Abstract
Purpose
To investigate the retinal sensitivity of highly myopic eyes with chorioretinal patchy atrophy (PA) using microperimetry.
Methods
Fifty-two eyes of 32 patients with high myopia were prospectively included. Twenty-two eyes of 16 patients had PA lesions; eyes without PA were analyzed as controls. Testing points on microperimetry in eyes with PA were designated as 3 zones: zone 1 as the PA lesion including its borders; zone 2 including testing points adjoining PA; zone 3 including all other testing points.
Results
In the PA group, the mean retinal sensitivity in zone 1 was 2.1 ± 2.8 dB, zone 2 = 8.3 ± 4.3 dB, and zone 3 = 9.4 ± 4.1 dB. Sensitivity in zone 1 was significantly reduced than zones 2 and 3 (P < 0.001). The mean retinal sensitivity in the PA group was lower than controls (6.5 ± 4.3 vs 13.9 ± 4.1 dB, P < 0.001), and combined zone 2 and 3 in the PA group also presented lower retinal sensitivity (8.8 ± 4.0 dB).
Conclusions
Eyes with PA generate patchy scotoma in PA lesions and reduced retinal sensitivity in regions beyond atrophic lesion on microperimetry. The presence of PA may be an indicator to reflect both significantly anatomical and functional alterations on myopic macular degeneration.
Keywords: high myopia, patchy atrophy, chorioretinal atrophy, myopic macular degeneration, microperimetry
High myopia is a major cause of visual impairment in developed countries,1,2 particularly in East Asia,3–5 because of several vision-threatening complications, such as myopic choroidal neovascularization (CNV), myopic foveoschisis, and myopic chorioretinal atrophy.6–8 The main alteration in high myopia is excessive axial length elongation of the globe. Additionally, posterior ocular segment deformities including posterior staphyloma and myopic macular degeneration may develop. According to the definition stated by the Meta-analysis for Pathologic Myopia (META-PM) study group, myopic macular degeneration is defined as five categories based on funduscopic findings: category 0, no maculopathy; category 1, tessellated fundus; category 2, diffuse chorioretinal atrophy; category 3, patchy chorioretinal atrophy; and category 4, macular atrophy; three additional features including lacquer cracks, myopic CNV, and Fuchs spots were defined as plus lesions.9 A tessellated fundus was suggested to be the first sign that high myopia had progressed to the myopic maculopathy. It can progress to diffuse atrophy or lacquer cracks, and may further progress to patchy atrophy (PA) and the formation of CNV.10
PA, classified as category 3, is a grayish-white chorioretinal atrophic lesion with well-defined borders in the macular area; it is of importance due to having a higher chance than tessellated fundus and diffuse atrophy to progress or develop myopic CNV and macular atrophy, causing severe visual impairment.6,9,10 Several studies have investigated the anatomical abnormalities of PA. PA is characterized by deformation of the sclera, complete loss of choriocapillaries, macular Bruch's membrane defects, and degeneration of the photoreceptors and the retinal pigment epithelium (RPE) when evaluated by optical coherence tomography (OCT).11,12 Fluorescein fundus angiography at 1 minute after dye injection showed relative hypofluorescence around PA, and turned into mild hyperfluorescence at the late phase.11 Progressive enlargement of PA could be observed during long-term follow-up of fundus photography or evaluated by fundus autofluorescence.10,13 Although previous studies have mostly focused on the anatomical aspect of PA, functional analysis of PA is currently not well established.
Best-corrected visual acuity (BCVA) has been the main indicator of visual function in retinal diseases; however, it cannot reflect comprehensive macular function because it is mainly based on responses from the foveal cones. Patchy atrophy does not commonly involve the foveal center when it develops initially.6,14 For assessment of macular disease especially when the foveal center is not involved, microperimetry is gaining acceptance as an effective clinical investigation. Microperimetry may provide additional information regarding fundus-correlated visual functional testing, severity and extent of macular disease, compensation for ocular movement, precise assessment of fixation stability and location, and detect subtle changes in visual function when visual acuity measurements do not identify changes.15–17
To our knowledge, there is limited functional analysis of eyes with PA. Thus, the purpose of this study was to evaluate the retinal sensitivity of highly myopic eyes with PA and to compare PA cases with highly myopic eyes not presenting with PA by means of microperimetry.
Materials and Methods
Participants
This is a prospective, cross-sectional study of highly myopic patients from the Kaohsiung Chang Gung Memorial Hospital. The aims and examinations were explained to the subjects before the study, and informed consent was obtained from all subjects in agreement with the Declaration of Helsinki for research involving human subjects. Institutional Review Board/Ethics Committee approval was prospectively obtained from the Committee of Medical Ethics and Human Experiments of Chang Gung Memorial Hospital.
This study included participants who were 18 years of age or older and diagnosed with high myopia (refractive error ≤–5.00 diopters (D)). PA was defined as unifocal or multifocal areas of chorioretinal and RPE atrophy with well-defined borders on color fundus photography, confirmed by spectral-domain optical coherence tomography (SDOCT).9,11,12 Eyes with PA of any size and eyes with foveal-involving PA were also included in this study. Exclusion criteria were other macular diseases (e.g., age-related macular degeneration, myopic traction maculopathy, foveoschisis, hereditary retinal dystrophies), history of CNV, or other potentially confounding diseases (e.g., significant cataract, diabetic retinopathy, glaucoma, trauma, uveitis).
