Abstract
Relapse remains the worst life-threatening complications after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with acute myeloid leukemia (AML), whose prognosis has been historically dismal. Given the rapid development of genomics and immunotherapies, the interference strategies for AML recurrence have been changing these years. More and more novel targeting agents that have received the U.S. Food and Drug Administration (FDA) approval for de novo AML treatment have been administrated in the salvage or maintenance therapy of post-HSCT relapse. Targeted strategies that regulate the immune microenvironment of and optimize the graft versus leukemia (GVL) effect of immune cells are gradually improved. Such agents not only have been proven to achieve clinical benefits from a single drug, but if combined with classic therapies, can significantly improve the poor prognosis of AML patients who relapse after allo-HSCT. This review will focus on currently available and promising upcoming agents and also discuss the challenges and limitations of targeted therapies in the allogeneic hematopoietic stem cell transplantation community.
KEY WORDS: AML, Targeted therapy, Relapse, Immune microenvironment, Allogeneic hematopoietic stem cell transplantation, Oncogenic effectors, Metabolism, Surface markers
Graphical abstract
For recurrence after allo-HSCT, novel agents not only target oncogenic effectors, key metabolism or surface markers of leukemia cells, but also target the immune microenvironment to improve the graft versus leukemia (GVL) effect and reduce graft versus host disease (GVHD).
1. Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the backbone therapy for patients with intermediate or high-risk acute myeloid leukemia (AML) who are eligible for intensive therapy. Relapse still represents the major cause of treatment failure and up to 50% of AML patients finally relapse after allo-HSCT, about 72%–85% of relapses occur in the first year1, 2, 3. Their prognoses are generally poor, many of which can neither tolerate nor respond to conventional treatments. According to reports, the median overall survival (OS) after hematological relapse is only 4–6 months2,4,5, and 1-year OS rate is about 20%5, 6, 7, 8. Furthermore, even with donor cell therapy can only rescue a minority of patients in the long run. The 2-year OS rates of AML patients who relapsed after allo-HSCT and received palliative therapy, donor lymphocyte infusion (DLI), or second transplantation were 29.7%, 27.6% and 17%–22%, respectively2,5. The dismal success of salvage therapies means that novel strategies are needed to prevent and/or treat relapse after allo-HSCT.
Although a number of factors come into play, including resistance to traditional treatments, relapse indicates that the leukemia cells have managed to escape from the control of donor immune sytsem9. Leukemia cells make themselves “invisible” to donor-derived T cells by losing genomic human leukocyte antigen (HLA) or downregulating major histocompatibility complex (MHC) class II genes10,11. Besides loss of HLA leading to less alloantigen recognition, regulatory T cell (Treg) infiltrating and leukemia-specific T cells display exhaustion markers are the other arm of immune escape. Exhausted CD8+T cells accumulate in the bone marrow of relapsing patients after allo-HSCT, of whom 67.8% expressed one or more immune inhibitory receptors (IRs)12,13. Donor natural killer (NK) cells are the first reconstituted immune cells, both the donor and the host present all killer-cell immunoglobulin-like receptors (KIRs) in the form of donor's14,15. Especially, allografts from KIR2DS1 or KIR B positive donor have stronger anti-leukemia effect16, 17, 18.
Giving the rapid improving of deep sequencing techniques, the genetic driver mutations in AML are better understood and more and more novel targeting agents are synthesized. While these new developments in U.S. Food and Drug Administration (FDA) approval are welcome, more than 7 new targeted agents have received FDA approval for the treatment of AML during last three years19. Not only single agents but also the combination with conventional therapies has obviously improved the outcomes of high-risk AML patients after allo-HSCT. In addition, targeted immunotherapy, such as checkpoint inhibitors, engineering donor lymphocytes and chimeric antigen receptor (CAR) T cells, have been administrated to treat and/or prevent recurrence. This review will not only focus on the directly/indirectly targeted therapies to leukemia cells, but also clarify targeted strategies that interfere with the immune microenvironment and optimize the graft versus leukemia (GVL) effect of immune cells. Giving the rapid evolution of this field, we have selected relevant articles mainly based on the intention of current applicability.
2. Targeting leukemia cells
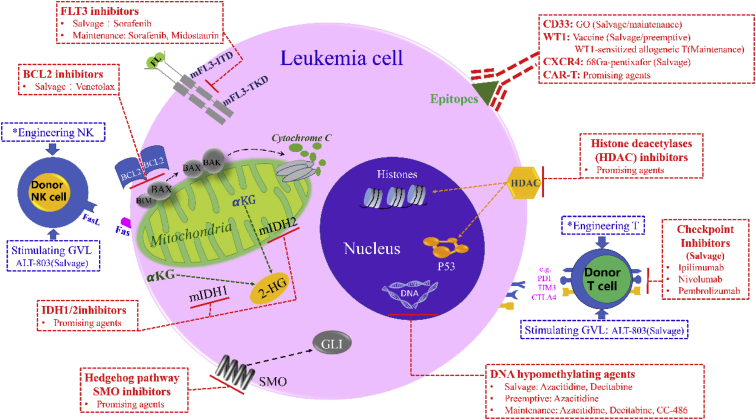
Recently, more and more novel agent winds have filled the sail of targeted therapy boats to leukemia cells, which don't just “direct hit” against all hematopoietic cells20. Targeted therapies aim to leukemia cells can be divided into three groups. Firstly, targeted agents act on oncogenic effectors of recurrent AML-associated mutations. Examples of such agents include fms-related tyrosine kinase 3 (FLT3), B-cell leukemia/lymphoma-2 (BCL-2), isocitrate dehydrogenase 1/2 (IDH1/2) and hedgehog signaling pathway inhibitors. Secondly, novel agents disrupt key metabolism of leukemia cells without directly damaging DNA. Examples include DNA hypomethylating agents and histone deacetylases inhibitors. A final group consists of leukemia epitope-targeting agents. Such immunotherapeutic strategies include antibody conjugate cytotoxic agents and antibody-based cellular therapies. Most agents will be formally introduced in the review.
2.1. Targeting oncogenic effectors of leukemia cells
2.1.1. FLT3 inhibitors
FLT3, a cytokine receptor (CD135) belonging to the receptor tyrosine kinase class III, which takes a pivotal role in myeloid and lymphoid cell proliferation and survival. It generally includes two mutations: FLT3 internal tandem duplications (FLT3-ITDs) and point mutations in the tyrosine kinase activating loop of the kinase domain FLT3-TKD19. Such mutations have been reported in approximately one third of patients with AML and are associated with higher relapse rates21. Sorafenib, midostaurin, quizartinib, gliteritinib and crenolanib are designed to target FLT3 and have been used to interfere with the relapse of FLT3 positive AML after allo-HSCT.
2.1.1.1. First generation FLT3 inhibitors
Sorafenib has been used to treat relapsed FLT3-ITD positive AML following allo-HSCT. In a large registered study, 409 relapsed FLT3-ITD positive patients after allo-HSCT were analyzed. There were five arms in the study. The complete remission (CR) and 1-year OS of DLI arm were 22% and 17%, respectively, which increased to 67% and 47% when used in combination with sorafenib22. The studies from European Society for Bone Marrow Transplantation (EBMT) and China showed similar results that sorafenib combined with DLI obviously improved the OS and leukemia free survival (LFS) of relapsed FLT3-ITD positive patients following allo-HSCT23,24. As a preventive or maintenance medication after allo-HSCT, sorafenib decreased the 3-year incidence of relapse (CIR) of FLT3-ITD positive patients from more than 50%–15% in a series of retrospective studies24, 25, 26, 27, 28, 29, 30. For the safety of sorafenib as a prophylactic agent, a prospective study depicted that the 3-year OS was 76% and the most common 3/4 adverse events were hepatic enzymes (23%) and thrombocytopenia (17%)31. In a randomized phase 3 trial, the other first generation FLT3 inhibitor, midostaurin or placebo, was used for 717 patients from induction therapy to maintenance therapy due to their capability of targeting both TKD and ITD mutations, and 57% of the patients discontinued the trial therapy because of allo-HSCT. Although patients who achieved CR1 and received allo-HSCT in both groups did not reach the median OS, the OS in the midostaurin group was significantly longer than that in the placebo group, at 69.8 and 21.8 months, respectively32. After that, as a prophylactic agent in a phase 2 hypothesis-generating trial, midostaurin controlled the post-HSCT 2-year CIR of FLT3 positive patients to 13.3%33.
2.1.1.2. The next generation FLT3 inhibitors
As the next generation FLT3 inhibitors, quizartinib, gliteritinib and crenolanib were more targeted than the first generation, and thereby off-target-associated toxicities and side effects were decreased. Quizartinib demonstrated acceptable tolerability for de novo or post-HSCT AML patients in the phase 1 dose escalation studies, and the most common grade 3/4 adverse events were neutropenia, thrombocytopenia, and anemia34,35. For patients with relapsed/refractory (R/R) FLT3-ITD positive AML, the rate of quizartinib monotherapy bridging allo-HSCT reached 32%, which was significantly higher than 11% of salvage chemotherapy36, especially in the 60 mg/day group37. For R/R FLT3-mutated patients, another inhibitor, gilteritinib, had an overall response rate (ORR) of 80% in the phase 1 clinical trial and was well tolerated38. In a phase 3 trial, 371 R/R FLT3-mutated patients were randomly assigned in a 2:1 ratio to receive either gliteritinib (at a dose of 120 mg/day) or salvage chemotherapy. Gilteritinib resulted in significantly longer LFS (2.8 vs. 0.7 months) and higher CR percentage (21% vs. 10.5%) than salvage chemotherapy39. As a potent typeI pan-FLT3 inhibitor, crenolanib retains the activity against FLT3-TKD mutation, which is a candidate for patients resistant to the other FLT3 inhibitors. Cumulatively, a high ORR (28%) was achieved with crenolanib monotherapy for such patients40,41. Since the phase 2/3 clinical trials of the next generation FLT3 inhibitors for maintenance therapy after allo-HSCT are being recruited, and none of them use a first-generation inhibitor as a control, so it is impossible to determine whether they are superior to the first-generation agents.
2.1.2. BCL-2 inhibitors
BCL-2 is an anti-apoptotic protein which binds to the BH3 and inhibits the apoptosis of hematologic malignancies. As a BCL-2 inhibitor, venetoclax competitively binds to the BH3 domain of BCL2, releases BH3-only proteins and induces apoptosis42. A phase 2 study demonstrated that venetoclax monotherapy for R/R AML or unfit for intensive chemotherapy patients achieved an ORR of 19% with an acceptable safety profile43. Currently, venetoclax has being investigated in a series of phase 1 studies in combination with low-dose cytarabine and decitabine or azacitidine44. For older de novo AML patients (median 75 years), venetoclax in combination with azacytidine achieved the CR/CRi rate up to 85%, compared with the 51% for conventional therapy (P = 0.0019)45. Such two-drug combination treatments reduced succinate dehydrogenase glutathionylation, impaired the tricarboxylic acid cycle, and depleted ATP in leukemia stem cells46. In addition, only a few patients who relapse after allo-HSCT have achieved CR after combination therapy with venetoclax and low-dose cytarabine or DNA hypomethylating agents47,48. Currently, there are two initiated prospective studies to evaluate the efficacy of venetoclax in combination with azacitidine to improve relapse free survival (RFS) in AML patients when given as maintenance or preemptive therapy following allo-HSCT. For patients presenting WBC above 25 G, uric acid above 7.5 mg/dL, or creatinine above 124 mmol/L, physicians need to know that the initiation of venetoclax-based therapy may elevate the risk of tumor lysis syndrome (TLS)49.
