Highlights
-
•
Remdesivir has been found to be potent in vitro inhibitor of RNA viruses including SARS-CoV-2, but its in vivo potency is still under investigation.
-
•
This study report the clinical and biological features of five patients hospitalized with COVID-19 and treated with remdesivir for compassionate use.
-
•
For two patients, viral loads in nasopharyngeal samples decreased, despite active replication in the lower respiratory tract area.
-
•
The treatment had to be interrupted in four of the five patients, because of ALT elevation and/or renal failure.
Keywords: SARS-CoV-2 viral load, Remdesivir, Antiviral therapy, Viral pneumonia, Case reports
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified as the virus responsible for the coronavirus disease 2019 (COVID-19) outbreak worldwide. Data on treatment are scare and parallels have been made between SARS-CoV-2 and other coronaviruses. Remdesivir is a broad-spectrum antiviral with efficient in vitro activity against SARS-CoV-2. Evidence of clinical improvement in patients with severe COVID-19 treated with remdesivir is controversial. The aim of this study was to describe the clinical outcomes and virological monitoring of the first five COVID-19 patients admitted to the intensive care unit of Bichat-Claude Bernard University Hospital, Paris, France, for severe pneumonia related to SARS-CoV-2 and treated with remdesivir. Quantitative reverse transcription PCR was used to monitor SARS-CoV-2 in blood plasma and the lower and upper respiratory tract. Among the five patients treated, two needed mechanical ventilation and one needed high-flow cannula oxygen. A significant decrease in SARS-CoV-2 viral load in the upper respiratory tract was observed in most cases, but two patients died with active SARS-CoV-2 replication in the lower respiratory tract. Plasma samples were positive for SARS-CoV-2 in only one patient. Remdesivir was interrupted before the initialy planned duration in four patients, two because of alanine aminotransferase elevations (3 to 5 normal range) and two because of renal failure requiring renal replacement. This case series of five COVID-19 patients requiring intensive care unit treatment for respiratory distress and treated with remdesivir, highlights the complexity of remdesivir use in such critically ill patients.
Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as responsible for the coronavirus disease 2019 (COVID-19) outbreak that started in China (Zhu et al., 2020). Treatment options investigated during previous severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics, both caused by other viruses of the Betacoronavirus genus (SARS-CoV-1 and MERS-CoV, respectively), have been proposed as possible therapeutic agents for SARS-CoV-2, with controversial results. These agents include ribavirin and interferon alpha 2b (IFNα-2b) (Martinez, 2020), lopinavir/ritonavir (Chu et al., 2004, Arabi et al., 2020), and hydroxychloroquine (Savarino et al., 2006, Chen et al., 2020, Gautret et al., 2020, Wang et al., 2020a).
Remdesivir, a nucleotide analogue prodrug with broad-spectrum antiviral activity (Sheahan et al., 2017, Mulangu et al., 2019), has shown promising activity against SARS-CoV-2 in vitro (Wang et al., 2020a). Two case reports (Holshue et al., 2020, Kujawski et al., 2020) and a recent clinical study of 54 patients (Grein et al., 2020) showed encouraging results in COVID-19 patients. Conversely, a randomized study did not show any significant clinical benefit, although it probably lacked power (Wang et al., 2020b).
Five patients were treated with compassionate-use remdesivir in our centre in Paris, France. Early findings in two of these patients have been described previously (Lescure et al., 2020). Here we describe the complete follow-up, drug tolerance, and virological monitoring of these five patients.
Case presentations
Participants and sources of data
All patients admitted to the Bichat-Claude Bernard University Hospital, Paris, France, between January 24 and March 1, 2020, diagnosed with COVID-19 and treated with remdesivir (Gilead Sciences), were enrolled. The indication criteria for compassionate-use remdesivir were defined by the French national regulatory authorities and French Ministry of Health: signs of severe illness at diagnosis or subsequent clinical worsening (respiratory symptoms or general signs). Since March 22, all patients requiring antiviral treatment have been enrolled in the Discovery Study (2020-000936-23). The Institutional Review Board of Bichat-Claude Bernard University Hospital approved this report and waived the need for informed consent from individual patients, due to the retrospective chart review design and absence of identifying images or personal/clinical details that could compromise anonymity.