Procedures
All participants underwent a complete ophthalmic examination, including BCVA, intraocular pressure measurement, slit-lamp biomicroscopy, indirect ophthalmoscopy, and axial length measurement. Color fundus photographs (20°) were obtained after pupil dilation. Visual acuity was converted to a logarithm of minimal angle of resolution (logMAR) for analysis.
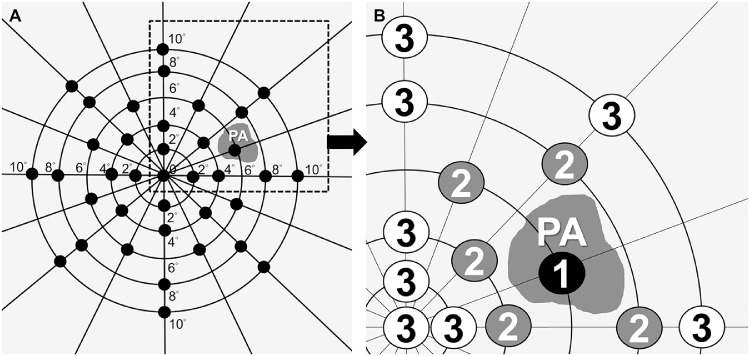
Retinal sensitivity measurements were obtained with microperimetry (MP-1, Nidek Inc., Fremont, CA), using the manufacturer-specified instructions for testing. Pupils were dilated with 1 drop each of tropicamide 1% and phenylephrine 2.5% at least 30 minutes before microperimetry. Because participants were kept in mesopic conditions before testing, the testing was performed in a darkened and quiet room. For microperimetry, a 2° red cross was used as the fixation target, and a 30-second fixation test was performed. The 4-2 staircase threshold strategy was used, and the test stimulus color was white; the stimulus was set to Goldmann size III with a duration of 200 ms against a white background with 1.27 cd/m2 illumination. The maximum possible sensitivity of the stimulus was 20 dB of attenuation. A custom grid designed to assess the macular region was used and consisted of 37 stimulus testing points with coverage of 20 degrees from fixation. Because PA has been defined as unifocal or multifocal chorioretinal atrophic lesions, it is to be expected that microperimetry might not be able to measure the retinal sensitivity of PA region precisely. Therefore, fixation was considered unstable if less than 75% of the fixation points were inside the 4° diameter circle.18 Microperimetry was retested immediately if less than 75% of the fixation points were inside the 4° diameter circle, and the four crosses or a ring would be used as an alternative fixation target when retested to increase test accuracy.19 In the PA group, testing points of microperimetry measurements superimposed on color fundus image were further designated into three zones based on their relative distance to the PA for analysis. Testing points were designated as zone 1 if it falls within the areas or at the border of patchy atrophy and were designated as zone 2 or 3 solely based on their relative distance to the PA lesion on the microperimetry image superimposed on color fundus image. Zone 2 was defined as the perilesional region, which included testing points immediately adjoining PA. Zone 3 was defined as the extralesional region, which included all other testing points which were separated from PA by at least one testing point or more than 2° away from PA (Fig. 1). The grading process was performed by two graders (J.L. and P.C.W.) according to the definitions described previously, and if there were disagreements between the two graders, they were resolved through consensus. In cases where PA coalesced with parapapillary atrophy, testing points located within the parapapillary atrophy were also designated as zone 1. A similar classification was previously described by another study in age-related macular degeneration-related geographic atrophy.20
Figure 1.
Schematic illustration of microperimetry sensitivity map in eye with PA. (A) PA and a testing grid consisted of 37 points located at 0, 2, 4, 6, 8, and 10° from fixation. (B) The testing points of microperimetric measurements were designated as three zones based on their relative distance to PA. Zone 1: PA lesion including its borders; zone 2 (perilesional region): testing points immediately adjoining PA; zone 3 (extralesional region): testing points which were separated from PA by at least one testing point or more than 2° away from PA.
The SDOCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) images were acquired with 25 horizontal raster scans covering a 20 × 20° oblong rectangle after microperimetry. Eyes with unifocal or multifocal areas of degeneration of the photoreceptors, RPE/Bruch's membrane, and loss of choriocapillaries were considered as PA cases.11,12 Eyes without chorioretinal and RPE/Bruch's membrane atrophy but with any reported value for retinal sensitivity measurement were analyzed as controls and were compared with PA cases. The OCT images were also used to exclude other macular disease and CNV when RPE detachment or fluid presented.
The measurement of PA area in eyes with PA was performed using ImageJ software (available at http://imagej.nih.gov/ij). Proportions of PA on color fundus image (with a resolution of 1392 × 1038 pixels) were calculated.