2.1.3. IDH1/2 inhibitors
Somatic mutations within the conserved active site of isocitrate dehydrogenases (IDH) 1 and 2 occur in 6%–10% and 8%–19% of patients with AML, respectively. These mutations cause accumulation of the oncogenic metabolite R-2-hydroxyglutarate (2-HG), 2-HG competitively inhibits α-ketoglutarate-dependent enzymes, leading to DNA and histone hypermethylation and impairing hematopoietic differentiation50,51. Ivosidenib and enasidenib are oral targeted IDH1 and IDH2 inhibitors, respectively52. In a phase 1 study of IDH1-mutated R/R AML, administration ivosidenib at a dose of 500 mg daily achieved durable CR (21.6%, 9.3 months) and ORR (41.6%, 6.5 months) with a low frequency of grade 3 or higher adverse events52. For IDH2 mutation R/R AML patients, enasidenib was well tolerated and produced an ORR of 40 % in a phase 1 trial50,53. Almost 20% patients attained CR, 43.1% red blood cell and 40.2% platelet transfusion-dependent patients achieved transfusion independence51. In addition, isocitrate dehydrogenase differentiation syndrome (IDH-DS) is a potentially lethal and recognizable complication. In a retrospective study of IDH-DS in 281 R/R AML patients, researchers recognized that approximately 12% of the patients were identified as IDH-DS, prompt diagnosis and systemic administration corticosteroids were effective for them54.
2.1.4. Hedgehog signaling pathway inhibitors
The Hedgehog (Hh) signaling pathway should be silenced in adults19, however, overexpression of Hh pathway components is observed in myeloid leukemia cells resistant to chemotherapy, especially in leukemia stem cells55. Aberrant Hh pathway releases transmembrane protein smoothened (SMO), which activates glioma (GLI)-associated proteins56. GLI is translocated into the nucleus and binds the promoter region of target DNA to express specific oncogenes, such as those encoding c-MYC, BCL2, and SNAIL55. Glasdegib is an oral small molecule inhibitor of SMO, which prevents SMO from translocating into the primary cilia56. In a phase 1b study, glasdegib was administered once daily in 28-day cycles in combination with low-dose cytarabine (arm A), decitabine (arm B) or cytarabine/daunorubicin (arm C), and the recommendation dose for glasdegib was 100 mg/day57. The following phase 2 study showed that 46.4% of patients achieved CR, and the median OS was 14.9 months. The most common treatment-related adverse events (≥50% patients) were diarrhea and nausea56. In a randomized phase 2 trial of AML or high-risk myelodysplastic syndrome (MDS) unsuitable for intensive chemotherapy, patients were randomized (2:1) to glasdegib (100 mg/day) and low dose cytarabine (LDAC) or LDAC (20 mg, Bid) only arms. The CR rate and median OS of the two arms were 17% vs. 2.3% (P < 0.05) and 8.8 months versus 4.9 months (P = 0.0004), respectively58. In a randomized phase 3 clinical trial, the efficacy of glasdegib maintenance vs. clinical observation after allo-HSCT will be evaluated until December 2026.
2.2. Targeting key metabolism of leukemia cells
2.2.1. DNA hypomethylating agents (HMAs)
HMA not only leads to hypomethylation of the promoter of silenced tumor suppressor genes, but also has the ability to enhance antigenicity and regulate immune checkpoints, and can induce CD8+T cells to respond to tumor antigen after transplantation, thereby increasing GVL response without increasing the risk of graft versus host disease (GVHD)59, 60, 61. Azacytidine (AZA) and decitabine (DEC) are not really novel agents, but as the representative of HMA, clinicians have been very enthusiastic about their application in recurrence patients after allo-HSCT. In a series of retrospective studies, salvage therapies with AZA or DEC for morphological recurrence rescued very limited patients. Even with DLI booster immunotherapy, only 3%–27% of patients achieved CR, and the 2-year OS was between 11% and 29%60, 61, 62, 63, 64, 65. In order to enhance the antitumor efficacy of HMA, 29 relapsed patients post-HSCT were treated with sequential AZA (75 mg/m2, 7 days) followed by escalating doses of LEN from Day 10–30 in the VIOLA trial. Almost 47% of patients achieved a major clinical response and 40% achieved a CR/CRi after LEN/AZA therapy66. Because HMA was safe for long-term use, AZA monotherapy was given as an MRD-guided preventive treatment in a phase 2 trial to prevent morphological recurrence. A total of 198 patients were screened in the study, 60 of whom developed MRD (CD34+ cell donor chimerism<80%, fusion or mutant gene>1%) during the 24-month screening period, and 53 of them were treated. Six months after initiating AZA treatment, 58% of patients were free of relapse and alive, while 75% of patients survived more than 12 months67. For initiating CD8+ T cell response to tumor antigens, AZA maintenance therapy in high-risk AML patients after allo-HSCT was well tolerated and has the capacity to reduce the relapse risk68,69. In a series of preventive treatments, low dose AZA in combination with DLI reduced the CIR of high-risk patients without increasing the cumulative incidence of GVHD70. CC-486 is an oral AZA, which can be used more conveniently. In a prospective phase 1/2 dose-finding study, CC-486 maintenance therapy for high-risk AML patients post-HSCT was generally well tolerated with low relapse rates (21%) and low GVHD (10%)71. Although the double-blind, phase 3, randomized study of CC-486 has just been initiated on June 14, 2019, for patients who need long-term home medication after allo-HSCT, their compliance to it will be better than traditional AZA and more likely to benefit from it.
2.2.2. Histone deacetylases inhibitors (HDACi)
Myeloid oncoproteins aberrantly recruit histone deacetylases (HDAC), causing chromatin remodeling and inhibiting the expression of tumor suppressor genes such as TP5372. Panobinostat (PAN) is a potent oral HDACi, which can modulate the acetylation of histone proteins and protein chaperones in malignant cells73. In the phase 1/2 PANOBEST trial, PAN was prophylactically administrated after allo-HSCT for AML or MDS. At 2 years after the first PAN dose, the cumulative incidence of relapse, non-relapse mortality and OS across all dose levels was 20%, 5% and 81%, respectively. About 52% of patients experienced reversible PAN-related G3/4 adverse events and 29% of patients experienced GVHD74. PAN (30 mg)+AZA (75 mg/m2) or AZA monotherapy was administered to high-risk MDS, CMML or AML with low burden (<30%) blasts in a phase 1b/2b trial. Although patients in PAN + AZA arm achieved better CR/CRi (27.5% vs. 14.3%), which resulted in more grade 3/4 adverse events (97.4% vs. 81.0%) and treatment-related deaths (13.2% vs. 4.8%)73. Lower dose PAN (20 mg) in combination with idarubicin and cytarabine (3 + 7) as inducing therapy for younger patients (median age, 55.5) with high-risk AML demonstrated tolerable toxicity and improved CR/CRi (60.9%)75. For older AML patients (median age, 69), the CR/CRi of PAN with “3 + 7” strategy was 32%, which was significantly associated with an increase in histone acetylation in peripheral blood mononuclear cells (PBMCs) after treatment76. In March 2020, a randomized multicenter phase 3 study was initiated to assess the efficacy of PAN 20 mg oral three times weekly every second week vs. standard of care following allo-HSCT. Since February 2019, another HDACi (vorinastat) combined with low-dose AZA as post-HSCT maintenance treatment has been studied in a phase 1 dose-escalation clinical trial, and the results will be announced after December 2021.
2.3. Targeting leukemic surface markers
2.3.1. Targeting CD33/CD44/CD123
2.3.1.1. Monoclonal antibodies
Some epitopes such as CD33, CD44 and CD123 are always expressed on leukemic blasts, but are not presented on normal hematopoietic stem cells. Especially, CD33 is expressed on leukemia cells in 90% of AML patients77,78. Clinical trials of humanized monoclonal antibodies against CD33 (SGN-33), CD44 (RG7356), and CD123 (CSL360) have generally shown good tolerability and limited antileukemia activity, but only when the burden of leukemia is small and in case of long-term infusion77,79, 80, 81. To improve the efficacy of antibodies against CD33 positive blasts, the researchers constructed a series of TandAbs composed of anti-CD33 and anti-CD3, which can redirect cytotoxic immune cells toward CD33 positive blasts and induce effective dose-dependent cytolysis in vitro and in xenograft models82.
2.3.1.2. Antibody conjugate cytotoxic agents
Antibody conjugate cytotoxic agents provide a method for delivering cytotoxic agents to leukemia cells, thereby increasing dose intensity while reducing toxicity83. Gemtuzumab ozogamicin (GO) consists of a humanized anti-CD33 monoclonal antibody and the DNA intercalator calicheamicin, which received accelerated FDA approval in 2000 as a new AML monotherapy, but withdrew from the market in 2010 due to safety considerations, and gained full FDA and EMA approval for CD33 positive AML first-line and relapse treatment in 2017 and 2018, respectively19. A meta-analysis of 5 phase-3 trials comprising 3325 AML patients reminded that GO significantly reduced the relapse rates and improved OS in the cytogenetic favorable and intermediate risk groups without increasing toxicity84. A series of studies found that GO monotherapy or in combination with conventional therapies can improve the recurrence rates or OS of pediatric patients (1 month–30 years), younger (18–65 years) or older patients (62–88 years) in de novo or R/R AML85, 86, 87, 88. There is a highly variable in the proportion of CD33-positive cells and the expression of CD33 per cell in patients with CD33 positive AML83,89. Patients with higher CD33 expression, NPM1 mutation, or CD33 single nucleotide polymorphism (rs12459419 = CT/TT) may benefit more from GO, especially at a lower dose (3 mg/m2)90, 91, 92, 93. In a small sample study, cytarabine and GO (9 mg/m2) were used as post-HSCT salvage therapy for AML patients, but only 25% of patients survived more than one year and all relapsed94. In a study of CD33-positive leukemia children receiving reduced-intensity conditioning (RIC) transplantation and using CD33 as maintenance therapy, patients were given two doses of GO (8 weeks apart) in a dose-escalation design (4.5, 6, 7.5, and 9 mg/m2) 6 days after allo-HSCT. The 1- and 5-year OS probabilities were 78% and 61%, respectively, and no toxicity that might be directly or directly related to GO was observed95. The data of phase 1 clinical trial for the preventive use of GO by patients receiving RIC allo-HSCT will be released as early as December 2020.
