Abstract
Context
The relevance of hyponatremia has been acknowledged by guidelines from the United States (2013) and Europe (2014). However, treatment recommendations differ due to limited evidence.
Objective
In hyponatremia following pituitary surgery—caused by the syndrome of inappropriate antidiuretic hormone (SIADH) secretion—we compared fluid restriction with the pharmacological increase of water excretion by blocking the vasopressin 2 receptors with tolvaptan at a low and a moderate dose.
Design
Prospective observational study.
Setting
Neurosurgical Department of a University hospital with more than 200 surgical pituitary procedures per year.
Patients
Patients undergoing pituitary surgery and developing serum sodium below 136 mmol/L. The diagnosis of SIADH was established by euvolemia (daily measurement of body weight and fluid balance), inappropriately concentrated urine (specific gravity), and exclusion of adrenocorticotropic and thyroid-stimulating hormone deficiency.
Intervention
Patients were treated with fluid restriction (n = 40) or tolvaptan at 3.75 (n = 38) or 7.5 mg (n = 48).
Main Outcome Measures
Treatment efficacy was assessed by the duration of hyponatremia, sodium nadir, and length of hospitalization. Safety was established by a sodium increment below 10 mmol/L per day and exclusion of side effects.
Results
Treatment with 7.5 mg of tolvaptan resulted in a significant attenuation of hyponatremia and in a significant overcorrection of serum sodium in 30% of patients. The duration of hospitalization did not differ between treatment groups.
Conclusions
Tolvaptan at a moderate dose is more effective than fluid restriction in the treatment of SIADH. Overcorrection of serum sodium may be a side effect of tolvaptan even at low doses.
Keywords: hyponatremia, vasopressin, antidiuretic hormone, fluid restriction, tolvaptan, pituitary surgery
Hyponatremia of any cause represents the most common electrolyte disturbance of hospitalized patients, affecting up to 30% [1, 2]. To date, diagnosis and treatment of hyponatremia are not considered ideal [3, 4], and the respective guidelines established in the United States [5] and Europe [6] document existing controversies resulting from the lack of evidence.
In at least 30% of cases, hyponatremia is a result of the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) [7], first described by Bartter and Schwartz [8]. Antidiuretic hormone (ADH) also known as arginine vasopressin (AVP) is released from the posterior pituitary and responds to increased serum osmolality to retain water in the nephrons. In SIADH, unregulated AVP secretion either from the posterior pituitary gland or an abnormal nonpituitary source results in antidiuresis, eventually resulting in hyponatremia.
Disorders affecting the pituitary and/or the hypothalamus are known to be associated with dysregulation of AVP/ADH secretion, such as SIADH [9, 10]. Adenomas are the most common pituitary tumors, with an overall estimated prevalence of 14% in autopsy studies and 22% in radiological studies [11]. Complications of pituitary surgery comprise anterior pituitary insufficiency of 5% to 19% [12, 13] and, due to the inevitable manipulation of the neurohypophysis, disturbances of water, electrolytes, and osmoregulation, including 18% to 23% presenting with hyponatremia [14-16]. Available data on the pathogenesis of hyponatremia after pituitary surgery point towards a bi- or triphasic release pattern of AVP/ADH. Following an early secretional arrest, an unregulated AVP release from the affected posterior pituitary or from degenerating magnocellular neurons may occur some days after surgery, finally returning to a residual AVP secretion [17]. The timing of delayed hyponatremia is similar to the interphase that is seen after experimentally induced [18] or clinically observed diabetes insipidus [19]. Hyponatremia resulting from impaired aquaresis [20] may be further amplified, since water intake in man is not appropriately reduced, unlike in animals [21].
Abnormal water and electrolyte handling in hyponatremia creates an osmotic gradient promoting the shift of water into brain cells [22], potentially causing cerebral edema [23]. Even in asymptomatic patients, hyponatremia may be responsible for adverse effects like gait instability [24]. Unless hyponatremia is corrected promptly and effectively, morbidity and mortality increase through seizures or elevation in intracranial pressure [25].
Current treatment recommendations for the management of hyponatremia are based on the differentiation of acute and chronic hyponatremia, the underlying etiology, and the existence of symptoms [5], while historically treatment was targeted at relief of symptoms and consisted of fluid restriction [26, 27]. In a single-center study, following pituitary surgery, a universal 1 L per day of fluid restriction for 1 week postoperatively was used quite successfully to prevent hospital readmission due to hyponatremia following early discharge [28]. However, AVP receptor antagonists (vaptans) have been assessed to treat SIADH after surgery for Cushing disease [29]. Given the lack of evidence-based postoperative practices in pituitary surgery regarding SIADH treatment, a recent audit revealed that fluid restriction is used most often (46/74, 62.2%), while a minority of physicians apply additional therapies such as NaCl tablets (25.7%), hypertonic saline (6.7%), or vaptans (17.6%) [30].
Vaptans, the specific AVP receptor antagonists, represent a novel therapeutic option in SIADH. In the kidney, ADH/AVP acts on vasopressin V2 receptors of the principal cells of the cortical and medullary collecting tubules to increase water permeability, thereby mediating an antidiuretic response. While randomized prospective trials show a consistent benefit of vaptans over placebo [31, 32], there exists no head to head trial with fluid restriction [33]. In neurosurgery, and specifically following pituitary surgery, as yet only case reports on the use of vaptans for hyponatremia have been published [16, 34-36].
The purpose of this prospective study was to evaluate the efficacy of fluid restriction versus tolvaptan at a low (3.75 mg) and a moderate (7.5 mg) dose in the treatment of hyponatremia due to SIADH following pituitary surgery with regard to the interval to re-establish normonatremia. While a rapid correction of chronic hyponatremia leaves the patient vulnerable to the risk of osmotic demyelination syndrome (ODS) [37], case reports demonstrate that even a careful correction protocol in hyponatremia following pituitary surgery can be associated with severe neurological deficits due to ODS [38-40]. Hence, safety was assessed by the occurrence of serum sodium overcorrection (>10 mmol/L per 24 hours).