Statistical Analysis
Baseline characteristics of the highly myopic eyes with and without PA were analyzed using descriptive statistics. In some patients, both eyes were included for analysis; thus, mixed model analysis was used for analyzing factors associated with the presence of PA. Pearson's correlation was used to analyze the relationship between the number of testing points in zone 1 and BCVA in eyes with PA. The mean retinal sensitivity of zones 1 to 3 in the PA group was calculated for every eye separately and the averages of these means were compared across PA cases by using one-way analysis of variance and post hoc comparisons by the Bonferroni method. Comparison of retinal sensitivity between eyes with PA and eyes without PA was analyzed by conditional logistic regression after matching for age, axial length, spherical equivalence, and lens status with the Mahalanobis distance within propensity score calipers method. When analyzing factors associated with retinal sensitivity in specific regions of eyes with and without PA, univariate mixed model analyses were first performed, and then all of the significant factors were included in a multivariable model for analysis. One-way analysis of variance (ANOVA) and post hoc analysis using the Bonferroni method was performed when analyzing the mean retinal sensitivity among different categories based on the definition of META-PM,9 and when comparing the mean retinal sensitivity among control group, PA group, and regions outside of atrophic lesion in the PA group. A P value <0.05 was considered statistically significant. Analyses were carried out using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) and SPSS (Statistical Package for Social Science, version 22; SPSS Inc, Armonk, NY).
Results
Demographics of Study Participants
Fifty-two eyes of 32 patients with high myopia (21 [65.6%] women and 11 men) were included in this study. Thirty-three (63.5%) eyes were female and 19 eyes were male. The mean age of the 32 patients was 53.2 ± 12.4 years, with a range of 30 to 81 years. The mean refractive error of 52 eyes was –12.37 ± 4.41 D, with a range of –6.25 to –23 D. Thirty (57.6%) eyes were phakic and 22 eyes were pseudophakic. The mean axial length was 30.4 ± 2.1 mm, with a range of 26.6 to 35.3, and the mean Snellen BCVA was 20/72 ± 20/66 (logMAR 0.56 ± 0.52). Baseline demographics of highly myopic eyes with and without PA are summarized in Table 1. Older age, worse BCVA, more myopic spherical equivalent, and reduced retinal sensitivity were significant factors associated with the presence of PA (Table 2).
Table 1.
Baseline Demographics of Highly Myopic Eyes With and Without Patchy Atrophy
| Eyes Without PA | Eyes With PA | |
|---|---|---|
| No. of eyes | 30 | 22 |
| Age (yr) | 51.3 ± 12 | 57.4 ± 13.1 |
| Sex (female/male) | 18/12 | 15/7 |
| BCVA (logMAR) | 0.39 ± 0.45 | 0.77 ± 0.53 |
| Lens status (phakia/pseudophakia) (no.) | 19/11 | 11/11 |
| Axial length (mm) | 30.3 ± 2.1 | 30.7 ± 2.0 |
| Spherical equivalent (D) | −11.42 ± 3.92 | −13.76 ± 4.81 |
| Total retinal sensitivity (dB) | 13.9 ± 4.1 | 6.5 ± 4.3 |
| Retinal sensitivity of the center (0°) (dB) | 12.3 ± 6.1 | 6.8 ± 5.8 |
| Retinal sensitivity of 2-degree ring (dB) | 13.9 ± 7.6 | 7.6 ± 5.5 |
Table 2.
Factors Associated With Patchy Atrophy Using Mixed Model Analysis
| Coefficient | Standard Error | P Value | |
|---|---|---|---|
| Age (yr) | 0.013 | 0.006 | 0.030 |
| Sex (female = 0, male = 1) | −0.118 | 0.169 | 0.491 |
| BCVA (logMAR) | 0.372 | 0.112 | 0.002 |
| Lens status (phakia = 0, pseudophakia = 1) | 0.305 | 0.157 | 0.059 |
| Axial length (mm) | −0.002 | 0.036 | 0.959 |
| Spherical equivalent (D) | −0.035 | 0.017 | 0.046 |
| Retinal sensitivity (dB) | −0.059 | 0.010 | <0.001 |
Characteristics of PA on SDOCT and Microperimetry
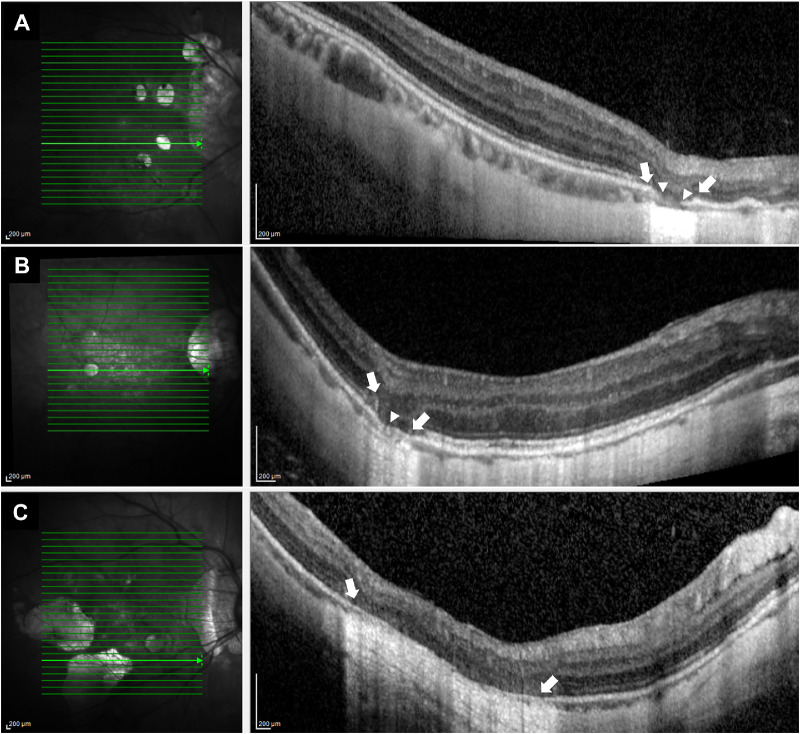
Twenty-two eyes of 16 patients presenting with PA on color fundus photography and confirmed by SDOCT were designated as the PA group. The PA lesions on SDOCT present as defects in the outer retina and the RPE/Bruch's membrane complex, with the inner retina directly in contact to the sclera (Fig. 2). A higher light reflectance was observed at regions with RPE/Bruch's membrane defects. At the edges of PA, the remnants of the Bruch's membrane with the loss of the RPE layer could be observed (Fig. 2A). SDOCT also showed that the choroid beneath the PA lesion was thinned or absent (Figs. 2B, 2C).