Vadastuximab talirine (SGN-CD33A, 33 A) is an antibody−drug conjugate consisting of pyrrolobenzodiazepine dimers and an anti-CD33 monoclonal antibody. In a phase 1 clinical trial, 33 A monotherapy was infused to CD33-positive AML patients at a dose of 40 mg/kg, and 28% of them achieved CR/CRi, of which 14% were MRD-negative96. In an expansion arm of this study, 33 A subsequently in combination with HMAs were provided to de novo CD33-positive older AML patients (median 75 years), and 70% of them achieved CR/CRi, of whom 51% were MRD-negative. In addition, the 30- and 60-day mortality rates were 2% and 8%, respectively97. In the terminated phase 1/2 trial (Table 1), 33 A was used as maintenance therapy after allo-HSCT. Unpublished data showed that the most common adverse events were neutropenia and thrombocytopenia.
Table 1.
Summary of clinical trials for novel agents targeting leukemia cells or immune microenvironment following allo-HSCT.
| Target | Agent | Intervention strategy | Therapy regimen | Identifier (phase) | Status | Complete date | With results |
|---|---|---|---|---|---|---|---|
| Oncogenic effectors | |||||||
| FLT3 | Sorafenib | Salvage | +/−DLI | NCT02867891 (no) | Completed | 12/2016 | Yes |
| Maintenance | Mono | NCT01398501 (1) | Completed | 8/2016 | Yes | ||
| Maintenance | Mono | NCT02474290 (2/3) | Completed | 8/2019 | Yes | ||
| Maintenance | Mono | NCT01578109 (no) | Active, not recruiting | 12/2025 | Yes | ||
| Maintenance | Mono | NCT03247088 (1/2) | Recruiting | 7/2022 | No | ||
| Midostaurin | Preemptive | Mono | NCT03951961 (2) | Not yet Recruiting | 12/2023 | No | |
| Maintenance | Mono | NCT01477606 (2) | Active, not recruiting | 6/2020 | Yes | ||
| Maintenance | Mono | NCT01883362 (2) | Completed | 4/2018 | No | ||
| Quizartinib | Salvage/maintenance | Mono | NCT02039726 (3) | Active, not recruiting | 12/2020 | Yes | |
| Maintenance/preemptive | Mono | NCT01468467 (1) | completed | 3/2015 | Yes | ||
| Gliteritinib | Maintenance | Mono | NCT02997202 (3) | Recruiting | 4/2025 | No | |
| Crenolanib | Maintenance | Mono | NCT02400255 (2) | Recruiting | 6/2021 | No | |
| BCL-2 | Venetolax | Maintenance/preemptive | +AZA | NCT04161885 (3) | Recruiting | 8/2024 | No |
| Maintenance/preemptive | +AZA | NCT04128501 (2) | Not yet Recruiting | 10/2022 | No | ||
| IDH1 | Ivosidenib | Maintenance | Mono | NCT03564821 (1) | Recruiting | 7/2024 | No |
| IDH2 | Enasidenib | Maintenance | Mono | NCT03515512 (1) | Recruiting | 5/2024 | No |
| Maintenance | Mono | NCT03728335 (1) | Recruiting | 12/2020 | No | ||
| SMO | Glasdegib | Maintenance | Mono | NCT04168502 (3) | Not yet Recruiting | 12/2026 | No |
| Key metabolism | |||||||
| DNA methylation | Azacitidine | Salvage | Mono | NCT01083706 (2) | Completed | 12/2013 | Yes |
| Salvage | Mono | NCT02017457 (2) | Completed | 11/2019 | Yes | ||
| Salvage | Mono | NCT00422890 (3) | Completed | 12/2009 | Yes | ||
| Salvage | +DLI | NCT00795548 (2) | Completed | 8/2011 | Yes | ||
| Salvage | +IFN | NCT04078399 (no) | Recruiting | 5/2020 | No | ||
| Salvage | +Chemo + DLI | NCT01390311 (1) | Completed | 4/2015 | Yes | ||
| Salvage | +LEN | ISCRCTN98163167 (1) | Completed | 12/2018 | Yes | ||
| Salvage | +LEN + DLI | NCT02472691 (2) | Active, not recruiting | 9/2020 | No | ||
| Salvage | +Chemo + DLI | NCT01369368 (1/2) | Recruiting | 10/2020 | No | ||
| Preemptive | Mono | NCT01462578 (2) | Active, not recruiting | 8/2020 | Yes | ||
| Preemptive | Mono | NCT03850418 (2) | Recruiting | 2/2024 | No | ||
| Preemptive | +DLI | NCT01541280 (2) | Completed | 7/2015 | Yes | ||
| Maintenance/preemptive | +/-DLI | NCT02458235 (2) | Active, not recruiting | 4/2020 | No | ||
| Maintenance | Mono | NCT01995578 (2) | Recruiting | 11/2021 | No | ||
| Maintenance | Mono | NCT00350818 (1) | completed | 8/2010 | No | ||
| Maintenance | Mono | NCT00887068 (3) | completed | 8/2018 | No | ||
| Maintenance | +Nivolumab | NCT04128020 (1) | Recruiting | 10/2022 | No | ||
| Maintenance | +APR-246 | NCT03931291 (2) | Recruiting | 9/2021 | No | ||
| Maintenance | +Valproic Acid | NCT02124174 (2) | Recruiting | 1/2021 | No | ||
| Maintenance | Mono | NCT01168219 (2) | Active, not recruiting | 11/2015 | Yes | ||
| Decitabine | Salvage | +DLI | NCT01758367 (1/2) | Unknown | 6/2018 | Yes | |
| Salvage | +2nd HSCT | NCT00002832 (1/2) | Completed | 3/2002 | No | ||
| Salvage | +Ruxolitinib + DLI | NCT04055844 (2) | Recruiting | 1/2025 | No | ||
| Preemptive | Mono | NCT03663751 (2) | Recruiting | 3/2021 | No | ||
| Preemptive | +DLI | NCT03662087 (2/3) | Recruiting | 12/2022 | No | ||
| Maintenance | Mono | NCT01809392 (2/3) | Unknown | 12/2015 | No | ||
| Maintenance | Mono | NCT01277484 (1) | Unknown | 12/2015 | Yes | ||
| Maintenance | Mono | NCT00986804 (1) | Completed | 2/2016 | Yes | ||
| Maintenance | +DLI | NCT03771222 (2) | Not yet Recruiting | 12/2021 | No | ||
| Guadecitabine | Salvage/preemptive | +DLI | NCT02684162 (2) | Recruiting | 6/2021 | No | |
| Maintenance | Mono | NCT03454984 (2) | Not yet Recruiting | 3/2022 | No | ||
| CC-486 | Maintenance | Mono | NCT01835587 (1/2) | Completed | 5/2017 | Yes | |
| Maintenance | Mono | NCT04173533 (3) | Recruiting | 6/2024 | No | ||
| Histone deacetylases | Panobinostat | Maintenance | Mono | NCT01451268 (1/2) | Unknown | 4/2018 | Yes |
| Maintenance | Mono | NCT04326764 (3) | Recruiting | 10/2023 | No | ||
| Vorinostat | Maintenance | +AZA | NCT03843528 (1) | Recruiting | 12/2021 | No | |
| Epitopes | |||||||
| CD33 | BI 836858 | Salvage | +F16IL2 | NCT03207191 (1) | Unknown | 12/2019 | No |
| GO | Salvage | Mono | NCT00044733 (2) | Completed | 9/2004 | Yes | |
| Maintenance | Mono | NCT01020539 (1) | Active, not recruiting | 12/2020 | No | ||
| Maintenance | Mono | NCT02117297 (2) | Recruiting | 12/2022 | No | ||
| 33 A | Maintenance | Mono | NCT02614560 (1/2) | Terminated | 9/2017 | No | |
| CD38 | Daratumumab | Salvage | +DLI | NCT03537599 (1/2) | Recruiting | 9/2021 | No |
| WT1 | DC vaccine | Salvage/preemptive | +DLI | NCT00923910 (1/2) | Completed | 11/2016 | Yes |
| Preemptive | +DEC | NCT01483274 (1) | Completed | 6/2015 | No | ||
| WT1-sensitized T | Maintenance/preemptive/salvage | +/−(FLU + CTX) | NCT01640301 (1/2) | Active, not recruiting | 10/2030 | Yes | |
| Salvage/preemptive | Mono | NCT00620633 (1) | Active, not recruiting | 2/2021 | No | ||
| CTL | Salvage/preemptive | Mono | NCT00052520 (1/2) | Completed | 6/2013 | No | |
| Maintenance | Mono | NCT02895412 (1) | Recruiting | 12/2020 | No | ||
| CD123 | Anti-CD123-CART | Salvage | Mono | NCT03114670 (1) | Recruiting | 3/2021 | No |
| Immune microenvironment | |||||||
| CTLA-4 | Ipilimumab | Salvage | +DLI | NCT00060372 (1) | Completed | 4/2008 | Yes |
| Salvage | Or Nivolumab | NCT01822509 (1) | Active, not recruiting | 12/2018 | Yes | ||
| Salvage | +LEN | NCT01919619 (2) | Recruiting | 6/2021 | No | ||
| Maintenance | +/-Nivolumab | NCT02846376 (1) | Recruiting | 7/2023 | No | ||
| PD-1 | Nivolumab | Salvage | Mono | NCT03146468 (2) | Recruiting | 6/2020 | No |
| Salvage | +/-Ipilimumab | NCT03600155 (1) | Recruiting | 6/2022 | No | ||
| Salvage | +Tocilizumab | NCT03588936 (1) | Recruiting | 8/2022 | No | ||
| Maintenance | Mono | NCT02985554 (1) | Recruiting | 3/2022 | No | ||
| Maintenance | Mono | NCT04361058 (1) | Recruiting | 4/2025 | No | ||
| Pembrolizumab | Salvage | Mono | NCT02981914 (1) | Recruiting | 2/2029 | No | |
| Salvage | Mono | NCT03286114 (1) | Recruiting | 10/2021 | No | ||
| Engineering donor T | CD25hi Treg depleted | Salvage | Mono | NCT00675831 (1) | Completed | 1/2013 | Yes |
| Salvage | +Ipilimumab | NCT03912064 (1) | Recruiting | 5/2024 | No | ||
| CD8+CD44hi | Salvage | Mono | NCT01523223 (1) | Completed | 10/2016 | Yes | |
| CD45RA-delepted | Maintenance | Mono | NCT03849651 (2) | Recruiting | 7/2025 | No | |
| Engineering donor NK | Purified NK | Salvage | +FLU + Ara-C or DEC | NCT04220684 (1) | Not yet Recruiting | 12/2021 | No |
| Salvage | +CTX + FLU | NCT00526292 (2) | Completed | 7/2015 | Yes | ||
| Maintenance | Mono | NCT00569283 (1) | Completed | 12/2008 | Yes | ||
| Maintenance | Mono | NCT01795378 (1/2) | Completed | 5/2015 | Yes | ||
| Maintenance | Mono | NCT00823524 (1/2) | Completed | 2/2013 | Yes | ||
| Maintenance | Mono | NCT03300492 (1/2) | Recruiting | 1/2023 | No | ||
| Maintenance | Mono | NCT04166929 (2) | Recruiting | 1/2022 | No | ||
| Maintenance | Mono | NCT02727803 (2) | Recruiting | 5/2021 | No | ||
| Maintenance | Mono | NCT01904136 (1/2) | Recruiting | 4/2021 | No | ||
| CD4- iNKT | Maintenance | Mono | NCT03605953 (no) | Not yet Recruiting | 4/2021 | No | |
| CIML NK | Salvage | Chemo + DLI+ | NCT03068819 (1) | Recruiting | 11/2024 | No | |
| Maintenance | +ALT-803 | NCT02782546 (2) | Recruiting | 2/2022 | No | ||
| DC | DC/AML fusion cells | Maintenance | +DEC | NCT03679650 (1) | Recruiting | 8/2024 | No |