1.Material and Methods
Patients in the Department of Neurosurgery, University of Erlangen-Nürnberg, undergoing surgery for sellar lesions were prospectively included. All procedures performed involving human participants were in accordance with the ethics standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards. The protocol of this study was approved by the local Ethics Committee (Re.-No. 103_12 BC) and has been registered at ClinicalTrials.gov (ID NCT04119206). Informed written consent was given by the patient or the next-of-kin in each case. Exclusion criteria comprised those below 18 years of age, pregnancy, and a drug intolerance.
A.Patients
Baseline information included age, gender, body mass index, and clinical presentation. The preoperative work-up comprised an ophthalmological examination, 1.5-Tesla magnetic resonance imaging with 2-mm axial, coronal, and sagittal sections revealing tumor localization, extension, and invasive behavior. Microsurgery was always performed by the same experienced neurosurgeon (M.B.). Macroscopic delineation and invasiveness, surgical manipulation of the pituitary stalk and extent of tumor resection, surgical complications, and intraoperative cerebrospinal fluid (CSF) leak were documented. The tumor and the dura of the floor of the sella were examined histopathologically, including proliferation rate, regressive changes, or atypical findings. Routine laboratory work-up included creatinine, urea, and alanine aminotransferase.
Pre- and postoperative endocrine testing of the pituitary function comprised basal values of cortisol, growth hormone, insulin-like growth factor 1, thyroid-stimulating hormone (TSH), free thyroxine, total triiodthyronine, luteinizing hormone [41], follicle-stimulating hormone, testosterone [42], estradiol, and prolactin (PRL). Insufficiency of the corticotropic axis was determined by a short synacthen test (adrenocorticotropic hormone; ACTH), stimulated with 0.25 mg of synacthen (Syntropin, Novartis Pharma, Nuremberg, Germany) intravenously, and serum cortisol measurement at 0 and 30 minutes.
B.Surveillance
Postoperatively, all patients were transferred to the general floor and hospitalized for a minimum of 10 days. Fluid intake, body weight, and urine volume with specific gravity were documented daily, along with blood pressure and heart rate 3 times a day. Blood samples for the assessment of electrolytes were taken on days 1, 2, 3, 5, 7, and 9 in the morning at 6:00 am. Assessment of endocrine function including basal values and dynamic testing was performed on day 7 and after 12 weeks. No routine hormonal replacement was performed in patients who did not suffer preoperatively from a deficiency. Patients in whom the neurosurgeon suspected intraoperative pituitary compromise received hydrocortisone substitution (100 mg on day 1, 80 mg on day 2, 60 mg on day 3, 30 mg on day 4, 25 mg maintenance dosage starting on day 5). On the first postoperative day, levels of cortisol and ACTH were tested in patients with Cushing disease. If they demonstrated an insufficiency postoperatively, they were treated with the hydrocortisone substitution protocol.
C.Analytical Methods
Venous blood samples were drawn in the morning between 6:00 and 8:00 am into precooled tubes. Clotted samples were promptly centrifuged at 3000g for 15 minutes at 4°C, and then plasma was frozen at –80°C until analysis.
An automated system (Immulite®2000, Diagnostic Products Corporation) was used for the following serum hormone measurements: Serum cortisol levels >10.5 µg/dL, with an assay sensitivity of 0.20 µg/dL, was considered normal [43]; a cortisol response peak to the ACTH test higher than 18 µg/dL was considered adequate; insulin-like growth factor of 135 to 485 ng/mL (18-30 years), 120 to 397 ng/mL (31-40 years), 113 to 306 ng/mL (41-50 years), 100 to 250 ng/mL (51-60 years), and 92 to 229 ng/mL (>60 years) (assay sensitivity of 20 ng/mL); LH >0.25 U/L (follicular phase) and >20 U/L (menopausal) in women, and >1 U/L in men (assay sensitivity of 0.05 mIU/mL); follicle-stimulating hormone >0.25 U/L (follicular phase) and >30 U/L (menopausal) in women, and >1 U/L in men (assay sensitivity of 0.1 mIU/mL); testosterone >90 ng/dL in men (assay sensitivity of 15 ng/dL); estradiol >60 pg/mL (follicular phase) and >10 pg/mL (menopausal) in women (assay sensitivity of 15 pg/mL); PRL <500 µU/L (assay sensitivity of 0.16 µU/L). PRL serum concentrations were considered normal <360 ng/mL in men and <530 ng/mL in women. TSH serum concentrations were considered normal >0.45 µU/mL, free thyroxine >0.77 ng/dL, and fT3 >0.8 ng/mL.
D.Study design
Treatment of hyponatremia was initiated as soon as serum sodium dropped below 136 mmol/L, and SIADH was confirmed by euvolemia (daily measurement of body weight and fluid balance), an inappropriately concentrated urine (bedside test of specific gravity), and exclusion of an ACTH or TSH deficiency. Patients with newly diagnosed ACTH deficiencies after day 5 were treated with a hydrocortisone maintenance dosage of 25 mg. The established treatment regimen of hyponatremia consisted of a restriction of fluid intake <1L (fluid restriction). In a second cohort of patients, fluid restriction was replaced by a low dosage of tolvaptan (tolvaptan 3.75 mg), and in a third cohort by a moderate dosage (tolvaptan 7.5 mg). Whenever patients were symptomatic or suffered from severe hyponatremia, they were additionally treated with 100 mL of 3% NaCl intravenously. The pharmacy of the university hospital provided 3.75 and 7.5 tablets after pestling the 15-mg formulation and coating. Primary study endpoints of efficacy were the duration of hyponatremia, serum sodium nadir, and length of hospitalization.
In order to avoid a serum sodium overcorrection, we established a tight follow-up regimen. The serum sodium concentration was rechecked after 12 hours at 6:00 pm. Patients whose serum sodium concentration further dropped below 132 mmol/L were treated with a second tablet of tolvaptan; serum sodium was measured the next day at 6:00 am. Patients whose serum sodium was increased by not more than 5 mmol/L underwent the next blood check on the next day at 6:00 am. Tolvaptan was discontinued in patients whose serum sodium increased by more than 5 mmol/L within the following 12 hours; they were treated with 1 L of tea/water or a 500-mL 5% glucose infusion. Study endpoint of safety was the occurrence of symptoms and an overcorrection of the serum sodium concentration above 10 mmol/L per 24 hours.