Figure 2.
Representative SDOCT images of highly myopic eye with PA. (A) Right fundus of a 40-year-old woman with PA. Her refractive error was -12.0 D and the axial length was 31.9 mm. Central macula had 25 scanned lines examined by SDOCT. Horizontal SDOCT section along the green scanned line in the left image shows defects in the outer retina and the RPE/Bruch's membrane in the area of PA (between arrows). The remnants of the Bruch's membrane are observed near the edge of the RPE/Bruch's membrane defect (between arrowheads). (B) Right fundus of a 51-year-old man with PA. His refractive error was -15.0 D and the axial length was 30.5 mm. The left image shows that the outer retina is not present in the area of PA (between arrows), and the RPE/Bruch's membrane is degenerated (arrowhead). (C) Right fundus of a 53-year-old woman with multifocal PA. The refractive error was -15.5 D and the axial length was 29.4 mm. The left image shows that the outer retina and RPE/Bruch’s membrane are absent in the area of PA. The inner retina appears to be directly attached to the sclera, and a higher light reflectance is observed in the region of RPE/Bruch's membrane defects (between arrows).
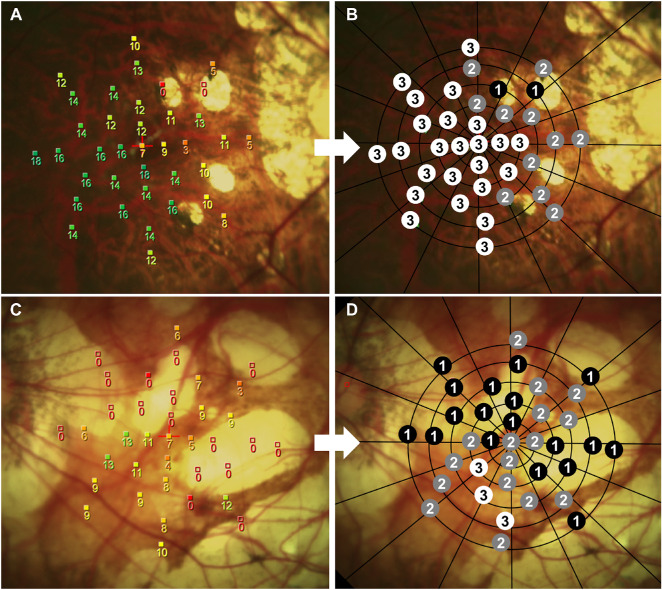
In the PA group, the mean PA area accounted for 9.4 ± 8.0% of the microperimetry color fundus image. Testing points of microperimetric measurements were designated as zones 1 to 3 for analysis (Fig. 3). The mean number of testing points within zones 1 to 3 was 11.4 ± 7.3, 10.7 ± 2.7, and 15.2 ± 8.0, respectively (P = 0.074). In eyes with PA, the number of testing points within zone 1 was not correlated with BCVA (r = 0.368, P = 0.121). Comparison of retinal sensitivity among zones 1 to 3 confirmed patchy scotoma in the PA lesion (one-way ANOVA F2, 54, 0.05 = 17.443, P < 0.001), that zone 1 (PA lesion) had the lowest mean retinal sensitivity (2.1 ± 2.8 dB) than zones 2 and 3 (8.3 ± 4.3 and 9.4 ± 4.1 dB, respectively). Post hoc comparisons by the Bonferroni method showed that the mean retinal sensitivity of zone 1 was significantly different from the other zones (P < 0.001), whereas there was no difference between zones 2 and 3 (P = 1.000).
Figure 3.