| MiHA-loaded PD-L-silenced DC | Maintenance/preemptive | Mono | NCT02528682 (1/2) | Recruiting | 12/2020 | No | |
| CIK | IL-15 activated CIK | Preemptive | Mono | NCT02752243 (1/2) | Recruiting | 3/2022 | No |
| Maintenance | Mono | NCT03669172 (1/2) | Recruiting | 11/2021 | No | ||
| IL-15 | ALT-803 | Salvage | Mono | NCT01885897 (1/2) | Active, not recruiting | 6/2020 | Yes |
| N-803 | Maintenance | Mono | NCT02989844 (2) | Suspended (COVID-19) | 1/2022 | No | |
Complete date, actual or estimated study completion date; Salvage, salvage therapy for patients with hematological relapse; Maintenance, preventive therapy for high-risk patients without any sigh of underlying disease; Preemptive, preemptive therapy for patients with minimal residual disease; Mono, monotherapy; +/−, the clinical trial has two arms, monotherapy or in combination with other regimens; DLI, donor lymphocyte infusion; AZA, azacytidine; IFN, interferon; Chemo, chemotherapy; LEN, lenalidomide; APR-246, agent targeted TP53; 2nd HSCT, the second allo-HSCT; CC-486, oral azacytidine; BI 836858, a human anti-CD33 antibody; F16IL2, F16 antibody fused to human IL-2; GO, gemtuzumab ozogamicin; 33 A, vadastuximab talirine; DEC, decitabine; WT1-sensitized T, WT1-sensitized allogeneic T-lymphocytes; CTL, donor CD8+cytotoxic T lymphocyte clones specific for WT1; Tocilizumab, interleukin-6 receptor antagonist; FLU, fludarabine; Ara-C, cytarabine; CTX, Cyclophosphamide; iNKT, invariant NKT cells; CIML NK, cytokine-induced memory-like (CIML) natural killer cell; DC, dendritic cells; CIK, cytokine induced killer cells; ALT-803 or N-803, interleukin-15 (IL-15) super agonist complex; No, results unavailable.
2.3.2. Targeting Wilms’ tumor antigen 1 (WT1)
WT1 is a non-polymorphic intracellular protein that promotes proliferation and carcinogenesis in AML, and is overexpressed 10–1000 times in leukemia cells compared to normal CD34 positive cells98,99. In a phase 1 pilot study, WT1-directed peptide vaccination was injected subcutaneously to 16 heavily pretreated AML and MDS patients, which exhibited a protective immune response (IR) and was well tolerated98. In a phase 2 study, injection of the WT1 peptide vaccine stimulated a specific IR in patients with AML and was associated with the survival in excess of 5 years100. After dendritic cells (DCs) were electroporated with WT1 mRNA and injected as a vaccine into AML patients with CR1, they induced the response of WT1-specific CD8+ T, reduced the relapse rate by 25%, and were well tolerated101. In a small pilot study, HLA-A2 positive recipients were vaccinated with a WT1 peptide-loaded donor-derived DC vaccine every two weeks and given a DLI every four weeks to increase specific GVL effect, which was well tolerated and no grade 3 or higher adverse events directly related to the vaccine were found102. Because WT1-specific (HLA-A∗ 24: 02) TCR-T cells can survive in vivo and retain a specific immune response to WT1103, Chapuis et al.99. isolated a high-affinity WT1-specific TCR (TCRC4) and inserted it into EBV-specific donor CD8+ T cells to make the TTCR-C4 cells. Twelve high-risk patients after allo-HSCT received at least one preventive infusion of TCRC4 (total 21 infusions). Compared with 88 patients in a concurrent comparative group, the median 44-month RFS was 100% vs. 54% without increasing the risk of chronic GVHD. However, the data as salvage or preemptive treatment have not yet been released.
2.3.3. Targeting chemokine (C-X-C motif) receptor 4 (CXCR4)
CXCR4 is a key player of AML blasts, which binds with CXCL12 for retention of leukemia cells in the protective bone marrow (BM) microenvironment104,105. The CXCR4 antagonist BL-8040 induced the robust mobilization of AML blasts from the BM and induced the apoptosis of AML cells by the upregulation of miR-15a/miR-16-1 resulting in downregulation of BCL-2, MCL-1 and cyclin-D1. Synergized treatment with a BCL-2 inhibitor or FLT3 inhibitor prolonged the survival of AML-bearing mice and reduced their MRD104. In a preclinical study, a humanized CXCR4 immunoglobulin G1 antibody-PF-06747143 bind to CXCR4 and inhibited CXCL12-mediated signaling pathway. PF-06747143 monotherapy or synergy with standard drugs has shown strong antitumor effects in a chemo-resistant AML patient-derived xenograft model106. CX-01 is a low-anticoagulant heparin, which can disrupt CXCL12-mediated cell sequestration in the bone marrow. In a pilot study, 12 naive AML patients were treated by CX-01 in combination with “3 + 7” inducing chemotherapy. Eleven patients (92%) achieved morphologic CR after one cycle of induction, and the median disease-free survival was 14.8 months. No CX-01-associated serious adverse events were observed107. Pentixafor is an effective CXCR4 antagonist. Three patients with AML relapse after the first allo-HSCT were given targeted endoradiotherapy consisting of 68 Ga-pentixafor, and all of them achieved leukemia clearance and successfully bridged to the second allo-HSCT105. Leukemia stem cells always employ CXCL12/CXCR4 to home toward the protective marrow niches106. In a phase 1 study, one dose plerixafor was injected to 12 patients before the first dose of fludarabine and busulfan to make sure more leukemic stem cells deletion before allo-HSCT. The engraftment rate was 100%, the median OS was 67 months, and only 2 patients (17%) relapsed108.
2.3.4. CAR T cell therapy
CAR T cell therapy has gained 90% of response rates in CD19+ ALL and CLL, which combines a monoclonal antibody of tumor epitope to intracellular T-cell receptor signaling domain by taking advantage of a single-chain variable fragment (scFv). Because of the greater heterogeneity of AML, one of the major challenges in translating the amazing clinical success of CAR T cells in ALL into AML is to find suitable leukemia cell surface markers109. Over the past five years, studies have been changing from using traditional AML antigens (such as CD33, CD123, CD44, TIM-3 or CD13) to using normal hematopoietic stem cells that do not express, while leukemia cells or leukemia stem cells overexpress antigens (e.g., folate receptor β, CLL-1, NKG2D, CD7 or FLT3) to reduce the off-target toxicities109, 110, 111, 112, 113, 114, 115. The efficacy of CAR T cells is often related to its durability in vivo. Zheng et al.116 improved the persistence of CAR T cells in vivo by combining the PI3K inhibitor LY294002. AML is characterized by the presence of heterogeneous blast cells, and almost no antigen is present on every leukemia cell. Researchers tried to use dual targeting CAR T cells (such as anit-CD33/CD123 or anti-CD13/TIM3) for targeting leukemia cells to reduce antigen escape-associated relapse117,118. In addition to selecting tumor-specific targets, directly reducing tumor-associated antigens expression in normal hematopoietic stem cells can indirectly increase the specificity of tumor-associated antigens. For example, CD33 is expressed on leukemia cells in 90% of AML patients. However, almost all normal myeloid cells also express CD33119. The researchers generated CD33 knockout (KO) hematopoietic stem and progenitor cells (HSPC) and demonstrated that they can be implanted and differentiated normally in immunodeficient mice and rhesus monkeys119,120. CD33-ablated HSPC were impervious to CD33-targeted immunotherapy (CAR T or GO), allowing for efficient elimination of CD33 positive blasts without myelotoxicity, which provided new ideas for the application of CD33-targeted immunotherapy in combination with auto/allo HSCT.
3. Targeting immune microenvironment
With the recognition of immune-escaping driving to two-thirds of relapse post-HSCT, it seems valuable to rapidly translate it into personalized medication. For example, second transplantation from different donor for patients with genomic HLA loss1,121,122, CPIs for patients with upregulation of IRs and inducing moderate chronic GVHD for those with downregulation of HLA class II genes10, 11, 12. However, in clinical practice, this translation will be subject to many restrictions, for example, alternative donor unavailable and relapse with active GVHD, etc. DLI is still an attractive treatment option for patients with high risks of recurrence or recurrence. Unfortunately, approximately 31.6%–66% of recipients obtained II−IV acute GVHD after DLI123, 124, 125, 126, 50% obtained chronic GVHD, and 26% of patients eventually died of GVHD related complications127,128. Using engineered donor T cells or NK cells to improve GVL effect and reduce GVHD is a promising strategy that is currently being extensively studied129. In addition, this review will describe strategies for activating endogenous IL-15 expression in the immune microenvironment to improve the GVL effect of immune cells and avoid systemic toxicity130.