E.Statistical Analysis
One year before the study began, we established the baseline of the estimated incidence of hyponatremia in pituitary surgery. Out of 233 patients, 40 patients (17.2%) developed mild hyponatremia, 22 patients (9.4%) moderate, and 8 patients (3.4%) severe hyponatremia (Fig. 1). Despite the established fluid restriction, serum sodium was below the threshold of 136 mmol/L for a mean of 4.2 ± 2.6 days (respectively below 133 mmol/L for 2.8 ± 2.1 days <133 mmol/L). To demonstrate a 50% reduction by a treatment with a probability of a type I error of 0.05 and a power of 80%, the minimum sample size to be included was 24 (resp. 35) patients per group. Parametric variables are given as mean and standard deviation, nonparametric data as median and range. SPSS statistical software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Group comparisons for categorized variables were performed using the chi-square test and the Kruskal–Wallis test for continuous variables. Significance was accepted at P < .05.
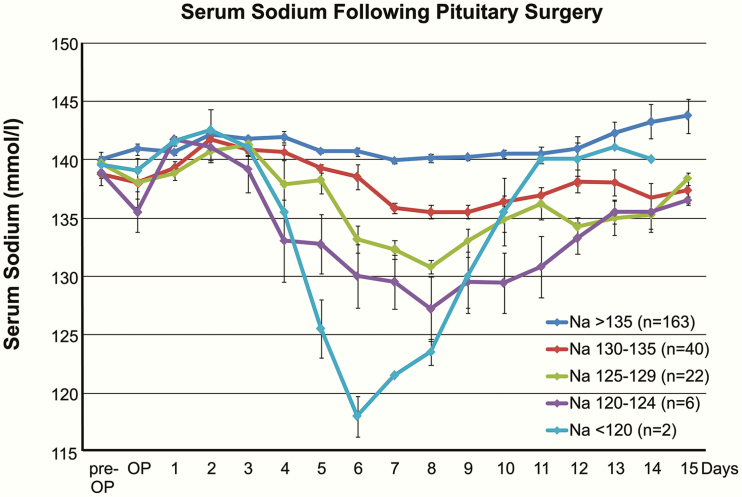
Figure 1.
Serum sodium following pituitary surgery. Data from the prestudy period demonstrate the relative incidence of severe, moderate, and mild hyponatremia as well as the biphasic course of serum sodium following manipulation of the pituitary. Mean serum sodium is given ± standard error of mean.
2.Results
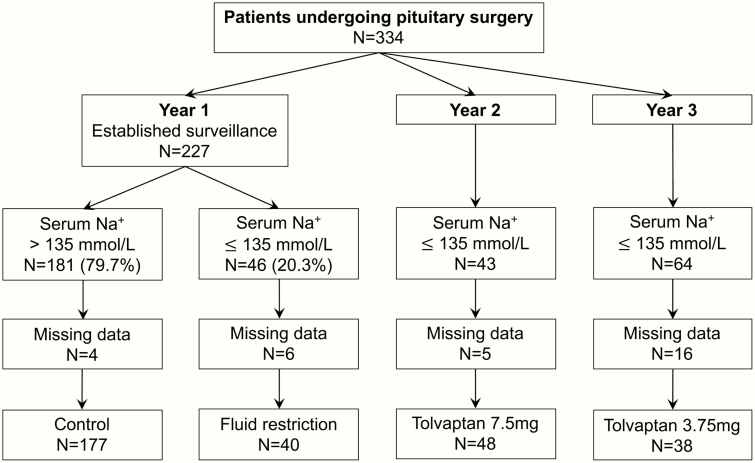
Over a 3-year period, a total of 334 patients undergoing surgery for sellar lesions were included prospectively (Fig. 2). In the first year of the study, 46 out of 227 patients (20.3%) developed hyponatremia during the postoperative course and were treated with fluid restriction as described above. The second cohort of 43 patients with postoperative hyponatremia was treated with 3.75 mg of tolvaptan, and the third cohort of 64 patients was treated with 7.5 mg of tolvaptan. Due to missing data, 31 patients were excluded, resulting in a total of 303 patients included in the final analysis.
Figure 2.
CONSORT diagram of patient recruitment.
A.Clinical Presentation and Radiological Findings
The age and gender distribution as well as the body mass index of the different treatment groups are presented in Table 1. A total of 151 patients (49.8%) were diagnosed because of a pituitary dysfunction; in 64 patients (21.1%) it was an incidental finding, and in 27 patients (8.9%) temporal visual deterioration was present. No relevant difference in preoperative routine laboratory assessment of liver or kidney function was evident. Due to the cohort study design, recruiting patients to the different treatment groups sequentially over 3 years, some shift in the patient’s diagnosis was evident. The prevalence of macroadenoma causing anterior pituitary deficiency decreased, while more patients with ACTH producing microadenoma were treated (Table 1).
Table 1.