Representative microperimetry sensitivity images of highly myopic eye with patchy atrophy (PA). (A) Microperimetry sensitivity map of a 35-year-old woman's right eye with small area of PA. (B) Retinal sensitivity in PA lesion is severely reduced, and regions beyond the PA are less affected. The testing points of microperimetric measurements were designated as zones 1 to 3 for analysis. (C) Microperimetry sensitivity map of a 73-year-old woman's left eye with large area of PA. (D) Eighteen of 37 testing points were designated as zone 1 because of a large area of PA and peripapillary atrophy.
Retinal Sensitivity in Eyes with and without PA
The PA group had lower retinal sensitivity than the control group (Table 1). Although the mean retinal sensitivity in the control group was 13.9 ± 4.1 dB, the PA group (zones 1 to 3 combined) showed severely reduced retinal sensitivity (6.5 ± 4.3 dB). Moreover, the retinal sensitivity outside of the PA lesion (zones 2 and 3 combined) in the PA group (8.8 ± 4.0 dB) was also significantly lower than that in the control group (one-way ANOVA F2, 68, 0.05 = 21.600, P <0.001) (Fig. 4). After matching age, axial length, spherical equivalent value, and lens status (phakic vs pseudophakic), the mean retinal sensitivity in the PA group remained lower than the control group (5.4 ± 4.0 and 12.7 ± 4.9 dB, respectively) with a trend toward statistical significance (P = 0.050).
Figure 4.
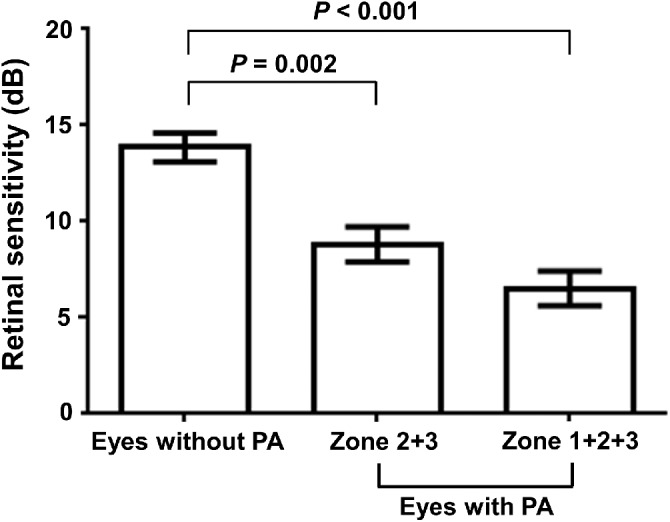
Comparison of retinal sensitivity between highly myopic eyes with and without PA. Retinal sensitivity in eyes without PA was analyzed as control. Eyes with PA (combined zones 1 to 3) showed significantly reduced retinal sensitivity than eyes without PA (P < 0.001), and the retinal sensitivity outside of the PA lesion (combined zones 2 and 3) in eyes with PA was also lower than that in eyes without PA (P = 0.002). Error bars refer to standard error of mean (SEM).
Mixed model analysis was used to determine factors associated with retinal sensitivity of specific regions (zones 1, 2, and 3) in eyes with PA and eyes without PA (Tables 3, 4, and 5). Univariate analysis revealed that older age, pseudophakia, and the presence of PA are significant factors associated with decreased retinal sensitivity in both atrophic region (zone 1) and relatively nonatrophic regions (zones 2 and 3). Further analysis using a multivariable model revealed that the presence of PA was the only associated factor with decreased retinal sensitivity in zone 1 (P < 0.001) and the relatively nonatrophic regions (zones 2 and 3, P = 0.008 and 0.021, respectively).
Table 3.
Mixed Model Analysis of Factors Associated With Retinal Sensitivity (Model 1, Dependent Variable: Mean Retinal Sensitivity in Eyes Without PA and Zone 1 in Eyes With PA)
| Univariate Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Coefficient | Standard Error | P Value | Coefficient | Standard Error | P Value | |
| Age (yr) | −0.234 | 0.073 | 0.003 | −0.062 | 0.047 | 0.199 |
| Sex (female = 0, male = 1) | 1.216 | 2.334 | 0.606 | |||
| Lens status (phakia = 0, pseudophakia = 1) | −5.832 | 2.031 | 0.007 | −1.647 | 1.335 | 0.228 |
| Axial length (mm) | −0.636 | 0.576 | 0.278 | |||
| Presence of patchy atrophy (eyes without PA = 0, zone 1 in eyes with PA = 1) | −11.652 | 1.090 | <0.001 | −10.689 | 1.088 | <0.001 |
Table 4.
Mixed model analysis of factors associated with retinal sensitivity (Model 2, dependent variable: mean retinal sensitivity in eyes without PA and Zone 2 in eyes with PA)
| Univariate Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Coefficient | Standard Error | P Value | Coefficient | Standard Error | P Value | |
| Age (yr) | −0.186 | 0.056 | 0.003 | −0.091 | 0.060 | 0.142 |
| Sex (female = 0, male = 1) | 0.109 | 1.837 | 0.953 | |||
| Lens status (phakia = 0, pseudophakia = 1) | −4.776 | 1.469 | 0.002 | −2.405 | 1.633 | 0.149 |
| Axial length (mm) | −0.764 | 0.403 | 0.068 | |||
| Presence of patchy atrophy (eyes without PA = 0, zone 2 in eyes with PA = 1) | −4.195 | 1.202 | 0.001 | −3.267 | 1.181 | 0.008 |
Table 5.