3.1. Immune checkpoint inhibitors (CPIs)
CTLA-4 and PD-1 are the well-known immune checkpoints for tumor immune escape131. CTLA-4 and PD-1 interact with ligands B7-1/B7-2 and PD-L1/PD-L2, respectively, and can inhibit the complete activation and proliferation of T cells132,133. Recently, monoclonal antibodies directed against immune checkpoints have benefited some patients who have relapsed after allo-HSCT. In the dose-escalation study, human anti-CTLA4 monoclonal-ipilimumab was infused in 29 patients (2 AML) who had relapsed more than 90 days after allo-HSCT or DLI. Ipilimumab did not result in clinically significant GVHD, and a response was observed in three lymphoma patients134. In the following phase 1/1b study, of the 22 patients receiving the 10 mg/kg dose, 23% had a complete response, including 4 patients with extramedullary AML and 1 patient with secondary AML. Because of GVHD, 4 patients had to discontinue further use of ipilimumab133. In a small sample study, the anti-PD1 antibody−nivolumab was given to 3 AML patients who had relapsed after allo-HSCT and failed standard therapies. One patient achieved an ongoing CR, one patient experienced disease stabilization and the side effects were tolerable135. In a review of 28 papers comprising 176 patients using CPIs (ipilimumab or nivolumab) after allo-HSCT, the ORR was 54% (CR 33%, PR 21%), 23% of patients developed GVHD and 28% of mortality was GVHD related132. However, Christopher et al.10 reported that the expression of PD1 on T cells was undetectable in 15 AML patients who had relapsed after allo-HSCT. Therefore, it will be valuable to determine the prevalence of immune checkpoints expression to predict whether these patients will respond to CPIs.
3.2. Engineering DLI
DLI is one of the standard treatments for recurrence after allo-HSCT. It can play a GVL effect by identifying minor histocompatibility antigens and leukemia antigens. However, the long-term efficacy of DLI in AML patients who had relapsed after allo-HSCT was only 15%–29%, and most of them were accompanied by significant GVHD136. More and more studies have been using engineering DLI to improve GVL effect and reduce GVHD. In a phase 1 study, CD25+ regulatory T cells were depleted from donor lymphocytes to avoid their inhibition of GVL effect. Compared to unmodified DLI, Treg depletion was associated with better response rate (62.5% vs. 28.6%) and improved event-free survival (27% vs. 0%) without excessive GVHD136. CD8+CD44hi memory T cells may have a more specific GVL effect, which were isolated and purified and given to fifteen patients in a phase 1 study. Ten patients achieved response (7 CR, 1 PR, 2 SD) for at least 3 months after infusion, and the incidence of GVHD was low137. CD200 is usually high expressed on leukemia cells, which binds to CD200 receptor on T-cell and negatively regulates T-cell function. Scientists used the costimulatory receptor-CD28 to replace the cytoplasmic tail of CD200R immunomodulatory fusion protein (IFP), and transduced the CD200R-CD28 IFP to CD8 T cells. It can eradicate blasts more efficiently than wild-type cells, and does not require interleukin 2 to maintain in vivo activity138. Donor NK cells treatment is the other arm to control blasts escaping. In a phase 2 study, haploidentical NK cells were successfully isolated and infused to AML patients who had relapsed following allo-HSCT. Patients with higher expression of KIR2DL2/2DL3/2DS2 at 14 days after haploidentical NK cell infusion achieved better survival139. Especially, allografts from donors who were positive for KIR2DS1 had more powerful anti-leukemia effect than negative. Donor KIR3DS1 was associated with reduced mortality16,17. In a phase 4 study, 84 AML patients with CR1 received histamine dihydrochloride and low-dose IL-2 for 18 months or until relapse/death. Patients carrying HLA-21 M harbored better educated NKG2A + cells and displayed superior capacity to degranulate lytic granules against KIR ligand matched blasts140. A series of clinical trials using engineered NK cells, such as invariant NKT cells or cytokine-induced memory-like (CIML) NK cells, will provide more and more options for the preventive and salvage therapy of recurrence after allo-HSCT.
3.3. Stimulating antitumor immunity (IL-15)
IL-15 is a λc-chain cytokine with unique properties to stimulate antitumor immunity, including stimulation of NK cells and CD8+ memory T cells141. In a mouse model of relapse after allo-HSCT, systemic IL-15 infusion often increases GVL activity with severe GVHD22. Therefore, strategies are focused on activating endogenous IL-15 expression to avoid the toxicity from a systemic administration. In a large registered study, the FLT3 inhibitor-sorafenib was used to treat relapsed FLT3-ITD positive AML patients after allo-HSCT, which can indirectly activate the IL-15 transcription and induce the leukemia cells to produce IL-15. Thus, a strong GVL effect was promoted without inducing obvious GVHD22. Compared to the cytotoxic chemotherapy arm, sorafenib obviously improved the CR (18.5% vs. 32.5%) and 1-year OS (10.5% vs. 22%) of patients. AML blasts always lack expression of the costimulatory molecule CD80. In a preclinical study, 32Dp210 murine AML cells were engineered to express heterodimeric IL-15/IL-15Ra together with CD80 and tested as irradiated cell vaccines, which markedly increased IL-15 stability and secretion. Compared to the 100% relapse and death of control group, the OS of vaccination group was 50%141. In a multicenter phase 1 trial, IL-15 superagonist complex ALT-803 was administered to 33 patients who relapsed >60 days after allo-HSCT. ALT-803 was well tolerated and stimulated the activation, proliferation and expansion T cells without increasing Treg cells. Responses were observed in 19% of evaluable patients, including 1 patient who achieved a CR for 7 months142.
4. Future research questions and challenges
Once recurrence after allo-HSCT happens, patients and physicians have to face the problem of making a choice to change the dismal prognosis. Leukemia cells always acquire new oncogenic mutations, and patients may not tolerate or become resistant to conventional cytotoxicity chemotherapy11,143. Unavailable donor, HLA loss or active GVHD are always greatly limiting the application and the efficacy of traditional DLI and second transplantation10,11,121,144. Compared with traditional treatments after allo-HSCT, the novel agents have a good response, well tolerance and low toxicity, which are summarized in Fig. 1. However, scientists and clinicians need to discover new approaches to prevent and treat recurrence after allo-HSCT according to the following rules. First, we must discard the notion that donor cell booster therapy is the only definitive therapy and pay more attention to the development of targeted drugs for AML. For example, we should focus on the epigenetic therapeutic targets currently being evaluated in AML: the MLL rearrangements145,146, the lysine demethylase LSD1147, the protein methyltransferases EZH2, DOT1L148, and PRMT5149,150, and the BET bromodomain proteins146,151, 152, 153, 154, 155. Second, if safety and effectiveness are shown, it is best to use these drugs in advance in a preventive or preemptive manner, rather than just “watch and wait” for the miraculous GVL effect until the disease recurs. For example, the 3-year OS of sorafenib for post-transplant prophylaxis is 76%–84.6%, compared to 50.9% for controls22,27,156. Unfortunately, clinical trials using novel agents as maintenance treatment after allo-HSCT are scarce, and many uses are based on the off-protocol access. The clinical trials using novel agents are summarized in Table 1. Third, a suitable synergistic strategy can achieve better therapeutic effects without generating additional toxicity, such as coupling FLT3 inhibitors with DLI or combining HMA with venetoclax or LEN. Finally, personalized treatment plans should be systematically formulated according to the epigenetics and immune response of AML patients, from induction therapy to maintenance treatment after allo-HSCT. However, because of the numerous and complex complications that may be encountered, clinical studies involving new drugs for de novo or R/R AML patients often exclude patients receiving allo-HSCT.
Figure 1.
Summary of novel agents that have been used to prevent or treat relapse after allo-HSCT. Salvage; salvage therapy for patients with hematological relapse; Maintenance, preventive therapy for high-risk patients without any sigh of underlying disease; Preemptive, preemptive therapy for patients with minimal residual disease; Promising agents, the agents in clinical trial; ∗, engineering NK or T cells are introduced in Table 1.
5. Conclusions
At present, more and more AML patients who have relapsed after allo-HSCT benefit from novel targeted agents. Clinicians must abandon the notion that anti-tumor immune effects induced by DLI or second transplantation are the only way to cure relapse after allo-HSCT, just as allo-HSCT is the definitive treatment for high-risk AML. For more individualized and systematic treatment based on the epigenetics and immunophenotype of AML patients, it should be implemented from induction chemotherapy to maintenance therapy after allo-HSCT. Scientists and clinicians must conduct more clinical trials to test novel agents earlier in allo-HSCT recipients to ensure how they can be successfully applied to improve patient outcomes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81302043) and the Collaborative Innovation Center of Hematology, China. We thank Dr. Stephanie J. Lee of Fred Hutchinson Cancer Research Center for her guidance in selecting the subject of the review.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Weiwei Jin searched for papers and wrote the draft. Wei Shi, Linghui Xia, and Yu Hu revised and edited the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Jan M., Leventhal M.J., Morgan E.A., Wengrod J.C., Nag A., Drinan S.D. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv. 2019;3:2199–2204. doi: 10.1182/bloodadvances.2019000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurosawa S., Fukuda T., Tajima K., Saito B., Fuji S., Yokoyama H. Outcome of 93 patients with relapse or progression following allogeneic hematopoietic cell transplantation. Am J Hematol. 2009;84:815–820. doi: 10.1002/ajh.21555. [DOI] [PubMed] [Google Scholar]
- 3.Schuler E., Boughoufala S., Rautenberg C., Nachtkamp K., Dienst A., Fenk R. Relapse patterns and treatment strategies in patients receiving allogeneic hematopoietic stem cell transplantation for myeloid malignancies. Ann Hematol. 2019;98:1225–1235. doi: 10.1007/s00277-019-03670-6. [DOI] [PubMed] [Google Scholar]
- 4.Sauer T., Silling G., Groth C., Rosenow F., Krug U., Gorlich D. Treatment strategies in patients with AML or high-risk myelodysplastic syndrome relapsed after Allo-SCT. Bone Marrow Transplant. 2015;50:485–492. doi: 10.1038/bmt.2014.300. [DOI] [PubMed] [Google Scholar]
- 5.Schmid C., de Wreede L.C., van Biezen A., Finke J., Ehninger G., Ganser A. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica. 2018;103:237–245. doi: 10.3324/haematol.2017.168716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devillier R., Crocchiolo R., Etienne A., Prebet T., Charbonnier A., Furst S. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma. 2013;54:1228–1234. doi: 10.3109/10428194.2012.741230. [DOI] [PubMed] [Google Scholar]
- 7.Bejanyan N., Weisdorf D.J., Logan B.R., Wang H.L., Devine S.M., de Lima M. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454–459. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piemontese S., Boumendil A., Labopin M., Schmid C., Ciceri F., Arcese W. Leukemia relapse following unmanipulated haploidentical transplantation: a risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol. 2019;12:68. doi: 10.1186/s13045-019-0751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeiser R., Vago L. Mechanisms of immune escape after allogeneic hematopoietic cell transplantation. Blood. 2019;133:1290–1297. doi: 10.1182/blood-2018-10-846824. [DOI] [PubMed] [Google Scholar]
