ABSTRACT
Background
Current evidence on associations between intakes of linoleic acid (LA), the predominant n–6 (ω-6) fatty acid, and mortality is inconsistent and has not been summarized by a systematic review and meta-analysis.
Objective
The aim was to perform a systematic review and meta-analysis of prospective cohort studies to examine associations between LA intake and mortality.
Methods
We conducted a comprehensive search of MEDLINE and EMBASE databases through 31 July 2019 for prospective cohort studies reporting associations of LA (assessed by dietary surveys and/or LA concentrations in adipose tissue or blood compartments) with mortality from all causes, cardiovascular disease (CVD), and cancer. Multivariable-adjusted RRs were pooled using random-effects meta-analysis.
Results
Thirty-eight studies reporting 44 prospective cohorts were identified; these included 811,069 participants with dietary intake assessment (170,076 all-cause, 50,786 CVD, and 59,684 cancer deaths) and 65,411 participants with biomarker measurements (9758 all-cause, 6492 CVD, and 1719 cancer deaths). Pooled RRs comparing extreme categories of dietary LA intake (high vs low) were 0.87 (95% CI: 0.81, 0.94; I2 = 67.9%) for total mortality, 0.87 (95% CI: 0.82, 0.92; I2 = 3.7%) for CVD mortality, and 0.89 (95% CI: 0.85, 0.93; I2 = 0%) for cancer mortality. Pooled RRs for each SD increment in LA concentrations in adipose tissue/blood compartments were 0.91 (95% CI: 0.87, 0.95; I2 = 64.1%) for total mortality, 0.89 (95% CI: 0.85, 0.94; I2 = 28.9%) for CVD mortality, and 0.91 (95% CI: 0.84, 0.98; I2 = 26.3%) for cancer mortality. Meta-regressions suggested baseline age and dietary assessment methods as potential sources of heterogeneity for the association between LA and total mortality.
Conclusions
In prospective cohort studies, higher LA intake, assessed by dietary surveys or biomarkers, was associated with a modestly lower risk of mortality from all causes, CVD, and cancer. These data support the potential long-term benefits of PUFA intake in lowering the risk of CVD and premature death.
Keywords: linoleic acid, dietary polyunsaturated fatty acid, biomarkers, mortality, cardiovascular disease
Introduction
The current US dietary guidelines recommend higher intakes of PUFAs in place of SFAs for the prevention of cardiovascular disease (CVD) (1). The health impact of linoleic acid (LA; 18:2n–6), an n–6 PUFA constituting >85–90% of dietary PUFA intake in the United States (2), is of critical public health importance but still contentious. Scientific evidence behind the dietary recommendations on PUFA intake has been largely based on studies of CVD endpoints. Meta-analyses of prospective cohort studies and randomized controlled trials (RCTs) suggest that higher LA intake, in replacement of SFAs or carbohydrate, has moderate benefits for the prevention of coronary artery disease (CAD) (3-5). Pooling studies of prospective cohorts reported inverse associations between LA concentrations in biological tissues and risk of type 2 diabetes and CVD (6, 7). In contrast, other meta-analyses (8) and some RCTs (9, 10) yielded null or positive associations between LA intake and CVD outcomes. However, some of the n–6 RCTs need to be interpreted with caution due to substantial dropouts, intermittent treatment, short duration of trials, and potential confounding of LA interventions by the atherogenic trans fat (11). Furthermore, concerns have also been raised on the theoretical proinflammatory and thrombogenic properties of LA (12). However, current RCTs do not support that LA intake increases concentrations of inflammatory biomarkers (13, 14).
In contrast to the many studies on CVD, the impact of LA on long-term mortality risk is less studied. Low-quality evidence from a few RCTs suggested little effect of dietary n–6 PUFAs on mortality (5, 15); however, such evidence is not generalizable to contemporary settings and the general population as most trials were conducted decades ago in participants with chronic diseases. Prospective cohort studies are, therefore, of high importance in examining associations between n–6 PUFAs and mortality risk. However, the evidence from prospective cohort studies on LA intake and mortality has been inconsistent. In addition, nutritional findings from observational studies are often questioned due to their common reliance on self-reported dietary assessments, which are subject to measurement errors. Because LA cannot be produced endogenously, its concentrations in the circulation/adipose tissues depend largely on dietary intakes from vegetable oils, nuts, seeds, and other foods (16), making these biomarkers useful tools for objective assessment of LA intake (17, 18).
To address the current controversy surrounding the relation between LA intake and mortality risk, we performed a systematic review and meta-analysis of prospective cohort studies to examine the associations between dietary intakes and biomarkers of LA with mortality from all causes, CVD, and cancer.
Methods
Literature search
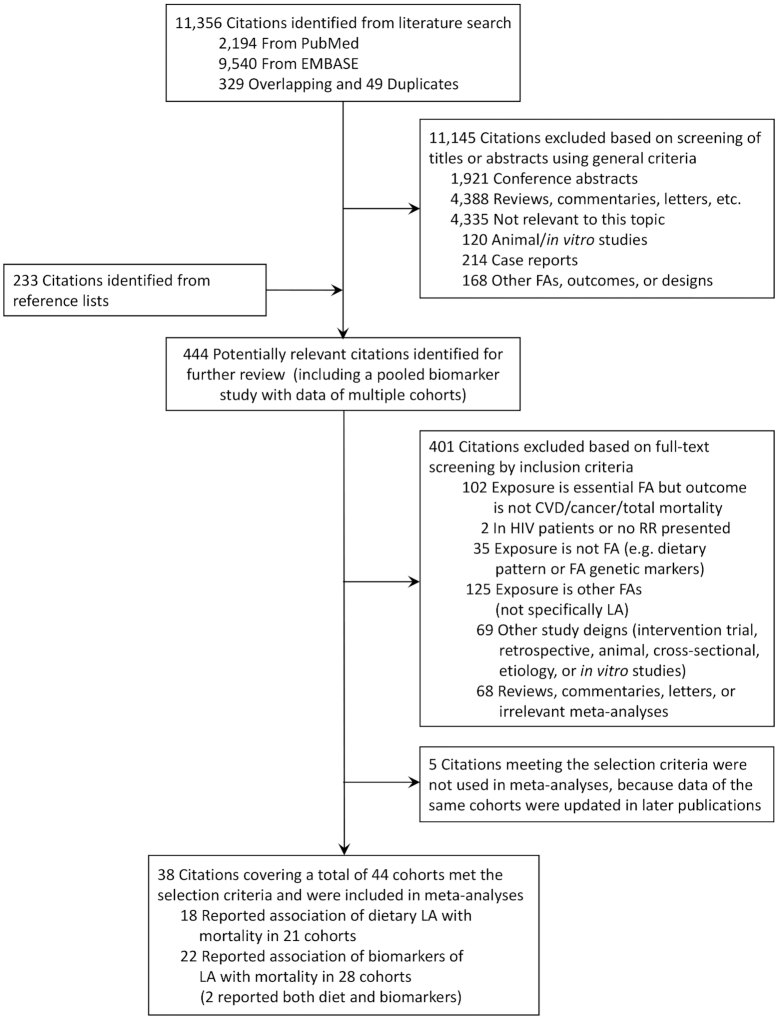
The present review was conducted following standard guidelines of Meta-analysis of Observational Studies in Epidemiology (19) and Preferred Reporting Items for Systematic Reviews and Meta-analysis (20). We performed a systematic literature review search in PUBMED and EMBASE through 31 July 2019 to identify studies reporting associations between LA intake, assessed either by self-reported dietary surveys or LA concentrations in biological tissues (i.e., LA biomarkers) and mortality from all causes, CVD, and cancer (i.e., primary outcomes of the study). A computer-based search combined search terms related to the exposure (i.e., “fatty acids” or “linoleic acids” or “α-linolenic acids”) and outcomes (“mortality” or “death”), with restrictions to “human” and language restriction to English. The search was supplemented by scans of the reference list of relevant articles, a hand-search of relevant/key journals, and correspondence with authors. A flow chart of the literature search and article selection is shown in Figure 1.
FIGURE 1.
Flow diagram of study screening. CVD, cardiovascular disease; FA, fatty acid; LA, linoleic acid.
Selection of articles
The titles and abstracts of the identified articles were screened by one investigator (JL). Potentially relevant articles were selected and reviewed in full text independently by two investigators (JL and MG-F) to determine eligibility, and discrepancies were resolved by consultation with FBH. The articles were considered for inclusion if they met the following criteria: 1) were original investigations; 2) were prospective cohort studies or nested case-control/case-cohort studies of adults (aged >18 y); and 3) reported multivariable-adjusted risk estimates for associations between LA (dietary intakes or biomarker concentrations) and ≥1 mortality outcome, including all-cause mortality, CVD mortality (i.e., fatal CAD, myocardial infarction, ischemic heart disease, or stroke; death from CAD, stroke, or other CVD causes; and CVD mortality), and cancer mortality (i.e., fatal/lethal cancer of different types, death from cancer, and cancer mortality). We additionally excluded studies in which the number of cases was <10. If multiple articles reported data on the same cohort, the record with the most up-to-date follow-up, the largest number of cases, maximum adjustment for covariates, and/or maximum amount of study information (depending on the comparison between records) was used.
