Abstract
We collected environmental surface samples prior to and after disinfection of a quarantine room to evaluate the stability of SARS-CoV-2 during the incubation period of an imported case traveling to Qingdao, China. Overall, 11 of 23 (47.8%) of the first batch of environmental surface samples (within 4 h after case confirmation) were tested positive for SARS-CoV-2. Whereas only 2 of 23 (8.7%) of the second batch of environmental samples (after first disinfection) were tested positive for SARS-CoV-2. The majority of samples from the bedroom (70%) were positive for SARS-CoV-2, followed by 50% of samples from the bathroom and that of 33% from the corridor. The inner walls of toilet bowl and sewer inlet were the most contaminated sites with the highest viral loads. SARS-CoV-2 was widely distributed on object surfaces in a quarantine room of a later diagnosed COVID-19 case during the incubation period. Proper disinfection is crucial to minimize community transmission of this highly contagious virus.
Keywords: SARS-CoV-2, COVID-19, Incubation period, Environmental contamination, Disinfection
Graphical abstract
1. Introduction
The pandemic of coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been sweeping across over 200 countries and regions, causing nearly 480 thousand deaths worldwide (WHO, 2020). At present, several surface sampling studies have indicated positive results for SARS-CoV-2 detection, however, they only focused on intensive care units and general wards of hospitals where confirmed patients stayed for several days (Faridi et al., 2020; Guo et al., 2020; Ong et al., 2020). Thus, there has been limited data regarding the contamination of dwelling environment during the incubation period.
In China, where the outbreak was first reported (WHO, 2020), thanks to series of aggressive measures such as strict traffic control, universal masking and highly restrictive form of social distancing, there has been a dramatic decrease of domestic cases (Chen et al., 2020). However, the country is now facing the threat of imported infections (Xu et al., 2020). A thorough understanding of environmental contamination by SARS-CoV-2 in quarantine rooms for overseas travelers would help expand knowledge of viral transmission route and facilitate proper infection control precautions.
2. Methods
On March 24, 2020, an overseas student (20-year-old Chinese male) arrived in Qingdao, China from the United States. He had no fever or other symptoms on arrival and was sent to a designated hotel for a 14-day centralized quarantine. He is the only person in the quarantine room, and there was no other confirmed COVID-19 case ever staying in this room. His nasopharyngeal samples obtained on March 24, 25 and 28 were all negative for SARS-CoV-2. On the twelfth day of the quarantine (April 5), the patient developed symptoms including fever and chills. He was immediately transferred to the local hospital and nasopharyngeal swabs, sputum specimens and fecal specimens taken on admission were all positive for SARS-CoV-2.
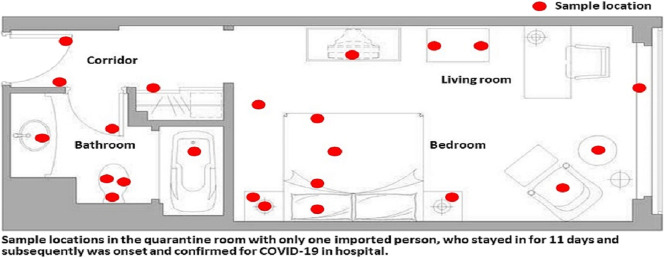
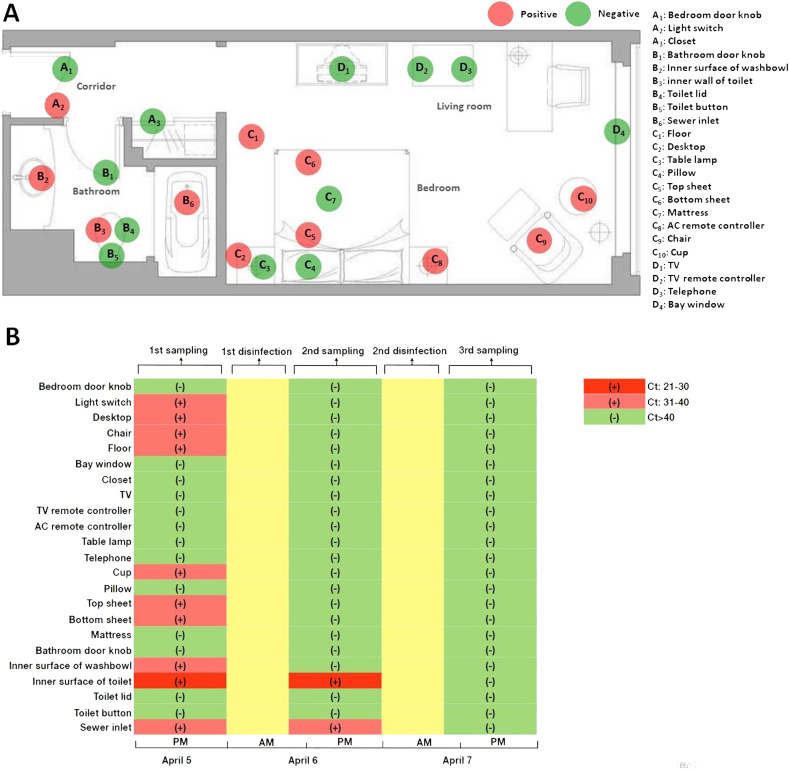
