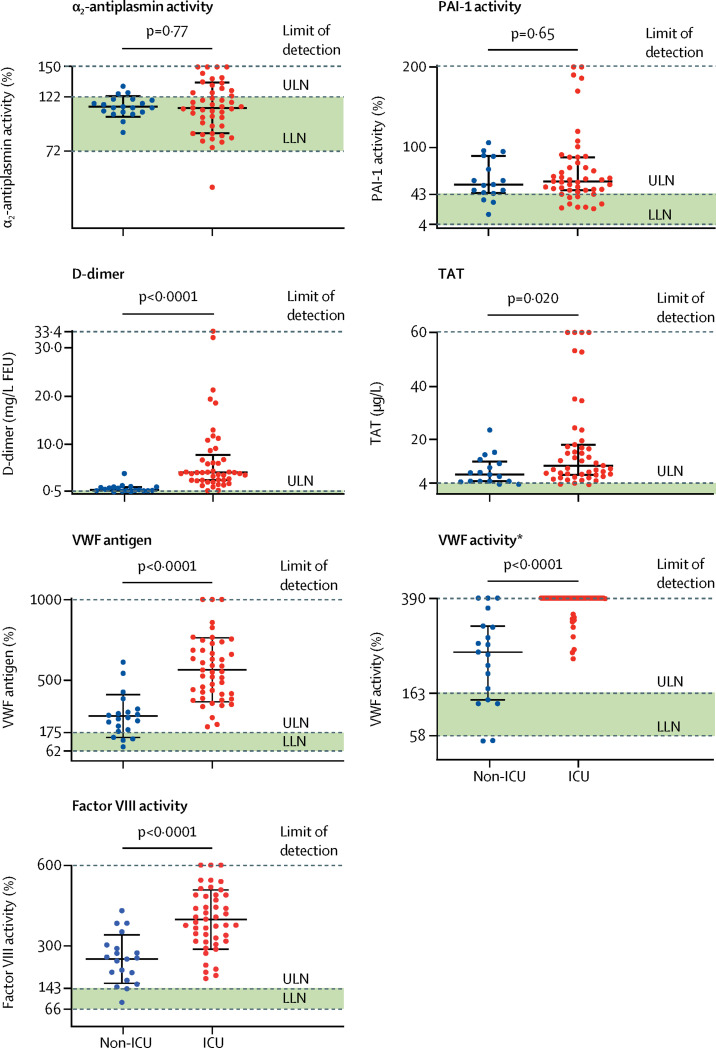
In terms of the measurement of coagulation factors, endogenous anticoagulants, and fibrinolytic enzymes (figure 1 ; table 2 ), there were near-universal elevations in PAI-1, with preserved α2-antiplasmin activity among both ICU and non-ICU patients. Non-ICU patients had elevated VWF antigen, VWF activity, and factor VIII activity, with 16 (80%) patients, 15 (75%) patients, and 18 (90%) patients above the normal range, respectively. These levels were further elevated in ICU patients, with 35 (73%) ICU patients having VWF activities above the limit of detection (figure 1). For endogenous anticoagulants, antithrombin activity, protein C activity, and protein S activity in ICU and non-ICU patients were generally preserved (figure 1; table 2). Five (7%) patients had an antithrombin activity below 70%, all of whom were in the ICU and had cirrhosis or bacterial or fungal sepsis. D-dimer and TAT concentrations were elevated in the entire study cohort, with significantly higher values in ICU patients compared with non-ICU patients (figure 1; table 2). D-dimer and TAT were also significantly correlated in the entire study cohort (r=0·49; p=0·0001; appendix).
Figure 1.