Procedures
All patients received remdesivir via intravenous infusion with a loading dose of 200 mg and a maintenance daily dose of 100 mg for a maximum duration of 14 days. All nasopharyngeal and bronchoalveolar samples were collected in universal transport medium (Virocult, Sigma) and transported to the laboratory within 24 h. All quantitative reverse transcription PCR (RT-qPCR) tests were performed according to the World Health Organization recommended procedure (Corman et al., 2020) after extraction on MagNA Pure (Total NA Large Volume Kit, Roche Diagnostics) from 200 μl of transport medium and amplification on an ABI 7500 instrument (Life Technologies). Quantification was done using a standardized RNA transcript control obtained from the European Virus Archive programme. Bacterial and mycological investigations were conducted on a separate sample without virological transport medium using the usual procedures for bacterial growth. Bronchoalveolar lavage fluid was also tested with an multiplex PCR assay (FilmArray Pneumonia assay; BioFire, BioMérieux) for virus and bacteria detection, and by fungal culture with mass spectrometry identification for Aspergillus detection. All samples were processed in a biosafety level 3 laboratory (BSL3).
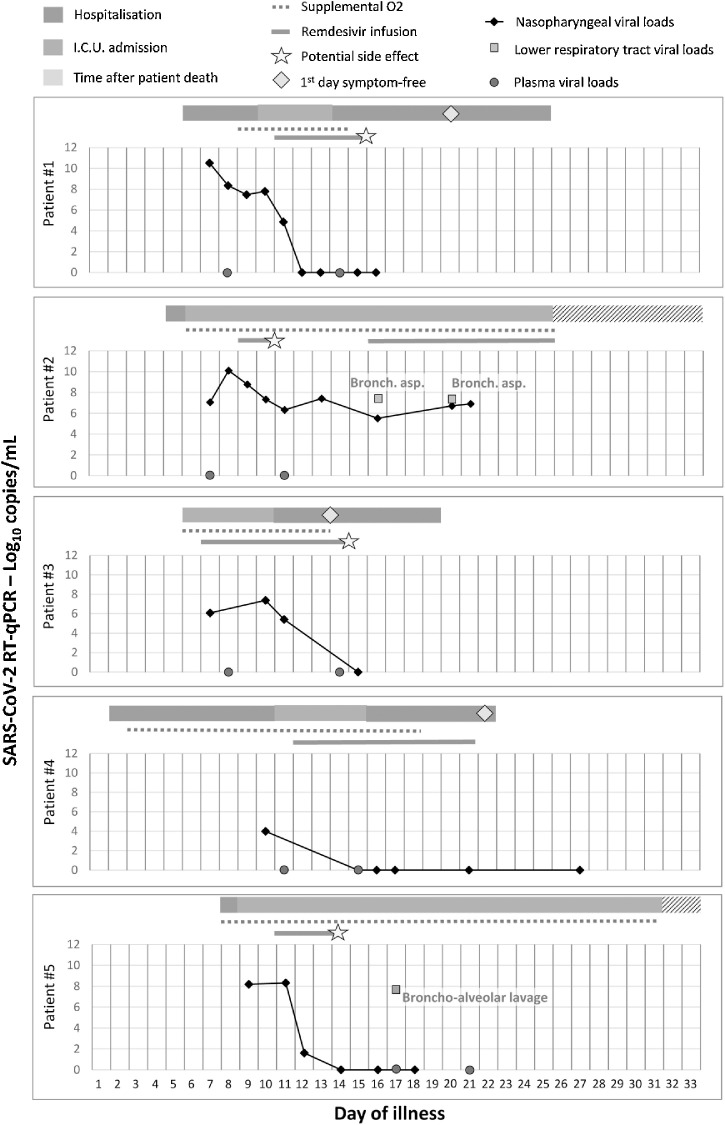
Results (Figure 1)
Figure 1.
Clinical and viral evolution of the case patients.
For each patient, the type of hospital ward is indicated by the coloured rectangles, supplemental O2 requirement by the dashed blue line, and remdesivir infusion by the red line. Viral load is shown with black diamonds, nasopharyngeal swabs with a black line, lower respiratory tract samples (when available) with green squares, and plasma samples with red circles. The viral load was estimated by cycle threshold (Ct) values; the lower the Ct value, the higher is the viral load. A sample is negative above a Ct value of 40.
