Abstract
Purpose
This study aims at exploring alterations of major metabolites and metabolic pathways in retinopathy of prematurity (ROP) infants and identifying biomarkers that may merit early diagnosis of ROP.
Methods
We analyzed targeted metabolites from 81 premature infants (<34 weeks of gestational age), including 40 ROP cases (15 males and 25 females, birth weight 1.263 ± 0. 345 kg, gestational age 31.20 ± 4.62 weeks) and 41 cases (30 males, 11 females, birth weight 1.220 ± 0.293 kg, gestational age 30.96 ± 4.17 weeks) of well-matched non-ROP controls. Metabolites were measured by ultra-performance liquid chromatography-tandem mass spectrometry. Standard multivariate and univariate analysis was performed to interpret metabolomic results.
Results
Glycine, glutamate, leucine, serine, piperidine, valine, tryptophan, citrulline, malonyl carnitine (C3DC), and homocysteine were identified as the top discriminant metabolites. In particular, discriminant concentrations of C3DC and glycine were also confirmed by univariate analysis as statistically significant different between ROP and non-ROP infants.
Conclusions
This study gained an insight into the metabolomic aspects of ROP development. We suggest that higher blood levels of C3DC and glycine can be promising biomarkers to predict the occurrence, but not the severity of ROP.
Keywords: retinopathy of prematurity, metabolomics, biomarkers, metabolic pathways, amino acids
Retinopathy of prematurity (ROP) is a common premature complication, in which the development of immature retinal vasculature is disrupted by higher oxygen environment encountered at birth. This can arrest developing retinal vasculature, which lead to neovascularization and retinal detachment, both are potential threats for vision loss.1 With improvement in the medical standards and neonatal care, the survival rate of premature infants in low-income countries is growing rapidly.2 Every year, ROP causes approximately 100,000 childhood blindness around the world,3,4 which makes it a critical factor affecting the quality of life of premature infants.5–7 Current screening methods and treatment tactics for ROP are greatly limited,8,9 new preventive strategy and therapeutic targets are urgently required.
The retina is one of the most energy-demanding tissues in the human body, and its energy consumption is comparative to that of a proliferating tumor.10,11 Studies have revealed that the occurrence and development of retinal diseases is related to abnormal metabolism.12 Amino acids, for instance, are known to play a major role in retinal vascular development, function, cell survival, and neurotransmission. The location, synthesis, and degradation enzymes of retinal amino acid can vary greatly in different diseases, such as retinal detachment,13 retinal degeneration,14 and retinopathy of prematurity.15,16 Furthermore, it has also been proposed that nutritional intervention can be beneficial for ROP. For example, dietary supplements, such as long-chain polyunsaturated fatty acids, can inhibit the formation of retinal neovascularization and prevent the occurrence of ROP.17–23 They revealed that in vivo metabolism intervention can benefits future prevention and treatment of ROP.
Cell metabolism is the process of nutrient being converted to energy and metabolic by-product. The retina is known to metabolize in a similar manner as cancer cells, which prioritize using aerobic glycolysis to generate energy, a process known as the Warburg effect.24 Alteration in metabolism during development can influence cell survival, migration, and growth, especially for endothelial cells (ECs). As a vascular disease, metabolomic aspect of ROP has been hugely overlooked. Until now, it has remained unclear as to which metabolites and/or pathways are affected in ROP.
To our knowledge, only a few studies have focused on ROP metabolic changes. Most of them focused on nutrition intervention, such as omega-3 long-chain polyunsaturated fatty acids, insulin-like growth factor I, erythropoietin, and carotenoids.25–29 Although some have explored the effect of single metabolites, such as arginine, glutamine, cysteine, hypoxia-inducing factor 1, and peroxisome proliferator-activated receptor γ coactivator-1α.23,30–33 Although these studies have identified the impact of a specific metabolites on the occurrence of ROP, they failed to establish connections between metabolites or relate to a specific metabolomic pathway. Moreover, ROP is multifactorial; alteration of a single metabolite may not be enough to explain fully the development of ROP. Advances in technology have made possible the universe detection of genes, messenger RNA, proteins, and metabolites. Hartnett et al. has explored genomic variations of several susceptible genes in ROP infants.34 Additionally, a more recent study has used plasma proteomics technique to identify potential therapeutic/diagnostic biomarkers for ROP.35,36 Mass spectrometry is instrumental in analyzing multiple metabolite in a single run. The amino acid and acylcarnitine analysis of dried blood spots using mass spectrometry contributes to current advancements in neonatal screening.37
The goal of this study is to identify potential blood metabolic biomarkers and provide a new insight into a better understanding of the underlying mechanism of ROP. This was achieved by taking advantage of ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) to generate and compare targeted metabolome profile of blood spot samples from premature infants with and without ROP.
Methods
Study Design
This is a prospective study with data collected between January 2016 and September 2017 from the Sixth Affiliated Hospital of Sun Yat-Sen University and Shenzhen ROP Collaborative Group (33 hospitals in Shenzhen, China). This study received ethical approved authorized by Medical Ethics Committees from all hospitals and adhered to the tenets of the Declaration of Helsinki. Signed consent forms for enrollment were obtained from all families. Infants were enrolled if they were born preterm, defined as delivered between 20 weeks to 34 weeks from gestation. The heel blood samples of infants were collected in 3 days after birth and 30 minutes after feeding using “newborn blood collection card” and store in −20°C. A total of eighty-one preterm infants were included in this study. The presence of ROP was determined by specialized ophthalmologists from Shenzhen Eye Hospital. Participants will be excluded from the study if (1) requested by participants’ families; (2) participants were diagnosed with congenital metabolomic diseases; (3) occurrence of severe complications, including but not limited to, sepsis, necrotizing enterocolitis, neonatal respiratory distress syndrome, and severe metabolic disorder; (4) the mother of the participant had a history of medication and/or serious disease, such as gonorrhea, syphilis, or AIDS during pregnancy; or (5) death.
Diagnosis of ROP
The diagnostic and therapeutic criteria of ROP follow the international classification38,39 and the screening guidelines for retinopathy of prematurity in China (2014).40 All infants were screened using binocular indirect ophthalmoscope (Heine, Bavaria, Germany) and RetCam (Natus Retcam3, California, United States) until the end of the follow-up. Every infant was examined by two experienced retina specialists independently and the eligibility of participation was confirmed by both the specialists. Infants with any zone ROP were included in this study.