Preoperative patient characteristics
| Serum Na+ > 135 mmol/L | Serum Na+ ≤ 135 mmol/L | |||
|---|---|---|---|---|
| Control (N = 177) | Fluid restriction (N = 40) | Tolvaptan 3.75 mg (N = 38) | Tolvaptan 7.5 mg (N = 48) | |
| Age (years; mean ± SD) | 51.8 ± 17.1 | 46.4 ± 18.1 | 50.9 ± 16.1 | 56.1 ± 15.2; bP = .011 |
| Male:female | N = 99:78 | N = 17:23 | N = 10:28; aP < .001 | N = 13:35; aP < .001 |
| Clinical findings BMI (mean ± SD) Incidental finding Pituitary insufficiency Temporal visual defect ALT (U/L; mean ± SD) Creatinine (mg/dL; mean ± SD) | 28.5 ± 6.1 N = 30 N = 94 N = 10 29.8 ± 3.7 0.85 ± 0.25 | 24.9 ± 5.6 N = 11 N = 16 N = 2 22.3 ± 1.4 0.86 ± 0.26 | 26.4 ± 4.3 N = 3; bP = .036 N = 21 N = 9; aP = .002 28.4 ± 2.9 0.81 ± 0.15 | 25.9 ± 5.8 N = 20; aP < .001 N = 20 N = 6 24.7 ± 2.1 0.77 ± 0.18; bP = .049 |
| MRI tumor location Intrasellar Suprasellar Parasellar Tumor size Anterior–posterior (mm; mean ± SD) Lateral (mm; mean ± SD) Cranio-caudal (mm; mean ± SD) Microadenoma Giant adenoma | N = 43 N = 89 N = 45 20.9 ± 11.0 18.8 ± 8.9 19.6 ± 9.0 N = 34 N = 43 | N = 13 N = 21 N = 6 20.2 ± 11.8 17.4 ± 9.8 23.6 ± 21.4 N = 13 N = 10 | N = 15 N = 14 N = 9 17.9 ± 12.3 16.1 ± 10.4 19.5 ± 18.8 N = 14; aP = .042 N = 5 | N = 17 N = 25 N = 6 16.4 ± 9.5; aP = .003 15.3 ± 7.7; aP = .012 17.7 ± 14.3; aP = .008 N = 17; aP = .031 N = 6 |
| Pituitary insufficiency Corticotropic Somatotropic Thyreotropic Gonadotropic Diabetes insipidus | N = 21 N = 59 N = 15 N = 50 N = 0 | N = 5 N = 11 N = 4 N = 9 N = 1 | N = 2; aP = .002 N = 6; aP = .001 N = 1 N = 13 N = 0 | N = 0; aP = .007, bP = .041 N = 6; aP = .008 N = 3; bP = .048 N = 11 N = 1 |
| Hormone excess ACTH GH Prolactin | N = 14 N = 41 N = 39 | N = 6 N = 6 N = 14 | N = 10; aP = .002 N = 3; aP = .001 N = 11 | N = 9; aP = .007 N = 13 N = 6; bP = .014 |
Abbreviations: SD, standard deviation; BMI, body mass index; ALT, alanine transaminase; MRI, magnetic resonance imaging; ACTH, adrenocorticotropic hormone; GH, growth hormone.
aComparison tolvaptan versus fluid restriction.
bComparison tolvaptan 3.75 versus 7.5mg.
B.Surgical Treatment
Twenty-three patients (7.6%) underwent tumor resection via a transcranial approach, in the remaining 280 a standardized transsphenoidal procedure was performed. Some minor differences between treatment groups regarding the percentage of resurgery (10.5-36.7%), accomplished total resection (61.6-79.2%), and intraoperative cerebrospinal fluid (CSF) leak (18.4-45%) was present (Table 2). Hyponatremia following surgery of sellar lesions most likely results from a changed ADH release pattern due to manipulation of the pituitary stalk. However, there was no difference regarding the surgical approach or an intraoperatively observed manipulation of the pituitary stalk (12.5-20%) comparing hyponatremic patients with normonatremic controls.
Table 2.
Intraoperative and histological findings
| Serum Na+ > 135 mmol/L | Serum Na+ ≤ 135 mmol/L | |||
|---|---|---|---|---|
| Control (N = 177) | Fluid restriction (N = 40) | Tolvaptan 3.75 mg (N = 38) | Tolvaptan 7.5 mg (N = 48) | |
| Surgical procedure Transsphenoidal Transcranial Resurgery Total resection Delineation Invasiveness Pituitary stalk manipulation CSF leak | N = 165 N = 12 N = 65 N = 109 N = 132 N = 55 N = 34 N = 59 | N = 38 N = 2 N = 9 N = 26 N = 33 N = 8 N = 8 N = 18 | N = 34 N = 4 N = 4; aP = .002 N = 26 N = 32 N = 8 N = 6 N = 7 | N = 43 N = 5 N = 10; aP = .038 N = 38; aP = .024 N = 41 N = 11 N = 6 N = 15; bP = .012 |
| Histology Null cell Prolactinoma Somatotropic Gonadotropic Corticotropic Thyreotropic Rathke cleft cyst Craniopharyngioma Meningioma Metastases | N = 23 N = 15 N = 48 N = 55 N = 24 N = 7 N = 3 N = 1 N = 1 N = 1 | N = 5 N = 13 N = 5 N = 12 N = 7 N = 0 N = 3 N = 0 N = 1 N = 0 | N = 3 N = 4 N = 4 N = 9 N = 10 N = 0 N = 2 N = 2; aP = .001 N = 4; aP = .001 N = 0 | N = 7 N = 1 N = 4 N = 12 N = 5 N = 1 N = 3 N = 1 N = 7; aP < .001 N = 2 |
| Serum sodium, mmol/L, mean ± SD Preoperative Intraoperative | 139.7 ± 2.1 140.3 ± 2.8 | 139.5 ± 2.2 138.8 ± 2.6 | 139.5 ± 2.0 139.6 ± 2.2 | 139.5 ± 2.3 138.8 ± 2.4 |
Abbreviations: CSF; cerebrospinal fluid; SD, standard deviation.
aComparison tolvaptan versus fluid restriction.
bComparison tolvaptan 3.75 versus 7.5mg.
C.Histological Findings
In 278 patients (91.7%), the histological examination revealed an adenoma, in 11 Rathke cleft cyst (3.6%), in 4 craniopharyngioma (1.3%), in 13 meningioma (4.3%), and in 3 metastases (1.0%). As mentioned above, due to the longitudinal study design, there was a slight shift of diagnoses, with a moderately increased prevalence of nonadenoma in both tolvaptan groups.
D.Hyponatremia and Treatment
In 6 patients (2.0%), a new ACTH, and in 10 patients (3.3%), a new TSH deficiency occurred postoperatively, which were treated with appropriate substitution therapies as described above (Table 3). In 21 of the 39 patients with Cushing disease (54%), serum cortisol levels were normalized postoperatively and were treated with a temporary substitution therapy.
Table 3.