Comparisons of select haemostatic factors in ICU vs non-ICU patients
Datapoints indicate individual measurements, whereas horizontal bars show mean (SD) for α2-antiplasmin, VWF antigen, and factor VIII and median (IQR) for PAI-1, D-dimer, TAT, and VWF activity. Green shaded areas indicate the normal range of values. ICU=intensive care unit. ULN=upper limit of normal. LLN=lower limit of normal. FEU=fibrinogen equivalent units. PAI-1=plasminogen activator inhibitor-1. TAT=thrombin-antithrombin complexes. VWF=von Willebrand factor. *Thick horizontal bar denotes patients who had measurements above the limit of detection of the assay.
Table 2.
Coagulation parameters
| Standard reference range, or measurements from the control cohort (n=13) | ICU (n=48) | Non-ICU (n=20) | p value | |
|---|---|---|---|---|
| D-dimer, mg/L FEU | <0·55 | 4·2 (2·6–6·9) | 0·7 (0·4–1·2) | <0·0001 |
| TAT, μg/L | <4 | 10·6 (7·1–18·4) | 7·2 (4·7–11·3) | 0·020 |
| Antithrombin activity | 70–133% | 102% (32) | 111% (13) | 0·12 |
| Protein C activity | 81–145% | 121% (97–150) | 106% (92–130) | 0·46 |
| Protein S activity | 62–166% | 100% (30) | 93% (22) | 0·32 |
| α2-antiplasmin activity | 72–122% | 112% (24) | 113% (10) | 0·77 |
| PAI-1, ng/mL | 4–43 | 58 (47–88) | 54 (44–89) | 0·65 |
| VWF antigen | 62–175% | 565% (199) | 278% (133) | <0·0001 |
| VWF activity | 58–163% | 390% (390–390) | 260% (145–323) | <0·0001 |
| Factor VIII activity | 66–143% | 398% (111) | 251% (90) | <0·0001 |
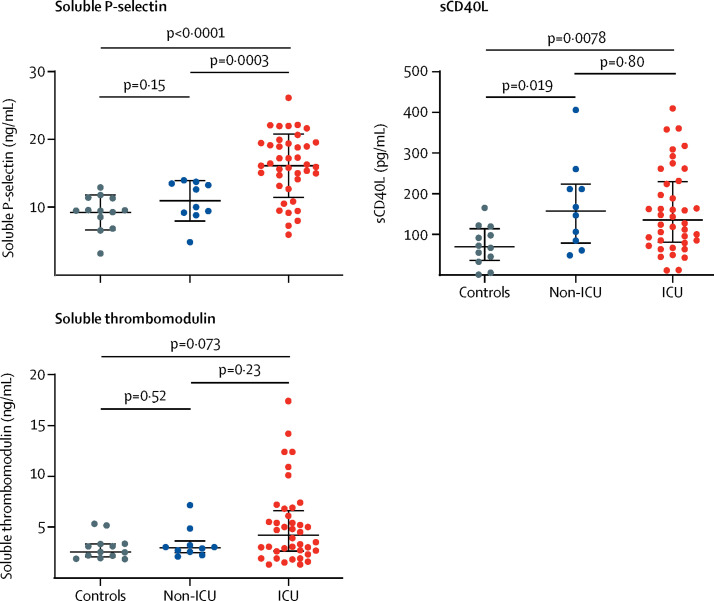
| Soluble P-selectin, ng/mL* | 9·5 (8·5–11·3)† | 15·9 (4·8) | 11·2 (3·1) | 0·0014 |
| Soluble thrombomodulin, ng/mL* | 2·5 (2·2–3·3)† | 4·2 (2·6–6·5) | 3·0 (2·6–3·2) | 0·23 |
| sCD40L, pg/mL* | 67 (33–98)† | 136 (82–228) | 157 (85–211) | 0·80 |
Data are mean (SD) or median (IQR), unless specified otherwise. p values are for comparison between ICU and non-ICU patients. ICU=intensive care unit. FEU=fibrinogen equivalent units. TAT=thrombin-antithrombin complex. PAI-1=plasminogen activator inhibitor-1. VWF=von Willebrand factor. sCD40L=soluble CD40 ligand.