Mixed Model Analysis of Factors Associated With Retinal Sensitivity (Model 3 Dependent Variable: Mean Retinal Sensitivity in Eyes Without PA and Zone 3 in Eyes With PA)
| Univariate Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Coefficient | Standard Error | P Value | Coefficient | Standard Error | P Value | |
| Age (yr) | −0.158 | 0.055 | 0.008 | −0.070 | 0.060 | 0.259 |
| Sex (female = 0, male = 1) | −0.003 | 1.738 | 0.999 | |||
| Lens status (phakia = 0, pseudophakia = 1) | −4.184 | 1.466 | 0.007 | −2.187 | 1.678 | 0.202 |
| Axial length (mm) | −0.506 | 0.460 | 0.282 | |||
| Presence of patchy atrophy (eyes without PA = 0, zone 3 in eyes with PA = 1) | −4.068 | 1.279 | 0.003 | −3.081 | 1.290 | 0.021 |
When analyzed by region, the mean retinal sensitivity was lower in the superior region of the macula than in the inferior region (12.9 ± 3.9 vs 14.6 ± 4.7, respectively; P = 0.001) in the control group, whereas in the PA group, the mean retinal sensitivity was not statistically different in the superior and inferior regions (6.7 ± 4.5 vs 6.7 ± 5.2, P = 0.996).
We further subdivided the control group based on the definition of META-PM9; 15 (50%) eyes were classified as having no myopic maculopathy (category 0), and another 15 eyes had either tessellated fundus (category 1) or chorioretinal diffuse atrophy (category 2). Comparisons of the mean retinal sensitivity among different categories and post hoc tests by the Bonferroni method (one-way ANOVA F2, 49, 0.05 =25.288, P < 0.001) found that eyes with PA (category 3) had the lowest mean retinal sensitivity (6.5 ± 4.3 dB), whereas the mean retinal sensitivity in category 0 (15.7 ± 2.6 dB), 1 or 2 (12.0 ± 4.5 dB) revealed reduced retinal sensitivity with increasing category.
Discussion
In this prospective study, we evaluated the retinal sensitivity of highly myopic eyes with PA by using microperimetry. Although previous studies have focused on the anatomical abnormality of PA, our study reveals that the presence of PA lesions have a severe impact on patient's visual functions, which has not previously been well shown by other studies, therefore the clinical importance of PA may be underestimated. Our results showed that PA was associated with patchy scotoma on microperimetry, with retinal sensitivity at the PA lesions being severely reduced when compared with regions outside of the atrophic lesion. Furthermore, we found retinal sensitivity was also significantly reduced in regions outside of the PA lesions when compared with eyes without PA.
Classification of myopic macular degeneration based on fundus photography was initially proposed in 1998,21 and recently modified in a long-term natural history study.6 Among different categories of myopic macular degeneration, eyes with lacquer cracks and diffuse atrophy may not have significant vision deterioration (Snellen BCVA 20/36 ± 20/44 and 20/40 ± 20/50, respectively), but eyes presented worse BCVA when PA progressed (Snellen 20/58 ± 20/52).10 Our results are consistent with the previous study10 in that we found not only worse BCVA, but also reduced retinal sensitivity in the PA group compared to those without PA. Our study also found that retinal sensitivity was reduced with increasing category number, in which category 0 patients had relative normal retinal sensitivity, whereas patients with category 1 (tessellated fundus) and category 2 (chorioretinal diffuse atrophy) macular degenerations had lower retinal sensitivity compared with normal patients, and category 3 (PA) patients presented with the lowest retinal sensitivity out of all of the categories. Clinical importance of the classification of myopic macular degeneration by META-PM9 represents anatomical differences among different categories, and we found this classification may also reflect the grading of functional deterioration in both vision and retinal sensitivity.
When examined on OCT, PA lesions were found to have a lack of outer retina and RPE/Bruch's membrane with the choroid being thinning or absent.12 At the edges of the Bruch's membrane defects, the ends of Bruch's membrane were reported to be folded with the RPE layer upturned.12 Such changes in the RPE/Bruch's membrane were also observed on OCT in our study. Because the normal metabolism of photoreceptors require RPE, the defects of RPE/Bruch's membrane observed at the edges of the PA lesions are likely to result in the loss of healthy photoreceptors, which can partly explain the reduced retinal sensitivity in zone 2 of eyes with PA noted in this study. Choroidal thickness may also have an important impact on retinal sensitivity.22 In a recent study on OCT-based classification of myopic maculopathy, PA-related macular atrophy was found to have the thinnest choroidal thickness compared to all other categories of myopic macular degeneration.23 Choroidal thickness was reported to be positively correlated with visual acuity and retinal sensitivity.22,24 It was suggested that a thinned or absent choroid may result in a decrease in supply of oxygen and nutrients to the overlying RPE and outer retina, leading to abnormal photoreceptor signal generation or the loss of photoreceptors.25 Therefore, in eyes with PA, the thinning or the absence of choroid noted on OCT even at regions beyond the PA lesions may result in the loss of the normal choroid-RPE-photoreceptor functions, and can explain the widespread reduction in retinal sensitivity of zone 3 in eyes with PA observed in this study.