Postoperative results
| Serum Na+ > 135 mmol/L | Serum Na+ ≤ 135 mmol/L | |||
|---|---|---|---|---|
| Control (N = 177) | Fluid restriction (N = 40) | Tolvaptan 3.75 mg (N = 38) | Tolvaptan 7.5 mg (N = 48) | |
| Pituitary function New insufficiency Corticotrope Thyreotrope Normalized ACTH of Cushing | N = 3 N = 5 N = 9 of 14 | N = 1 N = 3 N = 3 of 6 | N = 2 N = 0 N = 7 of 10 | N = 0 N = 2 N = 2 of 9 |
| Hyponatremia (mmol/L) Mild (130-135) With symptoms Moderate (125-129) With symptoms Severe (<125) With symptoms Nadir Na+ (mean ± SD) No. of days Na+ ≤135 (median) No. of days Na+ ≤132 (median) Na+ increment per 24hr (median) No. of patients with Na+ increase >5 mmol/L per 24 hours >10mmol/L per 24 hours Related symptoms | 137.7 ± 2.5 | N = 18 N = 0 N = 15 N = 6 N = 7 N = 6 128.4 ± 3.6 4 (1-11) 2 (1-8) 3.5 (1-12) N = 17 N = 3 N = 4 | N = 14 N = 2 N = 15 N = 10 N = 9 N = 8 127.4 ± 3.4 3.5 (1-8) 2 (1-5) 5.2 (1.5-15); aP= < .001 N = 20 N = 14; aP= < .001 N = 5 | N = 25 N = 1 N = 17 N = 7 N = 6 N = 5 127.8 ± 3.3 3 (1-7); aP = .005, bP = .002 2 (1-6); aP = .005 7.8 (2-14); aP= < .001 N = 20 N = 21; aP= < .001 N = 5 |
| Treatment Cumulative dose (mg; median) Single dose ALT (U/L; median) Hospital stay (days; median) Cost per patient (Euro) | 23 (5-73) 10 (8-26) | 23 (14-189) 12 (9-19) | 7.5; (3.75-18.75) N = 15 (39%) 36 (19-46) 11 (9-24) 69.28 | 15 (7.5-30); bP < .001 N = 22 (46%) 38 (18-94) 11 (9-18) 107.76 |
Abbreviations: ACTH, adrenocorticotropic hormone; SD, standard deviation; ALT, alanine transaminase.
aComparison tolvaptan versus fluid restriction.
bComparison tolvaptan 3.75 versus 7.5mg.
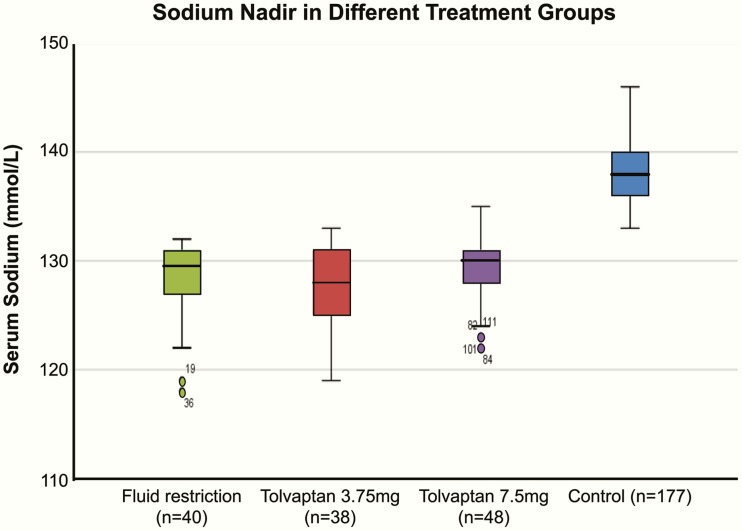
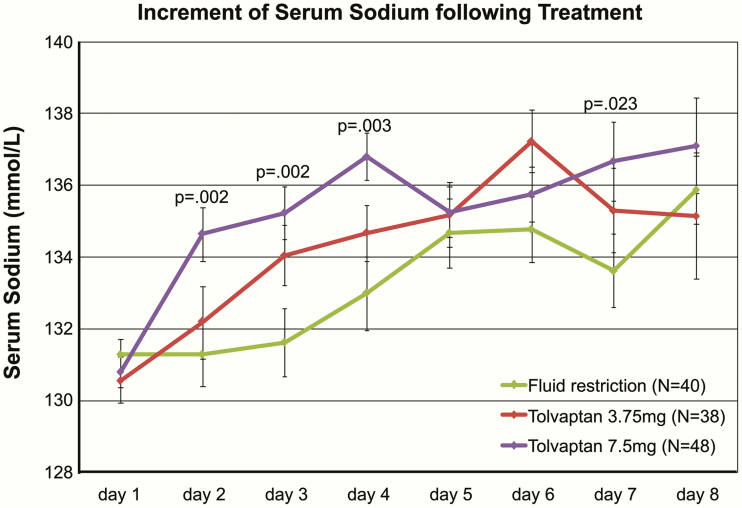
With regard to treatment efficacy, the mean nadir of the postoperative serum sodium concentration did not differ between the treatment groups (fluid restriction: 128.4, tolvaptan 3.75 mg: 127.4, tolvaptan 7.5 mg: 127.8 mmol/L; Fig. 3). Neither the percentage of mild, moderate, or severe hyponatremia nor the percentage of associated symptoms differed between treatment groups (Table 3). However, treatment with tolvaptan 7.5 mg resulted in a more rapid increase in mean sodium levels than fluid restriction following initiation of treatment (day 2 post treatment: P = .002; day 3 post treatment: P = .002; day 4 post treatment: P = .003; day 7 post treatment: P = .023; Fig. 4). Tolvaptan treatment reduced in a dose-dependent manner the duration of hyponatremia significantly compared with fluid restriction (≤135 mmol/L: fluid restriction: 4, range 1-11, tolvaptan 3.75 mg: 3.5, range 1-8, tolvaptan 7.5 mg: 3, range 1- 7 days, P = .005 vs fluid restriction, P = .002 vs tolvaptan 3.75mg; ≤132 mmol/L: fluid restriction: 2, range 1-8, tolvaptan 3.75 mg: 2, range 1-5, tolvaptan 7.5 mg: 2, range 1-6 days, P = .005; Table 3). Patients treated with tolvaptan 3.75 mg received a median cumulative dose of 9 mg (range 3.75-18.75 mg; single dose in 39% of patients), while patients treated with tolvaptan 7.5 mg received a significantly higher median cumulative dose of 15 mg (range 7.5-30 mg; P < .001; single dose in 46% of patients).
Figure 3.
Serum sodium nadir in different treatment groups following pituitary surgery.
Figure 4.
Increment of serum sodium following treatment of hyponatremia. P values give the comparison with fluid restriction. Mean serum sodium is given ± standard error of mean.