Data extraction
JL and MG-F independently extracted data on study characteristics, participant characteristics, follow-up duration, exposure assessments (e.g., methods and frequencies of self-reported dietary surveys, and tissue types and LA measurement assays), mortality (i.e., follow-up rate, case number, specific endpoints, and ascertainment methods), analysis strategies, and multivariable-adjusted risk estimates and precision. When multiple regression models were used in an article, risk estimates from the most-adjusted model were extracted. When several endpoints of cardiovascular or cancer mortality were reported, the endpoints with the most coverage were used (hierarchy: CVD mortality, mortality from specific CVD endpoints, and fatal CVD endpoints; cancer mortality, mortality from specific types of cancer, and fatal cancer of specific types). Study quality was assessed using the Newcastle-Ottawa Scale (NOS; scores ranged from 0 to 9) (Supplemental Table 1) (21).
Data synthesis
The included studies reported RRs or HRs for prospective cohort analyses or ORs for nested case-control studies; HRs and ORs were assumed to approximate RRs when events are rare. For studies of dietary LA intake, we pooled RRs comparing the highest with the lowest categories, because most studies reported risk estimates based on LA intake categories. For 1 study, data were collected from 2 articles (22, 23) that did not report 95% CIs; we calculated the SEs based on LA intake amounts, log RRs, or regression coefficients, and P values according to a previously reported method (4, 24). Because most biomarker studies evaluated mortality risk by continuous LA concentrations (which differed across studies due to differences in biological tissues and the number of total assayed fatty acids), we pooled RRs for each SD increment in LA concentrations. One study reported RRs stratified by the genotypes of a variant (25); we pooled RRs from all genotypes using a random-effects meta-analysis. For studies reporting RRs by categories of biomarker concentrations, we assumed that the association was linear and estimated RRs for each SD increment in biomarkers by dividing the log RRs comparing extreme tertiles, quartiles, or quintiles by 1.94, 2.30, or 2.56, respectively. For studies reporting RRs per interquintile range of biomarkers, we calculated RRs per SD increment in biomarkers by dividing the interquintile log RRs by 1.68. The conversion factors are equal to the numbers of SD between the medians of extreme categories or within the interquintile range, assuming that the exposure is normally distributed.
Forest plots were used to evaluate RRs and 95% CIs across studies. Potential heterogeneity among studies was assessed using the I2 statistics, and I2 <25%, 25–50%, 50–75%, and >75% were considered to represent none, low, medium, and high heterogeneity. Summary RRs and 95% CIs were calculated using an inverse-variance–weighted random-effects meta-analysis (26), allowing for heterogeneity between studies. The possibility of publication bias was evaluated using the Begg's test and funnel plots. Potential sources of heterogeneity, including study designs, geographic locations, sex, baseline age, population health status, exposure assessments, study quality, and follow-up duration, were examined by stratified meta-analysis and univariate meta-regressions.
We examined the dose–response relation between dietary LA intake and mortality using a dose–response random-effects meta-analysis [2-stage generalized least-square for trend in Stata (27)] and restricted cubic spline models using data from studies that provided doses and risk estimates for ≥3 LA intake categories. If numbers of participants and/or cases in a category were not provided, we used the average number across categories. When the median intake for a category was missing, we approximated it using the midpoint of higher and lower bounds; when the lowest/highest category was open-ended, we used the cutoff threshold for the lowest/highest category. The units of LA intake in each category were unified to the percentage of total calories. All analyses were performed using Stata version 15.0 (StataCorp), with a 2-tailed α of 0.05.
Results
The search strategy retrieved 11,356 unique records. After screening titles and abstracts, and an additional scan of the reference list and hand-search of key journals, 444 records were evaluated by full text. We identified 43 articles meeting the selection criteria but further excluded 5 because data in the same cohorts were updated in later publications. The final meta-analysis included 38 articles reporting prospective associations between LA intake (18 assessed diet and 22 assessed biomarkers; note that 2 articles assessed both diet and biomarkers) and mortality outcomes (Figure 1).
Dietary LA intake and mortality
Eighteen articles reported associations between dietary LA intake and mortality outcomes in 21 prospective cohorts, including 811,069 participants, 170,076 total deaths (11 studies), 50,786 CVD deaths (14 studies), and 59,684 cancer deaths (9 studies). Median follow-up durations ranged from 4.9 to 30.2 y. Most studies used food-frequency questionnaires (FFQs) to assess dietary intake (13 at baseline and 2 repeated), 3 studies used 24-h dietary recall (2 at baseline and 1 repeated), 2 used diet history, and the other used food records. Analyses in 9 cohorts specified the comparison macronutrient (i.e., carbohydrates) (Table 1). NOS scores ranged from 5 to 9, with data for 12 cohorts scoring ≥8 (Supplemental Table 1).
TABLE 1.
Characteristics of prospective cohort studies that evaluated the associations between dietary LA intake and mortality from all-causes, CVD, and/or cancer1
| Study name, location | First author, year (ref) | Mean follow-up, y | Total sample size, n | Total deaths, n | CVD deaths, n | Cancer death | Mortality type | Baseline age, y | Male,% | Dietary data | Mean/median of LA | Other baseline conditions | Covariates adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATBC, Finland | Pietinen, 1997 (28) | 6.1 | 21,930 | — | 635 | — | CAD | 56.6 | 100 | Baseline SFFQ | 6.9 energy-adjusted g/d | All smokers | Age, treatment group, smoking, BMI, blood pressure, intakes of energy, alcohol, and fiber (quintiles), education (<7, 7–11, >11 y), and physical activity (<1, 1–2, >2 times/wk), trans-, cis-MUFAs and SFAs |
| NHS, USA | Wang, 2016 (29) | 30.2 | 83,349 | 20,314 | 4000 | 7919 | All-cause, CVD, and cancer | 46 | 0 | Repeated SFFQ | 4.8% of energy | — | Age, race, marital status, BMI, physical activity, smoking status, alcohol consumption, multivitamin use, vitamin E supplement use, current aspirin use, family history of myocardial infarction, family history of diabetes, family history of cancer, history of hypertension, history of hypercholesterolemia, intakes of total energy and dietary cholesterol, percentage of energy intake from dietary protein, and menopausal status and hormone use in women; all models also included percentages of energy intake from remaining FAs (SFAs, PUFAs, MUFAs, trans-FAs, ω-6 PUFAs, ω-3 PUFAs, LA/ALA, AA, and marine ω-3 FAs) |
| Holmes, 1999 (30)2 | 13.1 | 1982 | 378 | — | — | All-cause | 54 | 0 | Repeated SFFQ | — | Breast cancer | Age, diet interval, calendar year of diagnosis, BMI, oral contraception use, menopausal status, postmenopausal hormone use, smoking, age at first birth and parity, number of metastatic lymph nodes, tumor size, and caloric intake | |
| Jiao, 2019 (31)2 | 11 | 9053 | 1945 | 481 | 297 | All-cause, CVD, and cancer | 72.2 | 0 | Repeated SFFQ | — | Type 2 diabetes | Age, sex, survey period, ethnicity, BMI at diagnosis, physical activity, smoking status, smoking pack years, alcohol consumption, multivitamin use, current aspirin use, family history of myocardial infarction, family history of diabetes, history of hypercholesterolemia, history of hypertension, duration of diabetes, total energy intake, dietary cholesterol, and percentage of energy from dietary protein and remaining FAs | |
| HPFS, USA | Wang, 2016 (29) | 19.8 | 42,884 | 12,990 | 3878 | 4192 | All-cause, CVD, and cancer | 53.2 | 100 | Repeated SFFQ | 5% of energy | — | Age, race, marital status, BMI, physical activity, smoking status, alcohol consumption, multivitamin use, vitamin E supplement use, current aspirin use, family history of myocardial infarction, family history of diabetes, family history of cancer, history of hypertension, history of hypercholesterolemia, intakes of total energy and dietary cholesterol, percentage of energy intake from dietary protein, and menopausal status and hormone use in women; all models also included percentages of energy intake from remaining FAs (SFAs, PUFAs, MUFAs, trans-FAs, ω-6 PUFAs, ω-3 PUFAs, LA/ALA, AA, and marine ω-3 FAs) |
