Abstract
Blastomycosis is a systemic fungal infection that most commonly affects dogs and humans. The disease is thought to be endemic in southern regions of Michigan, USA, but epidemiologic investigations have not been reported in detail for this state. The primary aims of this study were to investigate the prevalence and distribution of canine blastomycosis cases in Michigan and to identify risk factors for infection. Over 200 primary care veterinary clinics throughout the state were surveyed regarding blastomycosis prevalence, and demographic information was obtained from medical records of affected dogs that were evaluated at these clinics. A retrospective case control study was conducted for an additional 49 dogs with blastomycosis that were evaluated at specialty referral centers located in the southern mid-Michigan region. Prevalence rates were calculated for each county, and cases were mapped using geocoding software. Univariable and multivariable analyses were used to identify risk factors for infection. Prevalence rates were ≥100 cases per 100,000 dogs in five counties. Most blastomycosis cases originated from the Upper Peninsula or from a high-density area in the northern Lower Peninsula. Multivariable regression analysis identified travel or residence north of the 45th parallel as a strong risk factor for infection (P < .001). Blastomycosis was uncommon in southern counties. These results refute previous speculations and should be of value to both human and animal health. Given that many heightened risk areas are popular tourist destinations, practitioners across the USA should be mindful of the spatial distribution of blastomycosis in Michigan.
Keywords: dogs, Blastomyces sp, Upper Peninsula, systemic mycoses
Introduction
Blastomycosis is a systemic fungal infection that most commonly affects dogs, and to a lesser extent humans.1,2 The causative agent, Blastomyces dermatitidis, is a dimorphic fungus that exists as a hyphal form in the environment and as a yeast in tissue.3 The hyphal form produces infectious conidia that become aerosolized when soil is disrupted. Once inhaled, the conidia are phagocytized by alveolar macrophages and transform to the yeast phase. Pulmonary manifestations occur in greater than 80% and 90% of affected dogs and humans, respectively, and respiratory abnormalities are the most common reasons for clinical evaluation in both species.4,5 Lymphatic, ocular, osteoarticular, and dermatologic involvement also are common.4 Unfortunately, diagnoses are often delayed in both humans and dogs due to lack of clinician awareness and similarities with other disease processes.1 Mortality rates can approach 40% in dogs, and relapses occur in up to 20% of canine patients despite months of antifungal therapy.6,7 Approximately 15% of humans hospitalized with acute pulmonary blastomycosis develop acute respiratory distress syndrome (ARDS), and mortality rates in this subpopulation approach 50%.5 Disease incidence rates are estimated to be eightfold greater in dogs than in humans, and dogs likely serve as sentinels for human exposure.8
Blastomycosis is endemic in certain regions of the United States along the Ohio and Mississippi river valleys, around the Great Lakes, and in several southeastern states.1,9 The ecological niche of Blastomyces dermatitidis has been reported to include sites with an acidic pH, high organic content, and close proximity to waterways, although more recent studies have called into question the potential associations with soil type, pH, and organic matter content.9,10 Detailed epidemiologic investigations of blastomycosis in dogs have been reported for the states of Illinois, Louisiana, Tennessee, and Wisconsin,4,8–10 but most other investigations are non-controlled descriptive studies of limited numbers of affected animals.
Blastomycosis is considered to be endemic in the State of Michigan, and many resources suggest a distribution in southern regions of the state.1,2,11,12 These published distribution maps differ considerably from years of unpublished anecdotal observations made by the Internal Medicine Section of the Michigan State University College of Veterinary Medicine. However, detailed studies of blastomycosis in humans or dogs have not been reported for Michigan. A lack of information concerning prevalence rates, spatial distribution, and risk factors for disease in this region likely contributes to delayed diagnoses and poor patient outcomes. In support of this premise, many dogs diagnosed with blastomycosis have undergone multiple veterinary evaluations, often at multiple clinics, prior to definitive diagnosis.13,14 Similar problems exist for affected humans.1,3,15 The objectives of this study were to investigate the prevalence and distribution of canine blastomycosis cases in Michigan, USA. Furthermore, we sought to identify risk factors for infection. We hypothesized that blastomycosis in dogs would be most prevalent in the Upper Peninsula of Michigan, and that travel to these northern regions would be a significant risk factor for infection.
Methods
A two-part investigation was conducted consisting of (1) a survey of companion animal private practice clinics across the State of Michigan with subsequent medical record review, and (2) a retrospective case-control study of dogs with blastomycosis evaluated at three specialty referral centers located in the southern mid-Michigan area.
Phase 1 Study design
A list of small animal primary care veterinary clinics in the State of Michigan was obtained and stratified by county.16 Specialty or referral hospitals, wellness clinics associated with Animal Control or the Humane Society, mobile veterinary clinics, and spay-neuter exclusive practices were excluded. A sample size calculation suggested surveying 187 of the approximate 608 small animal clinics meeting above criteria to have 90% confidence in a representative sample with a 5% margin of error. Twenty clinics in southwestern Michigan initially were contacted with a response rate of 65%. In order to ensure appropriate geographic representation, and assuming a more conservative response rate of 60%, four clinics were randomly selected and contacted in each of Michigan's 83 counties. All clinics were contacted in counties with four or fewer clinics. Practices not responding after two contact attempts were excluded.