In regard to treatment safety, in those patients treated with tolvaptan, the median serum sodium increment was significantly higher (5.2, range 1.5-15mmol/L per 24 hours in tolvaptan 3.75 mg and 7.8, range 2-14mmol/L per 24 hours in tolvaptan 7.5 mg vs 3.5, range 1-12mmol/L in fluid restriction, each P < .001; Table 3). Although the patients treated with tolvaptan were not on fluid restriction, the serum sodium concentration was overcorrected in significantly more patients following tolvaptan treatment (>10 mmol/L per 24 hours: N = 14 in tolvaptan 3.75 mg; N = 21 in tolvaptan 7.5 mg vs N = 2 in fluid restriction, each P < .001; Table 3). However, overcorrection was graded exclusively on laboratory parameters. The percentage of symptoms like loss of appetite, nausea, headache, or fatigue was the same in the different treatment groups. No effect of tolvaptan on the liver function was observed. The treatment of hyponatremia did not affect the length of hospitalization (fluid restriction: 12, range 9-19 days; tolvaptan 3.75 mg: 11, range 9-24 days; tolvaptan 7.5mg: 11, range 9-18 days).
3.Discussion
With the development of AVP receptor antagonists, a specific treatment for SIADH has become available. Prospective trials demonstrated a benefit of vaptans over placebo [31, 32], but no head to head trial of vaptans with fluid restriction exists [33]. The manipulation of the neurohypophyseal stalk in pituitary surgery typically produces a bi- or triphasic response of AVP/ADH release (secretional arrest, first phase), unregulated release from stored vesicles (SIADH resulting in hyponatremia, second phase), and either subsequent normalization of ADH release or permanent diabetes insipidus (third phase) [17, 44, 45]. Therefore, hyponatremia following pituitary surgery provides a replicable model of SIADH. Nevertheless, counteracting the antidiuretic AVP/ADH effect with a specific antagonist may result in iatrogenic diabetes insipidus causing a too rapid correction of hyponatremia. Case reports of severe neurological deficits due to ODS even in elective pituitary surgery have been published [38-40]. Hence, we initiated a prospective study comparing the efficacy and safety of the AVP antagonist tolvaptan at a low (3.75 mg) and a moderate (7.5 mg) dose with our established treatment regimen of fluid restriction in SIADH following pituitary surgery.
There are 3 new findings A. Tolvaptan at a moderate dose is more effective as fluid restriction in re-establishing normonatremia; B. tolvaptan is less safe than fluid restriction in regard to overcorrection of hyponatremia in a dose-dependent manner; C. There was no difference of the duration of hospitalization between the pharmacological treatment with tolvaptan and fluid restriction.
At our institution, a high-volume neurosurgical department of a university hospital, we perform more than 200 pituitary procedures in a standardized fashion annually. The around 25% incidence of hyponatremia following pituitary surgery found in our study represents the upper limit of data from the literature, mostly because of the tight postoperative inpatient surveillance of our patients not waiting for symptoms and requiring readmission [16, 28]. Secondly, the threshold for intervention based on the level of hyponatremia varies among authors and most start at a serum sodium of 125-129 mmol/L (64.5%) [30]. At our institution, we use the definition of hyponatremia as a serum sodium concentration below 135 mmol/L [1, 2], and apply this threshold for intervention irrespective of clinical symptoms thereby accounting for the relatively high incidence of hyponatremic patients with a preference for women [46], but no association to age [47] or tumor size [48].
The treatment of hyponatremia in general is not ideal [3, 4], as documented by different guidelines established in the United States [5] and in Europe [6]. In pituitary surgery, 1 L of free water restriction is used for the prevention of hospital readmission due to SIADH [28], and complies with general recommendations that are mainly symptomatic and consist of fluid restriction [26, 27]. Only a minority of neurosurgeons use vaptans (7%) [30], although AVP receptor antagonists have been assessed in a literature review on neurosurgical and neurological adults [35], as well as in patients after Cushing disease surgery [29] and transsphenoidal pituitary surgery [16, 34].
Antagonizing the excess AVP secretion in SIADH—causing water retention and dilutional hyponatremia—with specific receptor antagonists like tolvaptan offers a novel treatment option, while restricting the fluid intake remains purely symptomatic. On the other hand, the complete blockage of AVP/ADH action causes diabetes insipidus. Hence, a careful titration of tolvaptan is crucial. The dilutional hyponatremia in SIADH creates an osmotic gradient potentially causing cerebral edema [22] with subsequently increased morbidity and mortality [25]. Vice versa, even in hyponatremia developing within days after pituitary surgery, reversing the osmotic gradient may be hazardous and leaves the patient at the risk of ODS [37-40]. As demonstrated in Fig. 1, following the secretional AVP arrest with a slight increase in serum sodium around day 2, the subsequent sodium nadir occurs between day 6 and day 8, that is, around 4 days later, thereby not meeting the criteria of acute hyponatremia. A study comparing 15 mg of tolvaptan with a reduced starting dose of 7.5 mg suggests that the latter may also be effective and safe to correct hyponatremia in SIADH [49].
Tolvaptan (Samsca®) is the only vasopressin V2 receptor antagonist approved by the European Medicines Agency for the treatment of hyponatremia resulting from SIADH [50]. Tolvaptan has been evaluated in several studies [51-53]. The affinity of tolvaptan for the human V2 receptor is 1.8 times that of native AVP. In healthy adult subjects, the oral administration of 7.5- to 120-mg doses of tolvaptan increased the urine excretion rate within 2 hours of dosing. Tolvaptan has linear pharmacokinetics for doses of 7.5 to 60 mg, and following a single oral dose of 7.5 to 60 mg, the daily urine volume increased dose dependently from 3 to 9 L. For all doses, the urine excretion rates returned to baseline levels after 24 hours. The terminal elimination half-life of tolvaptan is about 8 hours and steady-state concentrations of tolvaptan are obtained after the first dose. In line with the pharmacokinetics, tolvaptan resulted in a significant higher increment of the serum sodium concentration than fluid restriction, as well as in significantly more patients whose serum sodium was corrected above our limit of 10 mmol/L per 24 hours. All these effects were dose dependent. In the early studies to assess the safety of oral tolvaptan therapy in SIADH, an overcorrection (>12 mmol/L per 24 hours) after the first dose of 15 mg of tolvaptan was observed in 3 out of 51 patients (6%) [51]. In our patients, an overcorrection (>10 mmol/L per 24 hours) was found in 14 out of 38 patients (37%) after 3.75 mg of tolvaptan and in 21 out of 48 patients (44%) after 7.5 mg of tolvaptan (each P < .001 vs fluid restriction). Reviewing the accompanying symptoms in detail revealed that the diagnosis of “overcorrection” was exclusively based on laboratory parameter. No side effects with regard to liver function were assessed [54, 55]. The other common adverse effects of tolvaptan like dry mouth and thirst [31] may also occur with fluid restriction. The treatment of hyponatremia with tolvaptan did not affect the length of hospitalization compared with fluid restriction.
