To the Editor,
Patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may develop severe coronavirus disease (COVID-19) and acute respiratory failure. As the lung parenchyma is seriously damaged, opportunistic infections are feared. While invasive pulmonary mould superinfections are usually diagnosed primarily in highly immunocompromised patients, invasive aspergillosis has also been described in immunocompetent patients infected with influenza or SARS-CoV (responsible for the severe acute respiratory syndrome outbreak in 2002–2003). Moreover, we recently reported a case of invasive aspergillosis in an immunocompetent critically ill patient with severe COVID-19 [1]. Another study also suggested an increased risk of Aspergillus superinfection in COVID-19 patients [2].
To know whether or not COVID-19 is a factor promoting invasive pulmonary mould infections in immunocompetent individuals is of great medical interest. We report here the case of an immunocompetent diabetic patient with severe COVID-19 who developed a pulmonary fusariosis due to the common environmental mould Fusarium proliferatum.
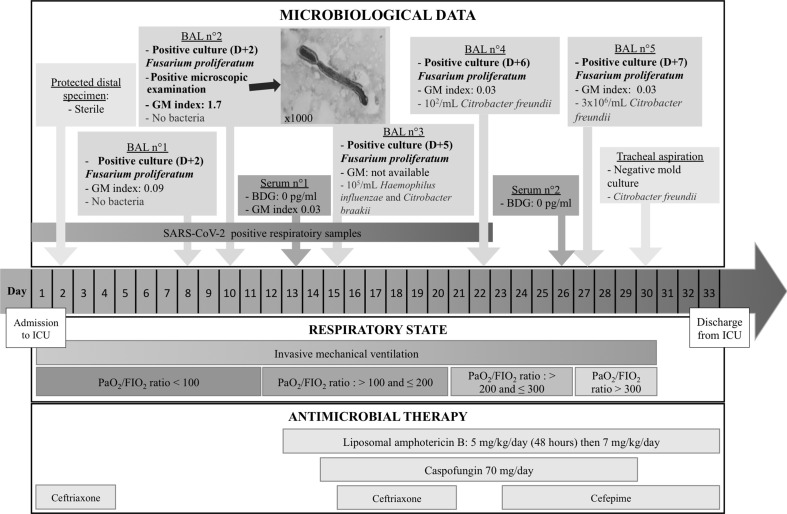
A 57-year-old man was sent to our hospital after 7 days of fever and cough worsening over the last 24 hr. On admission the patient presented signs of acute respiratory failure and was rapidly transferred to our intensive care unit (ICU) on March 26 (day 1) where he was intubated and placed under mechanical ventilation (Fig. 1 ). The patient had no history of any acquired or inherited immunodeficiency and no underlying respiratory disease. He was overweight (body mass index 30.6 kg/m2) with type 2 diabetes (blood glucose 11.6 mmol/L and glycosuria 1.4 mmol/L on admission), hypertension and a substituted hypothyroidism. SARS-CoV-2 viral RNA was detected in a tracheal aspiration on admission. The patient's condition worsened with a PaO2/FiO2 ratio <90 mmHg with a FiO2 of 100%. A protected specimen brush sample performed on day 2 remained sterile. While the patient's condition showed no improvement, a bronchoalveolar lavage (BAL) was performed on day 7. On day 9 this sample grew with 7 × 102/mL colony-forming unit (CFU) of mould while no bacteria were detected. The same day, a second BAL was performed: atypical fungal hyphae were detected on microscopic examination, testing for galactomannan antigen was positive (index: 1.7) and the sample grew with 103/mL CFU of mould. The different cultures were identified as Fusarium proliferatum using mass spectrometry (Bruker Microflex with MSI database) and a liposomal amphotericin B-based therapy was started on day 12 (5 mg/kg/day for 2 days then 7 mg/kg/day). Antifungal susceptibility testing determined using the gradient concentration strips method showed high minimal inhibitory concentrations (MICs) (>32 mg/L) for voriconazole, posaconazole and isavuconazole. The MIC value for amphotericin B was 2 mg/L.
Fig. 1.
Timeline for an immunocompetent patient who developed invasive pulmonary fusariosis during severe COVID-19. Day 1 is the day the patient was admitted to the intensive care unit. D, days; BAL, bronchoalveolar lavage; GM, galactomannan index determination; BDG, β-D-glucan dosage.
Considering the very serious condition of the patient, and despite the fact that voriconazole is suggested as first-line treatment (alone or in combination) [3], caspofungin was added, assuming a potential synergistic effect with liposomal amphotericin B [4,5]. To test this hypothesis in vitro, we used the checkerboard method to assess combinations of amphotericin B and caspofungin. The MIC of each drug alone and the combinations of the two were determined concomitantly on the same plate using a method adapted from the EUCAST procedure. Interaction was determined by calculating the fractional inhibitory concentration index (FICI) as follows: FICI = (MIC amphotericin B in combination/MIC amphotericin B alone) + (MEC caspofungin in combination/MEC caspofungin alone). Results showed no activity for caspofungin either alone or in combination (MEC > 32 mg/L). The MIC value for amphotericin B was 1 mg/L either alone or in combination. The FICI value was equal to 2, which is indicative of indifference. Moreover, as the patient's condition was beginning to improve, caspofungin was stopped on day 29. Three other BALs performed on day 15, day 22 and day 27 still grew with Fusarium proliferatum. Importantly, we noticed a gradual increase in the delay of culture positivity and a decrease in the number of colonies obtained. Galactomannan testing became negative, all of which indicated a reduction of the fungal load. The BALs performed on day 15 and day 27 also grew with a significant amount of bacteria, which led to specific antibiotic treatment. The patient's general condition slowly improved. He was extubated on day 30 then discharged to a post-ICU area. Liposomal amphotericin B administration was stopped on day 35.
Invasive aspergillosis is a well-recognized complication of severe influenza pneumonia and has been reported in patients with SARS-CoV and SARS-CoV-2 infections [1,2]. As fusariosis shares many common characteristics with aspergillosis [6], occurrence of fusariosis in immunocompetent critically ill patients with severe pulmonary damage is possible. If we draw a parallel between fusariosis and aspergillosis, the case we describe is a putative invasive fusariosis according to the classification by Blot et al. for the diagnosis of invasive aspergillosis in critically ill patients [7]. Serum β-glucan and galactomannan testing in serum were negative. Here again a resemblance can be seen with what is observed during invasive aspergillosis in immunocompetent patients where blood tests are often negative while respiratory samples establish the diagnosis [7].
In conclusion, we have reported a case of pulmonary fusariosis in an immunocompetent patient with severe SARS-CoV-2 pneumonia. As the outbreak continues to spread worldwide, other reports are needed to assess the frequency of fungal superinfections during severe COVID-19.
Transparency declaration
Funding: None. The authors declare no competing interests.
Author contributions
C.P. and M.B. collected mycological data, performed antifungal susceptibility testing and the EUCAST checkboard method and participated in the writing. C.V. participated in the therapeutic decision, collected clinical data and participated in the writing. A.L. participated in the therapeutic decision and in the writing. A.M. participated in the therapeutic decision, collected clinical data and participated in the writing. A.F. participated in the therapeutic decision, collected data and wrote the paper.
Editor: E. Roilides
References
- 1.Blaize M., Mayaux J., Nabet C., Lampros A., Marcellin A.G., Thellier M., et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehler P., Cornely O.A., Bottiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. Covid-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nucci M., Marr K.A., Vehreschild M.J., de Souza C.A., Velasco E., Cappellano P., et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014;20:580–585. doi: 10.1111/1469-0691.12409. [DOI] [PubMed] [Google Scholar]
- 4.Vagace J.M., Sanz-Rodriguez C., Casado M.S., Alonso N., Garcia-Dominguez M., de la Llana F.G., et al. Resolution of disseminated fusariosis in a child with acute leukemia treated with combined antifungal therapy: a case report. BMC Infect Dis. 2007;7:40. doi: 10.1186/1471-2334-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arikan S., Lozano-Chiu M., Paetznick V., Rex J.H. In vitro synergy of caspofungin and amphotericin B against aspergillus and Fusarium spp. Antimicrob Agents Chemother. 2002;46:245–247. doi: 10.1128/AAC.46.1.245-247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci F., Nouer S.A., Capone D., Anaissie E., Nucci M. Fusariosis Semin Respir. Crit Care Med. 2015;36:706–714. doi: 10.1055/s-0035-1562897. [DOI] [PubMed] [Google Scholar]
- 7.Blot S.I., Taccone F.S., Van den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]