Environmental surface samples were obtained prior to and after disinfection of the quarantine room to evaluate the stability of SARS-CoV-2 during the patient's incubation period. As shown in Fig. 1 , a total of 23 sites were sampled from the quarantine room. The surface of the entire item was swabbed except for the pillow, the sheet, and the mattress by vigorously swabbing 10 times horizontally and vertically in a contact area of 100 cm2. All sites were sampled three times with a sterile polyester-tipped applicator pre-moistened in viral transport medium, and the first batch of environmental surface samples were taken within 4 h after case confirmation (on April 5); subsequent environmental samples were taken within 24 h after every disinfection (on April 6 and April 7, respectively). Once the patient was confirmed with COVID-19, sodium hypochlorite (containing 1000 mg/L available chlorine) was used to disinfect the patient's quarantine room based on the protocol of Chinese Centers for Disease Control and Prevention (China CDC, 2020). Detailed procedures of sample collection and room disinfection were described in Supplementary Materials. All samples were sent to Qingdao Municipal Center for Disease Control and Prevention for centralized RT- PCR testing for the detection of SARS-CoV-2. A Ct value less than 37 was defined as positive, and a Ct value of 40 or more was defined as a negative test. An equivocal result, defined as a Ct value between 37 and 40, was required confirmation by retesting. If the repeated Ct value was less than 40 and an obvious peak was observed, or if the repeated Ct value was less than 37, the retest was deemed positive.
Fig. 1.
Environmental sampling sites and its Ct values in the quarantine room.
This study was approved by the Ethics Commission of Municipal Centre of Disease Control and Prevention of Qingdao and written informed consent was waived considering the emergency of infectious disease.
3. Results
The predominant symptom at the onset of the illness was fever (highest temperature 37.8 °C), which lasted for 2 days. Other symptoms included chills, cough, sore throat and sputum production. The patient was in a stable condition during the course of the disease. Radiological and laboratory findings showed no specific changes of COVID-19. Overall, 11 of 23 (47.8%) of the first batch of environmental surface samples were tested positive for SARS-CoV-2, and the Ct values for frame 1ab (ORF1ab) ranged from 26 to 39, with a median of 35 (Supplementary Material, Table 1 ). Whereas only 2 of 23 (8.7%) of the second batch of environmental samples were tested positive for SARS-CoV-2. After repeated disinfection of the quarantine room, no environmental viral RNA was detected in the third sampling. Three functional areas of the quarantine room were detected positive for Reverse-Transcription PCR (RT-PCR) testing, including the corridor, the bathroom and the bedroom (except for the living room). The majority of samples taken from the bedroom (70%) were tested positive for SARS-CoV-2, followed by 50% of samples taken from the bathroom and that of 33% from the corridor. The inner walls of toilet bowl and sewer inlet were the most contaminated sites with the highest viral loads (as reflected by the reverse of Ct values). Interestingly, Ct value of the environmental sample taken from the inner wall of toilet bowl (Ct: 26) was in the middle of that of respiratory samples (nasopharyngeal swab Ct: 20; sputum specimen (Ct: 24) and stool specimen (Ct: 34) at the onset of the illness.
Table 1.