- 10.Christopher M.J., Petti A.A., Rettig M.P., Miller C.A., Chendamarai E., Duncavage E.J. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379:2330–2341. doi: 10.1056/NEJMoa1808777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toffalori C., Zito L., Gambacorta V., Riba M., Oliveira G., Bucci G. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25:603–611. doi: 10.1038/s41591-019-0400-z. [DOI] [PubMed] [Google Scholar]
- 12.Noviello M., Manfredi F., Ruggiero E., Perini T., Oliveira G., Cortesi F. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019;10:1065. doi: 10.1038/s41467-019-08871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L., Chang Y.J., Xu L.P., Zhang X.H., Wang Y., Liu K.Y. Reversal of T cell exhaustion by the first donor lymphocyte infusion is associated with the persistently effective antileukemic responses in patients with relapsed AML after allo-HSCT. Biol Blood Marrow Transplant. 2018;24:1350–1359. doi: 10.1016/j.bbmt.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni A., Labopin M., Lorentino F., Van Lint M.T., Koc Y., Gülbas Z. Killer cell immunoglobulin-like receptor ligand mismatching and outcome after haploidentical transplantation with post-transplant cyclophosphamide. Leukemia. 2019;33:230–239. doi: 10.1038/s41375-018-0170-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X.Y., Yu X.X., Xu Z.L., Cao X.H., Huo M.R., Zhao X.S. Donor and host coexpressing KIR ligands promote NK education after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:4312–4325. doi: 10.1182/bloodadvances.2019000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venstrom J.M., Pittari G., Gooley T.A., Chewning J.H., Spellman S., Haagenson M. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arima N., Nakamura F., Yabe T., Tanaka J., Fuji S., Ohashi K. Influence of differently licensed KIR2DL1-positive natural killer cells in transplant recipients with acute leukemia: a Japanese national registry study. Biol Blood Marrow Transplant. 2016;22:423–431. doi: 10.1016/j.bbmt.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Weisdorf D., Cooley S., Wang T., Trachtenberg E., Vierra-Green C., Spellman S. KIR B donors improve the outcome for AML patients given reduced intensity conditioning and unrelated donor transplantation. Blood Adv. 2020;4:740–754. doi: 10.1182/bloodadvances.2019001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohl S.R., Bullinger L., Rucker F.G. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20:1983. doi: 10.3390/ijms20081983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hevener K., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X., Zhu Y., Lin Y.C., Li M., Du J., Dong H. PRMT1-mediated FLT3 arginine methylation promotes maintenance of FLT3-ITD+ acute myeloid leukemia. Blood. 2019;134:548–560. doi: 10.1182/blood.2019001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew N.R., Baumgartner F., Braun L., O'Sullivan D., Thomas S., Waterhouse M. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–291. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazarbachi A., Labopin M., Battipaglia G., Djabali A., Passweg J., Socie G. Sorafenib improves survival of FLT3-mutated acute myeloid leukemia in relapse after allogeneic stem cell transplantation: a report of the EBMT Acute Leukemia Working Party. Haematologica. 2019;104:e398–e401. doi: 10.3324/haematol.2018.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xuan L., Wang Y., Chen J., Jiang E., Gao L., Wu B. Sorafenib therapy is associated with improved outcomes for FMS-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1674–1681. doi: 10.1016/j.bbmt.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Pratz K.W., Rudek M.A., Smith B.D., Karp J., Gojo I., Dezern A. A prospective study of peritransplant sorafenib for patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic transplantation. Biol Blood Marrow Transplant. 2020;26:300–306. doi: 10.1016/j.bbmt.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen F., Sun J., Yin C., Cheng J., Ni J., Jiang L. Impact of FLT3-ITD allele ratio and ITD length on therapeutic outcome in cytogenetically normal AML patients without NPM1 mutation. Bone Marrow Transplant. 2020;55:740–748. doi: 10.1038/s41409-019-0721-z. [DOI] [PubMed] [Google Scholar]
- 27.Chappell G., Geer M., Gatza E., Braun T., Churay T., Brisson J. Maintenance sorafenib in FLT3-ITD AML following allogeneic HCT favorably impacts relapse and overall survival. Bone Marrow Transplant. 2019;54:1518–1520. doi: 10.1038/s41409-019-0493-5. [DOI] [PubMed] [Google Scholar]
- 28.Bazarbachi A., Labopin M., Battipaglia G., Djabali A., Forcade E., Arcese W. Allogeneic stem cell transplantation for FLT3-mutated acute myeloid leukemia: in vivo T-cell depletion and posttransplant sorafenib maintenance improve survival. a retrospective acute leukemia working party-European society for blood and marrow transplant study. Clin Hematol Int. 2019;1:58–74. doi: 10.2991/chi.d.190310.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burchert A., Bug G., Finke J., Stelljes M., Rollig C., Wäsch R. Sorafenib as maintenance therapy post allogeneic stem cell transplantation for FLT3-ITD positive AML: results from the randomized, double-blind, placebo-controlled multicentre sormain trial. Blood. 2018;132:661. [Google Scholar]
- 30.Battipaglia G., Ruggeri A., Massoud R., El Cheikh J., Jestin M., Antar A. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3-mutated acute myeloid leukemia. Cancer. 2017;123:2867–2874. doi: 10.1002/cncr.30680. [DOI] [PubMed] [Google Scholar]
- 31.Brunner A.M., Li S., Fathi A.T., Wadleigh M., Ho V.T., Collier K. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175:496–504. doi: 10.1111/bjh.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone R.M., Mandrekar S.J., Sanford B.L., Laumann K., Geyer S., Bloomfield C.D. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlenk R.F., Weber D., Fiedler W., Salih H.R., Wulf G., Salwender H. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133:840–851. doi: 10.1182/blood-2018-08-869453. [DOI] [PubMed] [Google Scholar]
- 34.Sandmaier B.M., Khaled S., Oran B., Gammon G., Trone D., Frankfurt O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. 2018;93:222–231. doi: 10.1002/ajh.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altman J.K., Foran J.M., Pratz K.W., Trone D., Cortes J.E., Tallman M.S. Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol. 2018;93:213–221. doi: 10.1002/ajh.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes J.E., Khaled S., Martinelli G., Perl A.E., Ganguly S., Russell N. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20:984–997. doi: 10.1016/S1470-2045(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 37.Cortes J.E., Tallman M.S., Schiller G.J., Trone D., Gammon G., Goldberg S.L. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. 2018;132:598–607. doi: 10.1182/blood-2018-01-821629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usuki K., Sakura T., Kobayashi Y., Miyamoto T., Iida H., Morita S. Clinical profile of gilteritinib in Japanese patients with relapsed/refractory acute myeloid leukemia: an open-label phase 1 study. Canc Sci. 2018;109:3235–3244. doi: 10.1111/cas.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perl A.E., Martinelli G., Cortes J.E., Neubauer A., Berman E., Paolini S. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Savage S., Schultz A.R., Bottomly D., White L., Segerdell E. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat Commun. 2019;10:244. doi: 10.1038/s41467-018-08263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antar A.I., Otrock Z.K., Jabbour E., Mohty M., Bazarbachi A. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia. 2020;34:682–696. doi: 10.1038/s41375-019-0694-3. [DOI] [PubMed] [Google Scholar]
- 42.Uchida A., Isobe Y., Asano J., Uemura Y., Hoshikawa M., Takagi M. Targeting BCL2 with venetoclax is a promising therapeutic strategy for “double-proteinexpression” lymphoma with MYC and BCL2 rearrangements. Haematologica. 2019;104:1417–1421. doi: 10.3324/haematol.2018.204958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konopleva M., Pollyea D.A., Potluri J., Chyla B., Hogdal L., Busman T. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Canc Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das M. Venetoclax with decitabine or azacitidine for AML. Lancet Oncol. 2018;19:e672. doi: 10.1016/S1470-2045(18)30824-6. [DOI] [PubMed] [Google Scholar]
- 45.Pollyea D.A., Stevens B.M., Jones C.L., Winters A., Pei S., Minhajuddin M. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24:1859–1866. doi: 10.1038/s41591-018-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakada D. Venetolax with azacitidine drains fuel from AML stem cells. Cell Stem Cell. 2019;24:7–8. doi: 10.1016/j.stem.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Gaut D., Burkenroad A., Duong T., Feammelli J., Sasine J., Schiller G. Venetoclax combination therapy in relapsed/refractory acute myeloid leukemia: a single institution experience. Leuk Res. 2020;90:106314. doi: 10.1016/j.leukres.2020.106314. [DOI] [PubMed] [Google Scholar]
- 48.Liu B., Narurkar R., Hanmantgad M., Zafar W., Song Y., Liu D. Venetoclax and low-dose cytarabine induced complete remission in a patient with high-risk acute myeloid leukemia: a case report. Front Med. 2018;12:593–599. doi: 10.1007/s11684-018-0635-y. [DOI] [PubMed] [Google Scholar]
- 49.DiNardo C.D., Wei A.H. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135:85–96. doi: 10.1182/blood.2019001239. [DOI] [PubMed] [Google Scholar]
- 50.Amatangelo M.D., Quek L., Shih A., Stein E.M., Roshal M., David M.D. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130:732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein E.M., DiNardo C.D., Fathi A.T., Pollyea D.A., Stone R.M., Altman J.K. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133:676–687. doi: 10.1182/blood-2018-08-869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiNardo C.D., Stein E.M., de Botton S., Roboz G.J., Altman J.K., Mims A.S. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 53.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fathi A.T., DiNardo C.D., Kline I., Kenvin L., Gupta I., Attar E.C. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol. 2018;4:1106–1110. doi: 10.1001/jamaoncol.2017.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terao T., Minami Y. Targeting hedgehog (Hh) pathway for the acute myeloid leukemia treatment. Cells. 2019;8:312. doi: 10.3390/cells8040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortes J.E., Douglas Smith B., Wang E.S., Merchant A., Oehler V.G., Arellano M. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. 2018;93:1301–1310. doi: 10.1002/ajh.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savona M.R., Pollyea D.A., Stock W., Oehler V.G., Schroeder M.A., Lancet J. Phase Ib study of glasdegib, a hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high-risk MDS. Clin Cancer Res. 2018;24:2294–2303. doi: 10.1158/1078-0432.CCR-17-2824. [DOI] [PubMed] [Google Scholar]