The survey was completed by a single veterinarian employed at the selected practice via phone interview or email based on respondent preference. Information obtained from the survey included: address of the clinic, annual number of unique canine patients, and annual number of blastomycosis cases in dogs. If an exact unique annual canine caseload was unknown, participating clinics were asked to estimate weekly caseload by reviewing several weeks of appointment logs while also considering the possible effects of seasonal variation. The average estimated weekly caseload was then extrapolated for the entire year. Participating clinics were asked to provide medical record information from dogs diagnosed with blastomycosis including client address, patient demographics, date of evaluation, duration of clinical signs, diagnostic test results, and recent travel history when available.
Phase 2 Study design
A retrospective case-control study was conducted for dogs with blastomycosis that were evaluated at three veterinary specialty hospitals located in the southern mid-Michigan region (areas of Detroit, East Lansing, and Grand Rapids) between the years 2005 and 2018. Cases of blastomycosis in dogs were identified by medical record searches. All dogs diagnosed with blastomycosis based on cytologic or histologic identification of organisms in tissue samples, culture of Blastomyces spp. from lesions, or positive urine antigen testing accompanied by consistent clinical signs were included.14 Dogs with presumptive diagnoses based on clinical signs, diagnostic imaging findings, or serum antibody testing alone or in combination were excluded.2 An age matched control population of dogs that were evaluated during a similar time period was randomly selected from the hospital database with a ratio of three controls per blastomycosis case. Data retrieved from medical records included demographic information, home address, date of evaluation, duration of clinical signs, recent (4 month) travel history, and whether or not the dog was used for hunting. Owners of case and control dogs were contacted by phone to ensure accurate information concerning both travel history and hunting.
Data and statistical analysis
Descriptive statistics were reported for all data in both phases. County prevalence rates were calculated for phase 1 by dividing the annual number of affected dogs by the annual unique canine caseload of the participating clinics in each county. Available cases from this phase were geocoded and mapped according to home and travel addresses using QGIS 3.8 software (QGIS Development Team, 2016).17 A separate map was created for phase 2 in which all cases and controls were coded according to both home addresses and recent travel history. All instances of travel ≥ 25 miles from the home address within 4 months of evaluation were included in the map. If a specific address of travel was unknown, the center of the city or region to which travel occurred was used. Spatial clustering of blastomycosis cases were identified using SaTScan software (Harvard Pilgrim Health Care, Inc. Boston, MA, USA) as described elsewhere.9,18,19 Briefly, a circular window of variable radius moves across the map and clusters are identified by comparing the number of cases and controls within the window with the number expected, assuming that cases and controls are randomly distributed in the study area. Identification of areas with significantly high and low numbers of cases was done under the Bernoulli probability model assumption using a maximum spatial cluster size of 50% of the total population and a 100 kilometer (km) radius.
Statistical analyses also were conducted to determine major risk factors that may influence the occurrence of blastomycosis in dogs. Potential risk factors evaluated included sex, breed grouping, travel history, and whether the dog was used for hunting purposes. All factors were treated as categorical variables with sex classified as male or female and hunting classified as yes or no. Individual dogs were further classified according to the American Kennel Club guidelines into working, sporting, herding, and other breed categories.20 If a dog was listed as crossed with another breed, it was classified according to the initially listed breed (i.e., a Labrador Retriever cross would be classified as a Labrador Retriever). Travel history was treated as a dichotomous variable with time spent in Northern Michigan (north of the 45th parallel) classified as yes or no. This latitude was selected based on mapping results from phase 1. Given that the outcome of interest was categorical (blastomycosis present or absent) and four risk factors were being evaluated, a multiple variable logistic regression modeling approach was used for the analyses. A stepwise forward logistic regression was used, and the analysis was conducted in two stages. First, risk factors (sex, hunting, breed group, and travel or residence north of the 45th parallel) hypothesized to influence the outcome were analyzed using the univariable logistic regression approach. Second, factors that passed initial screening (P ≤ .05) were considered in the multivariable regression model with the exception of breed grouping which was not considered due to collinearity with hunting. Sex, which was not significant in univariable analysis, was forced in the multiple logistic regression given potential biologic importance. Odds ratios (OR) and 95% confidence intervals (CI) were reported for all possible associations. Analyses were performed with commercially available software (SAS, version 9.4, SAS Institute Inc, Cary, NC, USA), and for all analyses, P ≤ .05 was considered significant.