Measured in 40 ICU and ten non-ICU patients.
Median values measured in the control cohort (n=13).
Following the observation of elevated VWF parameters in patients with COVID-19, together with an even greater increase in these levels associated with critical illness, we further explored the role of endothelial cell injury and platelet activation using additional plasma biomarkers. We measured soluble P-selectin (a marker of endothelial cell and platelet activation), sCD40L (a marker of platelet and T-cell activation), and soluble thrombomodulin (a marker of endothelial cell activation) in 40 ICU and 10 non-ICU patients and in 13 non-COVID-19, non-hospitalised controls (table 2; figure 2 ). Both soluble P-selectin and sCD40L were significantly elevated in ICU patients compared with controls (figure 2). Soluble P-selectin concentrations were also significantly higher in ICU patients than in non-ICU patients (figure 2). The increase in soluble thrombomodulin in ICU patients compared with controls was not significant (figure 2). Concentrations of both soluble P-selectin (r=0·36; p=0·012) and soluble thrombomodulin (r=0·48; p=0·0005) were significantly correlated with VWF antigen (appendix), consistent with their common source from endothelial cells. Both VWF antigen and soluble thrombomodulin were significantly correlated with mortality among all patients (r=0·38 for both; p=0·0022 and p=0·0078, respectively), and soluble thrombomodulin remained significantly correlated with mortality when the analysis was restricted to ICU patients (r=0·37; p=0·024; appendix).
Figure 2.
Comparisons of endothelial cell and platelet activation markers in ICU patients, non-ICU patients, and controls
Datapoints indicate individual measurements, whereas horizontal bars show mean (SD) for soluble P-selectin and median (IQR) for sCD40L and soluble thrombomodulin. ICU=intensive care unit. sCD40L=soluble CD40 ligand.
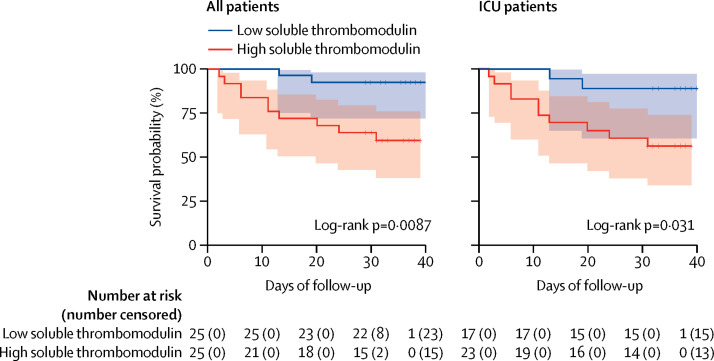
Of the 50 patients in whom soluble thrombomodulin was measured, the median value was 3·26 ng/mL (IQR 2·6–12·4), which was used to define two groups of 25 patients each: low soluble thrombomodulin (<3·26 ng/mL) and high soluble thrombomodulin (>3·26 ng/mL). 22 (88%) patients with low soluble thrombomodulin were discharged from the hospital compared with 13 (52%) patients with high soluble thrombomodulin (χ2 test p=0·0050). Among the 40 ICU patients in whom soluble thrombomodulin was measured, patients with low soluble thrombomodulin were also more likely than patients with high soluble thrombomodulin to be discharged from the hospital (14 [82%] of 17 patients vs 11 [48%] of 23 patients; χ2 test p=0·026). In a survival analysis, in-hospital mortality was significantly lower among patients with low soluble thrombomodulin compared with patients with high soluble thrombomodulin in the entire cohort (hazard ratio 5·9, 95% CI 1·9–18·4) and among ICU patients (4·5, 1·5–14·0; figure 3 ).
Figure 3.
Kaplan–Meier curve of survival and soluble thrombomodulin concentration
Data are shown for patients with low soluble thrombomodulin (<3·26 ng/mL) and high soluble thrombomodulin (>3·26 ng/mL). Shaded areas represent 95% CIs. ICU=intensive care unit.