Sample Collection and Metabolomic Data Acquisition
Eighty-one heel blood samples were collected and sent for UPLC-MS/MS analysis (QI000 Japanese Electronics, American Biological Application Systems Co., Ltd.). In brief, blood was collected by cards (Whatman 903 Filter Paper, America) that were punched as 3-mm-diameter circular pieces (Puncher, Perkin-Elmer, Turku, Finland) and placed in 96-well filter plates (0.45 µm, Millipore Inc., America). Each hole includes 100 µL marked methanol (Sigma Inc., America, (N,O-Bis(trimethylsilyl)trifluoroacetamide/Trimethylchlorosilane) containing stable isotope-labelled internal standards (Cambridge Isotope Labs Inc., America) and was left to stand for 20 minutes at room temperature. The homogenate was centrifugated (Ebender, 5147.C, Germany) at 14,000 rpm for 10 minutes with centrifugal radius of 11 cm and removed to the 96-hole polypropylene board (America, Fisher Inc.). The solution was dried (L-129A, Beijing Lai Heng Science and Trade Co., Ltd.) at 50°C and added 60 µL hydrochloric acid N-butyl alcohol at a 65°C constant temperature box for 15 minutes covered with Teflon film. Then, it was dried at 50°C and 80% acetonitrile (15.4 mol/L) was added. Instrumental parameters were as follows: mobile phase, eluted with isocratic elution of 80% acetonitrile (15.4 mol/L); the flow rate was 140 µL/min. Neutral amino acids were scanned with neutral loss scan, and the mass to load ratio (m/z) of neutral loss fragment was 102 Da. The scanning range was m/z 140 to m/z 280. Acylcarnitine analysis was done by using precursor scan. The daughter ions were m/z 85 Da fragment, and the scanning range from m/z 210 to m/z 502. Glycine, ornithine, arginine, and citrulline adopted multiresponse monitoring, and a sample test took approximately 2 minutes.
Data Processing and Analysis
According to the m/z of the mass spectrum peak, the concentration value of metabolites was automatically calculated by quantitative analysis software. After removing ratio substances, multivariate data analysis was conducted using SIMCA (v.14.1; Umetrics, Umeå, Sweden). Orthogonal partial least squares-discriminant analysis (OPLS-DA) was used to increase the class separation, flatten dataset, and find potential biomarkers.41 The quality of models was validated using two parameters: R2Ycum (goodness of fit) and cumulated Q² (Q²cum, goodness of prediction). A threshold of 0.5 is widely accepted in model classification to identify good (Q²cum ≥ 0.5) or poor (Q²cum < 0.5) predictive capabilities.
We validated the OPLS-DA model using permutation test (200 times) to reduce the risk of overfitting and possibilities of false-positive findings. Plots show correlation coefficients between the original Y and the permuted Y versus the cumR2Y and Q2. Fitted regression lines were also displayed, which connects the observed Q2 to the centroid of permuted Q2 cluster. The model was considered valid if (1) all Q2 values from the permuted data set to the left are lower than the Q2 value on the actual data set to the right and (2) the regression line has a negative value of intercept on the y-axis.42 To identify moderate and strong outliers, DModX test and Hoteling's T-squared test was performed. These tests were performed using SIMCA.
The variable importance in projection (VIP) values was then identified by supervised investigations. VIP is a readout of the contribution of each variable on the x-axis to the model. It is summed over all components and weighted to the Y accounted for by every single component.43 Therefore, VIP ranking reflects the metabolites’ contribution to the model. In this study, we select metabolites with VIP >1 as major discriminant metabolite to expand our selection, this criteria is similar to other studies.44,45
The receiver operating characteristic (ROC) analysis was then used to assess the ability of predicting potential metabolic biomarkers. To classify ROP infants, we calculated and compared area under the curve (AUC), sensitivity, and specificity values of identified principal components. This approach takes both predictive capability and statistical significance of individual metabolites in to account. The analysis was performed using SPSS, version 25.0.
Statistical Analysis
Demographic characteristics and distribution pattern of each metabolites were first determined by QQ- and PP-plots. Normally distributed variables were presented as mean ± SD, otherwise data were presented as median and mean ranks. Student's t-tests and Wilcoxon tests were then used to compare variables whose distribution followed and did not follow normal distribution, respectively, between ROP and non-ROP infants. Differences were considered as significant when P < 0.05. Analysis was performed using SPSS, version 25.0.
Results
Demographic Characteristics
Demographic characteristics of the cohort was described in Table 1. There was no statistically significant difference in mean gestational age between ROP (31.20 ± 4.62 weeks) and non-ROP (30.96 ± 4.17 weeks) groups (P = 0.657). Mean birth weight was also comparative (1.263 kg in ROP vs 1.220 kg for non-ROP, P = 0.546). Nor does multiple pregnancy differ significantly between groups (48% singleton in ROP vs 70% in non-ROP, P = 0.078). With regard to nutritional supply, we did not find statistically significant difference between two groups in feeding tactics at day 3 of life (80% breast fed and total parental nutrition [TPN] supplemented in ROP vs 95% in non-ROP, P = 0.084) and TPN or breast milk volume (TPN 105.4 ± 25 mLvs 103.2 ± 27 mL, ROP vs non-ROP, P = 0.707; breast milk 8.0 mL vs 8.0 mL, ROP vs non-ROP, P = 0.970). Noteworthy, none of them have insulin infusion. Additionally, oxygen administration is similar between groups (oxygen saturation, 92.5% vs 92.5%, respectively, P = 0.291; fraction of inspired O2 [FiO2], 30% vs 29%, respectively, P = 0.724). However, delivery mode (% cesarean section, 32.40% in ROP vs 65.50% in non-ROP, P = 0.008) and sex (35.5% male in ROP vs 73.2% in non-ROP, P = 0.002) differed significantly between the two groups.
Table 1.
Demographic Characteristics
| Variables | ROP | Non-ROP | P Value |
|---|---|---|---|
| Gestational age (weeks) | 31.20 ± 4.62 | 30.96 ± 4.17 | 0.657 |
| Birth weight (kg) | 1.263 ± 0.345 | 1.220 ± 0. 293 | 0.546 |
| Delivery mode (cesarean section %) | 12 (32.40) | 19 (65.50) | 0.008* |
| Multiple pregnancy (singleton %) | 18 (48.60) | 21 (70.00) | 0.078 |
| Sex (male %) | 15 (38.50) | 30 (73.20) | 0.002* |
| Feeding strategy (TPN and nontrophic feeding %) | 32 (80.00) | 39 (95.12) | 0.084 |
| TPN (mL) | 105.4 ± 25 | 103.2 ± 27 | 0.707 |
| Breast milk (mL) | 8.0 | 8.0 | 0.970 |
| Oxygen saturations (%) | 92.5 | 92.5 | 0.291 |
| FiO2 (%) | 30 | 29 | 0.724 |
Numbers were expressed as mean ± SD. Gestational age, birth weight, and TPN are normally distributed parameters whose significant differences (P value) are calculated using Student's t-test. Breast milk, oxygen saturation, and FiO2 are presented by median and statistical significance analyzed using Wilcoxon rank-sum test. Delivery mode, multiple pregnancy status, sex, and feeding strategy are analyzed by Chi-squared test. TPN = total parenteral nutrition.