| Richman, 2013 (32)2 | 8.4 | 4577 | 1064 | — | — | All-cause | 69 | 100 | Repeated SFFQ | 4.9% of energy | Prostate cancer | Age, energy, time since diagnosis treatment, Gleason sum, clinical stage, diagnostic prostate specific antigen, number of prostate-specific antigen screening tests prior to diagnosis, BMI, smoking, vigorous activity, and intake of calcium, alcohol, protein, saturated fat, monounsaturated fat, trans fat, LA/ALA/PUFA, long-chain ω-3 FAs, and prediagnostic intake of polyunsaturated fat based on the 1986 FFQ, high blood pressure at prostate cancer diagnosis, elevated cholesterol at prostate cancer diagnosis, diabetes mellitus at prostate cancer diagnosis, parental history of myocardial infarction before age 60, comorbidity of CVD, emphysema, COPD, or PD | |
| Jiao, 2019 (31)2 | 11 | 2211 | 557 | 165 | 154 | All-cause, CVD, and cancer | 73.1 | 100 | Repeated SFFQ | — | Type 2 diabetes | Age, sex, survey period, ethnicity, BMI at diagnosis, physical activity, smoking status, smoking pack years, alcohol consumption, multivitamin use, current aspirin use, family history of myocardial infarction, family history of diabetes, history of hypercholesterolemia, history of hypertension, duration of diabetes, total energy intake, dietary cholesterol, and percentage of energy from dietary protein, and remaining FAs | |
| MRFIT, USA | Dolecek, 1992 (22) | 10.5 | 6258 | 5223 | 232 | 132 | All-cause, CVD, and cancer | 35–57 | 100 | Repeated 24-h dietary recall | 14.6 g/d | High-CVD-risk population | Age, race, smoking, baseline diastolic blood pressure, HDL, LDL, alcohol |
| Dolecek, 1991 (23) | 10.5 | 6258 | 4393 | 232 | 132 | All-cause, CVD, and cancer | 35–57 | 100 | Repeated 24-h dietary recall | 14.6 g/d | High-CVD-risk population | Age, race, baseline smoking, diastolic blood pressure, HDL and LDL concentrations | |
| Italian elderly, Italy | Fortes, 2000 (33) | 5 | 162 | 53 | — | — | All-cause | 80 | 32 | Baseline SFFQ interviewed | 11.53 g/d | — | Age, sex, education, BMI, smoking, cognitive function, and chronic diseases |
| CSPOC-BC, USA | McEligot, 2006 (34) | 6.7 | 516 | 96 | — | — | All-cause | 64.8 | 0 | Baseline SFFQ | 8.49% of energy | Breast cancer | Age, stage of disease, BMI, parity, hormone replacement therapy uses, alcohol use, multivitamin use, and energy intake |
| KIHD, Finland | Laaksonen, 2005 (35) | 14.6 | 1551 | 220 | 78 | — | All-cause and CVD | 52 | 100 | Baseline 4-d food record | 3.5% of energy | — | Age, year of examination, smoking, alcohol consumption, adult socioeconomic status, moderate to vigorous leisure-time physical activity, plasma lipid-standardized α-tocopherol concentrations, plasma ascorbic acid, dietary total energy and energy-adjusted saturated fat and fiber intake, LDL cholesterol concentrations, systolic blood pressure, blood pressure medication, family history of ischemic heart disease, C-reactive protein concentrations, fasting concentrations of insulin and nonesterified FAs, and BMI |
| LIBCSP, USA | Khankari, 2015 (36) | 14.7 | 1463 | 485 | — | 210 | All-cause and breast cancer | 20–98 | 0 | Baseline SFFQ | 7.44 g/d | Breast cancer | Age, total energy intake (kcal/d) |
| ARIC-Men, USA | Farvid, 2014 (4) | 9.2 | 5240 | — | 51 | — | CAD | 54 | 100 | FFQ | 4.37% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| FMC-Men, Finland | Farvid, 2014 (4) | 10 | 2712 | — | 147 | — | CAD | 47 | 100 | Dietary history | 1.49% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| FMC-Women, Finland | Farvid, 2014 (4) | 10 | 2481 | — | 48 | — | CAD | 49 | 0 | Dietary history | 1.47% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| VIP-Men, Sweden | Farvid, 2014 (4) | 10 | 9521 | — | 38 | — | CAD | 52 | 100 | FFQ | 3.37% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| IWHS, USA | Farvid, 2014 (4) | 10 | 30,180 | — | 294 | — | CAD | 61 | 0 | FFQ | 5.28% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| IIHD, Israel | Farvid, 2014 (4) | 10 | 8272 | — | 165 | — | CAD | 48 | 100 | FFQ | 6.42% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| MDC, Sweden | Farvid, 2014 (4) | 15.6 | 20,674 | — | 1060 | — | CAD | 58 | 39 | FFQ + 7-d registration of cooked meals and cold beverages | 4.73% of energy | — | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFAs (or carbohydrate), MUFAs, PUFAs other than LA, fiber intake, hypertension, and education |
| Canada-BC, Canada | Goodwin, 2003 (37) | 6.1 | 477 | 52 | — | 51 | Breast cancer | 50.4 | 0 | FFQ | 15.1 g/d | Breast cancer | Age, BMI (quadratic), tumor stage, nodal stage, adjuvant hormone therapy, adjuvant chemotherapy, and total energy |
| NBSS, Canada | Jain, 1994 (38) | 4.9 | 678 | — | — | 76 | Breast cancer | 52.7 | 0 | Dietary history | — | Breast cancer | Age, total energy, smoking, and body weight |
| AARP-DHS, USA | Zhuang, 2019a4 (39) | 16 | 521,120 | 129,328 | 38,747 | 45,783 | All-cause, CVD, and cancer | 63 | 58.8 | FFQ | 5.96% of energy | — | Age, sex, BMI, race, education, marital status, household income, smoking, alcohol, physical activity, history of hypertension, history of hypercholesterolemia, perceived health condition, history of heart disease, stroke, diabetes mellitus, and cancer at baseline, multivitamin use, aspirin use, hormone use for women, intake of total energy, percentages of energy intake from protein, and remaining FAs |
| Örebro-PC, Canada | Epstein, 2012 (40) | >10 | 525 | 490 | — | 222 | Prostate cancer | 70.7 | 100 | FFQ | 7.9 g/d | Prostate cancer | Age, family history of prostate cancer, smoking status, calendar year, alcohol intake, and BMI |
| NHANES, USA | Zhuang, 2019b4 (41) | 9.1 | 36,032 | 4826 | 1299 | 1099 | All-cause, CVD, and cancer | 46.2 | 48.6 | One-day 24-h dietary recall | 14.8 g/d | — | Age, gender, race-ethnicity, BMI, education, marital status, physical activity, smoking, alcohol drinking status, history of hypertension, history of diabetes, family history of CVD, intake of total energy, vegetables, fruits, red meat and saturated fat |
| CHNS, China | Zhuang, 2019b4 (41) | 14 | 14,117 | 1007 | — | — | All-cause | 41.4 | 46 | Three-day, 24-h dietary recall | 9.2 g/d | — | Age, gender, BMI, education, marital status, residence, physical activity, smoking, alcohol drinking status, history of hypertension, history of diabetes, intake of total energy, vegetables, fruits, red meat, and saturated fat |
| InCHIANTI, Italy | Lelli, 2019 (42) | <9 | 927 | 318 | 114 | — | All-cause and CVD | 75 | 44 | FFQ | — | — | Age, sex, education, BMI, estimated glomerular filtration rate (CKD-EPI equation), caloric intake/body weight, smoke, hypertension, diabetes, alcohol, and oleic acid consumption |
1AARP-DHS, NIH–American Association of Retired Persons (AARP) Diet and Health Study; ALA, α-linolenic acid; ARIC-Men, male participants from the Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; Canada-PC, Canada Breast Cancer Study; CAD, coronary artery disease; CHNS, China Health and Nutrition Survey; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disorder; CSPOC-BC, Cancer Surveillance Program of Orange County–Breast Cancer; CVD, cardiovascular disease, FA, fatty acid; FFQ, food-frequency questionnaire; FMC, Finnish Mobile Clinic Health Study; HPFS, Health Professionals Follow-Up Study; IIHD, Ischemic Heart Disease Study; InCHIANTI, the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study; Italian elderly, a cohort of old people recruited in Italy; IWHS, Iowa Women's Health Study; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; LA, linoleic acid; LIBCSP, Long Island Breast Cancer Study Project; MDC, Malmo Diet and Cancer Cohort Study; MRFIT, Multiple Risk Factor Intervention Trial; NBSS, National Breast Screening Study; NHS, Nurses’ Health Study; Örebro-PC, Örebro Prostate Cancer Study; PD, Parkinson disease; ref, reference; SFFQ, semiquantitative food-frequency questionnaire; VIP-Men, male participants from the Västerbotten Intervention Program.
2The cohort reported in this paper is reported more in detail/updated in another paper, but due to specific reasons (e.g., specific baseline conditions), we included this paper in the stratification analysis.
3MRFIT was reported in two articles with the same follow-up period but different numbers of all-cause mortality cases. In reference 22 Table II, the number of cases of all-cause mortality was reported as 522; in reference 23 Table 2, it was reported as 439.
Zhuang 2019a refers to reference 53 while Zhuang 2019b refers to reference 55.