Case 1
A 31-year-old Chinese male originating from Wuhan and reporting flu-like symptoms for 6 days was diagnosed with COVID-19 on January 24, 5 days after arriving in Paris. He was immediately hospitalized with mild lymphopenia (1.00 × 109/l) and thrombocytopenia (1.46 × 109/l); there were no abnormalities on chest X-ray. RT-qPCR on nasopharyngeal samples was positive, with a SARS-CoV-2 viral load (VL) of 10.5 log10 copies/ml. On day 10 of illness, he was transferred to the intensive care unit (ICU) due to worsening oxygen saturation (PO2 = 58 mmHg; low-flow nasal cannula 4 l/min) and bilateral ground-glass and alveolar opacities on chest computed tomography (CT) scan with no increase in VL. Remdesivir was started on January 29, 2020 (day 11 of illness) and was stopped on day 15, due to alanine aminotransferase (ALT) elevation (195 IU/l versus 46 IU/l before remdesivir administration) and the presence of a maculopapular rash. A rapid decline in VL from a cycle threshold (Ct) value of 27.6 to undetectability was observed on day 2 of remdesivir infusion. The skin and liver abnormalities improved within 3 days after discontinuing treatment. The patient was discharged on February 12.
Case 2
An 80-year-old tourist originating from Hubei Province, with a past medical history of thyroid cancer, presented on January 25 with fever and diarrhoea of 4-day duration. A chest X-ray showed bilateral alveolar opacities, but he did not fulfil the COVID-19 case definition at that time. Airborne and contact precautions were observed and the diagnosis of COVID-19 was eventually made 3 days later. On January 26, acute respiratory failure with multiple organ failure triggered his admission to the ICU. Broad-spectrum antibiotic therapy was started and adapted for co-infection with a susceptible Acinetobacter baumannii (diagnosed by multiplex PCR and confirmed by tracheal aspirate culture) and Aspergillus flavus (tracheal aspirate culture). Remdesivir was started on January 29, but was discontinued on January 31, as the patient needed renal replacement therapy. The nasopharyngeal VL decreased from a Ct value of 21.0 before infusion to 28.9 on day 2 of infusion. A CT scan performed on January 31 showed bilateral alveolar condensations, ground-glass opacities, and pulmonary cysts. On February 5, because of the disease severity and the persistence of viral detection, remdesivir was re-initiated. Multiple organ failure persisted without any other co-infection identified. He died on February 14.
Case 3
A 39-year-old male airport worker, who was obese (body mass index = 33 kg/m2) and had obstructive sleep apnoea syndrome, was diagnosed with severe COVID-19 and admitted to the ICU on February 26. He had had a cough and fever since February 21. He presented acute respiratory failure (PaO2 = 74 mmHg; high-flow nasal cannula 40 l/min, 40%) and basal interstitial syndrome on chest X-ray. Remdesivir was started on February 27. Viral RNA levels increased slightly from a Ct value of 32.5 to 28.8 during the first 4 days of infusion, and started to decline on day 5 until undetectability. On March 1, he was referred to the infectious diseases ward, and he was weaned off oxygen on day 13 of illness. His VL was below the RT-qPCR limit of detection on day 14. Remdesivir was discontinued after eight administrations because of ALT elevation (116 IU/l versus 43 IU/l before remdesivir administration) and a maculopapular rash. These symptoms resolved 5 days after remdesivir discontinuation and the patient was discharged on day 20 of illness.
Case 4
A 76-year-old French male, with a history of chronic kidney injury (creatinine 115 μmol/l, normal range 50–70 μmol/l), was admitted on February 22 due to a cough and fever of 24-h duration; he was transferred to our centre on February 26 after a diagnosis of COVID-19. The patient presented an SpO2 of 92% on room air and showed posterior pulmonary ground-glass opacities on chest CT scan. On day 11 of illness, he was transferred to the ICU due to worsening oxygen saturation (PO2 = 69 mmHg; low-flow nasal cannula 3 l/min). The nasopharyngeal VL was already very low at Ct 38.5, but remdesivir was initiated on March 3 and discontinued on March 12, as the SARS-CoV-2 VL was constantly negative; there were no side effects. The patient was weaned off oxygen on day 19 of illness and he was discharged on day 23.