Limitations of the study result from the study design. Over the study period of 3 years, the diagnosis of patients admitted for pituitary surgery changed resulting in a significant difference of the distribution of diagnosis between groups. However, since the treatment of hyponatremia was initiated irrespective of the underlying diagnosis as long as SIADH was confirmed, we consider this difference irrelevant. More important is the group difference of the outcome measure “serum sodium overcorrection” verifying treatment safety [51]. In our study, we implemented a serum sodium relowering as soon as the treatment induced serum sodium increment was above 5 mmol/L within 12 hours in order to assure a tight titration of the AVP receptor antagonist between SIADH and diabetes insipidus and to avoid any possibility of ODS in this elective patient group. On the other hand, the instant relowering of the serum sodium resulted occasionally in a yo-yo–type effect of the serum sodium as seen between days 5 and 8 (Fig. 4).
Taken together, this is the first prospective head to head trial of the pharmacological treatment of SIADH with a vasopressin 2 receptor antagonist and fluid restriction demonstrating tolvaptan to be more effective and substantiating attention to its safety.
Acknowledgment
Financial Support: The presented study was supported in part by a research grant from Otsuka Europe, Inc. The authors have not received any honorarium in relation to this manuscript.
Clinical Trial Information: The study protocol was approved by the local Ethical Committee (Re.-No. 103_12 BC) Apr 24th, 2012 and has been registered at ClinicalTrials.gov (ID NCT04119206) on Oct 2nd, 2019.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- ALT
alanine transaminase
- AVP/ADH
arginine vasopressin/antidiuretic hormone
- BMI
body mass index
- CSF
cerebrospinal fluid
- GH
growth hormone
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- ODS
osmotic demyelination syndrome
- PRL
prolactin
- SD
standard deviation
- SIADH
syndrome of inappropriate secretion of antidiuretic hormone
- TSH
thyroid-stimulating hormone
Additional Information
Disclosure Summary: A.K. is on the Otsuka Pharmaceutical advisory board for tolvaptan and has received a research grant from Otsuka Europe, Inc.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172. [DOI] [PubMed] [Google Scholar]
- 2. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-S35. [DOI] [PubMed] [Google Scholar]
- 3. Huda MS, Boyd A, Skagen K, et al. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J. 2006;82(965):216-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657. [DOI] [PubMed] [Google Scholar]
- 5. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42. [DOI] [PubMed] [Google Scholar]
- 6. Spasovski G, Vanholder R, Allolio B, et al. ; Hyponatraemia Guideline Development Group Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170(3):G1-47. [DOI] [PubMed] [Google Scholar]
- 7. Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985;102(2):164-168. [DOI] [PubMed] [Google Scholar]
- 8. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806. [DOI] [PubMed] [Google Scholar]
- 9. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072. [DOI] [PubMed] [Google Scholar]
- 10. Kleindienst A, Hannon MJ, Buchfelder M, Verbalis JG. Hyponatremia in neurotrauma: the role of vasopressin. J Neurotrauma. 2016;33(7):615-624. [DOI] [PubMed] [Google Scholar]
- 11. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613-619. [DOI] [PubMed] [Google Scholar]
- 12. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40(2):225-36; discussion 236. [DOI] [PubMed] [Google Scholar]
- 13. Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008;63(4 Suppl 2):244-56; discussion 256. [DOI] [PubMed] [Google Scholar]
- 14. Kristof RA, Rother M, Neuloh G, Klingmüller D. Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: a prospective observational study. J Neurosurg. 2009;111(3):555-562. [DOI] [PubMed] [Google Scholar]
- 15. Bohl MA, Ahmad S, Jahnke H, et al. Delayed hyponatremia is the most common cause of 30-day unplanned readmission after transsphenoidal surgery for pituitary tumors. Neurosurgery. 2016;78(1):84-90. [DOI] [PubMed] [Google Scholar]
- 16. Jahangiri A, Wagner J, Tran MT, et al. Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J Neurosurg. 2013;119(6):1478-1483. [DOI] [PubMed] [Google Scholar]
- 17. Hensen J, Henig A, Fahlbusch R, Meyer M, Boehnert M, Buchfelder M. Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin Endocrinol (Oxf). 1999;50(4):431-439. [DOI] [PubMed] [Google Scholar]
- 18. Blair ET, Clemmer JS, Harkey HL, Hester RL, Pruett WA. Physiologic mechanisms of water and electrolyte disturbances after transsphenoidal pituitary surgery. World Neurosurg. 2017;107:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loh JA, Verbalis JG. Diabetes insipidus as a complication after pituitary surgery. Nat Clin Pract Endocrinol Metab. 2007;3(6):489-494. [DOI] [PubMed] [Google Scholar]
- 20. Olson BR, Gumowski J, Rubino D, Oldfield EH. Pathophysiology of hyponatremia after transsphenoidal pituitary surgery. J Neurosurg. 1997;87(4):499-507. [DOI] [PubMed] [Google Scholar]
- 21. Verbalis JG. An experimental model of syndrome of inappropriate antidiuretic hormone secretion in the rat. Am J Physiol. 1984;247(4 Pt 1):E540-E553. [DOI] [PubMed] [Google Scholar]
- 22. Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289-301. [DOI] [PubMed] [Google Scholar]
- 23. Kleindienst A, Dunbar JG, Glisson R, Marmarou A. The role of vasopressin V1A receptors in cytotoxic brain edema formation following brain injury. Acta Neurochir (Wien). 2013;155(1):151-164. [DOI] [PubMed] [Google Scholar]
- 24. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-71.e8. [DOI] [PubMed] [Google Scholar]
- 25. Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102(1):67-77. [DOI] [PubMed] [Google Scholar]