In normal individuals, the mean retinal sensitivity was found to be reduced significantly in the superior region of the macula compared to the inferior region but was similar in the nasal and temporal regions.26,27 In myopic individuals, retinal sensitivity was found to be significantly reduced in the entire macula and particularly in the superior outer quadrant compared to normal individuals.28 In highly myopic eyes without PA in our study, we also found that the retinal sensitivity in the superior region of the macula was significantly reduced which is consistent with the previous reports. Such difference, however, was not observed in eyes with PA in this study, which may be explained by the relatively random distribution of PA lesions without particular region predilections. The mechanism of different retinal sensitivity by regions is currently unclear, with age, choroidal thickness, and changes of overlying Bruch's membrane proposed as possible associated factors.12,22 Further studies may be needed to further evaluate the underlying mechanism contributing to regional differences in retinal sensitivity.
Several studies have suggested microperimetry for monitoring atrophic macular diseases, especially when there is no significant change or decline in visual acuity.16,20,29 For example, geographic atrophy (GA), an end-stage manifestation of nonexudative age-related macular degeneration,30 presents similar funduscopic findings to PA. GA has been described as a process that involves the loss of RPE and the associated choriocapillaries and overlying photoreceptors.31 In a recent systematic review, the retinal sensitivity of GA measured by microperimetry was considered to be the only functional tool that consistently detected progression in each cohort.17 In our study, we found that microperimetry was a useful tool in investigating eyes with PA and other categories of myopic macular degeneration because microperimetry was able to detect the subtle functional decline even when the fovea was not involved16 and may be able to reflect functional changes among the different categories of myopic macular degeneration. In the current study, retinal sensitivity in the nonatrophic regions of PA patients as measured by microperimetry was significantly reduced compared with patients without PA, which suggests that the presence of PA in highly myopic eyes may be an indicator of poorer visual outcome. Characterization of visual function in highly myopic eyes with PA and other categories of myopic macular degeneration with microperimetry may be important in determining the severity of each category of myopic macular degeneration, and allow physicians to provide suitable patient counseling or timely referral to low vision rehabilitation when encountering such patients.
This study has some limitations. First, the number of highly myopic eyes with PA was relatively small. The results of this study need to be confirmed in a larger series of patients. Second, our study was cross-sectional; however, PA in highly myopia may progress over time. In a long-term observational study, about 67.6% of eyes with PA showed enlargement of patchy areas, and 2.7% of eyes developed a CNV.6 Because of the limitation of our study design, the effects of PA progression on the changes in retinal sensitivity could not be evaluated. Thus, future longitudinal studies assessing the functional changes in eyes with PA would be important in providing further information regarding the prognosis of highly myopic patients because this may more clearly demonstrate the relationship between the decline in retinal sensitivity and the appearance or progression of PA lesions. Third, although the zone designation for the testing points was performed by two graders to reduce the bias of having the grading done by just one grader, the microperimetry sensitivity results were not masked to the graders on the fundus image. This may lead to a bias for the graders to be more inclined to give a point lying on the edge of the PA lesion a “zone 1” designation if it had a lower retinal sensitivity, or a “zone 2” designation if it had a higher retinal sensitivity. Although any discrepancies in zone designations were eventually resolved by consensus between the two graders, the previously mentioned bias could not be totally eliminated unless the retinal sensitivity results on the fundus image were masked to the graders. Therefore, this possible grading bias may need to be taken into consideration when interpreting the results of our study, and a better method would be performing similar gradings in a masked fashion in future studies. Last, in this study, microperimetry was retested immediately if less than 75% of the fixation points were inside the 4° diameter circle. The criteria and methods for retesting used in this study was done with the intent of improving testing accuracy in these patients whom we presume would have poorer fixation. This was solely based on our clinical experience and opinion that a possible learning effect might exist with improved testing accuracy after repeated testing. However, previous literatures have not clearly demonstrated the relationship between unstable fixation and measurement variability on microperimetry, and thus re-testing in patients who present with unstable fixation may not have been necessary.
In conclusion, this prospective study investigated PA, a type of myopic macular degeneration that can cause severe functional impairments on vision and retinal sensitivity. Highly myopic patients with PA may represent a group of patients with a more pronounced functional deterioration and are at a more severe stage of myopic macular degeneration.
Acknowledgments
The authors thank Hung-Yin Lai, MD for PA area measurement using ImageJ software and appreciate the Biostatistics Center, Kaohsiung Chang Kung Memorial Hospital, and Pei-Shan Ho, MS, PhD, Kaohsiung Medical University, for the statistics work.