P < .05.
Multivariate Analysis Using OPLS-DA
OPLS-DA Model Building and Validation
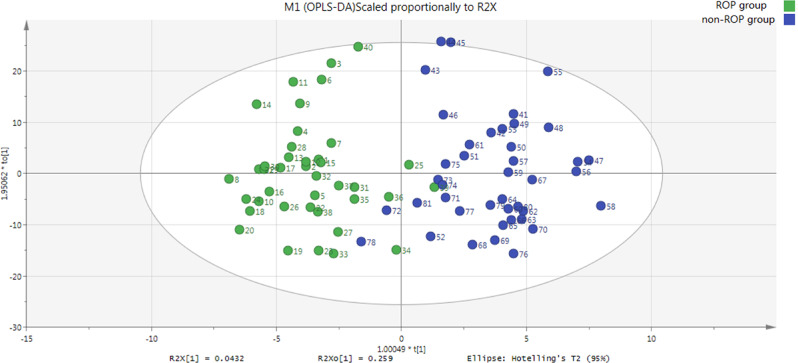
The OPLS-DA score plot revealed a clear and separate clustering between premature infants with and without ROP (Fig. 1). In addition, this OPLS-DA model has both R2Xcum (0.991) and R2Ycum (0.772) values exceeding 0.5, and that the first two principal components explained 56.3% of the variation of analyzed metabolites. This collectively suggest that this model fits the data very well and has good predictive ability.
Figure 1.
OPLS-DA score scatter plot of first two principal components (ROP and non-ROP groups). Clear separate clustering can be observed between ROP and non-ROP groups. R2Xcum = 0.991, R2Ycum = 0.772, Q2cum = 0.563. Green dots represent ROP (n = 40); blue dots represent non-ROP (n = 41).
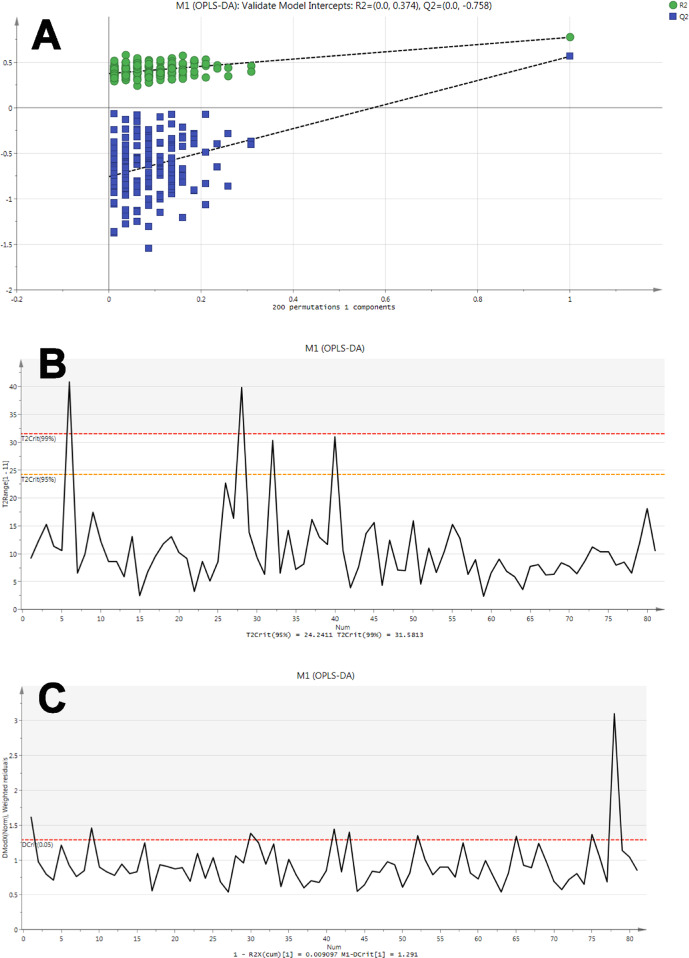
This model was then evaluated by permutation analysis (Fig. 2a). All permuted R2s were below or around 0.6 and all permuted Q2s were below 0, plus all R2s and Q2s are lower than the original values on the right. This suggests that the model fitting was valid, and this was unlikely to be built by chance.
Figure 2.
Validity tests for OPLS-DA model. (A) Permutation analysis plotting R2 and Q2 from 200 permutation tests in the OPLS-DA model. The y-axis shows R2 and Q2, whereas the x-axis shows the correlation coefficient of permuted and observed data. The two points on the right represent the observed R2 and Q2. Cluster of points on the left represents 200 permuted R2s and Q2s. Green and blue dots represent R2 and Q2 values, respectively. Dashed lines denote corresponding fitted regression lines for observed and permutated R2 and Q2. (B) Hoteling's T-squared test revealed that most samples did not show deviation, except participants 6 (T2 = 40.82) and 28 (T2 = 39.91) that exceeded 99% CI level and participants 32 (T2 = 30.33) and 40 (T2 = 31.04) that exceeded 95% CI level, all four of these deviators were from the ROP group. Red and orange horizontal dashed line denotes 99% and 95% CI level, respectively. (C) DModX test plot presented that most samples did not show severe deviation in DModX, the only moderate outlier being participant 78 (3.10). Red horizontal dashed line denotes 95% CI.
To identify strong and moderate outliers, we performed Hoteling's T-squared test (Fig. 2b) and DModX test (Fig. 2c), respectively. No strong outliers in the sample were identified, whereas participant 79 seems to show evident deviation in the DModX test.