- 58.Cortes J.E., Heidel F.H., Hellmann A., Fiedler W., Smith B.D., Robak T. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–389. doi: 10.1038/s41375-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gbolahan O.B., Zeidan A.M., Stahl M., Abu Zaid M., Farag S., Paczesny S. Immunotherapeutic concepts to target acute myeloid leukemia: focusing on the role of monoclonal antibodies, hypomethylating agents and the leukemic microenvironment. Int J Mol Sci. 2017;18:1660. doi: 10.3390/ijms18081660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeder T., Rachlis E., Bug G., Stelljes M., Klein S., Steckel N.K. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions—a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant. 2015;21:653–660. doi: 10.1016/j.bbmt.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Ueda M., El-Jurdi N., Cooper B., Caimi P., Baer L., Kolk M. Low-dose azacitidine with DNMT1 level monitoring to treat post-transplantation acute myelogenous leukemia or myelodysplastic syndrome relapse. Biol Blood Marrow Transplant. 2019;25:1122–1127. doi: 10.1016/j.bbmt.2018.12.764. [DOI] [PubMed] [Google Scholar]
- 62.Craddock C., Labopin M., Robin M., Finke J., Chevallier P., Yakoub-Agha I. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2016;101:879–883. doi: 10.3324/haematol.2015.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Motabi I.H., Ghobadi A., Liu J., Schroeder M., Abboud C.N., Cashen A.F. Chemotherapy versus hypomethylating agents for the treatment of relapsed acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplant. Biol Blood Marrow Transplant. 2016;22:1324–1329. doi: 10.1016/j.bbmt.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 64.Woo J., Deeg H.J., Storer B., Yeung C., Fang M., Mielcarek M. Factors determining responses to azacitidine in patients with myelodysplastic syndromes and acute myeloid leukemia with early post-transplantation relapse: a prospective trial. Biol Blood Marrow Transplant. 2017;23:176–179. doi: 10.1016/j.bbmt.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Schroeder T., Rautenberg C., Kruger W., Platzbecker U., Bug G., Steinmann J. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI—a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann Hematol. 2018;97:335–342. doi: 10.1007/s00277-017-3185-5. [DOI] [PubMed] [Google Scholar]
- 66.Craddock C., Slade D., De Santo C., Wheat R., Ferguson P., Hodgkinson A. Combination lenalidomide and azacitidine: a novel salvage therapy in patients who relapse after allogeneic stem-cell transplantation for acute myeloid leukemia. J Clin Oncol. 2019;37:580–588. doi: 10.1200/JCO.18.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Platzbecker U., Middeke J.M., Sockel K., Herbst R., Wolf D., Baldus C.D. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19:1668–1679. doi: 10.1016/S1470-2045(18)30580-1. [DOI] [PubMed] [Google Scholar]
- 68.Craddock C., Jilani N., Siddique S., Yap C., Khan J., Nagra S. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant. 2016;22:385–390. doi: 10.1016/j.bbmt.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Cheikh J., Massoud R., Fares E., Kreidieh N., Mahfouz R., Charafeddine M. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. 2017;52:918–921. doi: 10.1038/bmt.2017.31. [DOI] [PubMed] [Google Scholar]
- 70.Guillaume T., Malard F., Magro L., Labopin M., Tabrizi R., Borel C. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019;54:1815–1826. doi: 10.1038/s41409-019-0536-y. [DOI] [PubMed] [Google Scholar]
- 71.de Lima M., Oran B., Champlin R.E., Papadopoulos E.B., Giralt S.A., Scott B.L. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant. 2018;24:2017–2024. doi: 10.1016/j.bbmt.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wouters B.J., Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127:42–52. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Manero G., Sekeres M.A., Egyed M., Breccia M., Graux C., Cavenagh J.D. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with 30% blasts. Leukemia. 2017;31:2799–2806. doi: 10.1038/leu.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bug G., Burchert A., Wagner E.M., Kroger N., Berg T., Guller S. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial) Leukemia. 2017;31:2523–2525. doi: 10.1038/leu.2017.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeAngelo D.J., Walker A.R., Schlenk R.F., Sierra J., Medeiros B.C., Ocio E.M. Safety and efficacy of oral panobinostat plus chemotherapy in patients aged 65 years or younger with high-risk acute myeloid leukemia. Leuk Res. 2019;85:106197. doi: 10.1016/j.leukres.2019.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieduwilt M.J., Pawlowska N., Thomas S., Olin R., Logan A.C., Damon L.E. Histone deacetylase inhibition with panobinostat combined with intensive induction chemotherapy in older patients with acute myeloid leukemia: phase I study results. Clin Canc Res. 2019;25:4917–4923. doi: 10.1158/1078-0432.CCR-19-0171. [DOI] [PubMed] [Google Scholar]
- 77.Perl A.E. Vol. 2017. Hematology Am Soc Hematol Educ Program; 2017. The role of targeted therapy in the management of patients with AML. Hematology; pp. 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pollyea D.A., Jordan C.T. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129:1627–1635. doi: 10.1182/blood-2016-10-696039. [DOI] [PubMed] [Google Scholar]
- 79.He S.Z., Busfield S., Ritchie D.S., Hertzberg M.S., Durrant S., Lewis I.D. A phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma. 2015;56:1406–1415. doi: 10.3109/10428194.2014.956316. [DOI] [PubMed] [Google Scholar]
- 80.Gadhoum S.Z., Madhoun N.Y., Abuelela A.F., Merzaban J.S. Anti-CD44 antibodies inhibit both mTORC1 and mTORC2: a new rationale supporting CD44-induced AML differentiation therapy. Leukemia. 2016;30:2397–2401. doi: 10.1038/leu.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vey N., Delaunay J., Martinelli G., Fiedler W., Raffoux E., Prebet T. Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget. 2016;7:32532–32542. doi: 10.18632/oncotarget.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reusch U., Harrington K.H., Gudgeon C.J., Fucek I., Ellwanger K., Weichel M. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Canc Res. 2016;22:5829–5838. doi: 10.1158/1078-0432.CCR-16-0350. [DOI] [PubMed] [Google Scholar]
- 83.Godwin C.D., Gale R.P., Walter R.B. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31:1855–1868. doi: 10.1038/leu.2017.187. [DOI] [PubMed] [Google Scholar]
- 84.Hills R.K., Castaigne S., Appelbaum F.R., Delaunay J., Petersdorf S., Othus M. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daver N., Kantarjian H., Ravandi F., Estey E., Wang X., Garcia-Manero G. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30:268–273. doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tarlock K., Alonzo T.A., Gerbing R.B., Raimondi S.C., Hirsch B.A., Sung L. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: a report from the Children's Oncology Group. Clin Canc Res. 2016;22:1951–1957. doi: 10.1158/1078-0432.CCR-15-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Candoni A., Papayannidis C., Martinelli G., Simeone E., Gottardi M., Iacobucci I. Flai (fludarabine, cytarabine, idarubicin) plus low-dose Gemtuzumab Ozogamicin as induction therapy in CD33-positive AML: final results and long term outcome of a phase II multicenter clinical trial. Am J Hematol. 2018;93:655–663. doi: 10.1002/ajh.25057. [DOI] [PubMed] [Google Scholar]
- 88.Amadori S., Suciu S., Selleslag D., Aversa F., Gaidano G., Musso M. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 89.Wei A.H., Tiong I.S. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood. 2017;130:2469–2474. doi: 10.1182/blood-2017-08-784066. [DOI] [PubMed] [Google Scholar]
- 90.Burnett A., Cavenagh J., Russell N., Hills R., Kell J., Jones G. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 Trial. Haematologica. 2016;101:724–731. doi: 10.3324/haematol.2016.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pollard J.A., Loken M., Gerbing R.B., Raimondi S.C., Hirsch B.A., Aplenc R. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2016;34:747–755. doi: 10.1200/JCO.2015.62.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khan N., Hills R.K., Virgo P., Couzens S., Clark N., Gilkes A. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia. 2017;31:1059–1068. doi: 10.1038/leu.2016.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamba J.K., Chauhan L., Shin M., Loken M.R., Pollard J.A., Wang Y.C. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2017;35:2674–2682. doi: 10.1200/JCO.2016.71.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koren-Michowitz M., Maayan H., Apel A., Shem-Tov N., Yerushalmi R., Volchek Y. Salvage therapy with ARA-C and gemtuzumab ozogamicin in AML patients relapsing after stem cell transplantation. Ann Hematol. 2015;94:375–378. doi: 10.1007/s00277-014-2229-3. [DOI] [PubMed] [Google Scholar]
- 95.Zahler S., Bhatia M., Ricci A., Roy S., Morris E., Harrison L. A phase I study of reduced-intensity conditioning and allogeneic stem cell transplantation followed by dose escalation of targeted consolidation immunotherapy with gemtuzumab ozogamicin in children and adolescents with CD33+ acute myeloid leukemia. Biol Blood Marrow Transplant. 2016;22:698–704. doi: 10.1016/j.bbmt.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 96.Stein E.M., Walter R.B., Erba H.P., Fathi A.T., Advani A.S., Lancet J.E. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood. 2018;131:387–396. doi: 10.1182/blood-2017-06-789800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fathi A.T., Erba H.P., Lancet J.E., Stein E.M., Ravandi F., Faderl S. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132:1125–1133. doi: 10.1182/blood-2018-03-841171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brayer J., Lancet J.E., Powers J., List A., Balducci L., Komrokji R. WT1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am J Hematol. 2015;90:602–607. doi: 10.1002/ajh.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chapuis A.G., Egan D.N., Bar M., Schmitt T.M., McAfee M.S., Paulson K.G. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25:1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maslak P.G., Dao T., Bernal Y., Chanel S.M., Zhang R., Frattini M. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018;2:224–234. doi: 10.1182/bloodadvances.2017014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anguille S., Van de Velde A.L., Smits E.L., Van Tendeloo V.F., Juliusson G., Cools N. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shah N.N., Loeb D.M., Khuu H., Stroncek D., Ariyo T., Raffeld M. Induction of immune response after allogeneic Wilms' tumor 1 dendritic cell vaccination and donor lymphocyte infusion in patients with hematologic malignancies and post-transplantation relapse. Biol Blood Marrow Transplant. 2016;22:2149–2154. doi: 10.1016/j.bbmt.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tawara I., Kageyama S., Miyahara Y., Fujiwara H., Nishida T., Akatsuka Y. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130:1985–1994. doi: 10.1182/blood-2017-06-791202. [DOI] [PubMed] [Google Scholar]
- 104.Abraham M., Klein S., Bulvik B., Wald H., Weiss I.D., Olam D. The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression. Leukemia. 2017;31:2336–2346. doi: 10.1038/leu.2017.82. [DOI] [PubMed] [Google Scholar]