The authors alone are responsible for the content and writing of the paper
Disclosure: J. Lo, None; L.Y.-C. Poon, None; Y.-H. Chen, None; H.-K. Kuo, None; Y.-J. Chen, None; W.-Y. Chiang, None; P.-C. Wu, None
References
- 1. Cotter SA, Varma R, Ying-Lai M, et al.. Causes of low vision and blindness in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2006; 113: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 2. Buch H, Vinding T, La Cour M, et al.. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004; 111: 53–61. [DOI] [PubMed] [Google Scholar]
- 3. Iwase A, Araie M, Tomidokoro A, et al.. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006; 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 4. Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004; 111: 62–69. [DOI] [PubMed] [Google Scholar]
- 5. Liu HH, Xu L, Wang YX, et al.. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology. 2010; 117: 1763–1768. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi K, Ohno-Matsui K, Shimada N, et al.. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010; 117: 1595–1611. [DOI] [PubMed] [Google Scholar]
- 7. Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002; 109: 704–711. [DOI] [PubMed] [Google Scholar]
- 8. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 9. Ohno-Matsui K, Kawasaki R, Jonas JB, et al.. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015; 159: 877–883. [DOI] [PubMed] [Google Scholar]
- 10. Fang Y, Yokoi T, Nagaoka N, et al.. Progression of Myopic Maculopathy during 18-Year Follow-up. Ophthalmology. 2018; 125: 863–877. [DOI] [PubMed] [Google Scholar]
- 11. Ohno-Matsui K, Akiba M, Moriyama M, et al.. Intrachoroidal cavitation in macular area of eyes with pathologic myopia. Am J Ophthalmol. 2012; 154: 382–393. [DOI] [PubMed] [Google Scholar]
- 12. Ohno-Matsui K, Jonas JB, Spaide RF. Macular bruch nembrane holes in highly myopic patchy chorioretinal atrophy. Am J Ophthalmol. 2016; 166: 22–28. [DOI] [PubMed] [Google Scholar]
- 13. Miere A, Capuano V, Serra R, et al.. Evaluation of patchy atrophy secondary to high myopia by semiautomated software for fundus autofluorescence analysis. Retina. 2018; 38: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 14. Ito-Ohara M, Seko Y, Morita H, Imagawa N, Tokoro T. Clinical course of newly developed or progressive patchy chorioretinal atrophy in pathological myopia. Ophthalmologica. 1998; 212: 23–29. [DOI] [PubMed] [Google Scholar]
- 15. Markowitz SN, Reyes SV. Microperimetry and clinical practice: an evidence-based review. Can J Ophthalmol. 2013; 48: 350–357. [DOI] [PubMed] [Google Scholar]
- 16. Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 442–448. [DOI] [PubMed] [Google Scholar]
- 17. Wong EN, Chew AL, Morgan WH, Patel PJ, Chen FK. The use of microperimetry to detect functional progression in non-neovascular age-related macular degeneration: a systematic review. Asia Pac J Ophthalmol (Phila). 2017; 6: 70–79. [DOI] [PubMed] [Google Scholar]
- 18. Midena E, Radin PP, Pilotto E, et al.. Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry study. Semin Ophthalmol. 2004; 19: 55–61. [DOI] [PubMed] [Google Scholar]
- 19. Midena E. Perimetry and the Fundus: An Introduction to Microperimetry. Thorofare, NJ: SLACK Incorporated, 2007. [Google Scholar]
- 20. Meleth AD, Mettu P, Agron E, et al.. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011; 52: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tokoro T. Types of fundus changes in the posterior pole. In: Tokoro T. ed. Atlas of Posterior Fundus Changes in Pathologic Myopia. Tokyo: Springer-Verlag; 1998: 5–22. [Google Scholar]
- 22. Zaben A, Zapata MA, Garcia-Arumi J. Retinal sensitivity and choroidal thickness in high myopia. Retina. 2015; 35: 398–406. [DOI] [PubMed] [Google Scholar]
- 23. Fang Y, Du R, Nagaoka N, et al.. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology. 2019; 126: 1018–1032. [DOI] [PubMed] [Google Scholar]
- 24. Nishida Y, Fujiwara T, Imamura Y, et al.. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012; 32: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Zhang G, Zhou X, et al.. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. 2019; 60: 3074–3083. [DOI] [PubMed] [Google Scholar]
- 26. Midena E, Vujosevic S, Cavarzeran F, Microperimetry Study G. Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology. 2010; 117: 1571–1576. [DOI] [PubMed] [Google Scholar]
- 27. Springer C, Bultmann S, Volcker HE, Rohrschneider K. Fundus perimetry with the Micro Perimeter 1 in normal individuals: comparison with conventional threshold perimetry. Ophthalmology. 2005; 112: 848–854. [DOI] [PubMed] [Google Scholar]
- 28. Qin Y, Zhu M, Qu X, et al.. Regional macular light sensitivity changes in myopic Chinese adults: an MP1 study. Invest Ophthalmol Vis Sci. 2010; 51: 4451–4457. [DOI] [PubMed] [Google Scholar]
- 29. Chen FK, Patel PJ, Webster AR, et al.. Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina. 2011; 31: 371–379. [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Klein BE, Knudtson MD, et al.. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114: 253–262. [DOI] [PubMed] [Google Scholar]
- 31. Sunness JS, Gonzalez-Baron J, Applegate CA, et al.. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106: 1768–1779. [DOI] [PubMed] [Google Scholar]