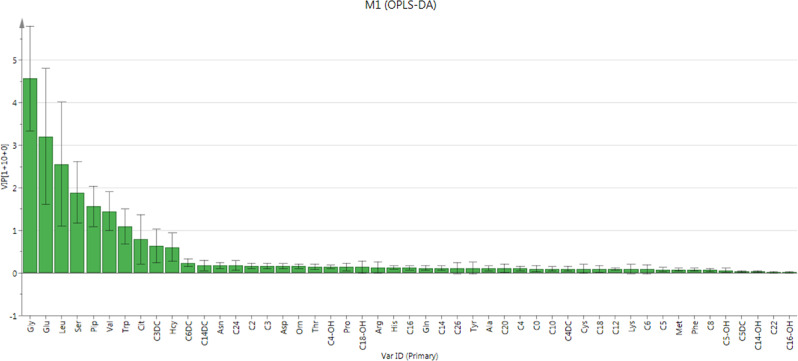
Contribution Analysis of All Metabolites to ROP
Contribution plot ranks metabolites by their contribution to the model (Fig. 3). The following top 10 metabolites were identified as potential discriminant metabolites for disease prediction (VIP > 1.0): glycine (VIP = 4.5689), glutamate (VIP = 3.2083), leucine (VIP = 2.5558), serine (VIP = 1.8946), piperidine (VIP = 1.5630), valine (VIP = 1.4493), and tryptophan (VIP = 1.0895). Citrulline (VIP = 0.7915), C3DC (VIP = 0.6336), and homocysteine (VIP = 0.6078) were also considered to expand our selection (0.5 < VIP < 1.0).
Figure 3.
Contribution plot from the OPLS-DA model including all metabolites. According to the plot, glycine, glutamate, leucine, serine, piperidine, valine, and tryptophan were considered important; citrulline, C3DC, and homocysteine were slightly below the “important” threshold. Metabolites to the right of homocysteine were unimportant in contributing to the OPLS-DA model. All metabolites analyzed in current study obtained positive contribution scores, which indicated higher levels in ROP.
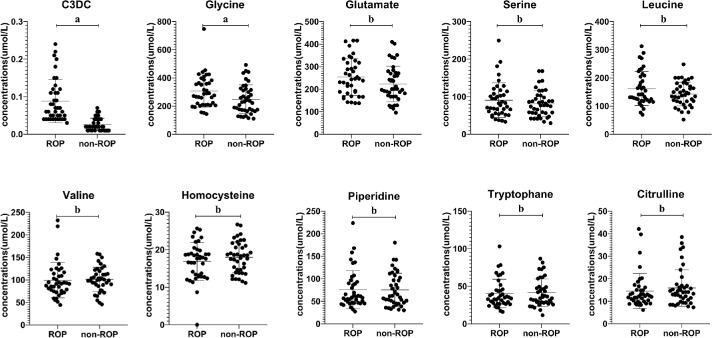
Cross Validation Using Univariant Analysis
To identify alterations in metabolites associated with development of ROP, univariant analysis was performed on all metabolites (not shown), for cross validation of our multivariate analysis using OPLS-DA, results of top 10 potential discriminant metabolites were shown here. Distribution patterns of each metabolites were assessed first to determine the type of significance test to be used. Among all, glycine, glutamate, leucine, valine, and homocysteine obeyed normal distribution (Table 2), whereas serine, piperidine, tryptophan, citrulline, and C3DC did not (Table 3). Scatter plots presented that glycine, glutamate, leucine, serine, and C3DC were higher in the ROP group compared with the non-ROP group. Valine, homocysteine, piperidine, tryptophan, and citrulline are reduced in the ROP group (Fig. 4). Notably, only glycine (P = 0.018) and C3DC (P < 0.001) presented statistically significant difference between ROP and non-ROP groups (Fig. 4). After adjusting for sex, the changes in C3DC and glycine levels remained significant. C3DC and glycine are still independent risk factors for ROP. The higher the value of C3DC, the higher the risk of ROP, indicating it is a strong risk factor (P < 0.001, Wald = 16.478, odds ratio [OR] = 4.846E+56, 95% confidence interval [CI] = 2.068E+29-1.136E+84); glycine is a low risk factor for ROP, and is positively correlated (P = 0.031, Wald = 4.669, OR = 1.014, CI = 1.001-1.027). With every 1 µmol/L increase in glycine, the risk of ROP is elevated by 1.4%. However, the level of C3DC and glycine had nothing to do with the severity of ROP after adjusting for sex, delivery mode, gestational age, and birth weight (i.e., nonproliferation vs proliferation [C3DC: P = 0.822, Wald = 0.051, OR = 12.477, CI = 0.000-4.231E+10]) and glycine: P = 0.707, Wald = 0.141, OR = 0.998, CI = 0.987-1.009). Our study provides the very first data indicating a potential association between increased C3DC/glycine and risk of ROP, but not the severity of ROP.
Table 2.
Blood Metabolites That Are Normally Distributed
| Material | Group | χ ± SD (µmol/L) | t Value | P Value |
|---|---|---|---|---|
| Glycine | ROP (+) | 306.47 ± 114.28 | 2.411 | 0.018 |
| ROP (−) | 248.29 ± 102.40 | |||
| Glutamate | ROP (+) | 253.69 ± 82.23 | 1.687 | 0.095 |
| ROP (−) | 223.38 ± 79.39 | |||
| Leucine | ROP (+) | 162.28 ± 60.13 | 1.676 | 0.098 |
| ROP (−) | 143.00 ± 42.07 | |||
| Valine | ROP (+) | 99.27 ± 39.05 | −0.246 | 0.807 |
| ROP (−) | 101.10 ± 27.08 | |||
| Homocysteine | ROP (+) | 16.86 ± 5.01 | −1.097 | 0.276 |
| ROP (−) | 17.98 ± 4.15 |
Glycine, glutamate, leucine, valine, and homocysteine obey normal distributions. Data expressed as χ ± standard deviation. Statistical significance evaluated using Student's t-test.
Table 3.
Blood Metabolites That Are Not Normally Distributed
| Material | Group | Median (µmol/L) | Mean Rank | P Value |
|---|---|---|---|---|
| Serine | ROP (+) ROP (−) | 79.94 76.94 | 42.85 39.20 | 0.485 |
| Piperidine | ROP (+) ROP (−) | 58.89 62.29 | 40.73 41.27 | 0.917 |
| Tryptophan | ROP (+) ROP (−) | 34.76 35.70 | 40.08 41.90 | 0.727 |
| Citrulline | ROP (+) ROP (−) | 12.27 13.97 | 38.38 43.56 | 0.321 |
| C3DC | ROP (+) ROP (−) | 0.06 0.02 | 57.98 24.44 | <0.001 |
Serine, piperidine, tryptophan, citrulline, and C3DC did not obey normal distributions. Data presented as median and mean ranks. Statistical significance analyzed using Wilcoxon rank-sum test.
Figure 4.