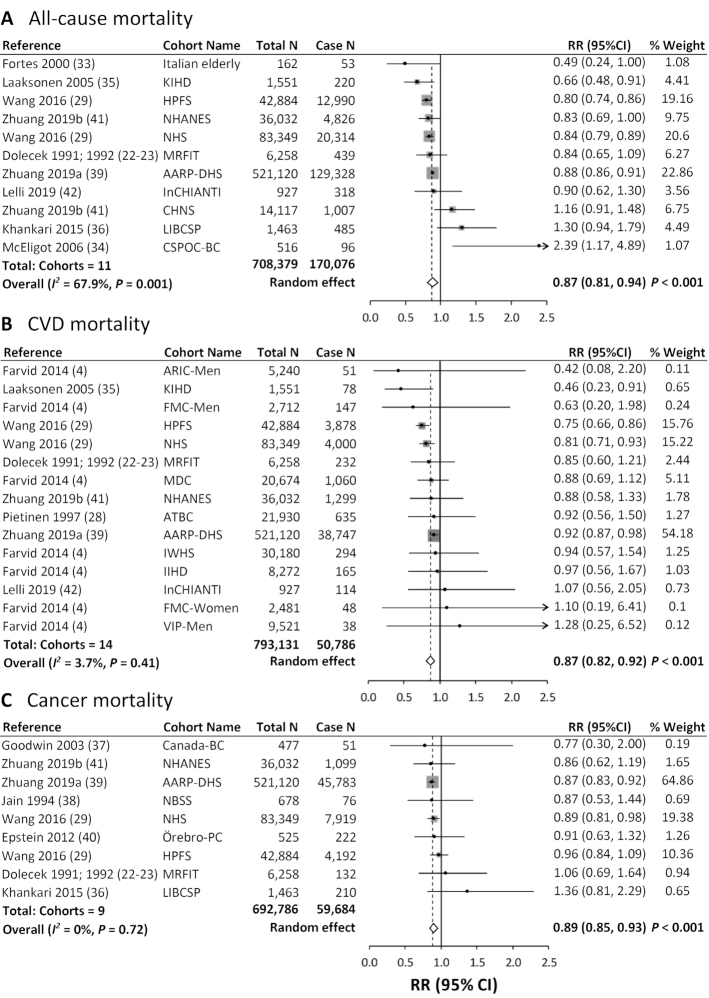
Comparing the highest with the lowest category of dietary LA intake, the pooled multivariable-adjusted RRs in random-effects meta-analysis were 0.87 (95% CI: 0.81, 0.94; I2 = 67.9%) for all-cause mortality, 0.87 (95% CI: 0.82, 0.92; I2 = 3.7%) for CVD mortality, and 0.89 (95% CI: 0.85, 0.93; I2 = 0%) for cancer mortality (Figure 2). For all-cause mortality, a sensitivity analysis excluding 1 study at a time suggested that no study contributed substantially to the heterogeneity (Supplemental Figure 1). In stratified meta-analyses, the inverse associations between dietary LA and total mortality, and between LA and CVD mortality, were stronger in studies with higher NOS scores (≥8), studies that assessed diet repeatedly, or studies with younger participants (baseline age <60 y), longer follow-up (≥10 y), or higher proportions of men (>50%) (Supplemental Table 2). Univariate meta-regressions suggested that baseline age and whether diet was assessed repeatedly during follow-up may be potential sources of heterogeneity for the associations between LA intake and CVD mortality (P < 0.05). We did not identify a significant source of heterogeneity for the associations between LA intake and total or cancer mortality, after examining baseline health status, geographic location, NOS scores, dietary assessment, baseline mean age, sex, and follow-up duration (Supplemental Table 2).
FIGURE 2.
Meta-analysis of the associations between dietary linoleic acid intake and mortality from all causes (A), CVD (B), and cancer (C) in prospective cohort studies. For each study, RR corresponds to the comparison of extreme quantiles of dietary linoleic acid intake; the area of the grey square is proportional to the weight of the study, which is the inverse of the variance of the log RR; dots and horizontal lines represent RRs and 95% CIs. Diamonds depict pooled estimates from random-effects inverse-variance–weighted meta-analyses. AARP-DHS, NIH–American Association of Retired Persons Diet and Health Study; ARIC-Men, male participants from the Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; Canada-BC, Canada Breast Cancer Study, CHNS, China Health and Nutrition Survey; CSPOC-BC, Cancer Surveillance Program of Orange County–Breast Cancer; CVD, cardiovascular disease; FMC, Finnish Mobile Clinic Health Study; HPFS, Health Professionals Follow-Up Study; IIHD, Ischemic Heart Disease Study; InCHIANTI, the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study; Italian elderly, a cohort of old people recruited in Italy; IWHS, Iowa Women's Health Study; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; LIBCSP, Long Island Breast Cancer Study Project; MDC, Malmo Diet and Cancer Cohort Study; MRFIT, Multiple Risk Factor Intervention Trial; NBSS, National Breast Screening Study; NHS, Nurses’ Health Study; Örebro-PC, Örebro Prostate Cancer Study; VIP-Men, male participants from the Västerbotten Intervention Program.
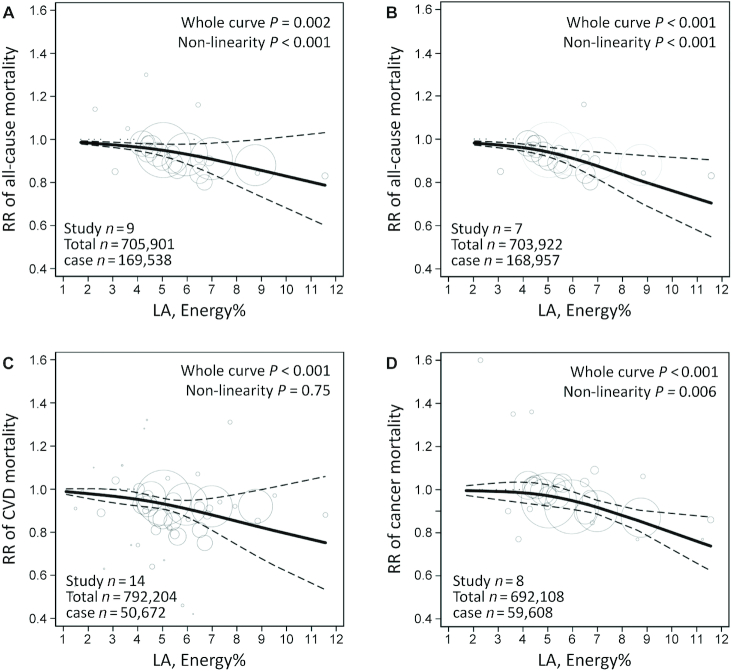
The estimated median intake across LA categories ranged from 1.1% to 11.6% of energy among studies that provided adequate data for dose–response analysis. We observed a nonlinear association between dietary LA and total mortality and between LA and cancer mortality (P < 0.05 for the overall shape of curve and nonlinearity). Compared with the lowest LA intake, the RRs of total mortality were 0.97 (95% CI: 0.89, 1.06) for 5% of energy and 0.88 (95% CI: 0.74, 1.05) for 10% of energy intake from dietary LA (Figure 3A); the RRs of cancer mortality were 0.96 (95% CI: 0.94, 0.98) for 5% of energy and 0.83 (95% CI: 0.78, 0.89) for 10% of energy intake from LA (Figure 3D). In a sensitivity analysis excluding 2 studies in which participants had cancer at baseline, the RRs of total mortality were 0.94 (95% CI: 0.86, 1.02) for 5% of energy and 0.81 (95% CI: 0.69, 0.96) for 10% of energy intake from LA, with the lowest LA intake as reference (Figure 3B). There was a linear association between dietary LA intake and CVD mortality (P < 0.001); the RR for each 5% increment of energy intake from LA was 0.93 (95% CI: 0.91, 0.95) (Figure 3C).
FIGURE 3.
Dose–response meta-analysis for associations between dietary LA intake and mortality from all causes in all studies (A) and excluding 2 studies in which participants had cancer at baseline (B), and between LA intake and mortality from CVD (C) and cancer (D), in prospective cohort studies. The pooled RR trend by LA intake dosage (solid line) and its 95% CIs (dashed lines) were obtained by a random-effects dose–response meta-analysis. Circles represent RRs according to LA categories from each study, inversely proportional to the variance of log RRs. CVD, cardiovascular disease; LA, linoleic acid.
Biomarkers of LA and mortality
Twenty-two articles reported associations between biomarkers of LA and mortality outcomes in 28 prospective cohorts, including 65,411 participants, 9758 total deaths (16 studies), 6492 CVD deaths (20 studies), and 1719 cancer deaths (7 studies). Median follow-up durations ranged from 2 to 31 y. GC or GLC were used to measure LA concentrations in adipose tissue (2 cohorts) or different blood compartments (plasma phospholipids, 10 cohorts; erythrocyte phospholipids, 4 cohorts; cholesteryl esters, 7 cohorts; total plasma/serum, 5 cohorts; and whole blood, 1 cohort; 1 cohort measured fatty acids in both plasma phospholipids and cholesteryl esters). The mean proportion of LA relative to the total assayed fatty acids ranged from 9.2% to 54.4%, based on the use of different tissue types (Table 2). NOS scores ranged from 5 to 9, with data for 15 cohorts scoring ≥8 (Supplemental Table 1).
TABLE 2.