- 105.Habringer S., Lapa C., Herhaus P., Schottelius M., Istvanffy R., Steiger K. Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics. 2018;8:369–383. doi: 10.7150/thno.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu S.H., Gu Y., Pascual B., Yan Z., Hallin M., Zhang C. A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies. Blood Adv. 2017;1:1088–1100. doi: 10.1182/bloodadvances.2016003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovacsovics T.J., Mims A., Salama M.E., Pantin J., Rao N., Kosak K.M. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018;2:381–389. doi: 10.1182/bloodadvances.2017013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michelis F.V., Hedley D.W., Malhotra S., Chow S., Loach D., Gupta V. Mobilization of leukemic cells using plerixafor as part of a myeloablative preparative regimen for patients with acute myelogenous leukemia undergoing allografting: assessment of safety and tolerability. Biol Blood Marrow Transplant. 2019;25:1158–1163. doi: 10.1016/j.bbmt.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 109.Lynn R.C., Poussin M., Kalota A., Feng Y., Low P.S., Dimitrov D.S. Targeting of folate receptor beta on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125:3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lynn R.C., Feng Y., Schutsky K., Poussin M., Kalota A., Dimitrov D.S. High-affinity FRbeta-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J., Chen S., Xiao W., Li W., Wang L., Yang S. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11:7. doi: 10.1186/s13045-017-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baumeister S.H., Murad J., Werner L., Daley H., Trebeden-Negre H., Gicobi J.K. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2019;7:100–112. doi: 10.1158/2326-6066.CIR-18-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomes-Silva D., Atilla E., Atilla P.A., Mo F., Tashiro H., Srinivasan M. CD7 CAR T Cells for the therapy of acute myeloid leukemia. Mol Ther. 2019;27:272–280. doi: 10.1016/j.ymthe.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jetani H., Garcia-Cadenas I., Nerreter T., Thomas S., Rydzek J., Meijide J.B. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32:1168–1179. doi: 10.1038/s41375-018-0009-0. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Xu Y., Li S., Liu J., Xing Y., Xing H. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J Hematol Oncol. 2018;11:60. doi: 10.1186/s13045-018-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng W., O'Hear C.E., Alli R., Basham J.H., Abdelsamed H.A., Palmer L.E. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32:1157–1167. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrov J.C., Wada M., Pinz K.G., Yan L.E., Chen K.H., Shuai X. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia. 2018;32:1317–1326. doi: 10.1038/s41375-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He X., Feng Z., Ma J., Ling S., Cao Y., Gurung B. Bi-specific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood. 2020;135:713–723. doi: 10.1182/blood.2019002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim M.Y., Yu K.R., Kenderian S.S., Ruella M., Chen S., Shin T.H. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173:1439–1453. doi: 10.1016/j.cell.2018.05.013. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borot F., Wang H., Ma Y., Jafarov T., Raza A., Ali A.M. Gene-edited stem cells enable CD33-directed immune therapy for myeloid malignancies. Proc Natl Acad Sci U S A. 2019;116:11978–11987. doi: 10.1073/pnas.1819992116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vago L., Perna S.K., Zanussi M., Mazzi B., Barlassina C., Stanghellini M.T. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 122.Ahci M., Toffalori C., Bouwmans E., Crivello P., Brambati C., Pultrone C. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood. 2017;130:1270–1273. doi: 10.1182/blood-2017-05-784306. [DOI] [PubMed] [Google Scholar]
- 123.He F., Warlick E., Miller J.S., MacMillan M., Verneris M.R., Cao Q. Lymphodepleting chemotherapy with donor lymphocyte infusion post-allogeneic HCT for hematological malignancies is associated with severe, but therapy-responsive aGvHD. Bone Marrow Transplant. 2016;51:1107–1112. doi: 10.1038/bmt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inamoto Y., Fefer A., Sandmaier B.M., Gooley T.A., Warren E.H., Petersdorf S.H. A phase I/II study of chemotherapy followed by donor lymphocyte infusion plus interleukin-2 for relapsed acute leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1308–1315. doi: 10.1016/j.bbmt.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bar M., Flowers M.E.D., Storer B.E., Chauncey T.R., Pulsipher M.A., Thakar M.S. Reversal of low donor chimerism after hematopoietic cell transplantation using pentostatin and donor lymphocyte infusion: a prospective phase II multicenter trial. Biol Blood Marrow Transplant. 2018;24:308–313. doi: 10.1016/j.bbmt.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warlick E.D., DeFor T., Blazar B.R., Burns L., Verneris M.R., Ustun C. Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:480–486. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eefting M., von dem Borne P.A., de Wreede L.C., Halkes C.J., Kersting S., Marijt E.W. Intentional donor lymphocyte-induced limited acute graft-versus-host disease is essential for long-term survival of relapsed acute myeloid leukemia after allogeneic stem cell transplantation. Haematologica. 2014;99:751–758. doi: 10.3324/haematol.2013.089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schroeder T., Czibere A., Platzbecker U., Bug G., Uharek L., Luft T. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27:1229–1235. doi: 10.1038/leu.2013.7. [DOI] [PubMed] [Google Scholar]
- 129.Boudreau J.E., Giglio F., Gooley T.A., Stevenson P.A., Le Luduec J.B., Shaffer B.C. KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J Clin Oncol. 2017;35:2268–2278. doi: 10.1200/JCO.2016.70.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moutuou M.M., Page G., Zaid I., Lesage S., Guimond M. Restoring T cell homeostasis after allogeneic stem cell transplantation: principal limitations and future challenges. Front Immunol. 2018;9:1237. doi: 10.3389/fimmu.2018.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simonetta F., Pradier A., Bosshard C., Masouridi-Levrat S., Dantin C., Koutsi A. Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2019;10:1034. doi: 10.3389/fimmu.2019.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ijaz A., Khan A.Y., Malik S.U., Faridi W., Fraz M.A., Usman M. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant. 2019;25:94–99. doi: 10.1016/j.bbmt.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davids M.S., Kim H.T., Bachireddy P., Costello C., Liguori R., Savell A. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bashey A., Medina B., Corringham S., Pasek M., Carrier E., Vrooman L. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Albring J.C., Inselmann S., Sauer T., Schliemann C., Altvater B., Kailayangiri S. PD-1 checkpoint blockade in patients with relapsed AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2017;52:317–320. doi: 10.1038/bmt.2016.274. [DOI] [PubMed] [Google Scholar]
- 136.Nikiforow S., Kim H.T., Daley H., Reynolds C., Jones K.T., Armand P. A phase I study of CD25/regulatory T-cell-depleted donor lymphocyte infusion for relapse after allogeneic stem cell transplantation. Haematologica. 2016;101:1251–1259. doi: 10.3324/haematol.2015.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muffly L., Sheehan K., Armstrong R., Jensen K., Tate K., Rezvani A.R. Infusion of donor-derived CD8+ memory T cells for relapse following allogeneic hematopoietic cell transplantation. Blood Adv. 2018;2:681–690. doi: 10.1182/bloodadvances.2017012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oda S.K., Daman A.W., Garcia N.M., Wagener F., Schmitt T.M., Tan X. A CD200R-CD28 fusion protein appropriates an inhibitory signal to enhance T-cell function and therapy of murine leukemia. Blood. 2017;130:2410–2419. doi: 10.1182/blood-2017-04-777052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shaffer B.C., Le Luduec J.B., Forlenza C., Jakubowski A.A., Perales M.A., Young J.W. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:705–709. doi: 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hallner A., Bernson E., Hussein B.A., Ewald Sander F., Brune M., Aurelius J. The HLA-B-21 dimorphism impacts on NK cell education and clinical outcome of immunotherapy in acute myeloid leukemia. Blood. 2019;133:1479–1488. doi: 10.1182/blood-2018-09-874990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi Y., Dincheva-Vogel L., Ayemoba C.E., Fung J.P., Bergamaschi C., Pavlakis G.N. IL-15/IL-15Ralpha/CD80-expressing AML cell vaccines eradicate minimal residual disease in leukemic mice. Blood Adv. 2018;2:3177–3192. doi: 10.1182/bloodadvances.2018019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ertz-Archambault N., Kosiorek H., Slack J.L., Lonzo M.L., Greipp P.T., Khera N. Cytogenetic evolution in myeloid neoplasms at relapse after allogeneic hematopoietic cell transplantation: association with previous chemotherapy and effect on survival. Biol Blood Marrow Transplant. 2017;23:782–789. doi: 10.1016/j.bbmt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Crucitti L., Crocchiolo R., Toffalori C., Mazzi B., Greco R., Signori A. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia. 2015;29:1143–1152. doi: 10.1038/leu.2014.314. [DOI] [PubMed] [Google Scholar]
- 145.Krivtsov A.V., Evans K., Gadrey J.Y., Eschle B.K., Hatton C., Uckelmann H.J. A Menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Canc Cell. 2019;36:660–673 e11. doi: 10.1016/j.ccell.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCalmont H., Li K.L., Jones L., Toubia J., Bray S.C., Casolari D.A. Efficacy of combined CDK9/BET inhibition in preclinical models of MLL-rearranged acute leukemia. Blood Adv. 2020;4:296–300. doi: 10.1182/bloodadvances.2019000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wingelhofer B., Somervaille T.C.P. Emerging epigenetic therapeutic targets in acute myeloid leukemia. Front Oncol. 2019;9:850. doi: 10.3389/fonc.2019.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brzezinka K., Nevedomskaya E., Lesche R., Steckel M., Eheim A.L., Haegebarth A. Functional diversity of inhibitors tackling the differentiation blockage of MLL-rearranged leukemia. J Hematol Oncol. 2019;12:66. doi: 10.1186/s13045-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Radzisheuskaya A., Shliaha P.V., Grinev V., Lorenzini E., Kovalchuk S., Shlyueva D. PRMT5 methylome profiling uncovers a direct link to splicing regulation in acute myeloid leukemia. Nat Struct Mol Biol. 2019;26:999–1012. doi: 10.1038/s41594-019-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fong J.Y., Pignata L., Goy P.A., Kawabata K.C., Lee S.C., Koh C.M. Therapeutic targeting of RNA splicing catalysis through inhibition of protein arginine methylation. Canc Cell. 2019;36:194–209 e9. doi: 10.1016/j.ccell.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saenz D.T., Fiskus W., Manshouri T., Mill C.P., Qian Y., Raina K. Targeting nuclear beta-catenin as therapy for post-myeloproliferative neoplasm secondary AML. Leukemia. 2019;33:1373–1386. doi: 10.1038/s41375-018-0334-3. [DOI] [PubMed] [Google Scholar]
- 152.Piya S., Mu H., Bhattacharya S., Lorenzi P.L., Davis R.E., McQueen T. BETP degradation simultaneously targets acute myelogenous leukemia stem cells and the microenvironment. J Clin Invest. 2019;129:1878–1894. doi: 10.1172/JCI120654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mill C.P., Fiskus W., DiNardo C.D., Qian Y., Raina K., Rajapakshe K. RUNX1-targeted therapy for AML expressing somatic or germline mutation in RUNX1. Blood. 2019;134:59–73. doi: 10.1182/blood.2018893982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Masse A., Roulin L., Pasanisi J., Penneroux J., Gachet S., Delord M. BET inhibitors impair leukemic stem cell function only in defined oncogenic subgroups of acute myeloid leukaemias. Leuk Res. 2019;87:106269. doi: 10.1016/j.leukres.2019.106269. [DOI] [PubMed] [Google Scholar]
- 155.Jiang Y., Hu T., Wang T., Shi X., Kitano A., Eagle K. AMP-activated protein kinase links acetyl-CoA homeostasis to BRD4 recruitment in acute myeloid leukemia. Blood. 2019;134:2183–2194. doi: 10.1182/blood.2019001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xuan L., Wang Y., Huang F., Jiang E., Deng L., Wu B. Effect of sorafenib on the outcomes of patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation. Cancer. 2018;124:1954–1963. doi: 10.1002/cncr.31295. [DOI] [PubMed] [Google Scholar]