Univariant analysis of the top 10 VIP. Concentration of the top 10 VIPs identified from the OPLS-DA model were compared between ROP and non-ROP premature infants. Only C3DC (P < 0.001) and glycine (P = 0.018) reached statistical significance between the two groups. For statistical significance analysis, Student's t-test was performed for glycine, glutamate, leucine, valine, and homocysteine; Wilcoxon rank-sum test was used for serine, piperidine, tryptophan, citrulline, and C3DC. “a” represents statistically significant differences (P < 0.05) “b” indicates differences are not significant.
ROC plot was drawn to assess the ability of predicting potential metabolic biomarkers in ROP (Fig. 5). AUC of all identified metabolites: citrulline (AUC = 0.564, CI = 0.438-0.691), glutamate (AUC = 0.601, CI = 0.478-0.725), glycine (AUC = 0.659, CI = 0.540-0.778), homocysteine (AUC = 0.554, CI = 0.428-0.680), valine (AUC = 0.573, CI = 0.446-0.699), leucine (AUC = 0.568, CI = 0.441-0.694), piperidine (AUC = 0.507, CI = 0.379-0.634), serine (AUC = 0.545, CI = 0.419-0.672), tryptophan (AUC = 0.523, CI = 0.396-0.649), and C3DC (AUC = 0.914, CI = 0.856-0.972) were greater than 0.5. Notably, AUC of C3DC is even greater than 0.8, indicating its good predictive ability. According to the hypothesis test (H0 = 0.5), AUCs of citrulline (P = 0.3213), glutamate (P = 0.1169), glycine (P = 0.1169), homocysteine (P = 0.4005), valine (P = 0.2610), leucine (P = 0.2944), serine (P = 0.4845), and C3DC (P < 0.01) were not obtained randomly. C3DC was exceptionally good at rejecting the null hypothesis. In this study, the accuracy of C3DC-based diagnosis is the best (AUC = 0.914, sensitivity = 97.5%, and specificity = 68.3%) among all potential discriminant metabolites, followed by glycine (AUC = 0.659, sensitivity = 92.5%, and specificity = 58.5%). However, diagnoses based on other metabolites were too weak to draw any significance. Collectively, these results suggest that C3DC and glycine can be potential biomarkers for ROP diagnosis.
Figure 5.
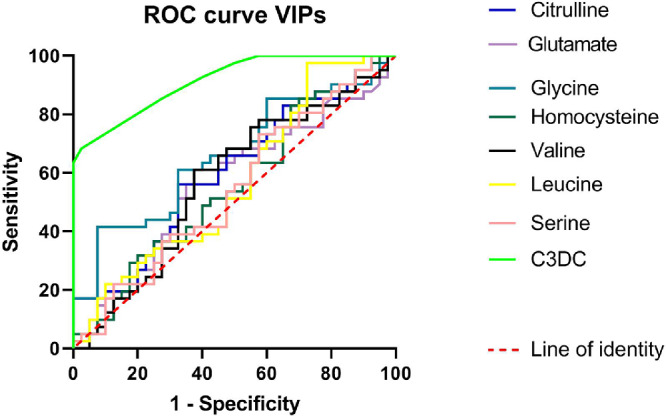
ROC curve of potential discriminant metabolites for ROP. C3DC is the best among all identified discriminant metabolites at predicting ROP, the diagnostic threshold of which is around 0.04 µmol/L, with high sensitivity (97.5%) and specificity (68.3%); followed by glycine (sensitivity = 92.5% and specificity = 58.5%), which is notably inferior at predicting than C3DC.
Discussion
To our knowledge, this is the first study investigating metabolomic alteration from blood of ROP and non-ROP infants using UPLC-MS/MS. Single-variant analysis may be insufficient and limited to reveal a comprehensive picture of the disease; we thus applied OPLS-DA to analyze our dataset and identified 10 metabolites that contributed largely to the separation between ROP and non-ROP. This highly significant OPLS-DA model had nearly perfect fit and predictivity. Our finding may shed lights on improving current ROP screening and diagnostic strategies.
We will next discuss the most significant metabolites (i.e., those with a VIP >1.0); because ROP is characterized by its vascular abnormality, mostly neovascularization, our discussion will focus on the implication of identified metabolite changes in the major vasculature component (i.e., endothelial cells).
Glycine, Serine, and Related Pathways
Proliferating and migrating endothelial cells require a large amount of metabolism and serine production.46 Although they are exposed to high level of oxygen in the blood, ECs produce most (80%) of their ATP through glycolysis.47 This metabolic signature is similar to that of tumor cells, which is known as the Warburg effect.48–51
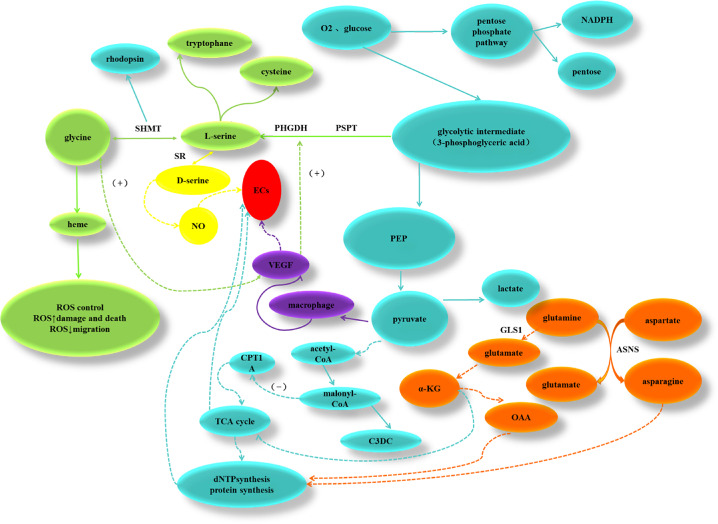
This highly active glycolysis process is vital for the synthesis of serine and glycine in ECs. Serine synthesis is dependent on the glycolytic metabolite 3-phosphoglycerate. Conversion to serine is catalyzed sequentially by phosphoglycerate dehydrogenase (PHGDH). Phosphoserine aminotransferase and phosphoserine phosphatase. Global mutations in the genes encoding these enzymes can lead to vascular and multiorgan abnormalities.52 Moreover, deletion of PHGDH is lethal to ECs.53 This revealed that ECs highly rely on serine synthesis pathway to survive.53 This is possibly because that active serine biosynthesis and metabolism provide essential substances for proliferating ECs and reduce the oxidative stress and reactive oxygen species level in Müller cells and ECs in the retina.49,54–56 Another isoform of serine, D-serine, can be converted from L-serine by serine racemase (SR). D-serine is a potent ligand for the N-methyl-D-aspartate receptor,57 activation of this receptor causes production of nitric oxide (Fig. 6), which is a proangiogenic factor.58 Deficiency in SR lowers nitric oxide and vascular endothelial growth factor (VEGF) levels and eventually attenuates choroidal neovascularization.59
Figure 6.