Characteristics of prospective cohort studies (including nested case-control and case-cohort studies) that evaluated the associations between LA biomarkers and mortality from all causes, CVD, and/or cancer1
| Study name, location | Sampling year | First author, year (ref) | Study design | Mean follow-up, y | Total sample size, n | Total deaths, n | CVD deaths, n | Cancer deaths, n | Mortality type | Age, y | Male, % | Tissue type | Measuring method | Mean/median of LA, %FA | Covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MP-1, Netherlands | 1987–1991 | de Goede, 2013 (43) | NCC | 12.5 | 444 | — | 222 | — | Fatal CAD | 50.5 | 70 | Cholesteryl esters | GC | 43.8 | Age, gender, cohort, enrollment date, smoking, BMI, educational level, alcohol intake, systolic blood pressure, total cholesterol |
| MP-2, Netherlands | 1993–1997 | de Goede, 2013 (43) | NCC | 12.5 | 114 | — | 57 | — | Fatal CAD | 51.7 | 79 | Cholesteryl esters | GC | 54.4c | Age, gender, cohort, enrollment date, smoking, BMI, educational level, alcohol intake, systolic blood pressure, total cholesterol |
| CHS, USA | 1992–1993 | Wu, 2014 (44) | Cohort | 9 | 2792 | 1994 | 678 | 411 | All-cause, CVD,2 and cancer | 74 | 36 | Phospholipids | GC | 19.7 | Age, sex, race, enrollment site, education, smoking status, prevalent diabetes, atrial fibrillation, and hypertension, leisure-time physical activity, BMI, waist circumference, alcohol use, and plasma phospholipid long-chain n–3 PUFAS (sum of ETA+DPA+DHA, % of total FAs) |
| 1992–1993 | Marklund, 2019 (7) | Cohort | 13 | 2907 | — | 832 | — | CVD | 72.1 | 36 | Phospholipids | GC-FID | 19.7 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs | |
| 60YO, Sweden | 1997–1999 | Marklund, 2015 (45) | Cohort | 14.5 | 1733 | 459 | — | — | All-cause and CVD2 | 60 | 47.2 | Cholesteryl esters | GC | 48.5 | Age, BMI, smoking, physical activity, education, alcohol intake, diabetes mellitus, drug-treated hypertension, and drug-treated hypercholesterolemia |
| 1997–1998 | Marklund, 2019 (7) | Cohort | 14.5 | 4150 | — | 69 | — | CVD | 60.3 | 48 | Cholesteryl esters | GC | 48.4 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs | |
| TRIUMPH, USA | 2005–2007 | Harris, 2013 (46) | Cohort | 2 | 1144 | 135 | — | — | All-cause | 59.5 | 65.8 | Erythrocytes | GC | 13.3 | GRACE score (based on age, heart rate, systolic blood pressure, renal function, congestive heart failure, ST-segment deviation, cardiac arrest, and elevated biomarkers) |
| ULSAM-70, Sweden | 1991–1995 | Iggman, 2016 (17) | Cohort | 14.8 | 853 | 605 | 251 | — | All-cause and CVD | 71 | 100 | Adipose tissue | GC | 12.7 | Age, analysis occasion, smoking, BMI, alcohol intake, physical activity, diabetes prevalence, systolic blood pressure, dyslipidemia, and hypertension treatment |
| ULSAM-50, Sweden | 1970–1973 | Warensjö, 2008 (47) | Cohort | 30.7 | 2009 | 1012 | 461 | — | All-cause and CVD | 50 | 100 | Cholesteryl esters | GC | 53.9 | Age, total cholesterol, BMI, smoking, physical activity, and hypertension |
| 1970–1973 | Kilander, 2001 (48) | Cohort | > 10 | 1990 | 630 | 301 | 216 | All-cause,2 CVD,2 and cancer | 50 | 100 | Cholesteryl esters | GC | 54 | Age | |
| NSCS-Men, Australia | 1996 | Miura, 2016 (49) | Cohort | 17 (Max) | 444 | 98 | — | — | All-cause | 50 | 100 | Phospholipids | GC | 239.75 μg/mL | Age, sex, smoking status, blood cholesterol, jaundice measure (proxy serum β-carotene concentration), and history of serious medical condition |
| NSCS-Women, Australia | 1996 | Miura, 2016 (49) | Cohort | 17 (Max) | 564 | 81 | — | — | All-cause | 49 | 0 | Phospholipids | GC | 257.25 μg/mL | Age, sex, smoking status, blood cholesterol, jaundice measure (proxy serum β-carotene concentration), and history of serious medical condition |
| WHIMS, USA | 1996 | Harris, 2017 (50) | Cohort | 14.9 | 6501 | 1851 | 617 | 462 | All-cause, CVD, and cancer | 70 | 0 | Erythrocytes | GC | 11.89 | Age, race, hormone therapy assignment, BMI, education, smoking pack-years, physical activity, weekly alcohol intake, waist circumference, region, family history of cancer, family history of CVD, aspirin use, high cholesterol requiring pills, and a history of hypertension, diabetes, CVD, and/or cancer |
| DIA Patient, Sweden | 1996–2010 | Huang, 2012 (51) | Cohort | 1.5 | 222 | 61 | — | — | All-cause | 57 | 61 | Phospholipids | GLC | 19.7 | Age, sex, comorbidities (composite score of diabetes and CVD), dialysis modality and protein-energy wasting (Subjective Global Assessment tool), and IL-6 |
| Old patients, Norway | 1994–1995 | Lindberg, 2008 (52) | Cohort | <3 | 254 | 101 | — | — | All-cause | 82.1 | 35 | Phospholipids | GC | 18.7 | Age, sex, assignment to Geriatric Evaluation and Management Unit treatment, Barthel Index, residence (private home or sheltered housing), current smoking status, history of CVD, and HDL-cholesterol, LDL-cholesterol, prealbumin, and α-tocopherol concentrations |
| KIHD, Finland | 1984–1989 | Virtanen, 2018 (53) | Cohort | 22.4 | 2480 | 1143 | 575 | 317 | All-cause, CVD, and cancer | 53 | 100 | Total serum | GC | 26.39 | Age, examination year, BMI, family history of diabetes, smoking, education, income, leisure-time physical activity, intake of alcohol, serum long-chain n–3 PUFAs, hypertension, family history of CVD, cancer, or diabetes, use of hypercholesterolemia, hypertension, or diabetes medications at baseline or during follow-up, and intakes of SFAs, MUFAs, trans-FAs, fiber, and fruit, berries, and vegetables |
| 1984–1989 | Laaksonen, 2005 (35)2 | Cohort | 14.6 | 1551 | 202 | 69 | — | All-cause and CVD | 52 | 100 | Total serum | GC | 27.9 | Age, year of examination, smoking, alcohol consumption, adult socioeconomic status, moderate to vigorous leisure-time physical activity, plasma lipid-standardized α-tocopherol concentrations, plasma ascorbic acid, dietary total energy and energy-adjusted saturated fat and fiber intake, LDL cholesterol, systolic blood pressure, blood pressure medication, family history of ischemic heart disease, C-reactive protein concentrations, fasting concentrations of insulin and nonesterified FAs, and BMI | |
| LURIC, Germany | 1997–2000 | Delgado, 2017 (54) | Cohort | 10 | 3259 | 975 | 614 | — | All-cause and CVD | 64.9 | 70 | Erythrocytes | GC | 11.5 | Age, gender, BMI, LDL cholesterol, HDL cholesterol, log triglycerides, hypertension, diabetes, smoking, and lipid-lowering therapy |
| EUROASPIRE- Finnish, Finland | 1991–1994 | Erkkila, 2003 (55) | Cohort | 5 | 415 | 35 | 18 | — | All-cause and CAD | 60.7 | 69 | Cholesteryl esters | GC | 48.72 | Age, sex, diagnostic category (coronary artery bypass grafting, or percutaneous transluminal coronary angioplasty compared with acute myocardial infarction or acute myocardial ischemia), energy intake, serum cholesterol, serum triacylglycerol, diabetes, BMI, and education |
