Abstract
Purpose
To investigate the relationship between proangiogenic and inflammatory cytokines in concurrent vitreous, aqueous, and plasma samples from patients with proliferative diabetic retinopathy (PDR).
Methods
Vitreous, aqueous, and plasma samples were analyzed using multiplex immunoassay for 10 PDR-related cytokines (IL-6, IL-8, TNF-α, monocyte chemoattractant protein-1 [MCP-1], macrophage inflammatory protein-1β [MIP-1β], VEGF receptor 1 [Flt-1], placental growth factor [PlGF], VEGF-A, VEGF-C, VEGF-D). A total of 17 patients with PDR and 7 controls were included. The primary outcome was correlation of cytokines in vitreous, aqueous, and plasma. The secondary outcome was the comparison of cytokine levels in controls and diabetics with and without recent anti-VEGF injection.
Results
The following factors were elevated in diabetics compared with controls: vitreous IL-6, IL-8, TNF-α, MCP-1, MIP-1β, PlGF, and VEGF-A; and aqueous IL-6, IL-8, PlGF, and VEGF-C (all P < 0.05). Vitreous and aqueous IL-8, PlGF, and VEGF-A were significantly correlated in patients with PDR (all P < 0.05). Plasma cytokines were not correlated with those in vitreous and aqueous (all P > 0.05). Vitreous and aqueous IL-6, IL-8, TNF-α, PlGF, and VEGF-A differed among controls and diabetics with and without recent anti-VEGF injection (all P < 0.05). In one-to-one comparisons, aqueous VEGF-A levels were lower in diabetic patients who had recent anti-VEGF injection compared with those who did not (P = 0.01).
Conclusions
In this proof-of-concept study, IL-8, VEGF-A, and PlGF demonstrated a strong correlation in vitreous and aqueous of patients with PDR. The aqueous may serve as a proxy for vitreous for some cytokines involved in PDR. Recent anti-VEGF injections decreased VEGF-A levels in aqueous, but did not significantly affect other cytokines, suggesting a role for other targeted therapies in PDR management.
Keywords: aqueous, vitreous, cytokines, diabetic retinopathy
Risk factors for diabetic retinopathy include diabetes duration, uncontrolled blood glucose and blood pressure, and dyslipidemia. However, in the Wisconsin Epidemiologic Study of Diabetic Retinopathy, only 9% to 10% of the retinopathy risk was accounted for by systemic factors.1 Further insight into mechanisms of diabetic retinopathy progression is warranted in managing this increasingly common disease.
The pathogenesis of proliferative diabetic retinopathy (PDR) involves disruption in the balance of proangiogenic and antiangiogenic factors, leading to neovascularization and its associated sight-threatening complications.2 Vitreous vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) are upregulated in PDR, whereas VEGF receptor 1 (Flt-1) is downregulated. Accordingly, PDR progression has decreased with anti-VEGF therapy.3 Prior studies also identified inflammatory markers in aqueous and plasma.4 The FAME trial showed that fluocinolone acetonide slowed PDR progression, suggesting that targeting inflammation may have a role in management.5
From a practical standpoint, aqueous and plasma are more accessible than vitreous; thus they could have utility as proxies for assaying the vitreous. Greater understanding of the molecular mechanisms of PDR may aid in determining response to therapy and developing novel treatments. There is evidence that homocysteine levels correlate in vitreous, aqueous, and plasma of patients with PDR.6 However, the extent to which other cytokines correlate in these fluids is not well characterized.
Methods
This study was in accordance with the tenets of the Declaration of Helsinki and approved by the institutional review board of the University of California, San Francisco (UCSF). Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. Patients with PDR and nondiabetic controls undergoing elective primary pars plana vitrectomy at two UCSF sites from May 2018 to February 2019 were recruited. All vitrectomies were performed by a single surgeon (JMS).
Exclusion criteria included history of prior vitrectomy, rhegmatogenous retinal detachment, retinal vascular occlusion, uveitis, advanced glaucoma, complicated anterior segment surgery, lens dislocation, and trauma. Clinical characteristics were collected from the medical record, including history of cataract surgery, recent intravitreal injection of anti-VEGF agents (within the last 90 days), and prior panretinal photocoagulation. Patients with recent anti-VEGF treatment had received bevacizumab, ranibizumab, or aflibercept.