Discussion
In this study of laboratory features of COVID-19-associated coagulopathy, we report several findings that are novel, to our knowledge, and suggest that endotheliopathy and platelet activation are important features of COVID-19 in hospitalised patients. Although previous studies have examined VWF elevations exclusively in the ICU setting,5, 11, 12 we report that VWF is also elevated in non-critically ill patients with COVID-19. We also show that critical illness is associated with further elevations in VWF, as well as increases in soluble P-selectin and sCD40L when compared with controls. Together, these results provide biochemical evidence that endotheliopathy and platelet activation are ubiquitous in COVID-19-associated coagulopathy and might play key roles in the progression of disease.
To our knowledge, our study is the first to suggest that a specific marker of endothelial cell injury, soluble thrombomodulin, is associated with hospital discharge status and segregates with survival in patients with COVID-19. This finding requires further validation. Soluble thrombomodulin is an endothelial transmembrane glycoprotein that is released upon endothelial disruption or injury.13 Among healthy individuals, circulating thrombomodulin is thought to be produced by physiological cleavage and shedding of membrane-bound thrombomodulin. By contrast, in hyperinflammatory states, elevated soluble thrombomodulin concentrations are thought to be secondary to direct endothelial cell damage.14 Additional effects of soluble thrombomodulin on the coagulation cascade and fibrinolysis in diseases such as diabetes have also been described.15 Although the relative contribution of thrombomodulin shedding and pathological release of thrombomodulin due to endothelial cell injury or death in COVID-19 will require further investigation, our observation that soluble thrombomodulin is a predictive marker of mortality in COVID-19, coupled with the observed increases in VWF and soluble P-selectin specifically in critically ill patients, support a model in which endotheliopathy is an important event in the transition to critical illness and death in patients with COVID-19.
Our finding of preserved antifibrinolytic α2-antiplasmin activity in patients with COVID-19, together with the preserved endogenous anticoagulant activity (antithrombin activity, protein C activity, and protein S activity) observed both here and in other studies, supports the notion that COVID-19-associated coagulopathy is mechanistically distinct from disseminated intravascular coagulation.5, 11, 16 PAI-1 has been linked to elevated IL-6 in other contexts and has been suggested to play a role in severe acute respiratory distress syndrome.17, 18 The finding of elevated PAI-1 in critically ill and non-critically ill patients with COVID-19 further supports a picture of endotheliopathy, given the role of endothelial cells as the primary source of PAI-1.19 Elevation of PAI-1 also suggests the possibility that classical fibrinolysis might be inhibited in COVID-19-associated coagulopathy. Measurements of tissue and urokinase-type plasminogen activator and plasminogen levels might help to further assess this, although the interpretation of such levels might not be relevant if the observed elevation in PAI-1 is enough to overcome a possible increase in plasminogen activator.
We therefore propose that COVID-19-associated coagulopathy is an endotheliopathy that results in augmented VWF release, platelet activation, and hypercoagulability, leading to the clinical prothrombotic manifestations of COVID-19-associated coagulopathy, which can include venous, arterial, and microvascular thrombosis. The factors responsible for this endotheliopathy and platelet activation are uncertain but could include direct viral infection of endothelial cells, collateral damage to the tissue as a result of immune infiltration and activation, complement activation, or any number of inflammatory cytokines believed to play a role in COVID-19 disease.3, 9, 10
A central role for endothelial cells and platelets has been characterised in other forms of critical illnesses, including septic shock, acute respiratory distress syndrome, and veno-occlusive disease following haematopoietic stem-cell transplantation, where increased markers of endotheliopathy and platelet activation are observed.20, 21, 22, 23, 24 Disseminated intravascular coagulation also involves substantial endothelial cell and platelet activation but is mechanistically distinct from COVID-19-associated coagulopathy, in part as a result of unmitigated activation of the coagulation cascade and resultant endogenous anticoagulant consumption in disseminated intravascular coagulation.25 In models of septic shock, elevated VWF antigen and activity can be accompanied by reductions in the ADAMTS13 metalloproteinase responsible for cleaving ultra-large VWF multimers into smaller VWF forms, leading to thrombotic microangiopathy via a mechanism distinct from disseminated intravascular coagulation.26 We did not measure ADAMTS13 concentrations in our ICU patients but would predict that they might be similarly reduced.