| EPIC-Norfolk, UK | 1993–1997 | Marklund, 2019 (7) | Cohort | 17.6 | 7016 | — | 951 | — | CVD | 63.1 | 49 | Phospholipids | GC-FID | 24.3 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| MCCS, Australia | 1990–1994 | Marklund, 2019 (7) | Cohort | 7.1 | 6265 | — | 282 | — | CVD | 56.3 | 46 | Phospholipids | GLC-FID | 20.1 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| MESA, USA | 2000–2002 | Marklund, 2019 (7) | Cohort | 8.6 | 2722 | — | 208 | — | CVD | 62.1 | 47 | Phospholipids | GC-FID | 21.5 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| SHHEC, UK | 1985–1986 | Marklund, 2019 (7) | Cohort | 23.6 | 4391 | — | 308 | — | CVD | 48.7 | 52 | Adipose tissue | GC | 9.2 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| AGES- Reykjavik, Iceland | 2002–2006 | Marklund, 2019 (7) | Cohort | 10 | 1195 | — | 162 | — | CVD | 76.6 | 39 | Phospholipids | GC-FID | 17.7 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| HS, Japan | 2002–2003 | Marklund, 2019 (7) | Cohort | 10.2 | 3103 | — | 98 | — | CVD | 60.9 | 42 | Total plasma | GC | 27 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| ARIC, USA | 1987–1989 | Marklund, 2019 (7) | Cohort | 22.7 | 3749 | — | 289 | — | CVD | 63.9 | 48 | Phospholipids | GC | 22 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| CCCC, Taiwan, China | 1992–2000 | Marklund, 2019 (7) | Cohort | 14.1 | 1838 | — | 306 | — | CVD | 60.6 | 55 | Total plasma | GC-FID | 15.6 | Age, sex, race, field or clinical center if applicable, BMI, education, smoking, physical activity, alcohol intake, diabetes status, treated hypertension, treated hypercholesterolemia, regular aspirin use, biomarker concentrations of ALA, ETA, sum of trans-18:1 FAs, and sum of trans-18:2 FAs |
| HSS, USA | 2000–2002 | Pottala, 2010 (56) | Cohort | 5.9 | 956 | 237 | — | — | All-cause | 67 | 82 | Whole blood | GC | 22.96 | No |
| PPSII, France | 1981–1985 | Zureik, 1995 (57) | Cohort | 9.3 | 3277 | — | — | 59 | Cancer | 36–52 | 100 | Cholesteryl esters | GC | 48.5 | Age, smoking, alcohol consumption, BMI, and serum cholesterol |
| FHS, USA | 2005–2008 | Harris, 2018 (58) | Cohort | 7.3 | 2500 | 350 | 58 | 146 | All-cause, CVD, and cancer | 66 | 43 | Erythrocytes | GC | 11.3 | Age, sex, BMI, marital status, education, employment, health insurance status, regular aspirin use, prevalent hypertension, cholesterol medication, prevalent diabetes, alcohol consumption, smoking, metabolic equivalents, total to HDL cholesterol ratio, systolic blood pressure, C-reactive protein, omega-3 index |
| 3C, France | 1999–2001 | Satizabal, 2018 (25) | Cohort | 8.1 | 1406 | 251 | — | — | All-cause | 74.6 | 39.4 | Total plasma | GC | 24.9 | Age, sex, systolic blood pressure, antihypertensive medications, BMI, smoking, diabetes mellitus, and atrial fibrillation |
| MRFIT, USA | 1973–1976 | Simon, 1998 (59) | NCC | 6.9 | 323 | — | — | 108 | Fatal cancer | 35–57 | 100 | Cholesteryl esters | GLC | 51.66 | Age, smoking status, date of randomization, clinical center, treatment assignment, alcohol intake, plasma cholesterol concentration, and diastolic blood pressure |
| 1973–1976 | Simon, 1998 (59) | NCC | 6.9 | 323 | — | — | 108 | Fatal cancer | 35–57 | 100 | Phospholipids | GLC | 21.24 | Age, smoking status, date of randomization, clinical center, treatment assignment, alcohol intake, plasma cholesterol concentration, and diastolic blood pressure | |
| InCHIANTI, Italy | 1998–2000 | Lelli, 2019 (42) | Cohort | < 9 | 927 | 318 | 114 | — | All-cause and CVD | 75 | 44 | Total serum | GC | 24.2 | Age, sex, education, BMI, estimated glomerular filtration rate (CKD-EPI), caloric intake/body weight, smoke, hypertension, diabetes, alcohol, and oleic acid consumption |
1AGES-Reykjavik, Age, Gene/Environment Susceptibility–Reykjavik Study; ALA, α-linolenic acid; ARIC, Atherosclerosis Risk in Communities; CCCC, Chin-Shan Community Cardiovascular Cohort Study; CHS, Cardiovascular Health Study; CVD, cardiovascular disease, CAD, coronary artery disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DIA Patient, a small cohort of dialysis patients in Sweden; DPA, docosapentaenoic acid; EPIC, European Prospective Investigation into Cancer and Nutrition; ETA, eicosatetraenoic acid; EUROASPIRE-Finnish, Finnish cohort of EUROASPIRE (European Action on Secondary and Primary Prevention by Intervention to Reduce Events); FA, fatty acid; FHS, Framingham Heart Study; FID, flame-ionization detection; GRACE, Global Registry of Acute Coronary Events; HS, Hisayama Study; HSS, Heart and Soul Study; InCHIANTI, longitudinal InCHIANTI study; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; LA, linoleic acid; LURIC, Ludwigshafen Risk and Cardiovascular Health Study; MCCS, Melbourne Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; MP-1 and MP-2, Monitoring Project on Cardiovascular Disease Risk Factors; MRFIT, Multiple Risk Factor Intervention Trial; NCC, nested case-control; NSCS, Nambour Skin Cancer Study; PPSII, Paris Prospective Study II; ref, reference; SHHEC, Scottish Heart Health Extended Cohort; TRIUMPH, Translational Research Investigating Underlying disparities in acute Myocardial Infarction Patients’ Health status study; ULSAM-50, Uppsala Longitudinal Study of Adult Men investigations, recruitment at 50 y old; ULSAM-70, Uppsala Longitudinal Study of Adult Men investigations, recruitment at 70 y old; WHIMS, Women's Health Initiative Memory Study; 3C, Three-City Study; 60YO, Stockholm old men and women; Old patients, A small cohort of frail, old patients in Norway.
2Have more updated/completed data shown in other articles and thus these records were not used in analyses.
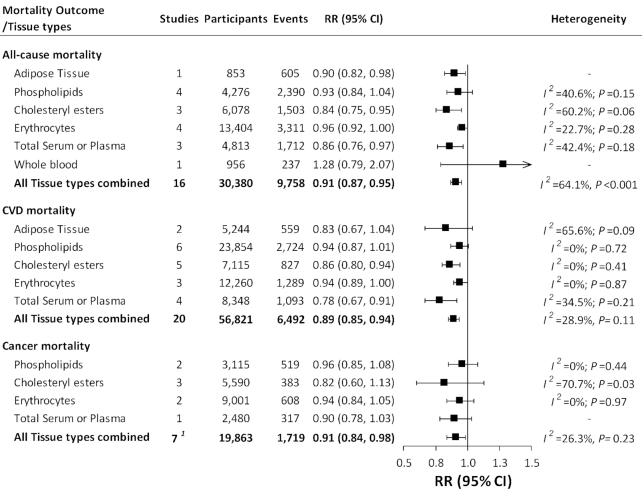
Combining findings from all tissue types, for each SD increment in LA concentrations, the pooled multivariable-adjusted RRs in random-effects meta-analysis were 0.91 (95% CI: 0.87, 0.95; I2 = 64.1%) for all-cause mortality, 0.89 (95% CI: 0.85, 0.94; I2 = 28.9%) for CVD mortality, and 0.91 (95% CI: 0.84, 0.98; I2 = 26.3%) for cancer mortality (Figure 4; Supplemental Figure 2). The pooled estimates varied by tissue types, but, in general, suggested an inverse association between LA biomarkers and mortality outcomes (Figure 4; Supplemental Table 3). Univariate meta-regressions suggested that the heterogeneity was not explained by the use of different tissue types, baseline health conditions, NOS scores, study design, or follow-up length. However, there was evidence of heterogeneity between studies of total mortality according to geographic location (P = 0.02) and sex (P = 0.008) and between studies of CVD mortality according to the average age at baseline (P = 0.02). Consistent with studies of dietary intake, the inverse associations between LA biomarkers and mortality outcomes were stronger in studies with a younger baseline age, a higher proportion of men, or a longer follow-up length (Supplemental Table 3; Supplemental Figure 3).
FIGURE 4.
Meta-analysis of the association between LA biomarkers and mortality from all causes, CVD, and cancer. Squares represent pooled RRs from random-effects inverse-variance–weighted meta-analyses, and horizontal lines represent 95% CIs. We present summary RRs and 95% CIs for each tissue and all tissue combined. 1The total study number is not the sum of study numbers for each of the tissues. This is because the MRFIT study (59) provided data on LA in phospholipid and cholesteryl ester for the same population; we used both data in the meta-analysis by tissue types but chose to use LA in phospholipid in the overall meta-analysis. Detailed forest plots presenting association estimates from each study and meta-analyses are provided in Supplemental Figure 2. CVD, cardiovascular disease; LA, linoleic acid; MRFIT, Multiple Risk Factor Intervention Trial.
Assessment of publication bias
Funnel plots and the Egger's tests suggested no evidence for publication bias for prospective cohort studies examining associations between LA intake (dietary studies, P ≥ 0.16; biomarker studies, P ≥ 0.49) and mortality from all causes, CVD, and cancer (Supplemental Figure 4).
Discussion
In this systematic review and meta-analysis of prospective cohort studies, we found that higher LA intake, assessed by either dietary surveys or biomarkers, was associated with a modestly lower risk of mortality from all causes, CVD, and cancer. These findings support potential long-term health benefits of LA in the prevention of premature death and are in line with current recommendations on PUFA consumption for CVD prevention.