Aqueous, vitreous, and plasma samples were collected on the day of surgery. Prior to initiating vitrectomy, at least 50 µL of aqueous was collected using a sterile 30-gauge needle on a 1-mL syringe. Then, before fluid infusion was turned on, at least 1 mL of nondilute vitreous sample was collected using 25- or 27-gauge vitrectomy. Whole blood was collected and spun down to isolate the plasma portion. All samples were stored at –80°C. Sandwich multiplex immunoassay was performed using customized MSD V-plex kits (Mesoscale Discovery, Rockville, MD, USA) according to the manufacturer's protocol. All samples were plated in duplicate. Levels of the following 10 cytokines were measured: IL-6, IL-8, TNF-α, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), Flt-1, PlGF, VEGF-A, VEGF-C, VEGF-D. Diabetic and nondiabetic samples were plated in randomized fashion. For the purposes of statistical analysis, zero values were treated as half of the manufacturer's lower limit of detection.
The Shapiro-Wilk test demonstrated that the data were not normally distributed. The Mann-Whitney U test for continuous variables and the χ2 test for categorical variables were used. For the primary outcome, the Spearman's rank correlation was used to determine the strength of the relationship between cytokine levels in vitreous, aqueous, and plasma. Outliers were excluded. For the secondary outcome, Kruskal-Wallis testing was performed for vitreous and aqueous cytokine levels among controls and diabetic patients subdivided by history of recent anti-VEGF injection. For one-to-one testing between these three groups, Bonferroni correction for multiple comparisons was applied, and a P value < 0.0167 was considered significant (0.05/3). Statistical analyses were performed using Stata version 15.0 (StataCorp, College Station, TX, USA).
Results
The final study group included 17 patients with PDR and 7 nondiabetic controls (Table). Five cases were excluded due to insufficient sample quantity. The mean and standard deviation of the subjects’ age was 55.5 ± 12.7 years. The nondiabetic controls were older than the patients with PDR (67.1 ± 5.1 years vs. 50.6 ± 11.7; P < 0.05).
Table.
Patient Characteristics
| Characteristic | Controls (n = 7) | Diabetics (n = 17) | P Value |
|---|---|---|---|
| Age, y | |||
| Mean ± SD (range) | 67.1 ± 5.1 (62–77) | 50.6 ± 11.7 (28–64) | < 0.05 |
| Median (IQR) | 65 (64–69) | 53 (48–59) | |
| Gender | 0.20 | ||
| Male | 3 (42.9%) | 12 (70.6%) | |
| Female | 4 (57.1%) | 5 (29.4%) | |
| Lens status | 0.20 | ||
| Phakic | 3 (42.9%) | 12 (70.6%) | |
| Pseudophakic | 4 (57.1%) | 5 (29.4%) | |
| Indication for vitrectomy | N/A | ||
| ERM | 4 (57.1%) | 1 (5.9%) | |
| Macular hole | 3 (42.9%) | 1 (5.9%) | |
| TRD | 3 (17.6%) | ||
| VH | 3 (17.6%) | ||
| TRD + VH | 9 (52.9%) | ||
| Hemoglobin A1c, % | - | N/A | |
| Mean ± SD (range) | 7.8 ± 2.5 (5.5–11.6) | ||
| Median (IQR) | 7.1 (6.0–8.9) | ||
| Recent intravitreal injection (within 90 days) | - | 6 | N/A |
| History of PRP | - | 8 | N/A |
The mean age of the controls was greater than that of diabetics because the indications for vitrectomy in controls predominantly affect older patients. There was no significant difference in gender or lens status between the two populations. ERM, epiretinal membrane;
IQR, interquartile range; N/A, not available; TRD, tractional retinal detachment; VH, vitreous hemorrhage.
Of the 10 cytokines examined, vitreous levels of IL-6, IL-8, TNF-α, MCP-1, MIP-1β, PlGF, and VEGF-A were significantly elevated in diabetics versus controls (Supplementary Table S1). Aqueous levels of IL-6, IL-8, PlGF, and VEGF-C were elevated in diabetics. There was no significant difference in levels of plasma factors between diabetics and controls.