Our findings of endotheliopathy and platelet activation point to antiplatelet therapy or endothelial cell modification as potential therapeutic targets in addition to traditional anticoagulation targeting thrombin generation. Thus far, no retrospective or prospective studies have identified a beneficial effect of aspirin on COVID-19 disease outcomes. However, dipyridamole, defibrotide, eculizumab, and other agents with endothelial cell-modifying effects might have therapeutic potential.27, 28, 29 In light of our preliminary finding that soluble thrombomodulin might predict mortality in patients with COVID-19, measurements of soluble thrombomodulin could be useful in identifying patients who might benefit the most from these therapies. However, the prognostic potential of this marker should be further validated.
Limitations of our study include the small sample size; the heterogeneity of patient groups, with a wide range of ages, comorbidities, length of hospitalisation; and the fact that each patient's laboratory measures for the purposes of this study were assessed once during their hospital stay, as well as limited generalisability of the findings in a single-centre study. Further studies incorporating serial sampling of relevant biomarkers over time will yield additional mechanistic insights into the biology of endotheliopathy in COVID-19. In addition, the widespread use of tocilizumab, particularly among ICU patients, might have led to an underestimation of the degree of endotheliopathy in ICU patients in this study, as previous literature has reported an association of IL-6 signalling with endothelial cell activation and VWF release, and other studies have shown that IL-6 blockade reduces TAT and PAI-1 concentrations.17, 30 In addition, five patients had blood samples drawn outside of the 0300–0700 h window, which could potentially affect PAI-1 concentrations given the circadian fluctuations of this enzyme; however, in all but one of these instances, PAI-1 was high, indicating that the time of PAI-1 measurement did not have an impact on PAI-1 concentration. Lastly, further evidence of platelet activation, beyond increased circulating concentrations of soluble P-selectin and sCD40L, will be needed to characterise the extent of platelet activation in COVID-19.
In summary, we did an expanded analysis of global haemostatic assays that, to our knowledge, have not been previously reported in both critically ill and non-critically ill patients with COVID-19. Through measurements of VWF, PAI-1, soluble thrombomodulin, soluble P-selectin, and sCD40L, we found that endotheliopathy and platelet activation might be important factors in the pathophysiology of COVID-19-associated coagulopathy. Additional studies are required to elucidate the factors that drive endotheliopathy in critical illness and to explore the possible therapeutic effects of adding antiplatelet or endothelial cell-modifying therapy.
Acknowledgments
Acknowledgments
This work was supported by a gift donation from Jack Levin to the Benign Hematology programme at Yale, and the National Institutes of Health grant (HL142818) to HJC. This study was presented in part as a late-breaking oral at the 25th (Virtual) Congress of the European Hematology Association. We would like to thank all front-line staff taking care of our community of patients affected by severe acute respiratory syndrome coronavirus 2 and are grateful for the multidisciplinary collaboration at the Yale University School of Medicine in the care of our community. We also thank Lauren Pischel (Yale University School of Medicine), Susan F McDonald, and Eric Andrewsen for their support and assistance.
Contributors
GG, ABP, MLM, CT, HMR, HJC, and AIL designed the study. C-HC, HZ, and PB did assays to measure haemostatic factors and endothelial cell and platelet biomarkers. AB, RDB, AJB, AD, SH, HM, NN, HMR, HJC, and AIL contributed to sample collection. NB, RDB, CSDC, SH, JH, JK, NN, CP, and JMS contributed valuable ideas and input during the study. GG, ABP, MLM, HJC, and AIL wrote the manuscript and all authors participated in editing the manuscript. GG, ABP, and MLM contributed equally as first authors. HJC and AIL contributed equally as senior investigators.