Previous prospective cohort studies examining the health effects of n–6 PUFAs mostly focused on CVD endpoints. Meta-analysis and pooling studies suggest that higher intakes of PUFAs or LA, in replacement of SFAs or carbohydrates, are associated with lower CAD incidence (4, 60); higher concentrations of LA biomarkers have also been associated with a lower risk of type 2 diabetes, CVD, and CVD mortality (6, 7). A null association between LA and CAD risk was reported in a meta-analysis examining dietary intake and biomarkers of various types of fats (8); however, this study was debated due to methodological problems and misleading interpretations (61), such as the omission of a pooling study and potentially erroneous data input (35, 60, 61).
Our study is the first meta-analysis examining the associations between LA intake and mortality risk. We found that higher LA intake, assessed by dietary surveys or biomarkers, is associated with a modestly lower risk of mortality from all causes, CVD, and cancer. Our work is in line with evidence on CVD, but considerably expands prior meta-analysis and pooling studies by emphasizing long-term mortality risk, combining dietary and biomarker data, and including the most recent publications (data on >60% of the cohorts were published in the last 5 y).
With respect to RCTs, although several meta-analyses came to divergent conclusions (5, 9, 35, 62), recent well-performed meta-analyses suggested that substituting n–6 PUFAs for SFAs or monounsaturated fat modestly reduced CAD risk (5, 62). However, there was little effect of LA intake on mortality (5). Several reasons may explain the lack of strong experimental evidence on LA intake and mortality. First, most RCTs on n–6 PUFAs and hard clinical endpoints were conducted decades ago, with limited sample sizes and intervention periods, in participants with chronic diseases. These studies are, therefore, not sufficiently powered to examine the effect on mortality or generalizable to contemporary settings and the general population. Second, some RCTs, especially those showing adverse effects of LA on CAD risk, need to be interpreted with caution (9). For example, the Sydney Diet Heart Study had a limited sample size (intervention group, n = 221) and follow-up length (39 mo), and the intervention was potentially confounded by trans fat (11). The Minnesota Coronary Survey had a high drop-out rate, short follow-up, intermittent intervention, and potential confounding by trans fat (9, 11). A meta-analysis excluding these 2 RCTs yielded a protective effect of n–6 PUFAs on CAD risk (11). Considering the practical challenges of conducting large RCTs on mortality endpoints, prospective cohort studies are still of high importance to examine long-term mortality risk in the general population.
Self-reported dietary intakes and nutrient biomarkers each have strengths and limitations. On the one hand, self-reported dietary intakes are subject to reporting errors and errors in the nutrient composition database. In our stratified analyses, studies that assessed diet repeatedly showed a more robust inverse association with total/CVD mortality, probably because repeated measurements can reduce random errors and are more representative of long-term diet. In addition, dietary LA comes from different food sources, including vegetable oils, nuts, seeds, and plant-based spreads (63). Prior studies (64) revealed that the same fat from different food sources showed different associations with mortality, which may be, in part, due to differences in other nutrients in the foods. Therefore, differences in the food sources of LA and in dietary patterns across different populations may contribute to the cross-population heterogeneity. On the other hand, LA concentrations in biological tissues have been shown to be reliable biomarkers (7, 65-67). However, LA measured in different types of tissues reflects intakes across different time windows (e.g., serum LA, short-term; phospholipid LA, 1–3 y; adipose tissue LA, long-term for years) (65, 67). In addition, tissue LA concentrations can be influenced by metabolism and variations in sample storage and laboratory assays (67), which may introduce between-study heterogeneity. In our meta-analysis, the consistent findings from dietary and biomarker studies are reassuring.
Our analysis suggested a potential nonlinear dose–response relation between dietary LA and total mortality, and between LA and cancer mortality, but no clear threshold effect was observed. Because heterogeneity among studies of CVD and cancer mortality is low, the moderate heterogeneity among studies of total mortality may be partially due to differences in the causes of mortality in different populations. Moreover, previous studies suggested that the conversion rate of LA to arachidonic acid (AA; 20:4n−6) is lower in men than in women (68), which may partially explain the stronger inverse association in men. The association between LA and mortality was stronger in younger populations, possibly due to the chronological decline in tissue LA concentrations (69). It is worth noting that, although we observed an inverse association between LA and cancer mortality, the association between LA and total mortality among cancer patients was nonsignificantly positive. More studies are required to elucidate the effects of LA on mortality in patients with chronic diseases.
Although the current US guidelines recommend ≥5–10% of energy from LA, other recommendations suggested limiting LA intake to <4% of energy (70, 71). One concern over the health benefit of LA is the lack of strong RCT evidence as discussed above. Another concern over the potentially deleterious effects of LA is the putative risk related to the conversion of LA to AA (a precursor for proinflammatory eicosanoids) and the competition between LA and n–3 PUFAs for the same metabolizing enzymes (16). However, conversion of LA to AA is suggested to be limited in humans (13). RCTs indicate that dietary LA and AA had no appreciable effects on inflammation, immune activation, and platelet function (14, 72, 73). Large prospective cohort studies also do not support an adverse association between AA biomarkers and risk of CVD and CVD mortality (7). On the contrary, RCTs and observational studies support that higher LA intakes reduce LDL cholesterol, increase insulin sensitivity, and are associated with lower concentrations of C-reactive protein, IL-6, and IL-1β (16).
Strengths of our study include the large number of participants in original studies and the examination of dose–response relations. The assessments of both dietary intakes and biomarkers of LA complemented each other, providing consistent findings. We included only prospective cohort studies, minimizing the influence of reverse causation and selection bias that is often present in cross-sectional or case-control studies.
Some limitations are worth noting. First, we observed a large variation in the study design, exposure assessment, study populations, and covariate adjustment across studies. Although we identified a few potential sources of heterogeneity, we were unable to identify other sources due to limited numbers of studies. Second, our analyses were based on observational studies and residual confounding cannot be completely ruled out. Finally, only 9 dietary studies prespecified the comparison macronutrients. However, because most studies were conducted in Western countries and the default comparison in a typical Western diet is largely refined carbohydrates and SFAs, our combined results are still comparable to previous studies substituting PUFAs for carbohydrates or SFAs.
In conclusion, in prospective cohort studies, higher LA intake, assessed by either dietary surveys or biomarkers, was significantly associated with a modestly lower risk of mortality from all causes, CVD, and cancer. These data support the current recommendations of replacing foods high in saturated fats or carbohydrates with foods rich in PUFAs for the prevention of CVD, and the potential long-term health benefits of LA intake in the prevention of premature death.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JL and FBH: designed the research, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis; JL, MG-F, YL, and FBH: conducted research and made critical revisions to the manuscript for important intellectual content; JL, MG-F, and FBH: performed systematic review and analyzed the data; JL: drafted the manuscript; and all authors: read and approved the final manuscript. FBH and YL have received research support from the California Walnut Commission. The other authors report no conflicts of interest.
Notes
Supported by NIH grants R01 HL60712 and P30 DK46200. JL is supported by the American Diabetes Association Fellowship Award (9-17-CMF-011). MG-F is supported by the American Diabetes Association Fellowship Award (1-18-PMF-029). The funding sources played no role in the design, collection, analysis, or interpretation of the data, or in the decision to submit the manuscript for publication.
Supplemental Tables 1–3 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, arachidonic acid; CAD, coronary artery disease; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; LA, linoleic acid; NOS, Newcastle-Ottawa Scale; RCT, randomized controlled trial.