Results
Phase 1
A total of 210 clinics completed the survey, and 80 of Michigan's 83 counties were represented. Twenty-two veterinary clinics (10.5%) reported one or more canine blastomycosis cases per year, and 19 of these 22 clinics were located in Michigan's Upper Peninsula or in Cheboygan and Emmet counties, which are located at the northernmost aspect of the Lower Peninsula. Thirteen counties had prevalence rates ≥20 cases per 100,000 dogs, and five counties had prevalence rates ≥100 cases per 100,000 dogs (Fig. 1). A lack of electronic medical records precluded comprehensive record searches; however, 20 clinics permitted study investigators to review available medical records from 119 affected dogs. Fifteen of these dogs were diagnosed based on cytologic or histologic organism identification, 92 dogs were diagnosed based on positive serum or urine antigen testing in conjunction with clinical signs, and 12 dogs were diagnosed based on both organism identification and urine antigen testing. Blastomycosis was diagnosed throughout the year, but approximately 70% of cases were diagnosed from June through November (Fig. 2). The majority of affected dogs were between two and eight years of age, and 56.3% of dogs were male whereas 43.7% of dogs were female. These and additional characteristics of affected dogs are presented in Table 1. Mapping results suggested that most cases were contracted in Northern Michigan (Fig. 3) as very few dogs resided in, or travelled to, southern regions. However, specific reference to travel history was not consistently recorded in medical records for most dogs.
Figure 1.
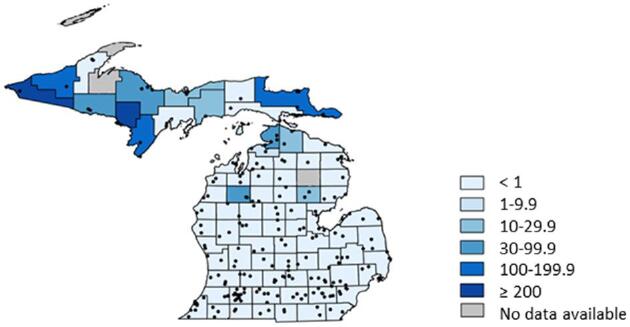
Intensity map depicting county prevalence rates for canine blastomycosis. Prevalence rates are reported as the number of cases per 100,000 dogs. Each black dot represents a responding veterinary clinic that participated in the state-wide survey. For the counties shaded in grey, data were unable to be obtained due to a lack of canine veterinary clinics.
Figure 2.
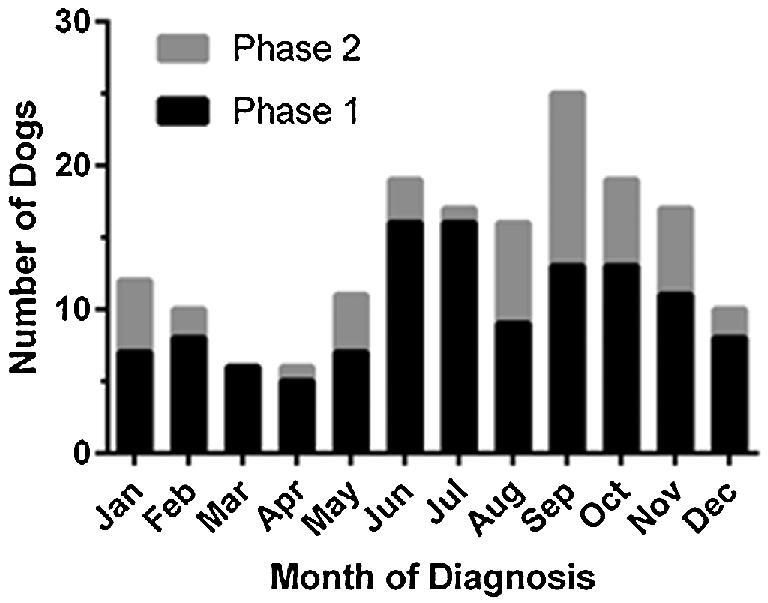
Bar graph depicting the distribution of blastomycosis cases (phase 1 and phase 2) by month of diagnosis. Blastomycosis was diagnosed throughout the year in both study phases, but most cases were diagnosed in summer and fall months.
Table 1.
Characteristics of blastomycosis affected dogs from both study phases.
