Sir,
The scientific and clinical communities are still reeling from the sudden retraction by the New England Journal of Medicine [1,2] and The Lancet [3,4] of coronavirus disease 2019 (Covid-19) papers by Mehra et al. The ripple effects of these retractions extend widely and include an otherwise important meta-analysis by Zhang and colleagues [5] published in Pharmacological Research before the recent retractions. Of specific concern are the Mehra et al. [1] mortality data on angiotensin converting enzyme inhibitors (ACEI) and angiotensin-II-receptor blockers (ARB) and mortality in Covid-19 patients.
We re-estimated the Zhang et al. [5] pooled estimates for mortality in Covid-19 patients and mortality in Covid-19 patients with antihypertensive indication after excluding the Mehra et al. [1] data. These re-estimations are necessary not only because of the retracted data themselves but also because of the relative weight of the Mehra et al. [1] data in the meta-analyses and the high heterogeneity observed in these analyses. Our re-estimations were performed using R Core Team (2020) (R Foundation for Statistical Computing, Vienna, Austria) and applied random effects models. Because of differences in the software packages used [6], small differences may be observed that may be real or may be due to computation and rounding.
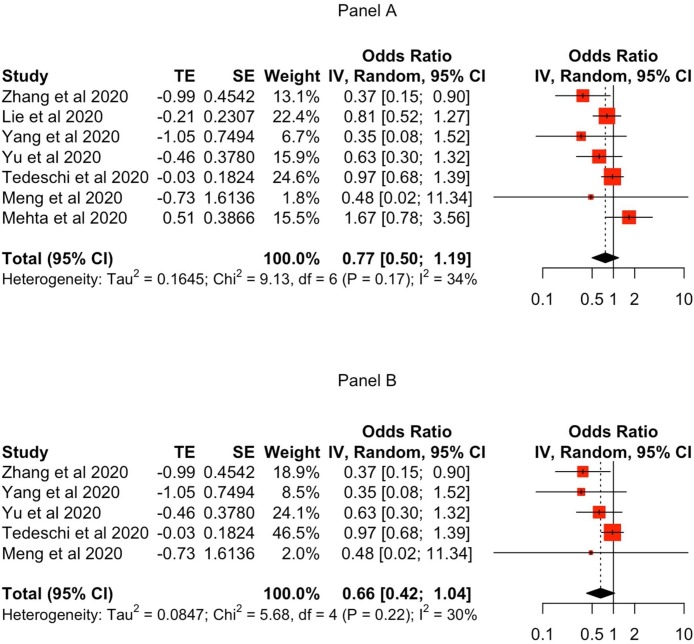
In the Zhang et al. [5] random effects meta-analysis of mortality in Covid-19 patients, the Mehra et al. [1] ACEI and ARB data had a combined weight of 30.81 %, heterogeneity was 70.7 %, and the pooled odds ratio (OR) estimate was 0.73 (95CI% = 0.50-1.07). After removing the Mehra et al. [1] data (Fig. 1 A), the seven residual studies yielded a pooled OR estimate of 0.77 (95CI% = 0.50-1.19). While nominally slightly higher, like Zhang et al. [5] this estimate was not statistically significant and therefore no altered risk of mortality is inferred. Of note, this result was derived from a less heterogeneous (34 %) set of seven studies.
Fig. 1.
Forest plot of re-estimated ACEI/ARB exposure and risk of mortality in (A) Covid-19 patients and (B) Covid-19 patients with antihypertensive indication.
As to mortality in patients with antihypertensive indication, Zhang et al. [5] reported a combined weight of 40.32 % for the Mehra et al. [1] data, heterogeneity of 74.8 %, and a pooled OR of 0.62 with a 95 %CI of 0.38-1.02 indicating no statistically significant risk of mortality in this subpopulation. However, our calculations including Mehra et al. [1] yielded slightly different results: combined weight of 41.9 % and a pooled OR of 0.64 with a 95 %CI of 0.41-0.98 indicating a statistically significant lower risk of mortality in this subpopulation (at a similar 75 % heterogeneity). When re-estimated following the removal of the Mehra et al. [1] data (Fig. 1B), the five remaining studies yielded a statistically non-significant pooled OR of 0.66 [95 %CI = 0.42-1.04) but with much lower heterogeneity (30 %) among studies.
The encouraging conclusion is that, apart from a small discrepancy between our re-estimations and the original Zhang et al. [5] results, there is no statistically significant mortality risk signal in Covid-19 patients receiving ACEI/ARB therapy - and this from a set of studies with much lower heterogeneity. Similarly, there is no statistically significant mortality risk signal in Covid-19 patients with the anti-hypertensive indication. However, caution is advised because excluding the Mehra et al. [1] study reduced the number of Covid-19 patients in the re-estimation by 1326 (61 %), including 54 deaths. Hence the risk estimates reported here must be considered preliminary and indicative. More primary observational studies and safety trials are necessary, the results of which should subsequently be included in a “living systematic review” that updates the mortality risk estimates as data from more studies accrue [7].
Submission considerations
This work has not been published before; is not under consideration elsewhere; and has been approved by both authors.
Authors' contributions
I.A. conceived the idea and A.A. extracted all the data from the relevant articles and performed the statistical analysis for this letter. Both I.A. and A.A. discussed and verified the accuracy of the presented data against the published studies, and jointly drafted the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgement
We would like to thank Zhang and colleagues for their meta-analysis. Our letter is intended to supplement their analysis.
References
- 1.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Retraction: cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020;382:2582. doi: 10.1056/NEJMoa2007621. N Engl J Med. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Mehra M.R., Ruschitzka F., Patel A.N. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31324-6. 1820 6. Epub 2020 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Yu J., Pan L.Y., Jiang H.Y. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol. Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochrane Community. Living systematic reviews. Available at https://community.cochrane.org/review-production/production-resources/living-systematic-reviews. Last accessed 25 June 2020. Stylefix.