For the primary outcome, Spearman's rank correlation between cytokine levels in different fluids was determined. There was a significant correlation between vitreous and aqueous IL-8 (r = 0.6321), PlGF (r = 0.8353), and VEGF-A (r = 0.6117) (Fig. 1; all P < 0.05). Because the vitreous and aqueous are thought to more freely communicate following cataract surgery, the correlation could have been explained by patients with pseudophakic status. Analyzing phakic patients only, similar correlations persisted (Supplementary Fig. S2; all P < 0.05).
Figure 1.
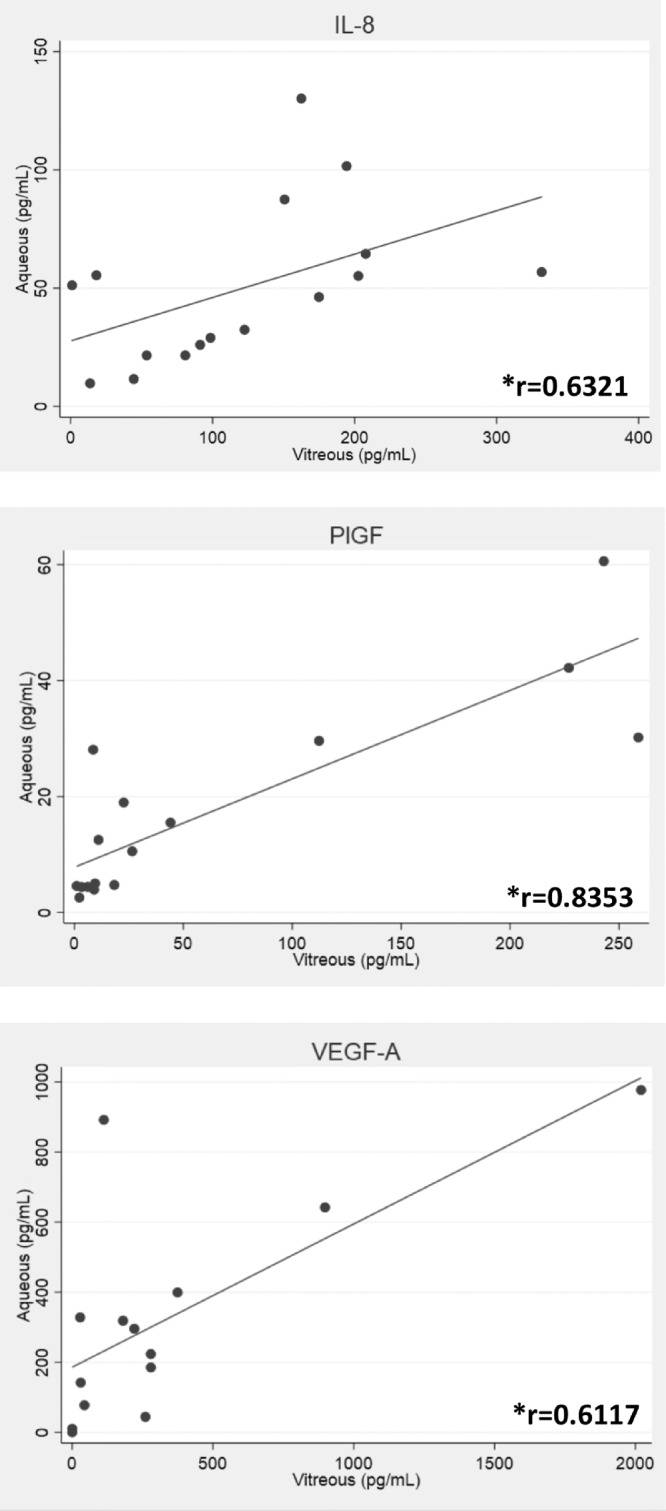
Correlation of vitreous and aqueous factors in diabetic patients. There was a significant correlation between vitreous and aqueous concentrations of the cytokines IL-8, PlGF, and VEGF-A. *P < 0.05.