| Number (%) | |||
|---|---|---|---|
| Variable | Phase 1 (n = 119) | Phase 2 (n = 49) | Total (n = 168) |
| Sex | |||
| Male | 67 (56.3) | 27 (55.1) | 94 (56.0) |
| Female | 52 (43.7) | 22 (44.9) | 74 (44.0) |
| Breed groups | |||
| Sporting | 70 (58.8) | 27 (55.1) | 97 (57.7) |
| Working | 11 (9.2) | 5 (10.2) | 16 (9.5) |
| Herding | 12 (10.1) | 5 (10.2) | 17 (10.1) |
| Others | 26 (21.8) | 12 (24.5) | 38 (22.6) |
| Individual breeds | |||
| Labrador Retrievers | 32 (26.9) | 11 (22.4) | 43 (25.6) |
| Golden Retrievers | 17 (14.3) | 10 (20.4) | 27 (16.1) |
| Border Collie | 4 (3.4) | 2 (4.1) | 6 (3.6) |
| German Shepherd | 5 (4.2) | 2 (4.1) | 7 (4.2) |
| Cocker Spaniel | 6 (5.0) | 0 (0) | 6 (3.6) |
| Age | |||
| 0–1.9 years | 12 (10.1) | 7 (14.3) | 19 (11.3) |
| 2–3.9 years | 34 (28.6) | 14 (28.6) | 48 (28.6) |
| 4–5.9 years | 19 (16) | 15 (30.6) | 34 (20.2) |
| 6–7.9 years | 23 (19.3) | 6 (12.2) | 29 (17.3) |
| ≥8 years | 29 (24.4) | 7 (14.3) | 36 (21.4) |
| Unknown | 2 (1.7) | 0 (0) | 2 (1.2) |
Figure 3.
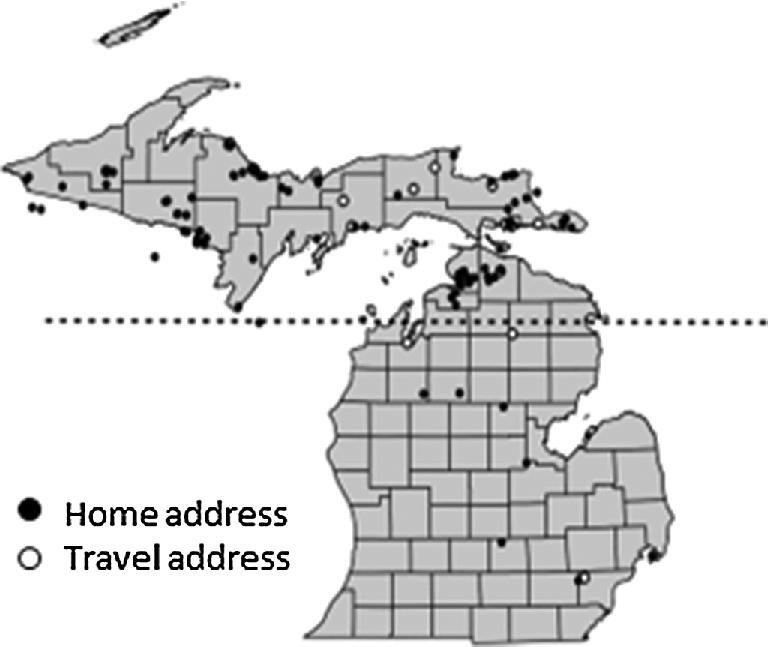
Distribution map depicting the home and travel addresses for the 119 phase 1 dogs diagnosed with blastomycosis. Black dots represent home addresses, and outlined dots with a white center represent available travel addresses. The dashed line represents the 45th parallel. Although travel history was not specifically mentioned in most of the medical records, the three dogs with the southernmost home addresses did have a reported travel history north of the 45th parallel.
Phase 2
A total of 49 dogs diagnosed with blastomycosis and 147 control dogs were included in phase 2. Characteristics of dogs with blastomycosis are presented in Table 1. Most common breeds in this population were Labrador Retrievers (n = 11) and Golden Retrievers (n = 10). Similar to blastomycosis affected dogs in phase 1, the majority cases in phase 2 were diagnosed with blastomycosis from June through November (Fig. 2), and the majority of cases were between 2 and 8 years of age. The mean ± standard deviation (SD) age for blastomycosis affected dogs was 4.3 ± 2.6 years and 4.3 ± 3.4 years for control dogs. Univariable analyses identified breed grouping, use of dogs for hunting, and travel or residence north of the 45th parallel as risk factors for infection (Table 2). Travel or residence north of the 45th parallel remained a risk factor in the multivariable logistic regression model (P < .001). Dogs residing or travelling north of the 45th parallel were over 13 times more likely to have blastomycosis as compared to those that did not (adjusted OR = 13.15; 95% CI = 5.61–30.83).
Table 2.
Characteristics and univariable analyses of case and control dogs from phase 2.
| Subjects (%) | |||
|---|---|---|---|
| Variable | Test n = 49 | Control n = 147 | Unadjusted odds ratio (95% CI)1 |
| Sex | |||
| Male | 27 (55.1) | 76 (51.7) | 1.15 (0.6–2.2) |
| Female | 22 (44.9) | 71 (48.3) | 0.87 (0.46–1.67) |
| Used for hunting | 6 (12.2) | 5 (3.4) | 3.96 (1.15–13.63)* |
| Breed groups2 | |||
| Sporting | 27 (55.1) | 30 (20.4) | 4.79 (2.4–9.56)** |
| Working | 5 (10.2) | 22 (15.0) | 0.65 (0.23–1.81) |
| Herding | 5 (10.2) | 8 (5.4) | 1.97 (0.61–6.35) |
| All others | 12 (24.5) | 87 (59.2) | 0.22 (0.11–0.46)** |
| North of 45th parallel3 | 24 (49.0) | 10 (6.8) | 13.15 (5.61–30.84)** |
The characteristics of the 49 dogs affected by blastomycosis and 147 randomly selected dogs with conditions other than blastomycosis (control population) are presented in the respective columns. For each breed grouping, the odds ratio and 95% confidence interval are reported for the individual breed category as compared to the other categories combined.