Case 5
A 70-year-old male with a past medical history of chronic obstructive pulmonary disease was diagnosed with COVID-19 on March 1. He had had a cough and fever since February 23 while taking non-steroidal anti-inflammatory drugs for renal lithiasis. He was admitted to the ICU on March 2 with acute respiratory distress syndrome. Remdesivir was started on March 4 (day 11 of illness) and discontinued on March 6 because of acute kidney injury (creatinine level up to 396 μmol/l) needing renal replacement therapy. The VL in nasopharyngeal samples decreased significantly from Ct 26 to undetectability on day 2 of remdesivir infusion. However, the SARS-CoV-2 VL was detectable in bronchoalveolar lavage on March 10. Cefotaxime was initiated because of a Haemophilus influenzae respiratory co-infection. Nevertheless, he developed multiple organ failure and refractory acute respiratory distress syndrome despite prone positioning and adapted mechanical ventilation. Dexamethasone and lopinavir/ritonavir were started on March 12. He died on March 24 (day 31 of illness).
Discussion
Of this case series of five COVID-19 patients requiring ICU treatment for respiratory distress and treated with remdesivir, three (patients 1, 3, and 4) had a favourable outcome despite the initial respiratory severity. They were weaned off oxygen between day 14 and day 19 of illness and were discharged between day 20 and day 26 of illness. Patients 2 and 5 died in the ICU on day 25 and day 31 of illness with multi-organ failure. While on remdesivir treatment, we observed a decrease in nasopharyngeal VL in all but patient 2, for whom the treatment was re-introduced after an early interruption, without any additional decrease in VL in the upper or lower respiratory tract. For patient 5, viral replication was still ongoing in the lower respiratory tract despite a concomitant undetectable VL in the nasopharyngeal area, highlighting the discrepancies between viral replication in the upper and lower respiratory tract among the most severe patients. Plasma samples were only positive for SARS-CoV-2 for patient 2.
As described in previous case reports (Grein et al., 2020, Kujawski et al., 2020), four of the five patients experienced major side effects while on remdesivir treatment: two suffered acute renal injury and two had a maculopapular rash with cytolytic hepatitis. Both kidney failure events could have been related either to remdesivir or to the SARS-CoV-2 infection. None of these patients received immunomodulatory drugs. Grein et al. (2020) described 53 COVID-19 patients treated with remdesivir, among whom 30 were on mechanical ventilation. After a median follow-up of 18 days after remdesivir initiation, a total of 25 (47%) were discharged; seven (13%) died and 10 were still on invasive mechanical ventilation. No virological data were available in that report. A recent randomized controlled study (Wang et al., 2020b) did not show any clinical of benefit for remdesivir treatment, but probably lacked power. Of note, 12% of patients in the remdesivir group discontinued remdesivir due to adverse events (compared with 5% in the placebo group).
In conclusion, the cases of the five patients presented herein highlight some difficulties with remdesivir infusion when administered in most patients with advanced disease. Particular attention should be paid to hepatic and kidney function when administering this treatment.
Author contributions
All authors have read and approved the manuscript. MD, VI, YY, JG, and XL wrote the manuscript and took care of the patients in the infectious and tropical diseases department. LD, DLP, and CR took care of the patients in the infectious and tropical diseases department. BV participated to the virological tests and wrote the manuscript. QLH and NHF participated in the virological tests. LB, JP, and PHW took care of the patients in the medical and infectious intensive care unit. LK assisted in obtaining and dispensing the drug.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval and consent to participate
This report was approved by the French Ethics Committee (NCT04262921).
Consent for publication
The Institutional Review Board of Bichat-Claude Bernard University Hospital approved this report and waived the need for informed consent from individual patients, due to the retrospective chart review design and absence of identifying images or personal/clinical details that could compromise anonymity.
Availability of data and materials
The datasets used and/or analysed in this study are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare that they have no competing interests.
References
- Arabi Y.M., Asiri A.Y., Assiri A.M., Aziz Jokhdar H.A., Alothman A., Balkhy H.H. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(January (1)):8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;(January) 2020.03.22.20040758. [Google Scholar]
- Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(March (3)):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V., Bleicker T., Brunink S., Drosten C. 2020. Diagnostic detection of 2019-nCoV by real-time RT-PCR — protocol and preliminary evaluation as of Jan 17, 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn=a9ef618c_2. [Cited 22 June 2020]. [Google Scholar]
- Mar Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;20(March) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;(April) doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv. 2020;(January) doi: 10.1038/s41591-020-0877-5. 2020.03.09.20032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;(March) doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;(March) doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(February (2)):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;(April) doi: 10.1016/S0140-6736(20)31022-9. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/abstract. [Cited 12 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed in this study are available from the corresponding author upon reasonable request.