Contributor Information
Jun Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Marta Guasch-Ferré, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Yanping Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Frank B Hu, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
References
- 1. USDA; US Department of Health and Human Services . 2015–2020 Dietary Guidelines for Americans. Washington (DC): US Department of Health and Human Services, 2015. [cited 2019 Feb 14]. Available from: https://health.gov/dietaryguidelines/2015/. [Google Scholar]
- 2. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJ, Hanson S, Jimoh OF, Ajabnoor SM, Deane KH et al.. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hooper L, Al-Khudairy L, Abdelhamid AS, Rees K, Brainard JS, Brown TJ, Ajabnoor SM, O'Brien AT, Winstanley LE, Donaldson DH et al.. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7:CD011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kroger J et al.. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marklund M, Wu JHY, Imamura F, Del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S et al.. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139(21):2422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG et al.. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 9. Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ. 2016;353:i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 12. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–88. [DOI] [PubMed] [Google Scholar]
- 13. Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46(2):269–80. [DOI] [PubMed] [Google Scholar]
- 14. Johnson GH, Fritsche K.. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112(7):1029–41., e1–15. [DOI] [PubMed] [Google Scholar]
- 15. Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 Fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104(11):1586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maki KC, Eren F, Cassens ME, Dicklin MR, Davidson MH. Omega-6 polyunsaturated fatty acids and cardiometabolic health: current evidence, controversies, and research gaps. Adv Nutr. 2018;9(6):688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iggman D, Arnlov J, Cederholm T, Riserus U. Association of adipose tissue fatty acids with cardiovascular and all-cause mortality in elderly men. JAMA Cardiol. 2016;1(7):745–53. [DOI] [PubMed] [Google Scholar]
- 18. Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–32S. [DOI] [PubMed] [Google Scholar]
- 19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 1 February, 2019]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 22. Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200(2):177–82. [DOI] [PubMed] [Google Scholar]
- 23. Dolecek TA, Granditis G.. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet. 1991;66:205–16. [DOI] [PubMed] [Google Scholar]
- 24. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 25. Satizabal CL, Samieri C, Davis-Plourde KL, Voetsch B, Aparicio HJ, Pase MP, Romero JR, Helmer C, Vasan RS, Kase CS et al.. APOE and the association of fatty acids with the risk of stroke, coronary heart disease, and mortality. Stroke. 2018;49(12):2822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. [DOI] [PubMed] [Google Scholar]
- 27. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 28. Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145(10):876–87. [DOI] [PubMed] [Google Scholar]
- 29. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, Rosner B, Willett WC. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA. 1999;281(10):914–20. [DOI] [PubMed] [Google Scholar]
- 31. Jiao J, Liu G, Shin HJ, Hu FB, Rimm EB, Rexrode KM, Manson JE, Zong G, Sun Q. Dietary fats and mortality among patients with type 2 diabetes: analysis in two population based cohort studies. BMJ. 2019;366:l4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richman EL, Kenfield SA, Chavarro JE, Stampfer MJ, Giovannucci EL, Willett WC, Chan JM. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern Med. 2013;173(14):1318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology. 2000;11(4):440–5. [DOI] [PubMed] [Google Scholar]
- 34. McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55(2):132–40. [DOI] [PubMed] [Google Scholar]
- 35. Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165(2):193–9. [DOI] [PubMed] [Google Scholar]
- 36. Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, Ahn J, Chen Y, Ahsan H, Terry MB et al.. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: a population-based follow-up study on Long Island, New York. Cancer. 2015;121(13):2244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodwin PJ, Ennis M, Pritchard KI, Koo J, Trudeau ME, Hood N. Diet and breast cancer: evidence that extremes in diet are associated with poor survival. J Clin Oncol. 2003;21(13):2500–7. [DOI] [PubMed] [Google Scholar]
- 38. Jain M, Miller AB, To T. Premorbid diet and the prognosis of women with breast cancer. J Natl Cancer Inst. 1994;86(18):1390–7. [DOI] [PubMed] [Google Scholar]
- 39. Zhuang P, Zhang Y, He W, Chen X, Chen J, He L, Mao L, Wu F, Jiao J. Dietary fats in relation to total and cause-specific mortality in a prospective cohort of 521 120 individuals with 16 years of follow-up. Circ Res. 2019;124(5):757–68. [DOI] [PubMed] [Google Scholar]
- 40. Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A, Wolk A, Hakansson N, Fall K, Andersson SO, Andren O. Dietary fatty acid intake and prostate cancer survival in Orebro County, Sweden. Am J Epidemiol. 2012;176(3):240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhuang P, Wang W, Wang J, Zhang Y, Jiao J. Polyunsaturated fatty acids intake, omega-6/omega-3 ratio and mortality: findings from two independent nationwide cohorts. Clin Nutr. 2019;38(2):848–55. [DOI] [PubMed] [Google Scholar]
- 42. Lelli D, Antonelli Incalzi R, Ferrucci L, Bandinelli S, Pedone C. Association between PUFA intake and serum concentration and mortality in older adults: a cohort study. Clin Nutr. 2019. pii:S0261–5614(19)30084–6. doi: 10.1016/j.clnu.2019.02.030. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Goede J, Verschuren WM, Boer JM, Verberne LD, Kromhout D, Geleijnse JM. N-6 and N-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: a nested case-control study and meta-analysis. PLoS One. 2013;8(5):e59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014;130(15):1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marklund M, Leander K, Vikstrom M, Laguzzi F, Gigante B, Sjogren P, Cederholm T, de Faire U, Hellenius ML, Riserus U. Polyunsaturated fat intake estimated by circulating biomarkers and risk of cardiovascular disease and all-cause mortality in a population-based cohort of 60-year-old men and women. Circulation. 2015;132(7):586–94. [DOI] [PubMed] [Google Scholar]
- 46. Harris WS, Kennedy KF, O'Keefe JH Jr, Spertus JA. Red blood cell fatty acid levels improve GRACE score prediction of 2-yr mortality in patients with myocardial infarction. Int J Cardiol. 2013;168(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88(1):203–9. [DOI] [PubMed] [Google Scholar]
- 48. Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer: a 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol. 2001;30(5):1119–26. [DOI] [PubMed] [Google Scholar]
- 49. Miura K, Hughes MCB, Ungerer JP, Green AC. Plasma eicosapentaenoic acid is negatively associated with all-cause mortality among men and women in a population-based prospective study. Nutr Res. 2016;36(11):1202–9. [DOI] [PubMed] [Google Scholar]
- 50. Harris WS, Luo J, Pottala JV, Espeland MA, Margolis KL, Manson JE, Wang L, Brasky TM, Robinson JG. Red blood cell polyunsaturated fatty acids and mortality in the Women's Health Initiative Memory Study. J Clin Lipidol. 2017;11(1):250–9. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang X, Stenvinkel P, Qureshi AR, Riserus U, Cederholm T, Barany P, Heimburger O, Lindholm B, Carrero JJ. Essential polyunsaturated fatty acids, inflammation and mortality in dialysis patients. Nephrol Dial Transplant. 2012;27(9):3615–20. [DOI] [PubMed] [Google Scholar]
- 52. Lindberg M, Saltvedt I, Sletvold O, Bjerve KS. Long-chain n-3 fatty acids and mortality in elderly patients. Am J Clin Nutr. 2008;88(3):722–9. [DOI] [PubMed] [Google Scholar]
- 53. Virtanen JK, Wu JHY, Voutilainen S, Mursu J, Tuomainen TP. Serum n-6 polyunsaturated fatty acids and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2018;107(3):427–35. [DOI] [PubMed] [Google Scholar]
- 54. Delgado GE, Marz W, Lorkowski S, von Schacky C, Kleber ME. Omega-6 fatty acids: opposing associations with risk—the Ludwigshafen Risk and Cardiovascular Health Study. J Clin Lipidol. 2017;11(4):1082–90., e14. [DOI] [PubMed] [Google Scholar]
- 55. Erkkila AT, Lehto S, Pyorala K, Uusitupa MI. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78(1):65–71. [DOI] [PubMed] [Google Scholar]
- 56. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: the Heart and Soul study. Circ Cardiovasc Qual Outcomes. 2010;3(4):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zureik M, Ducimetiere P, Warnet JM, Orssaud G. Fatty acid proportions in cholesterol esters and risk of premature death from cancer in middle aged French men. BMJ. 1995;311(7015):1251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harris WS, Tintle NL, Ramachandran VS. Erythrocyte n-6 fatty acids and risk for cardiovascular outcomes and total mortality in the Framingham Heart Study. Nutrients. 2018;10(12):E2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simon JA, Fong J, Bernert JT Jr, Browner WS for the MRFIT Research Group . Serum fatty acids and the risk of fatal cancer. Am J Epidemiol. 1998;148(9):854–8. [DOI] [PubMed] [Google Scholar]
- 60. Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S et al.. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willett WC, Stampfer MJ, Sacks FM. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med. 2014;161(6):453. [DOI] [PubMed] [Google Scholar]
- 62. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. USDA USDA Food Composition Databases. [cited 2019 Feb 20]. Available from: https://ndb.nal.usda.gov/ndb/. [Google Scholar]
- 64. Guasch-Ferre M, Zong G, Willett WC, Zock PL, Wanders AJ, Hu FB, Sun Q. Associations of monounsaturated fatty acids from plant and animal sources with total and cause-specific mortality in two US prospective cohort studies. Circ Res. 2019;124(8):1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baylin A, Campos H.. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17(1):22–7. [DOI] [PubMed] [Google Scholar]
- 66. Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE et al.. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19(4):938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arab L, Akbar J.. Biomarkers and the measurement of fatty acids. Public Health Nutr. 2002;5(6A):865–71. [DOI] [PubMed] [Google Scholar]
- 68. Decsi T, Kennedy K.. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94(6 Suppl):1914S–9S. [DOI] [PubMed] [Google Scholar]
- 69. Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids. 2013;88(4):257–63. [DOI] [PubMed] [Google Scholar]
- 70. Simopoulos AP, Leaf A, Salem N Jr.. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43(2):127–30. [DOI] [PubMed] [Google Scholar]
- 71. French Food Safety Agency Opinion of the French Food Safety Agency on the update of French population reference intakes (ANCs) for fatty acids. [cited 2019 Feb 20]. Available from: https://www.anses.fr/en/system/files/NUT2006sa0359EN.pdf. [Google Scholar]
- 72. Kusumoto A, Ishikura Y, Kawashima H, Kiso Y, Takai S, Miyazaki M. Effects of arachidonate-enriched triacylglycerol supplementation on serum fatty acids and platelet aggregation in healthy male subjects with a fish diet. Br J Nutr. 2007;98(3):626–35. [DOI] [PubMed] [Google Scholar]
- 73. Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Mackey BE, Kyle D. Effects of dietary arachidonic acid on human immune response. Lipids. 1997;32(4):449–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.