Confidence interval.
Those dogs who either resided or traveled north of the 45th parallel within the State of Michigan.
P = .02.
P < .001.
Forty-one of 49 cases resided in Michigan south of the 45th parallel, and 33 of these 41 dogs had travelled ≥25 miles from their primary residence within 4 months of evaluation. One hundred thirty-nine of 147 controls resided in Michigan south of the 45th parallel, and 54 of these 139 dogs had travelled ≥25 miles within 4 months of evaluation. A distribution map was created for home and travel addresses of both case and control dogs (Fig. 4). Cluster analysis identified a significant high risk area for blastomycosis (P < .001) that encompassed the majority of the eastern half of the Upper Peninsula and the northernmost aspect of the Lower Peninsula. A significant low risk area (P < .001) was identified in the south-central region of the state. Twenty-five cases were not reported to reside or travel north of the 45th parallel; however, 10 of these 25 dogs had traveled to states and provinces in which blastomycosis is considered endemic.2 This included Wisconsin (n = 2), Ohio (n = 2), Illinois (n = 2), Indiana (n = 1), Virginia (n = 1), Missouri (n = 1), Pennsylvania (n = 1), and Ontario (n = 3). Fifteen dogs did not reside in, or travel to, northern Michigan or regions known to be endemic for blastomycosis. Six of these 15 dogs spent time in or near the Huron-Manistee National Forest, but it is unknown if this region was the source of infection. The home and travel addresses of the remaining nine cases were scattered throughout the Lower Peninsula and appeared to represent isolated occurrences.
Figure 4.
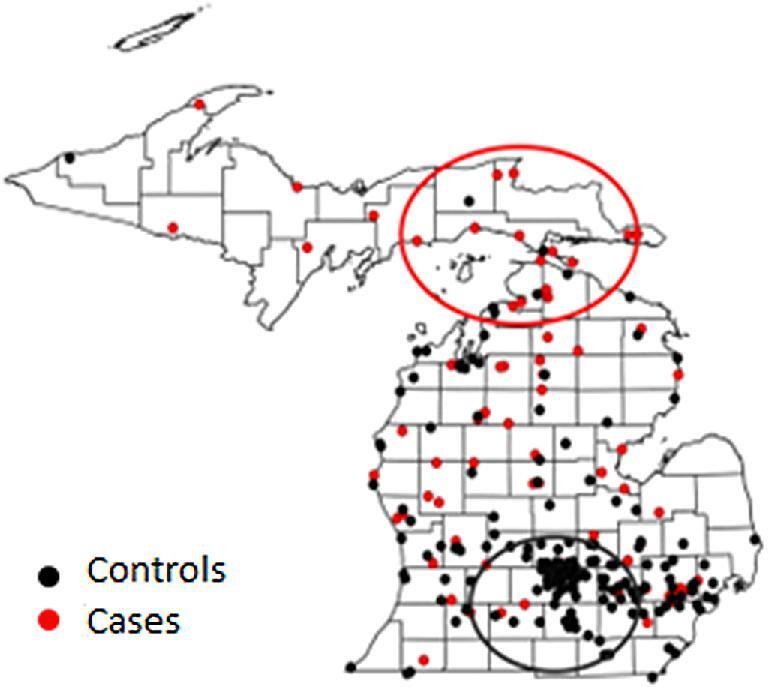
Distribution map of home and travel addresses for cases and controls in phase 2. Red dots represent the home and travel addresses for the 49 dogs with blastomycosis in study phase 2. Black dots represent the home and travel addresses for the 147 age matched control dogs with conditions other than blastomycosis. The red and black circles depict significant high-risk (P < .001) and low-risk areas (P < .001), respectively, as determined by cluster analysis.