Schematic diagram of the effect of specific metabolites and metabolic pathways on endothelial cells. Orange indicates amino acids that contributed to the respiratory pathways. Cyan shows conventional TCA cycle pathways. Green presents interconversion of glycine and serine. Abbreviations: α-KG = alpha-ketoglutarate; ASNS = asparagine synthase; CPT1A = carnitine palmitoyltransferase 1a; C3DC = malonyl carnitine; GLS1 = glutaminase 1; OAA = oxaloacetic acid; PEP = phosphoenolpyruvic acid; PSPT = phosphoserine phosphatase; SHMT = serine hydroxymethyltransferase; SR = serine racemase.
The level of plasma glycine maybe higher in ROP. Singh et al. used untargeted metabolite profiling research found that serine and glycine was more than 50% higher in the anti-hypoxia-inducing factor 1 (Roxadustat) treated group than the control group.60 In the meantime, serine and glycine are interconvertible, which is catalyzed by serine hydroxymethyl transferase. A recent study suggested that glycine could mimic the effect of VEGF, the well-known angiogenic cytokine for endothelial cells, on promoting angiogenesis (Fig. 6).61 VEGF-induced activation of its transporter, glycine transporter 1 (GlyT1), lead to influx of glycine into the cell, which can affect cellular metabolism. Furthermore, they found that dietary supplementation of glycine enhanced neovascularization both in vivo and in vitro.61 In addition, glycine can promote the biosynthesis of HEME, which is critical in controlling the level of reactive oxygen species and maintaining oxidative phosphorylation, thus providing a favorable environment for the vascular proliferation.46,53,55,62 Besides, glycine has been proved to be closely associated with rapid proliferation of cancer cells.63 Validity of glycine therapy has been verified in renal ischemia-reperfusion injury, in which glycine reduces the injury mainly by reducing initial hypoxic damage to the tissue and inhibiting consequent inflammatory response.64
C3DC
Another interesting finding was that the level of C3DC was most indicative of predicting ROP. C3DC is an odd-chain dicarboxylic acid acylcarnitine produced from malonyl-coenzyme A (CoA), the major regulator of energy homeostasis by inhibiting carnitine palmitoyltransferase 1A (CPT1A), an enzyme catalyzing the rate-limiting step of mitochondrial fatty acid β-oxidation.65 Most malonyl-CoA is converted back to acetyl-CoA by malonyl-CoA decarboxylase (Fig. 6). Patients with congenital deficiency of this enzyme presented elevated C3DC levels66; therefore, C3DC may be reflective of malonyl-CoA level.67 Higher serum C3DC level was also reported in cases of diabetes mellitus68,69 and chronic kidney disease.70 Nevertheless, angiogenesis wise, fatty acid oxidation is critical for ECs in that CPT1A knockdown diminishes angiogenesis.71 Additionally, accumulation of C3DC and malonyl-CoA leads to endothelial sprouting defects.71 However, it remains unclear whether malonyl-CoA or C3DC accumulation suppresses fatty acid oxidation and switches fuel utilization to glucose. In ROP, the observation that arrested ECs failed to sprout, repopulate, and continue developing normally can possibly be attributed to abnormal fatty acid metabolism or impaired mitochondria function. Collectively, these studies provide a reasonable explanation for our observation of higher C3DC in ROP infants.
This prospective study collected newborn bloodspot and our results revealed an elevation of malonyl carnitine at 3 days of age in ROP group. However, clinical features of ROP were usually noted around 1 to 3 months after birth. Our results suggested that signs of growth suppression can start much earlier than the diagnosis of disease; these early and mild events can lead to a more catastrophic growth deficit later.
There are three major limitations of our study. First, the size of our cohort was not very large (40 ROP and 41 non-ROPs), and participants enrolled were limited to southeast China. For a bigger picture of the ROP conditions nationwide, a multicenter study involving larger population across the country need to be carried out. The other being that this present study only looked at targeted metabolomics (i.e., amino acids and carnitine and its derivatives). To reveal a comprehensive network of metabolites, untargeted metabolomic study is urgently needed, which will detect more unknown metabolites and generate an unbiased view of the metabolome. Last, in this preliminary study, we simply explored the relationship of major metabolites and the occurrence of ROP. A larger cohort enrolling individuals at different stages of ROP will have to be carefully designed and studied in the future.
Considering the potential of metabolomics analysis on diagnosing and evaluating prognosis of ROP, there are still great challenges.72 The human metabolome needs to be comprehensively defined first, including the number of endogenous metabolites in the human body. It is necessary to establish a reliable spectral reference database for the identification and interpretation of metabolites. In addition, our interpretation of so-called "biologically relevant" is limited by our current knowledge of the biological system, which makes it even harder to gain and insight into how the metabolic spectrum contributing to a certain disease.
Our preliminary findings showed that metabolites, especially C3DC and glycine, are exceptionally good at predicting ROP, and can identify a few pathways likely to be affected by ROP. This study opened new possibilities of using metabolomic analysis to look at the development of the disease. Yet metabolomics is only part of the state-of-the-art “omics” technologies, which includes proteomics, metabolomics, transcriptomics, and genomics. These together will provide us a more in-depth knowledge on the development of ROP and could potentially merit future disease screening and diagnosis.
Acknowledgments
The authors thank the dedication of the Shenzhen ROP Screening Cooperative Group and the Sixth Affiliated Hospital of Sun Yat-Sen University, which include neonatal intensive care units of the following hospitals: Shenzhen People's Hospital, The Second People's Hospital of Shenzhen, Peking University Shenzhen Hospital, The University of Hongkong-Shenzhen Hospital, Shenzhen Children's Hospital, Shenzhen Maternal and Child Health Hospital, Shenzhen Luohu District People's Hospital, Shen Zhen Luohu Maternal and Child Health Hospital, Shenzhen Nanshan Hospital, Nanshan Maternity & Child Healthcare Hospital of Shenzhen, The People's Hospital of Baoan Shenzhen, Shenzhen Baoan Maternal and Child Health Hospital, Shenzhen Songgang People's Hospital, Shenzhen Baoan Shajing People's Hospital, Longgang District People's Hospital of Shenzhen, Shenzhen Longgang District Maternity & Child Healthcare Hospital, People's Hospital of New District Longhua Shenzhen, Shenzhen Gongming People's Hospital, and Shenzhen Guangming New District People's Hospital.