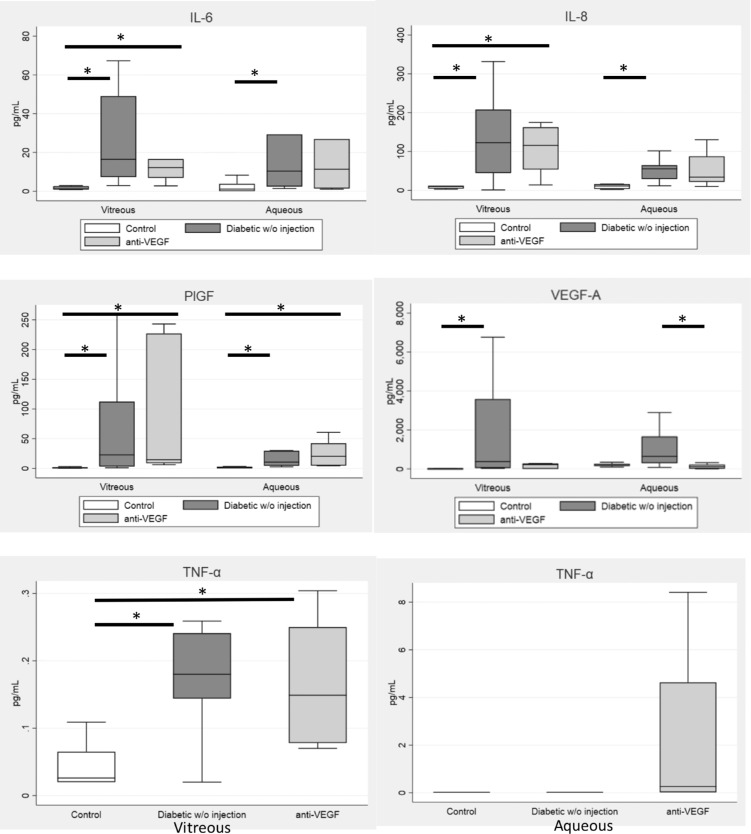
For the secondary outcome, diabetic patients were divided into those with and without recent anti-VEGF injection (within the past 90 days). Treatment with anti-VEGF occurred a mean of 55.8 days prior to sample collection, with a range of 18 to 88 days. Kruskal-Wallis testing demonstrated a significant difference between controls and diabetics with and without recent anti-VEGF injection for vitreous and aqueous IL-6, IL-8, TNF-α, PlGF, and VEGF-A (all P < 0.05). In one-to-one testing among the three patient groups, aqueous VEGF-A levels were lower in patients with recent anti-VEGF injection (P = 0.01) and were similar to those in nondiabetic controls (P = 0.28) (Fig. 2). Vitreous VEGF-A levels in patients with recent anti-VEGF injection were similar to those in nondiabetic controls (P = 0.1). In contrast, vitreous and aqueous IL-6, IL-8, TNF-α, and PlGF levels were indistinguishable in diabetics with and without recent anti-VEGF treatment (P > 0.0167).
Figure 2.
Cytokine levels among controls and diabetic patients with and without recent anti-VEGF injection. Kruskal-Wallis testing demonstrated a significant difference in vitreous and aqueous levels of all selected cytokines among controls and diabetics with and without recent anti-VEGF injection (all P < 0.05). In one-to-one comparisons, the vitreous and aqueous VEGF-A level was decreased in patients with recent anti-VEGF injection, whereas the other cytokine levels did not change. *P < 0.0167 (Bonferroni correction).
Discussion
In this pilot study with a small number of samples, inflammatory cytokines were found to be correlated in the vitreous and aqueous of patients with PDR. The correlations persisted even in phakic patients. These results suggest that the aqueous can serve as a reliable proxy for the vitreous regarding some, but not all, cytokines. Additional work is needed to determine why only certain factors are correlated between vitreous and aqueous.
Consistent with prior studies, VEGF-A was elevated in vitreous and aqueous of patients with PDR, and there was a strong correlation between vitreous and aqueous VEGF-A.7,8 Moreover, there was a correlation between vitreous and aqueous IL-8 and PlGF. Although TNF-α, MCP-1, MIP-1β, and VEGF-A were higher in vitreous, they were not significantly elevated in aqueous of diabetics versus controls. There are some possible explanations. Aqueous TNF-α levels were below the lower limit of detection in most samples (<0.040 pg/mL), so any difference between the two groups would not have been apparent. Aqueous MCP-1 and MIP-1β approached statistical significance and may have demonstrated a difference between diabetics and controls with larger sample sizes. The diabetic group included patients with and without recent anti-VEGF injections, and those with recent injection had lower levels of aqueous VEGF-A compared with those without recent injection (Fig. 2).