Discussion
This study is the first detailed epidemiologic investigation of canine blastomycosis reported for the State of Michigan, and one of few to be reported in the USA.8–10 Our results refute many of the distribution maps found throughout both human and veterinary blastomycosis literature.1,2,11,12 It is unknown why southern Michigan historically has been considered endemic for disease, but our results unequivocally document that most cases originate from northern Michigan. Owners of case and control dogs in the second study phase were contacted by phone to ensure that accurate and detailed information were available concerning travel history. Many previous studies of blastomycosis in humans and dogs have not considered travel history to the extent that was performed in our investigation,8,9 and this could have contributed to discrepancies between previous distribution maps and those reported herein. Cluster analysis from the second study phase identified the eastern Upper Peninsula and the northernmost Lower Peninsula as heightened risk areas (Fig. 4). These results are further supported by the county prevalence rates (Fig. 1) and distribution maps (Fig. 3) from the first study phase. Although western regions of the Upper Peninsula were not identified as heightened risk areas in the cluster analysis, it is probable that blastomycosis is endemic throughout most of this region too. Indeed, some of the highest county prevalence rates and many of the addresses of affected dogs from the first study phase were in these areas. Previous studies of blastomycosis in Wisconsin also have documented high prevalence rates in the regions adjacent to the western Upper Peninsula.10,21,22 Owners of dogs residing in or visiting the western Upper Peninsula that were seeking specialty or referral care for their pet would have been more likely to travel to specialty centers in Wisconsin rather than drive 6 to 10 hours to specialty centers in Michigan. This would account for the observation that only a few cases from our second study phase had home or travel addresses in the western regions of the Upper Peninsula.
Northern Michigan is a predominantly rural area. Although the Upper Peninsula comprises approximately 30% of Michigan's land mass, its population of 300,000 represents only 3% of the total state population.23 Our study is certainly of regional importance, but its significance is not limited to one region or one species. Northern Michigan is a popular tourist destination which attracts visitors from both within the state and across the USA.24 The Mackinac Bridge, which connects the Lower and Upper Peninsulas, is traversed over 2 million times during summer months alone.25 There are over 800,000 annual visitors to the Pictured Rocks National Lakeshore and over 1.5 million annual visitors to the Hiawatha National Forest, both of which are located in the Upper Peninsula.26,27 Petoskey, a coastal city located within the high-risk area in the northern Lower Peninsula, has a population of only 6000. Yet, over 750,000 tourists visit this community every year.28 These and other areas in northern Michigan are frequently visited by both residents and non-residents for camping, hunting, fishing, and hiking.29 These types of outdoor activities commonly involve dogs and are associated with increased exposure risk to Blastomyces dermatitidis.30 A lack of awareness that B. dermatitidis exists in these regions could lead to delayed or even incorrect diagnoses. For instance, in one of the highest prevalence counties in the Upper Peninsula, one clinic consistently diagnosed more than 10 canine blastomycosis cases every year whereas another clinic less than 10 miles away reported that no cases of blastomycosis had been diagnosed in recent years. Patient populations may have been different between these neighboring clinics, but it is also possible that a lack of awareness precluded diagnostic testing for blastomycosis. The updated prevalence and distribution maps from our study should heighten awareness in both human and veterinary health care providers, hopefully resulting in earlier disease detection and better patient outcomes.
Several possible risk factors for disease also were evaluated. In both univariable and multivariable analyses, travel or residence north of the 45th parallel was the strongest and most important predictor for blastomycosis occurrence. The use of dogs for hunting was also a significant risk factor in the univariable analysis, but this did not remain significant in the multivariable regression. Hunting is also a risk factor for blastomycosis in humans.30 Both humans and dogs that hunt are presumably more likely to have exposure to infectious conidia. However, a larger sample size would likely be needed to determine the overall importance of hunting as a risk factor for disease, and it was apparent that travel to heightened risk areas was a far more important risk factor in our study population. Similar to other reports, dogs in the sporting breed group, which includes Labrador Retrievers and Golden Retrievers, were at greater risk for disease than other breed groupings.9,31 This is not surprising as dogs in the sporting breed group are more likely to be involved in outdoor activities such as hunting.32 A dog belonging to a certain breed group does not necessarily translate to it being used for that purpose, but there is some degree of overlap and redundancy between the risk factors of hunting and breed grouping. Consequently, breed grouping was dropped from the multivariable regression.
The latency period of blastomycosis in dogs has been suggested to range from 5 to 12 weeks.2 We selected a 4 month time period when considering travel history in order to exceed this estimate by 1 month. However, exact latency periods are unknown, and one study limitation is that exposures could have occurred outside of this time period in some dogs. Proximity to waterways has been identified as a risk factor for blastomycosis in some studies, and another limitation of our investigation is that certain environmental factors such as this were not evaluated.9 Michigan has one of the greatest densities of lakes, ponds, rivers, and streams among the 50 states, and no location in the state is more than 6 miles from some type of inland water.33 As such, we did not think investigation of this potential risk factor would be of value for our region. An additional limitation of our study is that exact annual caseloads were unavailable for some clinics. Although the estimates provided by respondents are likely to approximate actual numbers, unaccounted seasonal variation in case numbers or other factors such as recall bias might have led to overestimation or underestimation of caseload for these clinics. Similarly, some clinics participating in the first study phase were unable to identify medical records from all affected dogs at their practice. This is a common challenge in many veterinary studies as most clinics do not utilize an electronic medical records system, and the clinics that do use electronic systems often lack the ability to specifically search the database for affected cases.34 It is also likely that some cases were missed in the second study phase if they were not appropriately coded in the medical record or if the diagnosis was made after the patient visit occurred. Even though some cases were not captured, we were able to utilize the records of nearly 170 dogs with blastomycosis in our study. Still, limited data were available for some specific regions and counties, and larger investigations of blastomycosis are needed to further refine the spatial distribution and risk factors for disease in Michigan.