Declaration of interests
NN reports research funding support from Janssen and GlaxoSmithKline and is an advisory board member for Sanofi, outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Johns Hopkins University Coronavirus resource center. COVID-19 case tracker. https://coronavirus.jhu.edu
- 2.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigada M, Bottino N, Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llitjos JF, Leclerc M, Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. published online April 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Kruip MJHA, van der Meer NJM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carsana L, Sonzogni A, Nasr A. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. 2020 doi: 10.1101/2020.04.19.20054262. published online April 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M, Verleden SE, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. published online May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Aguirre Y, Páramo JA. Endothelial cell and hemostatic activation in relation to cytokines in patients with sepsis. Thromb Res. 1999;94:95–101. doi: 10.1016/s0049-3848(98)00200-x. [DOI] [PubMed] [Google Scholar]
- 14.Nawroth PP, Häring HU. Thrombomodulin and coronary heart disease. Lancet. 1999;353:1722–1723. doi: 10.1016/S0140-6736(99)90039-9. [DOI] [PubMed] [Google Scholar]
- 15.Aso Y, Fujiwara Y, Tayama K, Takebayashi K, Inukai T, Takemura Y. Relationship between soluble thrombomodulin in plasma and coagulation or fibrinolysis in type 2 diabetes. Clin Chim Acta. 2000;301:135–145. doi: 10.1016/s0009-8981(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 16.Asakura H, Ontachi Y, Mizutani T. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29:1164–1168. doi: 10.1097/00003246-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Makrilakis K, Fragiadaki K, Smith J, Sfikakis PP, Kitas GD. Interrelated reduction of chemerin and plasminogen activator inhibitor-1 serum levels in rheumatoid arthritis after interleukin-6 receptor blockade. Clin Rheumatol. 2015;34:419–427. doi: 10.1007/s10067-014-2704-1. [DOI] [PubMed] [Google Scholar]
- 18.Gralinski LE, Bankhead A, 3rd, Jeng S. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4:1–12. doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handt S, Jerome WG, Tietze L, Hantgan RR. Plasminogen activator inhibitor-1 secretion of endothelial cells increases fibrinolytic resistance of an in vitro fibrin clot: evidence for a key role of endothelial cells in thrombolytic resistance. Blood. 1996;87:4204–4213. [PubMed] [Google Scholar]
- 20.Rubin DB, Wiener-Kronish JP, Murray JF. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayal S, Jaïs JP, Aguini N, Chaudière J, Labrousse J. Elevated circulating E-selectin, intercellular adhesion molecule 1, and von Willebrand factor in patients with severe infection. Am J Respir Crit Care Med. 1998;157:776–784. doi: 10.1164/ajrccm.157.3.9705034. [DOI] [PubMed] [Google Scholar]
- 22.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant. 2008;41:677–686. doi: 10.1038/sj.bmt.1705990. [DOI] [PubMed] [Google Scholar]
- 24.Johansen M, Johansson P, Ostrowski S. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost. 2015;41:16–25. doi: 10.1055/s-0034-1398377. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2018;131:845–854. doi: 10.1182/blood-2017-10-804096. [DOI] [PubMed] [Google Scholar]
- 26.Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16:646–651. doi: 10.1111/jth.13953. [DOI] [PubMed] [Google Scholar]
- 27.Yasmeen S, Akram BH, Hainsworth AH, Kruuse C. Cyclic nucleotide phosphodiesterases (PDEs) and endothelial function in ischaemic stroke. A review. Cell Signal. 2019;61:108–119. doi: 10.1016/j.cellsig.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Li Z, Liu S. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.04.008. published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diurno F, Numis FG, Porta G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 30.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.