As expected, vitreous and aqueous VEGF-A levels were suppressed in patients with recent anti-VEGF injections, and were similar to those in controls. However, there was no difference in other cytokines. This is in contrast with a prior study demonstrating increased IL-8 one week following anti-VEGF injection.9 In our patient population, anti-VEGF therapy was given a mean of 55.8 days prior to sample collection. One possibility is that a transient increase in IL-8 after injection diminishes over time.
Prior studies have identified elevated plasma cytokines in patients with PDR, but others have not.10,11 Overall the literature has been inconsistent. Some authors suggest that plasma VEGF levels may be influenced by systemic control of blood glucose.12 There was no increase in plasma cytokines in our samples, and patients were on systemic therapy for diabetes.
The pilot nature of this study should be emphasized. Limitations include its small sample size and the older age of the controls compared with diabetics. The indications for vitrectomy in nondiabetics include macular hole and epiretinal membrane, conditions that predominantly affect the elderly. It is not known whether age is a factor in cytokine levels, but no relationship has been found thus far.13
Conclusions
This proof-of-concept study demonstrated that in a small number of patients with PDR, cytokines elevated in the vitreous (IL-8, PlGF, and VEGF-A) were significantly correlated with those in the aqueous, suggesting that aqueous sampling can reliably reflect vitreous levels of these cytokines. Given that aqueous is relatively accessible compared with vitreous, analyzing the aqueous of patients with PDR may yield more data for future studies. Furthermore, diabetic patients with recent anti-VEGF injections had decreased VEGF-A levels, whereas other cytokine levels remained elevated, supporting the notion of targeting inflammation in PDR.
Supplementary Material
Acknowledgments
Supported in part by the National Eye Institute, Core Grant for Vision Research, EY002162, and 1R01EY024004 (JMS), Research to Prevent Blindness, Inc. (New York, NY, USA), and That Man May See, Inc. (San Francisco, CA, USA).
Disclosure: F. Wu, None; A. Phone, None; R. Lamy, None; D. Ma, None; S. Laotaweerungsawat, None; Y. Chen, None; T. Zhao, None; W. Ma, None; F. Zhang, None; C. Psaras, None; J.M. Stewart, None
References
- 1. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 2. Yu Y, Zhang J, Zhu R, et al.. The profile of angiogenic factors in vitreous humor of the patients with proliferative diabetic retinopathy. Curr Mol Med. 2017; 17: 280–286. [DOI] [PubMed] [Google Scholar]
- 3. Gross JG, Glassman AR, Jampol LM, et al.. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015; 314: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vujosevic S, Simó R.. Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci. 2017; 58: BIO68–BIO75. [DOI] [PubMed] [Google Scholar]
- 5. Wykoff CC, Chakravarthy U, Campochiaro PA, Bailey C, Green K, Cunha-Vaz J. Long-term effects of intravitreal 0.19 mg fluocinolone acetonide implant on progression and regression of diabetic retinopathy. Ophthalmology. 2017; 124: 440–449. [DOI] [PubMed] [Google Scholar]
- 6. Lim CP, Loo AV, Khaw KW, et al.. Plasma, aqueous and vitreous homocysteine levels in proliferative diabetic retinopathy. Br J Ophthalmol. 2012; 96: 704–707. [DOI] [PubMed] [Google Scholar]
- 7. Funatsu H, Yamashita H, Noma H, et al.. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 3–8. [DOI] [PubMed] [Google Scholar]
- 8. Aiello LP, Avery RL, Arrigg PG, et al.. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 9. Forooghian F, Kertes PJ, Eng KT, Agrón E, Chew EY. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci. 2010; 51: 2388–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012; 153: 307–313.e302. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Chen S, Jiang F, et al.. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One. 2014; 9: e110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baharivand N, Zarghami N, Panahi F, Dokht Ghafari MY, Mahdavi Fard A, Mohajeri A. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin Ophthalmol. 2012; 6: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi S, Adachi K, Suzuki Y, Maeno A, Nakazawa M. Profiles of inflammatory cytokines in the vitreous fluid from patients with rhegmatogenous retinal detachment and their correlations with clinical features. Biomed Res Int. 2016; 2016: 4256183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.