In summary, we established updated prevalence and distributions maps for canine blastomycosis in the State of Michigan and identified risk factors for infection. Contrary to previous reports, most blastomycosis infections are acquired in the Upper Peninsula or in the northernmost regions of the Lower Peninsula. Many of these areas are popular tourist destinations, and travel to these regions was the strongest predictor of disease in multivariable analysis. Given that dogs are sentinels for disease in humans, our results should be informative for veterinarians, physicians, and public health officials both within the state and across the USA.
Ethics approval
This study was reviewed by the Michigan State University Institutional Review Board (STUDY00002135) and deemed exempt from full review as questions pertaining to human health were not asked, and specific addresses and identifying information were not published. A questionnaire template designed by the Human Research Protection Program for non-human health related research was used for phone surveys, and informed consent was obtained from all participating individuals.
Acknowledgments
The authors would like to acknowledge Dr. Clinton Groover (Pickford Veterinary Hospital), Dr. Mike McDonald (Bay Pines Veterinary Clinic), Dr. Brian Scott (Iron Mountain Animal Hospital), and Dr. Tim Hunt (Bayshore Veterinary Hospital) amongst others for providing valuable insights on blastomycosis in the State of Michigan. The authors would also like to acknowledge Dr. William Schall (professor emeritus, Michigan State University) for providing preliminary data and design support and Dr. Amanda Tickner (Michigan State University Map Library) for providing mapping support.
Contributor Information
Leslie M Shelnutt, Department of Small Animal Clinical Sciences, Michigan State University College of Veterinary Medicine, East Lansing, Michigan, USA.
John B Kaneene, Center for Comparative Epidemiology, Michigan State University College of Veterinary Medicine, East Lansing, Michigan, USA.
Paulo A M Carneiro, Center for Comparative Epidemiology, Michigan State University College of Veterinary Medicine, East Lansing, Michigan, USA.
Daniel K Langlois, Department of Small Animal Clinical Sciences, Michigan State University College of Veterinary Medicine, East Lansing, Michigan, USA.
Funding
No funding to report.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of this paper.
References
- 1. Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016; 30: 247–264. [DOI] [PubMed] [Google Scholar]
- 2. Dedeaux A, Taboada J. Blastomycosis and histoplasmosis. In: Ettinger SJ, Feldman EC, Cote E. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 8th edn St. Louis, MO, USA: Elsevier, Inc., 2017: 1027–1032. [Google Scholar]
- 3. Chapman SW, Dismukes WE, Proia LA et al.. Clinical practice guidelines for the management of Blastomycosis: 2008 Update by the Infectious Disease Society of America. Clin Infect Dis. 2008; 46: 1801–1812. [DOI] [PubMed] [Google Scholar]
- 4. Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980–1995). J Am Vet Med Assoc. 1998; 213: 658–664. [PubMed] [Google Scholar]
- 5. Azar MM, Assi R, Relich RF et al.. Blasomycosis in Indiana: clinical and epidemiologic patterns of disease gleaned from a multicenter retrospective study. Chest. 2015; 148: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 6. Legendre AM, Rohrbach BW, Toal RL, Rinaldi MG, Grace LL, Jones JB. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med. 1996; 10: 365–371. [DOI] [PubMed] [Google Scholar]
- 7. Walton RA, Wey A, Hall KE. A retrospective study of anti-inflammatory use in dogs with pulmonary blastomycosis: 139 cases (2002–2012). J Vet Emerg Crit Care. 2017; 27: 439–443. [DOI] [PubMed] [Google Scholar]
- 8. Herrmann JA, Kostiuk SL, Dworkin MS, Johnson YJ. Temporal and spatial distribution of blastomycosis cases among humans and dogs in Illinois (2001–2007). J Am Vet Med Assoc. 2011; 239: 335–343. [DOI] [PubMed] [Google Scholar]
- 9. Chen T, Legendre AM, Bass C, Mays SE, Odoi A. A case-control study of sporadic canine blastomycosis in Tennessee, USA. J Mycol Med. 2008; 46: 843–852. [DOI] [PubMed] [Google Scholar]
- 10. Baumgardner DJ, Paretsky DP, Yopp AC. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Mycol Med. 1995; 33: 171–176. [DOI] [PubMed] [Google Scholar]
- 11. Legendre AM. Blastomycosis. In: Greene CE. Infectious Diseases of the Dog and Cat, 4th edn.St. Louis, MO, USA: Elsevier, Inc., 2012: 606–614. [Google Scholar]