- 26. Diringer MN, Zazulia AR. Hyponatremia in neurologic patients: consequences and approaches to treatment. Neurologist. 2006;12(3):117-126. [DOI] [PubMed] [Google Scholar]
- 27. Murphy T, Dhar R, Diringer M. Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit. Neurocrit Care. 2009;11(1):14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burke WT, Cote DJ, Iuliano SI, Zaidi HA, Laws ER. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary. 2018;21(1):25-31. [DOI] [PubMed] [Google Scholar]
- 29. Breshears JD, Jiang B, Rowland NC, Kunwar S, Blevins LS. Use of conivaptan for management of hyponatremia following surgery for Cushing’s disease. Clin Neurol Neurosurg. 2013;115(11):2358-2361. [DOI] [PubMed] [Google Scholar]
- 30. Eisenberg Y, Charles S, Dugas L, Agrawal N. Clinical practice patterns for postoperative water balance after pituitary surgery. Endocr Pract. 2019;25(9):943-950. [DOI] [PubMed] [Google Scholar]
- 31. Schrier RW, Gross P, Gheorghiade M, et al. ; SALT Investigators Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112. [DOI] [PubMed] [Google Scholar]
- 32. Berl T, Quittnat-Pelletier F, Verbalis JG, et al. ; SALTWATER Investigators Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peri A. Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab. 2013;98(4):1321-1332. [DOI] [PubMed] [Google Scholar]
- 34. Ichimura S, Fahlbusch R, Lüdemann W. Treatment of hyponatremia with tolvaptan in a patient after neurosurgical treatment of a pituitary tumor: case report and review of literature. J Neurol Surg Rep. 2015;76(2):e279-e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buckley MS, Patel SA, Hattrup AE, Kazem NH, Jacobs SC, Culver MA. Conivaptan for treatment of hyponatremia in neurologic and neurosurgical adults. Ann Pharmacother. 2013;47(9):1194-1200. [DOI] [PubMed] [Google Scholar]
- 36. Janneck M, Burkhardt T, Rotermund R, Sauer N, Flitsch J, Aberle J. Hyponatremia after trans-sphenoidal surgery. Minerva Endocrinol. 2014;39(1):27-31. [PubMed] [Google Scholar]
- 37. Wright DG, Laureno R, Victor M. Pontine and extrapontine myelinolysis. Brain. 1979;102(2):361-385. [DOI] [PubMed] [Google Scholar]
- 38. Ho PL, Chen YC, Teng CH, Wu CC, Huang P. Acute parkinsonism as an unexpected consequence of pituitary adenoma resection: a case report. Medicine (Baltimore). 2019;98(17):e15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tosaka M, Kohga H. Extrapontine myelinolysis and behavioral change after transsphenoidal pituitary surgery: case report. Neurosurgery. 1998;43(4):933-936. [DOI] [PubMed] [Google Scholar]
- 40. Nakano H, Ohara Y, Bandoh K, Miyaoka M. A case of central pontine myelinolysis after surgical removal of a pituitary tumor. Surg Neurol. 1996;46(1):32-36. [DOI] [PubMed] [Google Scholar]
- 41. Brabant G, von zur Mühlen A, Wüster C, et al. ; German KIMS Board Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60(2):53-60. [DOI] [PubMed] [Google Scholar]
- 42. Gittoes NJ, Bates AS, Tse W, et al. Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf). 1998;48(3):331-337. [DOI] [PubMed] [Google Scholar]
- 43. Boehnert M, Hensen J, Henig A, Fahlbusch R, Gross P, Buchfelder M. Severe hyponatremia after transsphenoidal surgery for pituitary adenomas. Kidney Int Suppl. 1998;64:S12-S14. [PubMed] [Google Scholar]
- 44. Ultmann MC, Hoffman GE, Nelson PB, Robinson AG. Transient hyponatremia after damage to the neurohypophyseal tracts. Neuroendocrinology. 1992;56(6):803-811. [DOI] [PubMed] [Google Scholar]
- 45. O’CONNOR WJ. The normal interphase in the polyuria which follows section of the supraoptico-hypophysial tracts in the dog. Q J Exp Physiol Cogn Med Sci. 1952;37(1):1-10. [DOI] [PubMed] [Google Scholar]
- 46. Tasdemir V, Oguz AK, Sayın I, Ergun I. Hyponatremia in the outpatient setting: clinical characteristics, risk factors, and outcome. Int Urol Nephrol. 2015;47(12):1977-1983. [DOI] [PubMed] [Google Scholar]
- 47. Chua M, Hoyle GE, Soiza RL. Prognostic implications of hyponatremia in elderly hospitalized patients. Arch Gerontol Geriatr. 2007;45(3):253-258. [DOI] [PubMed] [Google Scholar]
- 48. Staiger RD, Sarnthein J, Wiesli P, Schmid C, Bernays RL. Prognostic factors for impaired plasma sodium homeostasis after transsphenoidal surgery. Br J Neurosurg. 2013;27(1):63-68. [DOI] [PubMed] [Google Scholar]
- 49. Harbeck B, Lindner U, Haas CS. Low-dose tolvaptan for the treatment of hyponatremia in the syndrome of inappropriate ADH secretion (SIADH). Endocrine. 2016;53(3):872-873. [DOI] [PubMed] [Google Scholar]
- 50. Jamookeeah C, Robinson P, O’Reilly K, et al. Cost-effectiveness of tolvaptan for the treatment of hyponatraemia secondary to syndrome of inappropriate antidiuretic hormone secretion in Sweden. BMC Endocr Disord. 2016;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verbalis JG, Adler S, Schrier RW, Berl T, Zhao Q, Czerwiec FS; SALT Investigators Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164(5):725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gheorghiade M, Orlandi C, Burnett JC, et al. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of vasopressin antagonism in heart failure: outcome study with tolvaptan (EVEREST). J Card Fail. 2005;11(4):260-269. [DOI] [PubMed] [Google Scholar]
- 53. Lanfear DE, Sabbah HN, Goldsmith SR, et al. ; EVEREST trial investigators Association of arginine vasopressin levels with outcomes and the effect of V2 blockade in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2013;6(1):47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torres VE, Chapman AB, Devuyst O, et al. ; TEMPO 3:4 Trial Investigators Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watkins PB, Lewis JH, Kaplowitz N, et al. clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of Clinical Trials Database. Drug Saf. 2015;38(11):1103-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]