Supported by Shenzhen Science and Technology Innovation Commission basic discipline layout project, China (JCYJ20170817112542555), and Medical and Health Projects of Sanming.
Disclosure: Y. Yang, None; Z. Wu, None; S. Li, None; M. Yang, None; X. Xiao, None; C. Lian, None; W. Wen, None; H. He, None; J. Zeng, None; J. Wang, None; G. Zhang, None
References
- 1. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012; 367: 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quinn GE, Barr C, Bremer D, et al.. Changes in course of retinopathy of prematurity from 1986 to 2013: comparison of three studies in the United States. Ophthalmology. 2016; 123: 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoppe G, Yoon S, Gopalan B, et al.. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc Natl Acad Sci USA. 2016; 113: E2516–E2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liegl R, Hellstrom A, Smith LE. Retinopathy of prematurity: the need for prevention. Eye Brain. 2016; 8: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013; 74(Suppl 1): 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mora JS, Waite C, Gilbert CE, Breidenstein B, Sloper JJ. A worldwide survey of retinopathy of prematurity screening. Br J Ophthalmol. 2018; 102: 9–13. [DOI] [PubMed] [Google Scholar]
- 7. Painter SL, Wilkinson AR, Desai P, Goldacre MJ, Patel CK. Incidence and treatment of retinopathy of prematurity in England between 1990 and 2011: database study. Br J Ophthalmol. 2015; 99: 807–811. [DOI] [PubMed] [Google Scholar]
- 8. Sternberg P Jr., Durrani AK. Evolving concepts in the management of retinopathy of prematurity. Am J Ophthalmol. 2018; 186: xxiii–xxxii. [DOI] [PubMed] [Google Scholar]
- 9. Yang CS, Wang AG, Sung CS, et al.. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond). 2010; 24: 14–20. [DOI] [PubMed] [Google Scholar]
- 10. Rajala RV, Gardner TW. Burning fat fuels photoreceptors. Nat Med. 2016; 22: 342–343. [DOI] [PubMed] [Google Scholar]
- 11. Rajala RV, Rajala A, Kooker C, Wang Y, Anderson RE. The Warburg effect mediator pyruvate kinase M2 expression and regulation in the retina. Sci Rep. 2016; 6: 37727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nivison-Smith L, Chua J, Tan SS, Kalloniatis M. Amino acid signatures in the developing mouse retina. Int J Dev Neurosci. 2014; 33: 62–80. [DOI] [PubMed] [Google Scholar]
- 13. de Souza CF, Kalloniatis M, Polkinghorne PJ, Mcghee CN, Acosta ML. Functional and anatomical remodeling in human retinal detachment. Exp Eye Res. 2012; 97: 73–89. [DOI] [PubMed] [Google Scholar]
- 14. Gibson R, Fletcher EL, Vingrys AJ, et al.. Functional and neurochemical development in the normal and degenerating mouse retina. J Comp Neurol, 2013; 521: 1251–1267. [DOI] [PubMed] [Google Scholar]
- 15. Downie LE, Hatzopoulos KM, Pianta MJ, et al.. Angiotensin type-1 receptor inhibition is neuroprotective to amacrine cells in a rat model of retinopathy of prematurity. J Comp Neurol. 2010; 518: 41–63. [DOI] [PubMed] [Google Scholar]
- 16. Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol. 2007; 504: 404–417. [DOI] [PubMed] [Google Scholar]
- 17. Cekmez F, Canpolat FE, Cetinkaya M, et al.. IGF-I and visfatin levels in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012; 49: 120–124. [DOI] [PubMed] [Google Scholar]
- 18. Connor KM, Sangiovanni JP, Lofqvist C, et al.. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007; 13: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellstrom A, Ley D, Hansen-Pupp I, et al.. IGF-I in the clinics: use in retinopathy of prematurity. Growth Horm IGF Res. 2016; 30-31: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellstrom A, Ley D, Hansen-Pupp I, et al.. New insights into the development of retinopathy of prematurity—importance of early weight gain. Acta Paediatr. 2010; 99: 502–508. [DOI] [PubMed] [Google Scholar]
- 21. Lofqvist CA, Najm S, Hellgren G, et al.. Association of retinopathy of prematurity with low levels of arachidonic acid: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018; 136: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palanisamy K, Nareshkumar RN, Sivagurunathan S, et al.. Anti-angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvasc Res. 2018; 122: 136–145. [DOI] [PubMed] [Google Scholar]
- 23. Shaw LC, Li Calzi S, Li N, et al.. Enteral Arg-Gln dipeptide administration increases retinal docosahexaenoic acid and neuroprotectin D1 in a murine model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2018; 59: 858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng SK, Wood JP, Chidlow G, et al.. Cancer-like metabolism of the mammalian retina. Clin Exp Ophthalmol. 2015; 43: 367–376. [DOI] [PubMed] [Google Scholar]
- 25. Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012; 31: 89–119. [DOI] [PubMed] [Google Scholar]
- 26. Gong Y, Fu Z, Liegl R, et al.. Omega-3 and omega-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr. 2017; 106: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellstrom A, Ley D, Hallberg B, et al.. IGF-1 as a drug for preterm infants: a step-wise clinical development. Am J Clin Nutr. 2017; 23: 5964–5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenhartova N, Matasova K, Lasabova Z, Javorka K, Calkovska A. Impact of early aggressive nutrition on retinal development in premature infants. Physiol Res. 2017; 66(Suppl. 2): S215–s226. [DOI] [PubMed] [Google Scholar]
- 29. Zielinska MA, Wesolowska A, Pawlus B, Hamulka J. Health effects of carotenoids during pregnancy and lactation. Nutrients. 2017; 9: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Hadidy A R, El-Mohandes EM, Asker SA, Ghonaim FM. A histological and immunohistochemical study of the effects of N-acetyl cysteine on retinopathy of prematurity by modifying insulin-like growth factor-1. Biotech Histochem. 2016; 91: 401–411. [DOI] [PubMed] [Google Scholar]
- 31. Narayanan S P, Xu Z, Putluri N, et al.. Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. Cell Death Dis. 2014; 5: e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saint-Geniez M, Jiang A, Abend S, et al.. PGC-1alpha regulates normal and pathological angiogenesis in the retina. Am J Pathol. 2013; 182: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vadlapatla RK, Vadlapudi AD, Mitra AK. Hypoxia-inducible factor-1 (HIF-1): a potential target for intervention in ocular neovascular diseases. Curr Drug Targets. 2013; 14: 919–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartnett ME, Morrison MA, Smith S, et al.. Genetic variants associated with severe retinopathy of prematurity in extremely low birth weight infants. Invest Ophthalmol Vis Sci. 2014; 55: 6194–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vahatupa M, Nattinen J, Jylha A, et al.. SWATH-MS proteomic analysis of oxygen-induced retinopathy reveals novel potential therapeutic targets. Invest Ophthalmol Vis Sci. 2018; 59: 3294–3306. [DOI] [PubMed] [Google Scholar]