- 12. Sykes JE, Merkel LK. Blastomycosis. In: Sykes JE. Canine and Feline Infectious Diseases, 1st edn.St. Louis, MO, USA: Elsevier, Inc., 2014: 574–586. [Google Scholar]
- 13. Mondada K, Fullmer J, Hungerford E, Novack K, Vickers K, Scalarone G. Blastomyces dermatitidis: Antibody detection in sera from dogs with blastomycosis with yeast lysate antigens produced from human and dog isolates. Vet Med Int. 2014; 2014: 376725 doi: 10.1155/2014/376725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spector D, Legendre AM, Wheat J et al.. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med. 2008; 22: 839–843. [DOI] [PubMed] [Google Scholar]
- 15. Chapman SW, Lin AC, Hendricks KA et al.. Endemic blastomycosis in Mississippi: Epidemiological and clinical studies. Semin Respir Infect. 1997; 12: 219–228. [PubMed] [Google Scholar]
- 16. Veterinarians in Michigan USA Veterinarians. http://www.usa-veterinarians.com/in/michigan. Accessed August 17, 2019. [Google Scholar]
- 17. QGIS Development Team (2019) QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org. Accessed August 17, 2019. [Google Scholar]
- 18. Kulldorff M and Information Management Services, Inc . SaTScanTM v9.6: Software for the spatial and space-time scan statistics. http://www.satscan.org/, 2009. [Google Scholar]
- 19. Kulldorff M, Nagarwalla N. Spatial disease clusters: Detection and Inference. Stat Med. 1995; 14: 799–810. [DOI] [PubMed] [Google Scholar]
- 20. List of breeds by group American Kennel Club. https://www.akc.org/public-education/resources/general-tips-information/dog-breeds-sorted-groups/. Updated January 2, 2019. Accessed August 17, 2019. [Google Scholar]
- 21. Baumgardner DJ, Steber D, Glazier R et al.. Geographic information system analysis of blastomycosis in northern Wisconsin, USA: waterways and soil. J Mycol Med. 2005; 43: 117–125. [DOI] [PubMed] [Google Scholar]
- 22. Baumgardner DJ, Paretsky DP, Baseman ZJ, Schreiber A. Effects of season and weather on blastomycosis in dogs: Northern Wisconsin, USA. J Mycol Med. 2011; 49: 49–55. [DOI] [PubMed] [Google Scholar]
- 23. U.S. Census Bureau, 2010 Census American Fact Finder. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF. Published 2010. Accessed August 17, 2019. [Google Scholar]
- 24. Pure Michigan continues to deliver economic impact Michigan Economic Development Corporation. https://www.michigan.org/pressreleases/pure-michigan-continues-deliver-economic-impact. Published 2019. Accessed August 17, 2019. [Google Scholar]
- 25. Monthly traffic statistics Mackinac Bridge Authority. http://www.mackinacbridge.org/fares-traffic/monthly-traffic-statistics/. Published 2019. Accessed August 17, 2019. [Google Scholar]
- 26. Bingham E. Pictured Rocks set a new visitors record in 2018. https://www.mlive.com/life/2019/03/pictured-rocks-set-a-new-visitors-record-in-2018.html. Published March 8, 2019. Accessed August 17, 2019. [Google Scholar]
- 27. Hiawatha National Forest City of Manistique. https://cityofmanistique.org/hiawatha-national-forest/. Published 2018. Accessed August 17, 2019. [Google Scholar]
- 28. Moroney K. Smithsonian Magazine names Petoskey among best small American towns. M Live. https://www.mlive.com/business/index.ssf/2013/03/smithsonian_magazine_names_pet.html. Published March 26, 2013. Accessed August 17, 2019. [Google Scholar]
- 29. Michigan's U.P. State Parks: Nature's playground from dawn to starry night Upper Peninsula Travel and Recreation Association. https://www.uptravel.com/recreation/state-parks/. Published 2019. Accessed August 17, 2019. [Google Scholar]
- 30. Lemke MA, Baumgardner DJ, Brummitt CF et al.. Blastomycosis in urban southeastern Wisconsin. WMJ. 2009; 108: 407–410. [PubMed] [Google Scholar]
- 31. Archer JR, Trainer DO, Schell RF. Epidemiologic study of canine blastomycosis in Wisconsin. J Am Vet Med Assoc. 1987; 190: 1292–1295. [PubMed] [Google Scholar]
- 32. Sporting Group American Kennel Club. https://www.akc.org/dog-breeds/sporting/. Accessed August 17, 2019. [Google Scholar]
- 33. Michigan Tourism Facts Pure Michigan. https://web.archive.org/web/20161015171535/http://www.michigan.org/michigan-tourism-facts/. Accessed August 17, 2019. [Google Scholar]
- 34. Krone LM, Brown CM, Lindenmayer JM. Survey of electronic veterinary medical record adoption and use by independent small animal veterinary medical practices in Massachusetts. J Am Vet Med Assoc. 2014; 245: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]