Distribution of Ct values of environmental surface samples among each functional area of the quarantine room.
| Functional area | Positive/total | Minimum | Median | Maximum |
|---|---|---|---|---|
| Corridor | 1/3 (33%) | 32 | 32 | 32 |
| Bathroom | 3/6 (50%) | 26 | 33 | 34 |
| Bedroom | 7/10 (70%) | 35 | 38 | 39 |
| Living room | 0/4 (0) | >40 | >40 | >40 |
4. Discussion
Our study demonstrated extensive environmental contamination of SARS-CoV-2 in a quarantine room of an overseas COVID-19 patient during the incubation period. The highly contagious virus has been detected in the air and on object surfaces of hospital wards for COVID-19 patients (Faridi et al., 2020; Guo et al., 2020; Ong et al., 2020). Experiments conducted under controlled laboratory conditions revealed that the virus could remain viable and infectious on object surfaces for up to several days (van Doremalen et al., 2020). However, there has been a paucity of data regarding environmental contamination by SARS-CoV-2 during the incubation period. Detectable virus in the indoor environment of the quarantine room as shown in this study indicates environmental contamination might happen before clinical manifestation. Strict adherence to personal protective equipment (PPE) and precautionary approaches are advised to safeguard staff working in the centralized quarantine facilities.
Furthermore, the inner wall of toilet bowl was the most contaminated site with viral loads in the middle of respiratory samples and stool specimens, suggesting the extent of environmental contamination was closely correlated to SARS-CoV-2 shedding route. Except for the major routes including respiratory droplets and close contact (Guan et al., 2020; Huang et al., 2020), several other routes have been proposed for possible transmission of SARS-CoV-2, particularly, prolonged fecal shedding of viral particles (Cheung et al., 2020; Xing et al., 2020). Of note, SARS-CoV-2 could still be found on the surfaces of toilet bowl and sewer inlet after the first disinfection, but all environmental samples taken at the same sites turned negative for the virus after the second disinfection. Different techniques for disinfection might present as one of the possible explanations. Disinfectant spray was used for the first disinfection campaign, whereas immersion disinfection was applied for the second time. Hence, disinfectant immersion might be more effective for object surfaces like toilet bowl and sewer inlet. Such results highlight the importance of proper disinfection for the contaminated environment to reduce the exposure risk of SARS-CoV-2.
The limitations of this study should also be addressed. We were unable to collect air samples of the quarantine room to evaluate aerosol distribution characteristics. Moreover, results from this study were based on only one case of COVID-19, and further investigations with a larger sample size would be warranted. We neither performed isolation of the virus to determine its viability on environmental surfaces.
Taken together, this study showed the widespread distribution of SARS-CoV-2 on object surfaces in a quarantine room of a later diagnosed COVID-19 case during the incubation period. Proper disinfection is necessary to minimize community transmission of this highly contagious virus. In face of the increasing risk of imported infections from overseas, strict adherence of protective measures is of paramount importance to control the spread of SARS-CoV-2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Xiaowen Hu:Conceptualization, Writing - original draft.Yuhan Xing:Formal analysis, Writing - original draft.Wei Ni:Formal analysis, Writing - original draft.Feng Zhang:Investigation, Writing - original draft.Sheyu Lu:Investigation.Zhaoguo Wang:Investigation.Ruqin Gao:Investigation, Writing - original draft.Fachun Jiang:Conceptualization, Writing - original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are deeply thankful to all health-care workers involved in the diagnosis and treatment of patients in Qingdao.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.140620.
Appendix A. Supplementary data
Appendix material
References
- Chen S., Yang J., Yang W., Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395(10226):764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W., Tan A.R., Yip C.C., Leung K.H., Yim-Fong Fung A., Zhang R.R., Cheng H.M., Zhang A.J., To K.K., Chan K.H., Yuen K.Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Center for Disease Control and Prevention (China CDC) Technical guideline for disinfection and sterilization. 2020. https://max.book118.com/html/2018/0225/154659177.shtm [Assessed 1 May, 2020]. Available from. [DOI] [PMC free article] [PubMed]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shansipour M., Jandaghi N.Z.S., Sadeghniat K., Nabizadeh R., Yunesian M., Momeniha F., Mokamei A., Hassanvand M.S., MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Qu C.Q., He J.X., Liu L., Shan H. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R.B., Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26(7) doi: 10.3201/eid2607.200885. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X.W., Ren L.L., Zhao J.P., Hu Y., Zhang L., Fan G.H., Xu J.Y., Gu X.Y., Cheng Z.S., Yu T., Xia J.A., Wei Y., Wu W.J., Xie X.L., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J.G., Wang G.F., Jiang R.M., Gao Z.C., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornbury N.J., Gerber S.I., Lloydsmith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Orgnization (WHO) Novel Coronavirus (COVID-19) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Assessed 25 June, 2020]. Available from.
- Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D., Tong J.N., Song X.F., Wing-Kin Wong G., Xing Q.S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.021. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Chen C., Zhu Z., Cui M., Chen C., Dai H., Xue Y. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int. J. Infect. Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix material