ABSTRACT
The effect of berry polyphenols on glucose metabolism has been evaluated in several studies; however, the results are conflicting. A systematic review and meta-analysis was therefore conducted to evaluate the effect of berry polyphenol consumption on glucose metabolism in adults with impaired glucose tolerance or insulin resistance. PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, CINAHL (EBSCO), and Scopus were searched for randomized controlled trials published by June 2019. Of the 3240 articles found, 21 met inclusion criteria. Study-specific effects were calculated as mean differences, which were pooled using fixed-effect, inverse-variance weighting. Overall, berry polyphenol consumption did not have a clear effect on biomarkers of glucose metabolism compared with placebo or no treatment. Although some analyses showed statistically significant effects, these effects were too small to be of clinical relevance. The review protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews as CRD42019130811.
Keywords: berry, polyphenols, glucose metabolism, diabetes, fasting blood glucose, glycated hemoglobin, HOMA-IR, insulin, systematic review, meta-analysis
Introduction
The International Diabetes Federation has declared that diabetes mellitus (DM) affects >425 million people worldwide (1). They also project that by 2030, the number of persons affected by DM will increase to 552 million (2). Diabetes is also a major cause of blindness, kidney failure, heart attack, stroke, and lower limb amputation. In addition, coupled with the growing global problem of overweight and obesity among the populace, and the strong association between obesity and the risk of diabetes, there is a cause for concern (3).
There are numerous observational and experimental accounts of suggested health benefits of phenolic compounds, and berry fruits are believed to be a significant source of these compounds in the diet (4–7). The most predominant berry polyphenols include tannins and flavonoids, with the latter group accounting for approximately two-thirds of all the phenolics in the diet (8, 9). Anthocyanins are a subgroup of flavonoids and are responsible for the bright colors of berries and some other plants. They are also reported to possess significant health-promoting effects (10).
Diets rich in polyphenols have been suggested to aid in the management of DM through various mechanisms. These include their potential ability to modulate carbohydrate and lipid metabolism, enhance insulin production, improve glucose uptake in muscles and adipocytes, reduce apoptosis and facilitate the protection and proliferation of pancreatic cells, improve adipose tissue metabolism, alleviate oxidative stress and stress-sensitive signaling pathways, and exert anti-inflammatory effects (11–15).
Although there are numerous reports on the positive effect of polyphenols on glucose metabolism and diabetes management, the results of human clinical trials have been inconsistent. Of 3 recent meta-analyses, 1 study showed that only 1 outcome related to glucose metabolism (HOMA-IR) was significantly impacted by anthocyanin consumption in patients with varying health status (16). The other 2 meta-analyses showed that the consumption of berries and polyphenols of various origins by a similar population significantly lowered several glucose-related parameters [BMI (in kg/m2), hemoglobin A1c (HbA1c), and fasting blood glucose (FBG)]; however, the studies differed in terms of which parameters were lowered (17, 18). In addition to showing conflicting results, these reviews included studies of different interventions in populations of varying health status and, for some outcomes, included only a limited number of studies. We therefore conducted a more targeted systematic review and meta-analysis of randomized trials, including only trials studying the effects of berry polyphenols, specifically (compared with no treatment or placebo) on glucose metabolism in adults with impaired glucose tolerance or insulin resistance, a high-risk and more homogeneous population. Furthermore, we conducted several subgroup analyses to examine whether effects varied by study quality, type of intervention, dose of polyphenol or anthocyanin, study duration, and restrictions on polyphenol from nonintervention food and medications affecting glucose metabolism or insulin. The previously mentioned studies only considered a possible effect from a maximum of 3 of these factors.
Methods
The systematic review and meta-analysis performed was selective for berry polyphenols. Berries considered include botanical berries such as blueberries, cranberries, gooseberries, lingonberries, Caucasian whortleberries, and currants; and nonbotanical berries such as strawberries, raspberries, blackberries, mulberries, cloudberries, and acai berries. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used to conduct this systematic review (19). The review protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews as CRD42019130811. The protocol was amended once, before any statistical analysis was performed.
Eligibility criteria
Published randomized trials were screened based on their titles, abstracts, and full texts to determine if they met the following inclusion criteria: 1) the study population was adults (aged ≥18 y) suffering from insulin resistance or impaired glucose tolerance, with the exception of pregnant and lactating women; 2) the intervention was sustained for at least 2 wk; 3) the intervention was oral berry supplementation as purified isolates, crude extracts, juices, or whole berries, where the source of the berry was botanical or nonbotanical as outlined previously; 4) the comparator was no treatment or a placebo void of polyphenols; 5) the paper reported at least 1 of the primary outcomes of HbA1c, HOMA-IR, FBG, fasting blood insulin, or postprandial glucose/postprandial serum insulin following an intervention duration of ≥2 wk with meals containing polyphenols; 6) the study design was parallel-group or crossover randomized controlled trial (RCT); and 7) the paper was published in English. These inclusion criteria address each component of the PICOS (Population, Intervention, Comparison, Outcome, Study) model, which was used to formulate the research question. Studies were excluded if participants had end-stage diseases (e.g., carcinoma or organ failure) or hormone-related disorders (e.g., polycystic ovary syndrome), if participants engaged in strenuous exercise, and if trials applied multistage interventions. Studies that included other biologically active dietary components (e.g., fish oil) or any other source of polyphenols in combination with the berry polyphenols of interest were also excluded.
Search strategy and study selection
The systematic literature search was performed using PubMed/MEDLINE, Cochrane Central Register of Controlled Trials, CINAHL (EBSCO), and Scopus from inception until June 2019. The search strategy was executed using Medical Subject Heading, Boolean operators, and text words (Supplemental Methods). The reference lists of retrieved systematic reviews and included studies were also hand searched to find additional articles. Retrieved articles were downloaded and merged in EndNote (version X9 for Windows; Clarivate Analytics) and Rayyan software (HBKU Research Complex) to enable the review process. All retrieved articles were read independently by 2 reviewers (TFR and AN). Any disagreements were discussed and resolved by consensus or by a third independent reviewer (PN) if necessary.
Risk of bias
Risk of bias in the included studies was assessed using Covidence systematic review software (Veritas Health Innovation), which is recommended by Cochrane as part of its author support toolkit. The methodological quality of the studies was assessed to determine if their risk of bias was low, high, or unclear as it relates to the methods used to generate a random sequence, conceal allocations, blind participants and personnel, blind outcome assessors, and to determine the chances of incomplete outcome data and selective outcome reporting. The risk of bias assessment was done in duplicate by TFR and AN, and discrepancies were settled by PN.
Data extraction
The following data from eligible studies were extracted and entered into a spreadsheet: general study characteristics (first author, year of publication, country, type of berries consumed, type of supplement administered, polyphenol and anthocyanin dose, and study duration), characteristics of the participants (study population, number of participants, percentage of male participants, and mean age or age range of participants and control group), primary outcome results, and funding. Secondary outcome data including weight, waist circumference, and BMI were also extracted provided all other inclusion criteria were met. Data extraction was done in duplicate by TFR and JB.
Statistical analyses
We calculated study-specific effects of berry polyphenol consumption by taking the difference in postintervention means between the intervention group and the control group. The original protocol stated that standardized mean differences would be used, but before the analysis was performed, this was changed to ordinary (unstandardized) mean differences because the included studies used the same measurement scales (20). If a study had multiple intervention groups, each receiving a different quantity of berry supplementation, we combined these groups by calculating an overall mean and SD so that the study provided only 1 effect estimate. This approach is recommended because a meta-analysis may be biased if the same study is included twice (21). If postintervention means were not available, we used change-from-baseline scores. For crossover studies, we used the postintervention and post-control means and analyzed these as independent groups, a conservative approach (22), because none of the included crossover studies reported information necessary to derive SDs of changes at the individual level.
For study-specific effects, SEs were calculated using group-specific SDs or SEs, without assuming equal variability within groups. If SDs or SEs were not available, P values or CIs were used. We calculated CIs for study-specific effects under the assumption of t-distributions, with degrees of freedom estimated using the Satterthwaite approximation.
Study-specific mean differences were pooled using fixed-effect, inverse-variance weighting. The original protocol stated that random-effect weighting would be used, but this was changed prior to the analysis because the fixed-effects model provides an estimate of the average effect among the included studies rather than the average effect in a conceptual population of studies (20). Another advantage of the fixed-effects model is that it does not assume that the included studies are randomly sampled from a population of studies (20). For the pooled effects, CIs were derived based on the normal distribution. Heterogeneity in effects across studies was assessed using the I2 statistic and the χ2 test based on the Q statistic. I2 was interpreted as indicative of low (<40), moderate (40–59%), or substantial (≥60%) heterogeneity, similar to the recommendations of the Cochrane handbook (20). Publication bias was assessed using funnel plots and Egger's test.
When necessary, blood glucose was converted from milligrams per deciliter to millimoles per liter (1 mg/dL = 1/18 mmol/L), and HbA1c was converted from the units of the Diabetes Control and Complications Trial (DCCT) to millimoles per mole [mmol/mol = 10.929 × (% DCCT – 2.15)] (23). Insulin was converted from picomoles per liter to micro international units per milliliter (1 μIU/mL = 6 pmol/L) (24). Body weight was converted from pounds to kilograms (1 kg = 0.4536 lb).
Subgroup analyses were conducted for the outcome of FBG because this was the most frequently reported outcome (>90% of included studies). The subgroups were defined according to the following study characteristics: polyphenol dose (low: <400 mg/d; medium: 400–899 mg/d; high: ≥900 mg/d), anthocyanin dose (low: <300 mg/d; high: ≥300 mg/d), study duration (<12 or ≥12 wk), risk of bias (low on all parameters or high/unclear on ≥1 parameter), type of intervention (fresh/dried berries or berry extract), restriction on the use of medication affecting glucose metabolism or insulin, and restriction of polyphenols from nonintervention foods and drinks. The subgroups based on polyphenol dose, anthocyanin dose, and duration were defined after inspection of the characteristics of the included studies, but all subgroups were defined independent of outcome results. Differences in treatment effects across subgroups were tested using the χ2Q statistic (25).
Four sensitivity analyses were performed to examine whether changes in the analysis would affect overall conclusions. First, pooled effects were recalculated using the DerSimonian–Laird method, which assumes random effects instead of fixed effects (26). Second, analyses were rerun based on change-from-baseline scores, instead of postintervention scores, because using change-from-baseline scores can increase statistical power (20). In instances in which change-from-baseline scores were not accessible, we used postintervention scores, as in the main analysis. Third, we examined the sensitivity of the results to changes in included studies by rerunning results with 1 study excluded at a time. Fourth, the effect on FBG was recalculated after excluding crossover trials, as these can be biased by carryover effects (22). These sensitivity analyses were not prespecified in the original protocol, but the first sensitivity analysis (assumption of random effects) was specified in the revised protocol. The 3 other sensitivity analyses were not prespecified.
All statistical analyses were performed in Stata (version 15). Forest plots were made using the user-written forestplot command.
Results
Literature search
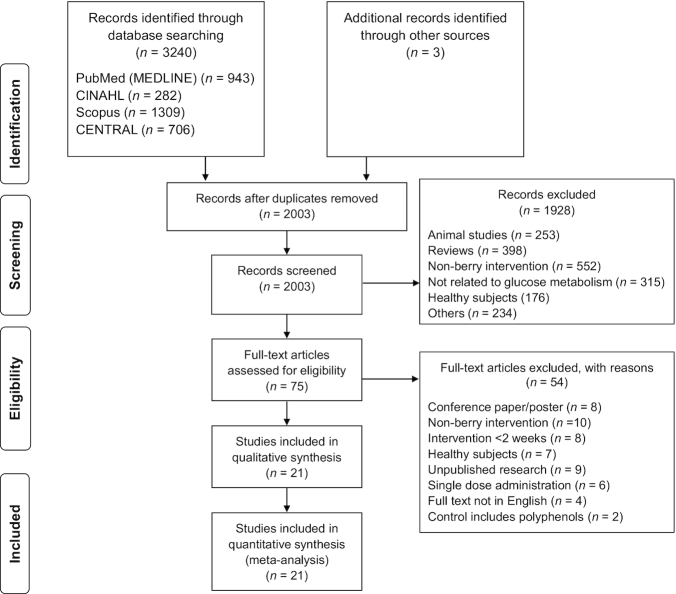
The detailed process of study selection is shown in Figure 1. The initial search yielded 3240 publications, and 3 articles were identified by hand search. Removal of duplicates resulted in 1240 articles being removed. The title and abstract of the remaining 2003 articles were screened, and this resulted in 75 articles for full-text review. An additional 54 articles were excluded for the following reasons: the full text was unavailable because it was a conference abstract or poster or because the study results were unpublished, the polyphenols used in the intervention were not of berry origin or were from a mixture of antioxidant-rich fruits, the study duration was less than the required minimum of 2 wk, the participants were healthy, only a single dose of polyphenol was administered, the full text was not available in English, or the control treatment contained polyphenols. With these exclusions, a total of 21 articles were included in the qualitative synthesis and in the meta-analysis.
FIGURE 1.
Flow diagram of included studies for the systematic review and meta-analysis of the effect of berry polyphenols on glucose metabolism. CENTRAL, Cochrane Central Register of Controlled Trials.
Study characteristics
The characteristics of the included studies are outlined in Table 1. The studies were published between 2006 and 2019 in the United States (n = 7), Iran (n = 5), China (n = 2), and a single study in each of the following countries: United Kingdom, Canada, India, Iraq, Korea, Russia, and Taiwan. The duration of the polyphenol intervention ranged from 4 to 24 wk.
TABLE 1.
Characteristics of the studies included in the systematic review and meta-analysis of the effect of berry polyphenols on glucose metabolism in adults with impaired glucose tolerance or insulin resistance1
| Intervention | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author and year (ref) | Country | RCT design | Participants | n | % men | Age,2 y | Type and dose | Polyphenol dose3 | Anthocyanin dose4 | Control treatment | Duration (wk) |
| Abidov 2006 (46) | Russia | 2-arm parallel | Type 2 DM | 42 | 0 | 46 | Blueberry leaf extract (750 mg/d tablets) | – | – | Unspecified placebo | 4 |
| An 2016 (41) | Korea | 3-arm parallel | Prediabetes | 39 | 30 | 20–80 | Black raspberry extract (900 or 1800 mg/d capsules) | – | – | Placebo capsules | 12 |
| Basu 2010 (27) | United States | 2-arm parallel | Metabolic syndrome | 48 | 8 | 50 | Dried blueberry powder beverage (equivalent to 350 g/d fresh berries or 50 g/d dried) | – | – | Water (960 mL/d) | 8 |
| Curtis 2019 (28) | United Kingdom | 3-arm parallel | Metabolic syndrome | 115 | 68 | 50–75 | Dried blueberry powder incorporated into a variety of food options (equivalent to 75 or 150 g/d of fresh berries; or 13 or 26 g/d dried) | 439 or 879 mg/d | 182 or 364 mg/d | Placebo powder identical in appearance, taste, and calorie and carbohydrate content | 24 |
| Dohadwala 2011 (29) | United States | Crossover | Coronary artery disease with high FBG | 44 | 68 | 62 | Cranberry juice (480 mL/d, 54% juice) | 835 mg/d | 94 mg/d | Placebo beverage identical in appearance, taste, and calorie content | 4 (berry) and 4 (control) (2 washout) |
| Kianbakht 2013 (42) | Iran | 2-arm parallel | Type 2 DM | 74 | 53 | 40–60 | Caucasian whortleberry extract (848 mg/d capsules) | – | 9.1 mg/d | Placebo capsules | 8 |
| Kim 2018 (37) | United States | 2-arm parallel | Metabolic syndrome | 37 | 30 | 18–65 | Acai berry beverage (equivalent to 162.5 g/d fresh berries) | 740.4 mg/d | 199.6 mg/d | Placebo beverage artificially colored and flavored | 12 |
| Lee 2008 (43) | Taiwan | 2-arm parallel | Type 2 DM | 30 | 16 | 50–75 | Cranberry extract (1500 mg/d capsules) | – | – | Placebo capsules | 12 |
| Li 2015 (38) | China | 2-arm parallel | Type 2 DM | 58 | 59 | 56–67 | Purified anthocyanins from mulberry and blackcurrant capsules (equivalent to 100 g/d fresh berries) | – | 320 mg/d | Placebo capsules | 24 |
| Al-Juhaishi 2018 (36) | Iraq | 2-arm parallel | Type 2 DM | 60 | 35–63 | Cranberry extract (500 mg/d capsules) | – | – | Nothing | 16 | |
| Mirfeizi 2016 (39) | Iran | 3-arm parallel | Type 2 DM | 75 | 27 | 30–65 | Caucasian whortleberry extract (1000 mg/d capsules) | – | 9.8 mg/d | Placebo capsules | 12 |
| Moazen 2013 (30) | Iran | 2-arm parallel | Type 2 DM | 36 | 36 | 35–60 | Dried strawberry powder drink (equivalent to 500 g/d fresh berries or 50 g/d dried) | 2006 mg/d | 154 mg/d | Placebo powder identical in flavor and macronutrients | 6 |
| Paquette 2017 (31) | Canada | 2-arm parallel | Overweight or obese and insulin resistant | 41 | 44 | 40–70 | Strawberry and cranberry extract beverage (112 g/d fresh berries) | 333 mg/d | – | Placebo beverage with matching taste and visual aspect | 6 |
| Riche 2017 (47) | United States | 2-arm parallel | Type 2 DM | 17 | 42 | 57 | Mulberry leaf extract (3000 mg/d capsules) | – | – | Placebo capsules | 12 |
| Schell 2019 (35) | United States | Crossover | Type 2 DM and elevated waist circumference | 22 | 20 | 54 | Frozen raspberries (equivalent to 250 g/d fresh berries) | 343 mg/d | 225 mg/d | Nothing | 4 (berry) and 4 (control) (2 washout) |
| Shidfar 2012 (44) | Iran | 2-arm parallel | Type 2 DM | 58 | 100 | 55 | Cranberry juice (240 mL/d) (% juice not reported) | – | – | Strawberry-flavored water | 12 |
| Stull 2015 (32) | United States | 2-arm parallel | Metabolic syndrome | 44 | 36 | 57 | Smoothie with blueberry power (equivalent to 280 g/d fresh berries or 45 g/d dried) | 1547.2 mg/d | 580.6 mg/d | Placebo smoothie identical in appearance, taste, and macronutrient content | 6 |
| Stull 2010 (33) | United States | 2-arm parallel | Obese, insulin-resistant adults | 32 | 16 | 51 | Smoothie with blueberry power (equivalent to 280 g/d fresh berries or 45 g/d dried) | 1462 mg/d | 668 mg/d | Placebo smoothie of equal sensory and nutritional value | 6 |
| Taghizadeh 2017 (40) | Iran | 2-arm parallel | Diabetic nephropathy | 60 | 23 | 45–85 | Mulberry extract (300 mg/d capsules) | – | – | Placebo supplement | 12 |
| Usharani 2013 (45) | India | 3-arm parallel | Type 2 DM | 60 | 66 | 30–68 | Indian gooseberry extract (500 or 1000 mg/d capsules) | – | – | Placebo capsule | 12 |
| Yang 2017 (34) | China | 2-arm parallel | Prediabetes or early untreated diabetes | 160 | 34 | 40–75 | Purified anthocyanins from bilberries and blackcurrants (capsules) | – | 320 mg/d | Placebo capsules | 12 |
DM, diabetes mellitus; FBG, fasting blood glucose; RCT, randomized controlled trial.
Values are minimum–maximum or mean.
Values are reported as total phenolics/polyphenols or as gallic acid equivalents.
Values are presented as total anthocyanins or as cyanidin-3-O-glucoside equivalents.
A total of 1172 participants were included in the 21 eligible studies, with 17–160 participants each. There was a mixture of both men and women in all studies with the exception of 1 study that used women participants only and another study for which the converse was true. Participants were aged 18 y or older, and their medical conditions included type 2 DM/prediabetes (n = 13), metabolic syndrome (n = 4), coronary artery disease with elevated FBG (n = 1), overweight/obesity and insulin resistance (n = 2), and diabetic nephropathy (n = 1).
The berry supplements used in the trials were of several forms, including fresh whole fruits, dried fruit powder, juice (neat and diluted), crude extracts, and purified isolates (Table 1). Most of these supplements were derived from blueberries (n = 5), cranberries (n = 4), raspberries (n = 2), Caucasian whortleberry (n = 2), or mulberries (n = 2). Each of the following berries was used by 1 study: acai berries, strawberries, and gooseberries. One study used a mixture of bilberries and blackcurrants, another used a mixture of mulberries and blackcurrants, and yet another used a combination of strawberries and cranberries.
Supplements were administered in the form of tablets (n = 1), capsules (n = 10), powders from dried fruits or extracts (n = 7) (which were incorporated into beverages or other food items), juices (n = 2), and frozen whole fruits (n = 1). Where polyphenol (n = 8) and anthocyanin (n = 11) contents were reported, daily intake was 333–2006 mg and 9–668 mg, respectively. Of the included 21 studies, 8 clearly outlined an imposition on any sources of polyphenol-rich foods in the diet throughout the trial (27–29, 30, 31, 32, 33, 34), whereas 1 study imposed a polyphenol restriction in the diet 48 h before each study visit (35). In addition, of the included studies that reported FBG outcome, all except 7 (29, 32, 36, 37, 38, 39, 40) placed restrictions on the use of medications affecting glucose metabolism or insulin.
Funding sources varied across the studies (Supplemental Table 1). Eight studies received nonindustry funding from universities, governments, or nonprofit organizations (30, 34, 39, 40, 41, 42, 43, 44), 2 received industry funding (28, 45), and 9 received both industry and nonindustry funding (27, 29,31,32, 33, 35, 38, 46, 47). Two studies did not disclose their source of funding (36, 37).
Supplemental Tables 2–9 provide the outcome data that were extracted for the meta-analysis. Postintervention means were available in 17 studies, and change-from-baseline means were available for 10 studies (6 studies reported both).
Data quality
The risk of bias assessment for all included studies is summarized graphically in Supplemental Figure 1. The individual parameters that constitute the risk of bias assessment revealed that there was a mixture of low (n = 9) and unclear (n = 12) risks of bias for the methods used to generate the randomization sequence. For the methods used to assess allocation concealment (selection bias) and the blinding of participants and personnel (performance bias), there were an equal number of low (n = 9), unclear (n = 9), and high risks of bias (n = 3) for both parameters for the 21 studies. Detection bias recorded the highest level of risk, with >25% of the included studies receiving a high risk of bias for the blinding of outcome assessment. One study had an unclear risk of attrition bias, and another had an unclear risk of reporting bias. No other biases were found within the studies. The details of the risk of bias assessment are outlined in Supplemental Table 10.
FBG
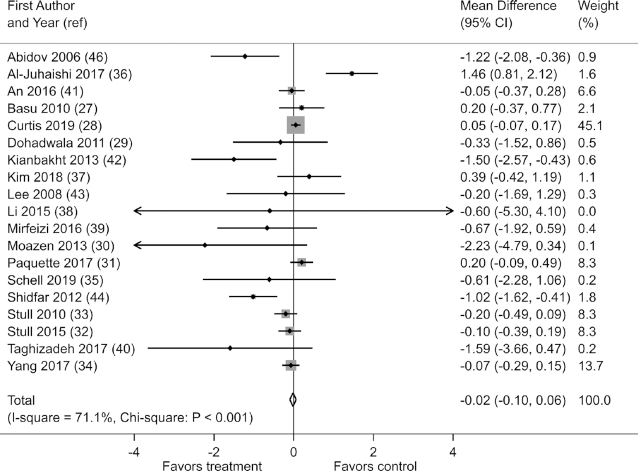
Effects on FBG were reported in 19 of the 21 studies. When the results of these studies were pooled, berry polyphenol consumption did not significantly reduce FBG [fixed-effects mean difference (MD): −0.02 mmol/L; 95% CI: −0.10, 0.06; I2 = 71.1%; χ2: P < 0.001)] (Figure 2). Similar results were observed in a sensitivity analysis assuming random effects (random-effects MD: −0.13 mmol/L; 95% CI: −0.34, 0.08; I2 = 71.1%; χ2: P < 0.001), in sensitivity analysis excluding crossover studies (fixed-effects MD: −0.01 mmol/L; 95% CI: −0.09, 0.07; I2 = 74.0%; χ2: P < 0.001), and in a sensitivity analysis of change-from-baseline scores (Supplemental Table 11).
FIGURE 2.
Effects of berry polyphenol consumption on fasting blood glucose (millimoles per liter) in adults with impaired glucose tolerance or insulin resistance. The total effect was estimated using fixed effect, inverse variance weighting.
Effects on FBG were considerably heterogeneous among studies in the main analysis (I2 = 71.1%; χ2: P < 0.001). This heterogeneity could not be explained, however, because subgroup analyses did not show significant differences in effect by study characteristics. A subgroup analysis by polyphenol dose was not possible to conduct because only 9 studies reported both polyphenol dose and effects on FBG. Nevertheless, among studies that reported polyphenol dose, those that used higher doses of polyphenol did not show significantly greater effects on FBG [low-dose MD: 0.18 mmol/L (95% CI: −0.10, 0.45); medium-dose MD: 0.06 mmol/L (95% CI: −0.01, 0.17); high-dose MD: −0.16 mmol/L (95% CI: −0.36, 0.03); P = 0.85 for interaction] (Supplemental Figure 2). A similar result was obtained when the studies were grouped by anthocyanin dose [low-dose MD: 0.02 mmol/L (95% CI: −0.09, 0.14); high-dose MD: −0.11 mmol/L (95% CI: −0.26, 0.03); P = 0.62 for interaction] (Supplemental Figure 3). Significant differences in effect on FBG were not observed in studies with a high or unclear risk of bias on at least 1 parameter [low risk of bias on all parameters: MD: 0.03 mmol/L (95% CI: −0.06, 0.13); high/unclear risk of bias on at least 1 parameter: MD: −0.13 mmol/L (95% CI: −0.27, 0.02); P = 0.85 for interaction], in studies that imposed restriction on the use of medications affecting glucose metabolism or insulin [medication restriction: MD: −0.03 mmol/L (95% CI: −0.12, 0.05); no medication restriction: MD: 0.10 mmol/L (95% CI: −0.13, 0.33); P = 0.33 for interaction], in studies using different forms of berry polyphenol [fresh/dried berries: MD: 0.00 mmol/L; (95% CI: −0.09, 0.10); berry extract: MD: 0.05 mmol/L (95% CI: −0.13, 0.24); P = 0.06 for interaction], in studies of different durations [duration <12 wk: MD: −0.10 mmol/L (95% CI: −0.25, 0.05); duration ≥12 wk: MD: 0.02 mmol/L (95% CI: −0.08, 0.11); P = 0.54 for interaction], or in studies restricting polyphenol intake from nonintervention foods and drinks [polyphenol restriction: MD: 0.00 mmol/L (95% CI: −0.08, 0.09); no polyphenol restriction: MD: −0.15 mmol/L (95% CI: −0.37, 0.06); P = 0.55 for interaction] (Supplemental Figures 4–8). A funnel plot suggested that smaller studies reported stronger effects on FBG (Supplemental Figure 9), although this observation was not confirmed by Egger's test (P = 0.12).
HbA1c
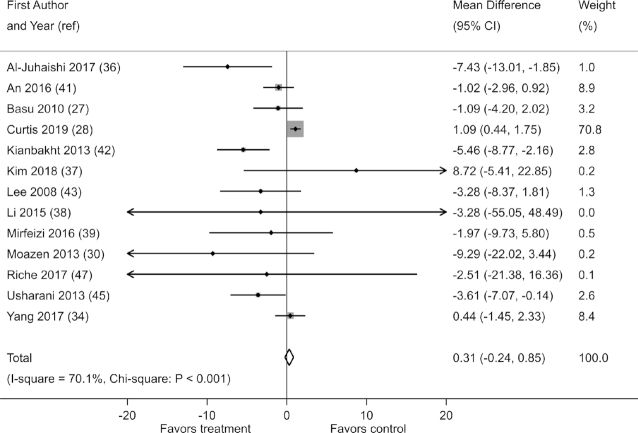
Effects on HbA1c were reported in 13 of the 21 studies. When pooled, these studies demonstrated no reduction in HbA1c with berry polyphenol consumption (fixed-effects MD: 0.31 mmol/mol; 95% CI: −0.24, 0.85) (Figure 3). However, there was substantial heterogeneity in results (I2 = 70.1%; χ2: P < 0.001), and smaller studies tended to report stronger effects, as evidenced by a funnel plot and Egger's test (P = 0.018) (Supplemental Figure 10).
FIGURE 3.
Effect of berry polyphenol consumption on HbA1c (millimoles per liter) in adults with impaired glucose tolerance or insulin resistance. The total effect was estimated using fixed-effect, inverse-variance weighting.
No effect on HbA1c was observed when studies of durations <12 wk (3 of 13 studies) were excluded (fixed-effects MD: 0.55 mmol/mol; 95% CI: −0.02, 1.11; I2 = 62.5%; χ2: P = 0.004). Sensitivity analyses demonstrated small but statistically significant reductions in HbA1c when random effects were assumed (random-effects MD: −1.83 mmol/mol; 95% CI: −3.56, −0.11; I2 = 70.1%; χ2: P < 0.001), when change-from-baseline scores were used (fixed-effects MD: −1.26 mmol/mol; 95% CI: −1.84, −0.67; I2 = 75.3%; χ2: P < 0.001) (Supplemental Table 11), and when the trial by Curtis et al. (28) was excluded (fixed-effects MD: −1.60 mmol/mol; 95% CI: −2.61, −0.60; I2 = 46.5%; P = 0.04). When the trial by Curtis et al. (28) was excluded, Egger's test no longer showed significant small-study effects (P = 0.28).
Fasting blood insulin
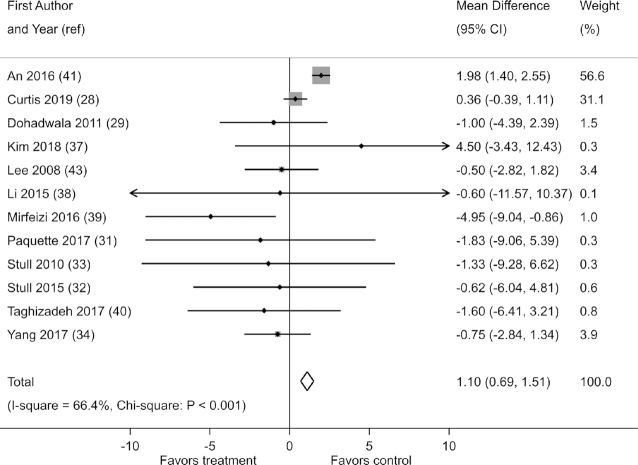
Effects on fasting blood insulin were reported in 12 of the 21 studies. When pooled, these studies showed that berry polyphenol consumption slightly increased fasting blood insulin (fixed-effects MD: 1.10 µIU/mL; 95% CI: 0.69, 1.51; I2 = 66.4%; χ2: P < 0.001) (Figure 4). Such an effect was, however, not confirmed in a sensitivity analysis based on the assumption of random effects (random-effects MD: −0.15 µIU/mL; 95% CI: −1.34, 1.04; I2 = 66.4%; χ2: P < 0.001). A similar discrepancy in the results of fixed-effect and random-effect analyses was observed in a sensitivity analysis of change-from-baseline scores (Supplemental Table 11). Excluding the trial by An et al. (41) weakened the evidence of an effect on fasting blood insulin (fixed-effects MD: −0.04 µIU/mL; 95% CI: −0.66, 0.59; I2 = 56%; χ2: P = 0.44).
FIGURE 4.
Effect of berry polyphenol consumption on fasting blood insulin (micro international units per milliliter)in adults with impaired glucose tolerance or insulin resistance. The total effect was estimated using fixed-effect, inverse-variance weighting.
In the main analysis, there was substantial heterogeneity in effect estimates (I2 = 66.4%; χ2: P < 0.001) (Figure 4). A funnel plot and Egger's test showed evidence of greater effects in small studies (P = 0.04) (Supplemental Figure 11).
HOMA-IR
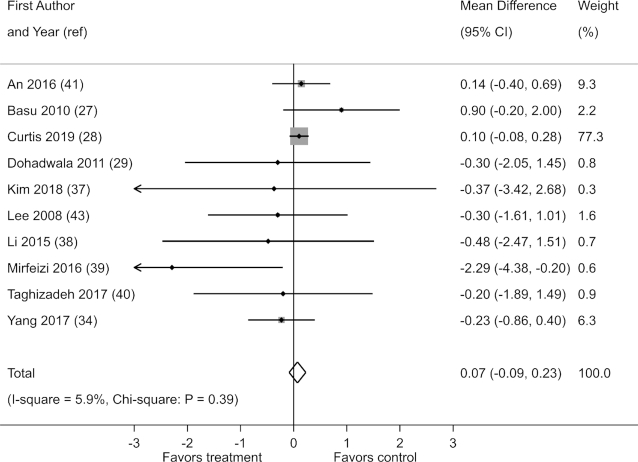
Results for HOMA-IR were reported in 10 of 21 studies. When the results of the studies were pooled, there was no evidence that berry polyphenol consumption reduces HOMA-IR (fixed-effects MD: 0.07; 95% CI: −0.09, 0.23) (Figure 5). There was also no evidence of heterogeneity (I2 = 5.9%; χ2: P = 0.39).
FIGURE 5.
Effect of berry polyphenol consumption on HOMA-IR in adults with impaired glucose tolerance or insulin resistance. The total effect was estimated using fixed-effect, inverse-variance weighting.
There was no reduction in HOMA-IR in a sensitivity analysis based on the assumption of random effects (random-effects MD: 0.05; 95% CI: −0.16, 0.26; I2 = 5.9%; χ2: P = 0.39). Furthermore, no reductions in HOMA-IR were observed in a sensitivity analysis of change-from-baseline scores (Supplemental Table 11). No statistically significant effect was observed when any 1 study was excluded from the analysis (data not shown). A funnel plot and Egger's test showed no evidence of greater effects on HOMA-IR in small studies (P = 0.20) (Supplemental Figure 12).
Postprandial blood glucose
Effects on postprandial blood glucose were reported in 5 of 21 studies. These studies showed substantial heterogeneity in effects (I2 = 92.9%; χ2: P < 0.001) (Figure 6). When pooled, the studies showed a reduction in postprandial blood glucose with berry polyphenol consumption (fixed-effects MD: −0.92 mmol/L; 95% CI: −1.41, −0.42). However, this result was not confirmed in a sensitivity analysis assuming random effects (random-effects MD: −0.60 mmol/L; 95% CI: −2.59, 0.39; I2 = 92.9%; χ2: P < 0.001). A discrepancy between the results of fixed-effects and random-effects analyses was also observed in a sensitivity analysis of change-from-baseline scores (Supplemental Table 11). Furthermore, there was no evidence that berry polyphenol consumption decreases postprandial blood glucose when the trial by Kianbacht et al. (42) was excluded (fixed-effects MD: 0.53 mmol/L; 95% CI: −0.12, 1.19; I2 = 70.9%; χ2: P = 0.016). Due to the small number of studies, a funnel plot and Egger's test did not provide meaningful information about small-study effects (P = 0.59) (Supplemental Figure 13).
FIGURE 6.
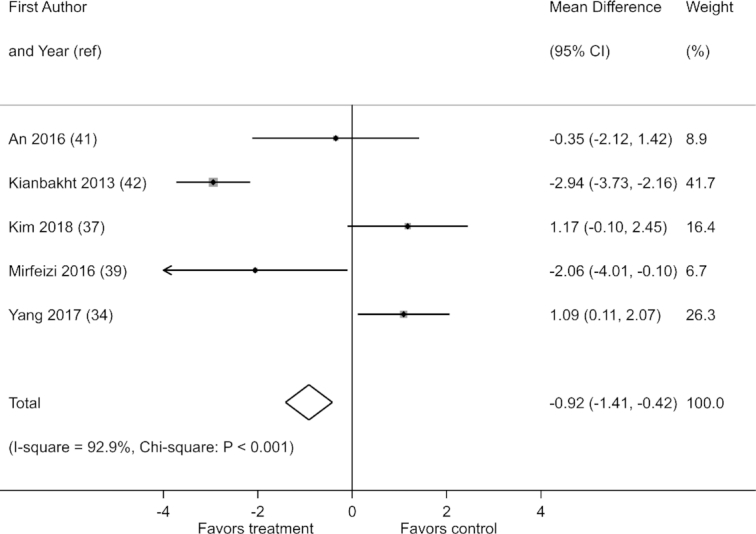
Effect of berry polyphenol consumption on postprandial blood glucose (millimoles per liter) in adults with impaired glucose tolerance or insulin resistance. The total effect was estimated using fixed-effect, inverse-variance weighting.
Postprandial insulin
None of the 21 studies reported effects on postprandial insulin.
Waist circumference
Effects on waist circumference were reported in 5 of the 21 studies. When pooled, these studies showed an increase in waist circumference with berry polyphenol consumption (fixed effects MD: 1.13 cm; 95% CI: 0.10, 2.17; I2 = 59.4%; χ2: P = 0.04) (Supplemental Figure 14). There was no significant evidence of such an effect in a sensitivity analysis based on random effects (random-effects MD: 1.04 cm; 95% CI: −0.93, 3.01; I2 = 59.4%; χ2: P = 0.04). A similar discrepancy between the results of fixed-effect and random-effect analyses was observed in a sensitivity analysis of change-from-baseline scores (Supplemental Table 11). Furthermore, no increase in waist circumference with berry polyphenol consumption and no heterogeneity were observed when the trial by An et al. (41) was excluded (fixed-effects MD: −0.12 cm; 95% CI: −1.48, 1.25; I2 = 0%; χ2: P = 0.52). Due to the small number of studies, a funnel plot was difficult to interpret but suggested no skewness (Supplemental Figure 15), which was confirmed by Egger's test (P = 0.89).
Weight
Effects on weight were reported in 7 of 21 studies. When pooled, these studies did not show a statistically significant reduction in weight with berry polyphenol supplementation (fixed-effects MD: −0.24 kg; 95% CI: −0.87, 0.38; I2 = 53.0; χ2: P = 0.05) (Supplemental Figure 16). Similar results were observed in sensitivity analyses based on random effects (random-effects MD: −0.52 kg; 95% CI: −2.09, 1.05; I2 = 53.0; χ2: P = 0.05) and on change-from-baseline scores (Supplemental Table 11) and exclusion of any 1 study (data not shown). There was moderate heterogeneity in effects (I2 = 53.0; χ2: P = 0.05). A funnel plot and Egger's test did not show evidence of small-study effects (P = 0.37) (Supplemental Figure 17).
BMI
Effects on BMI were reported in 9 of the 21 studies. Berry polyphenol consumption did not significantly reduce BMI (fixed-effects MD: −0.13; 95% CI, −0.30, 0.04; I2 = 11.9%; χ2: P = 0.34) (Supplemental Figure 18). This result was confirmed in sensitivity analyses based on random-effects (random-effects MD: −0.14; 95% CI: −0.35, 0.07; I2 = 11.9%; χ2: P = 0.34) or change-from-baseline scores (Supplemental Table 11). However, a significant reduction in BMI was observed when the study by Stull et al. (32) was excluded from the analysis (fixed-effects MD: −0.27; 95% CI: −0.49, −0.06; I2 = 0%; χ2: P = 0.69). Smaller studies did not show greater effects on BMI, as evidenced by a funnel plot and Egger's test (P = 0.81) (Supplemental Figure 19).
Discussion
This meta-analysis did not show clear evidence that berry polyphenol consumption improves glucose metabolism compared with placebo or no treatment in individuals with impaired glucose tolerance or insulin resistance. The results for most biomarkers were unstable, varying with the type of analysis performed and with the specific trials included in the analysis. Furthermore, none of these analyses showed an effect great enough to be of clinical relevance. Unstable results were also observed for weight-related outcomes.
The lack of effect on FBG is consistent with the results of a meta-analysis by Daneshzad et al. (16) but not with a meta-analysis by Huang et al. (17), which showed a reduction in FBG with berry interventions. Compared with Huang et al. (17), we included a larger number of trials (19 compared with 10), and we restricted the analysis to trials conducted in high-risk populations (patients with impaired glucose metabolism or insulin resistance). Such a restriction would be expected to produce a greater, not a weaker, effect. Furthermore, in the meta-analysis by Huang et al. (17), the mean effect on FBG was only 0.1 mmol/L in a healthier population. Thus, there is at best weak evidence that berry consumption reduces FBG, and the magnitude of this effect is too small to be of clinical relevance.
Although our main analysis did not show an effect on HbA1c, small reductions of 1.26–2.62 mmol/mol (0.12–0.24%) were observed in sensitivity analyses. Similarly, 3 previous meta-analyses reported that berry supplements, anthocyanins, or polyphenols (the latter 2 not limited to berry origin) reduced HbA1c by 0.20–0.53% (16–18), although with borderline significance in 1 of the meta-analyses (16). The similarity between these results and our results cannot be explained by an overlap in included trials because the largest of the previously stated meta-analyses (18) included 36 trials, but none of the 12 we included. Although the same meta-analysis showed evidence of publication bias, this was not clear in our analysis. Overall, the results of our and previous meta-analyses suggest that berry consumption can reduce HbA1c, and the changes seen are similar to those seen in blood glucose. The more stable effect on HbA1c is probably related to the fact that there is greater day-to-day variability in blood glucose. Yet, as for blood glucose, the effects on HbA1c are too small to be of clinical relevance.
The absence of effect on HOMA-IR is not consistent with the results of a previous meta-analysis, in which Daneshzad et al. (16) found that pure anthocyanin supplementation reduces HOMA-IR in adults. A direct comparison with our results is not possible because we examined the effects of polyphenols and not only anthocyanins, and these polyphenols were from berries only. However, we included a larger number of trials (12 instead of 3), and our results were confirmed in 3 sensitivity analyses. For these reasons, the evidence that berry polyphenol consumption reduces HOMA-IR is not compelling.
The unstable effect on fasting blood insulin in the current meta-analysis is consistent with the results of a previous meta-analysis of pure anthocyanin supplementation, which did not show strong evidence of an effect (16). Our analysis also showed an unstable effect on postprandial blood glucose, but this outcome was reported in only 4 trials. The 3 previously mentioned meta-analyses did not study effects on postprandial blood glucose (16–18), and currently available data are too limited to permit a conclusion about the effects of berry consumption on this biomarker. The same is true for postprandial blood insulin, which was neither reported in any of the trials we included nor examined in previous meta-analyses.
The lack of clear effects on glucose metabolism could not be explained by limited sample sizes or a limited number of published trials (i.e., statistical imprecision) because CIs were narrow for most pooled effects. A different explanation could be that effects depend on trial characteristics, as we observed considerable heterogeneity in effects on several outcomes. However, we could not find an explanation for this heterogeneity, despite conducting several subgroup analyses. Nevertheless, there were slightly, but not significantly, greater effects on FBG in studies that administered high doses of polyphenol. Slightly but not significantly greater effects were also observed in studies that had a high or unclear risk of bias on at least 1 parameter. We found no evidence of greater effects on FBG in trials of longer durations, trials that restricted polyphenol consumption from nonintervention sources, trials that restricted the use of medication that can impact glucose metabolism, or in trials administering extracts instead of fresh or dried berries. A possible source of heterogeneity that we did not explore is variation in levels of glycemic dysfunction across trials.
In contrast to the lack of clinically relevant effects in our meta-analysis, basic research has provided strong evidence that berry polyphenols have positive effects on glucose metabolism (11–13). Common berry polyphenols such as kaempferol, rutin, quercetin, and anthocyanins of the cyanidin, pelargonidin, and delfinidin types have been reported to impact glucose metabolism via various mechanisms (48–51). These flavonoids have demonstrated their ability to increase insulin secretion, insulin sensitivity, and glucose uptake, and these effects have been exhibited using various models. Some of the most commonly reported mechanisms through which they are able to elicit these effects include their ability to protect pancreatic β-cells by decreasing oxidative stress, prevent β-cell death via the mitochondrial pathway and NF-κB signaling, reduce caspase-3 activity in β-cells, improve cAMP signaling, lower TNF-α production, and influence the downregulation of inflammatory adipocytokines (48–53). Berry polyphenols have also been reported to be able to inhibit dipeptidyl-peptidase 4 and hyperglycemia, inhibit enzymes involved in the breakage of glucosidic linkages in carbohydrates, and inhibit intestinal glucose transporters (54–62). They have also been reported to increase the population of specific gut microbiota, which can improve glycemic control, and scavenge reactive oxygen species, which are involved in the progression of oxidative stress and the promotion of inflammatory processes, which in turn are associated with diabetes (63–66). The physicochemical properties of the compounds (among other factors), however, cause low solubility, poor stability, inadequate permeation rates, or low bioavailability, and as a result, the translation of effects in basic research to clinical outcome is challenging (67, 68).
Although the role of berry polyphenols in glucose metabolism is well documented in experimental research, the possibility of nonphenolic berry phytochemicals in crude plant extracts also impacting glucose metabolism cannot be ignored. In our analysis, 3 of the studies (46, 42, 45) that used fruit or leaf extracts as treatment reported the extracting solvent used, which in all 3 cases was polar (aqueous/hydroalcoholic). Some triterpenoids and the iminosugar, 1-deoxynojirimycin (present in mulberry leaves), are soluble in a polar medium and have also been reported to convey hypoglycemic or antidiabetic effects (69–72). This indicates that their interference in the studies that utilized berry extracts as treatment is a possibility. For the 2 studies that used pure anthocyanin isolates (38, 34), the possibility of this interference is nullified.
A limitation of the current meta-analysis is that many trials did not report polyphenol doses, and so it was difficult to search for a dose–response relation. A second and related limitation is that most trials did not examine the effects of pure berry polyphenol, so our analysis provides only indirect evidence of the effects of berry polyphenol. A third limitation is that there were not enough data to examine whether the effects of different types of berries differed; in addition, the duration of the included studies was relatively short, with a range of 4–24 wk. A fourth limitation is the limited information on the carbohydrate content of treatments administered, meaning that it was not possible to examine whether this impacted the outcome on biochemical variables linked to glucose metabolism. A final limitation is that the results of the review rely on the results of published studies, so any limitations of these studies are necessarily limitations of our review as well. The strengths of the current meta-analysis include the restrictions on study design (to only RCTs) and study population (to high-risk individuals), which are likely to increase the quality of evidence and the likelihood that any existing effects on glucose metabolism would have been detected. Other strengths include the relatively large number of trials included in the analyses of most main outcome measures and the large number of sensitivity analyses conducted, altogether increasing the reliability of the results.
In conclusion, this meta-analysis did not show statistically significant or clinically meaningful effects of berry polyphenol consumption on most biomarkers of glucose metabolism. Although some analyses did show a significant effect on HbA1c, these effects were also too small to be of clinical relevance. This conclusion is further supported by the fact that higher doses also were not associated with statistically significant or clinically meaningful effects. Future studies should examine whether supplements (as crude materials or pure isolates) with higher bioavailability are needed to observe an effect on glucose metabolism.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—TFR and AN: designed and conducted the review (conducted the systematic search and screened the articles); TFR and JB: extracted the data and drafted the paper; JB: performed the statistical analyses; PN and AN: critically reviewed the manuscript; TFR: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by the Kempe Foundation (grant SMK-1870). The funder played no role in the design, implementation, analysis, or interpretation of the data.
Author disclosures: The authors report no conflicts of interest.
Supplemental Methods, Supplemental Tables 1–11, and Supplemental Figures 1–19 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Data described in the manuscript will be made publically and freely available upon request without restriction.
Abbreviations used: DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; MD, mean difference; RCT, randomized controlled trial.
Contributor Information
Theresa F Rambaran, Email: theresa.rambaran@umu.se, Department of Public Health and Clinical Medicine, Section of Sustainable Health, Umeå University, Umeå, Sweden.
Jonathan Bergman, Department of Community Medicine and Rehabilitation, Unit of Geriatric Medicine, Umeå University, Umeå, Sweden.
Peter Nordström, Department of Community Medicine and Rehabilitation, Unit of Geriatric Medicine, Umeå University, Umeå, Sweden.
Anna Nordström, Department of Public Health and Clinical Medicine, Section of Sustainable Health, Umeå University, Umeå, Sweden; School of Sport Sciences, UiT Arctic University of Norway, Tromsö, Norway.
References
- 1. Piemonte L. About diabetes: type 2 diabetes. [Internet] Brussels (Belgium): International Diabetes Federation; 2019; [cited Aug 29, 2019; updated Jan 20, 2020]. Available from: https://idf.org/aboutdiabetes/type-2-diabetes.html. [Google Scholar]
- 2. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. [DOI] [PubMed] [Google Scholar]
- 3. WHO. Fact sheets: diabetes. [Internet] 2019; [cited Jul 13, 2019]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. [Google Scholar]
- 4. Ovaskainen M-L, Törrönen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138:562–6. [DOI] [PubMed] [Google Scholar]
- 5. El Gharras H. Polyphenols: food sources, properties and applications—a review. Int J Food Sci Technol. 2009;44:2512–8. [Google Scholar]
- 6. Campbell TF, McKenzie J, Murray J, Delgoda R, Bowen-Forbes CS. Rubus rosifolius varieties as antioxidant and potential chemopreventive agents. J Funct Foods. 2017;37:49–57. [Google Scholar]
- 7. Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87:323–31. [DOI] [PubMed] [Google Scholar]
- 8. Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–88. [Google Scholar]
- 9. Häkkinen S, Heinonen M, Kärenlampi S, Mykkänen H, Ruuskanen J, Törrönen R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res Int. 1999;32:345–53. [Google Scholar]
- 10. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anhê FF, Desjardins Y, Pilon G, Dudonné S, Genovese MI, Lajolo FM, Marette A. Polyphenols and type 2 diabetes: a prospective review. PharmaNutrition. 2013;1:105–14. [Google Scholar]
- 13. Edirisinghe I, Burton-Freeman B. Anti-diabetic actions of berry polyphenols—review on proposed mechanisms of action. J Berry Res. 2016;6:237–50. [Google Scholar]
- 14. Fenercioglu AK, Saler T, Genc E, Sabuncu H, Altuntas Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118–24. [DOI] [PubMed] [Google Scholar]
- 15. Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkanen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daneshzad E, Shab-Bidar S, Mohammadpour Z, Djafarian K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38:1153–65. [DOI] [PubMed] [Google Scholar]
- 17. Huang H, Chen G, Liao D, Zhu Y, Xue X. Effects of berries consumption on cardiovascular risk factors: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. 2016;6:23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palma-Duran SA, Vlassopoulos A, Lean M, Govan L, Combet E. Nutritional intervention and impact of polyphenol on glycohemoglobin (HbA1c) in non-diabetic and type 2 diabetic subjects: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2017;57:975–86. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011; [Internet]. Available from: http://www.handbook.cochrane.org. [Google Scholar]
- 21. Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011; [Internet]. Available from: http://www.handbook.cochrane.org. [Google Scholar]
- 22. Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011; [Internet]. Available from: http://www.handbook.cochrane.org. [Google Scholar]
- 23. Baynes JW, Dominiczak MH. Medical biochemistry. Philadelphia (PA): Elsevier; 2018. [Google Scholar]
- 24. Knopp JL, Holder-Pearson L, Chase JG. Insulin units and conversion factors: a story of truth, boots, and faster half-truths. J Diabetes Sci Technol. 2019;13:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konstantopoulos S, Hedges LV. Analyzing effect sizes: fixed-effects models. In: Cooper HHL, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed New York (NY): Russell Sage Foundation; 2009:279–93. [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 27. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, Evans M, Fernandez BO, Meiss MS, Minnion M et al.. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome: results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. 2019;109:1535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE et al.. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial. Ann Nutr Metab. 2013;63:256–64. [DOI] [PubMed] [Google Scholar]
- 31. Paquette M, Medina Larque AS, Weisnagel SJ, Desjardins Y, Marois J, Pilon G, Dudonne S, Marette A, Jacques H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br J Nutr. 2017;117:519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, Johnson WD, Cefalu WT. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2015;7:4107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140:1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, Ling W, Yang Y, Chen Y, Tian Z, Du Z, Chen J, Xie Y, Liu Z, Yang L. Role of purified anthocyanins in improving cardiometabolic risk factors in Chinese men and women with prediabetes or early untreated diabetes: a randomized controlled trial. Nutrients. 2017;9:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schell J, Betts NM, Lyons TJ, Basu A. Raspberries improve postprandial glucose and acute and chronic inflammation in adults with type 2 diabetes. Ann Nutr Metab. 2019;74:165–74. [DOI] [PubMed] [Google Scholar]
- 36. Al-Juhaishi AMR, Mousa TH, Mustafa R, Al-Shehristani RMM. Effect of cranberry in enhancing oral hypoglycemic agents in uncontrolled type-II diabetic patients. J Global Pharma Technol. 2019;10(8):319–24. [Google Scholar]
- 37. Kim H, Simbo SY, Fang C, McAlister L, Roque A, Banerjee N, Talcott ST, Zhao H, Kreider RB, Mertens-Talcott SU. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018;9:3097–103. [DOI] [PubMed] [Google Scholar]
- 38. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145:742–8. [DOI] [PubMed] [Google Scholar]
- 39. Mirfeizi M, Mehdizadeh Tourzani Z, Mirfeizi SZ, Asghari Jafarabadi M, Rezvani HR, Afzali M. Controlling type 2 diabetes mellitus with herbal medicines: a triple-blind randomized clinical trial of efficacy and safety. J Diabetes. 2016;8:647–56. [DOI] [PubMed] [Google Scholar]
- 40. Taghizadeh M, Soleimani A, Bahmani F, Moravveji A, Asadi A, Amirani E, Farzin N, Sharifi N, Naseri A, Dastorani M et al.. Metabolic response to mulberry extract supplementation in patients with diabetic nephropathy: a randomized controlled trial. Iran J Kidney Dis. 2017;11:438–46. [PubMed] [Google Scholar]
- 41. An JH, Kim DL, Lee TB, Kim KJ, Kim SH, Kim NH, Kim HY, Choi DS, Kim SG. Effect of Rubusoccidentalis extract on metabolic parameters in subjects with prediabetes: a proof-of-concept, randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2016;30:1634–40. [DOI] [PubMed] [Google Scholar]
- 42. Kianbakht S, Abasi B, Dabaghian FH. Anti-hyperglycemic effect of Vaccinium arctostaphylos in type 2 diabetic patients: a randomized controlled trial. Complementary Med Res. 2013;20:17–22. [DOI] [PubMed] [Google Scholar]
- 43. Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med. 2008;25:1473–7. [DOI] [PubMed] [Google Scholar]
- 44. Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci. 2012;17:355–60. [PMC free article] [PubMed] [Google Scholar]
- 45. Usharani P, Fatima N, Muralidhar N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab Syndr Obes. 2013;6:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abidov M, Ramazanov A, Jimenez Del Rio M, Chkhikvishvili I. Effect of Blueberin on fasting glucose, C-reactive protein and plasma aminotransferases, in female volunteers with diabetes type 2: double-blind, placebo controlled clinical study. Georgian Med News. 2006;(141):66–72. [PubMed] [Google Scholar]
- 47. Riche DM, Riche KD, East HE, Barrett EK, May WL. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): a randomized, placebo-controlled pilot study. Complement Ther Med. 2017;32:105–8. [DOI] [PubMed] [Google Scholar]
- 48. Dai X, Ding Y, Zhang Z, Cai X, Li Y. Quercetin and quercitrin protect against cytokineinduced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int J Mol Med. 2013;31:265–71. [DOI] [PubMed] [Google Scholar]
- 49. Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin Pharmacol Toxicol. 2006;98:97–103. [DOI] [PubMed] [Google Scholar]
- 50. Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H et al.. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–27. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic β-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670:325–32. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Zhen W, Maechler P, Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J Nutr Biochem. 2013;24:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zunino SJ, Storms DH, Stephensen CB. Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in nonobese diabetic mice. J Nutr. 2007;137:1216–21. [DOI] [PubMed] [Google Scholar]
- 54. Barrett A, Ndou T, Hughey CA, Straut C, Howell A, Dai Z, Kaletunc G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J Agric Food Chem. 2013;61:1477–86. [DOI] [PubMed] [Google Scholar]
- 55. Gonzalez-Abuin N, Martinez-Micaelo N, Blay M, Green BD, Pinent M, Ardevol A. Grape-seed procyanidins modulate cellular membrane potential and nutrient-induced GLP-1 secretion in STC-1 cells. Am J Physiol Cell Physiol. 2014;306:C485–92. [DOI] [PubMed] [Google Scholar]
- 56. Gonzalez-Abuin N, Martinez-Micaelo N, Margalef M, Blay M, Arola-Arnal A, Muguerza B, Ardevol A, Pinent M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014;5:2357–64. [DOI] [PubMed] [Google Scholar]
- 57. Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. [DOI] [PubMed] [Google Scholar]
- 58. Loureiro G, Martel F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: relation with obesity and type 2 diabetes. Food Rev Int. 2019;35:390–406. [Google Scholar]
- 59. McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J Agric Food Chem. 2005;53:2760–6. [DOI] [PubMed] [Google Scholar]
- 60. McDougall GJ, Stewart D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors. 2005;23:189–95. [DOI] [PubMed] [Google Scholar]
- 61. Serrano J, Casanova-Marti A, Gil-Cardoso K, Blay MT, Terra X, Pinent M, Ardevol A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016;7:483–90. [DOI] [PubMed] [Google Scholar]
- 62. Song P, Onishi A, Koepsell H, Vallon V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets. 2016;20:1109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015;2015:850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Esposito D, Chen A, Grace MH, Komarnytsky S, Lila MA. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J Agric Food Chem. 2014;62:7022–8. [DOI] [PubMed] [Google Scholar]
- 65. Grace MH, Esposito D, Dunlap KL, Lila MA. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J Agric Food Chem. 2014;62:4007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li C, Li X, Han H, Cui H, Peng M, Wang G, Wang Z. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: a meta-analysis of randomized, controlled trials. Medicine (Baltimore). 2016;95:e4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Conte R, Calarco A, Napoletano A, Valentino A, Margarucci S, Di Cristo F, Di Salle A, Peluso G. Polyphenols nanoencapsulation for therapeutic applications. J Biomol Res Ther. 2016;5(2). [Google Scholar]
- 68. Sorkin BC, Kuszak AJ, Bloss G, Fukagawa NK, Hoffman FA, Jafari M, Barrett B, Brown PN, Bushman FD, Casper S et al.. Improving natural product research translation: from source to clinical trial. FASEB J. 2020;34:41–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Choi J-W, Yoo Y-M, Kim M-Y, Nam J-H, Nugroho A, Park H-J. Anti-hyperglycemic and anti-hyperlipidemic effects of the triterpenoid-rich fractions from Rubus coreanus and Rubus crataegifolius and their main component, niga-ichigoside F1, in streptozotocin-induced diabetic rats. Nat Prod Sci. 2008;14:260–4. [Google Scholar]
- 70. Gan L, Zhao Y, Zhang J, Jiang F. Isolation and identification of triterpenoids from Rubus alceaefolius Poir. Zhongguo Zhongyao Zazhi. 1998;23:361–2., 383. [PubMed] [Google Scholar]
- 71. Sivakumar G, Vail DR, Nair V, Medina-Bolivar F, Lay JO. Plant-based corosolic acid: future anti-diabetic drug? Biotechnol J. 2009;4:1704–11. [DOI] [PubMed] [Google Scholar]
- 72. Vichasilp C, Nakagawa K, Sookwong P, Higuchi O, Luemunkong S, Miyazawa T. Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction. LWT: Food Sci Technol. 2012;45:226–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.