- 36. Zasada M, Suski M, Bokiniec R, et al.. An iTRAQ-based quantitative proteomic analysis of plasma proteins in preterm newborns with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2018; 59: 5312–5319. [DOI] [PubMed] [Google Scholar]
- 37. Rinaldo P, Tortorelli S, Matern D. Recent developments and new applications of tandem mass spectrometry in newborn screening. Curr Opin Pediatr. 2004; 16: 427–433. [DOI] [PubMed] [Google Scholar]
- 38. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005; 123: 991–999. [DOI] [PubMed] [Google Scholar]
- 39. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. Arch Ophthalmol. 1987; 105: 906–912. [PubMed] [Google Scholar]
- 40. FUNDUS GROUP C S O O. Chinese guideline for screening retinopathy of prematurity(2014). Chin J Ophthalmol. 2014; 50: 933–935. [Google Scholar]
- 41. Li F, Gonzalez FJ, Ma X. LC–MS-based metabolomics in profiling of drug metabolism and bioactivation. Acta Pharmaceutica Sinica B. 2012; 2: 118–125. [Google Scholar]
- 42. Mahadevan S, Shah SL, Marrie TJ, Slupsky CM. Analysis of metabolomic data using support vector machines. Anal Chem. 2008; 80: 7562–7570. [DOI] [PubMed] [Google Scholar]
- 43. Barnes S, Benton HP, Casazza K, et al.. Training in metabolomics research. II. Processing and statistical analysis of metabolomics data, metabolite identification, pathway analysis, applications of metabolomics and its future. J Mass Spectrom. 2016; 51: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li S, Liu H, Jin Y, et al.. Metabolomics study of alcohol-induced liver injury and hepatocellular carcinoma xenografts in mice. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879: 2369–2375. [DOI] [PubMed] [Google Scholar]
- 45. Zhang T, Wu X, Yin M, et al.. Discrimination between malignant and benign ovarian tumors by plasma metabolomic profiling using ultra performance liquid chromatography/mass spectrometry. Clin Chim Acta. 2012; 413: 861–868. [DOI] [PubMed] [Google Scholar]
- 46. Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014; 39: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997; 273: C205–213. [DOI] [PubMed] [Google Scholar]
- 48. Draoui N, De Zeeuw P, Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017; 7: 170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fitzgerald G, Soro-Arnaiz I, Bock K. The Warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Front Cell Dev Biol. 2018; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang H, Vandekeere S, Kalucka J, et al.. Role of glutamine and interlinked asparagine metabolism in vessel formation. Embo J. 2017; 36: 2334–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim B, Li J, Jang C, Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. Embo J. 2017; 36: 2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. El-Hattab AW, Shaheen R, Hertecant J, et al.. On the phenotypic spectrum of serine biosynthesis defects. J Inherit Metab Dis. 2016; 39: 373–381. [DOI] [PubMed] [Google Scholar]
- 53. Vandekeere S, Dubois C, Kalucka J, et al.. Serine synthesis via PHGDH is essential for heme production in endothelial cells. Cell Metab. 2018; 28: 573–587.e513. [DOI] [PubMed] [Google Scholar]
- 54. Kelly K, Wang JJ, Zhang SX. The unfolded protein response signaling and retinal Muller cell metabolism. Neural Regen Res. 2018; 13: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Zang QS, Liu Z, et al.. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol. 2011; 301: C695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang T, Gillies M C, Madigan MC, et al.. Disruption of de novo serine synthesis in Muller cells induced mitochondrial dysfunction and aggravated oxidative damage. Mol Neurobiol. 2018; 55: 7025–7037. [DOI] [PubMed] [Google Scholar]
- 57. Mothet JP, Parent AT, Wolosker H, et al.. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000; 97: 4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003; 9: 521–530. [DOI] [PubMed] [Google Scholar]
- 59. Jiang H, Wu M, Liu Y, et al.. Serine racemase deficiency attenuates choroidal neovascularization and reduces nitric oxide and VEGF levels by retinal pigment epithelial cells. J Neurochem. 2017; 143: 375–388. [DOI] [PubMed] [Google Scholar]
- 60. Singh C, Hoppe G, Tran V, et al.. Serine and 1-carbon metabolism are required for HIF-mediated protection against retinopathy of prematurity. JCI Insight. 2019; 4: e129398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo D, Murdoch CE. Vascular endothelial growth factor signaling requires glycine to promote angiogenesis. Sci Rep. 2017; 7: 14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Di Salvo ML, Contestabile R, Paiardini A, Maras B. Glycine consumption and mitochondrial serine hydroxymethyltransferase in cancer cells: the heme connection. Med Hypotheses. 2013; 80: 633–636. [DOI] [PubMed] [Google Scholar]
- 63. Jain M, Nilsson R, Sharma S, et al.. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012; 336: 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petrat F, Boengler K, Schulz R, De Groot H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br J Pharmacol. 2012; 165: 2059–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008; 28: 253–272. [DOI] [PubMed] [Google Scholar]
- 66. Salomons GS, Jakobs C, Pope LL, et al.. Clinical, enzymatic and molecular characterization of nine new patients with malonyl-coenzyme A decarboxylase deficiency. J Inherit Metab Dis. 2007; 30: 23–28. [DOI] [PubMed] [Google Scholar]
- 67. Hozyasz KK, Oltarzewski M, Dudkiewicz Z. Malonylcarnitine in newborns with non-syndromic cleft lip with or without cleft palate. Int J Oral Sci. 2010; 2: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mai M, Tonjes A, Kovacs P, et al.. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS One. 2013; 8: e82459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun L, Liang L, Gao X, et al.. Early Prediction of Developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care. 2016; 39: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 70. Wang F, Sun L, Sun Q, et al.. Associations of plasma amino acid and acylcarnitine profiles with incident reduced glomerular filtration rate. Clin J Am Soc Nephrol. 2018; 13: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schoors S, Bruning U, Missiaen R, et al.. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015; 520: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lains I, Gantner M, Murinello S, et al.. Metabolomics in the study of retinal health and disease. Prog Retin Eye Res. 2018; 69: 57–79. [DOI] [PubMed] [Google Scholar]