ABSTRACT
Background
Protein intake recommendations advise ≥0.8 g/kg body weight (BW)/d, whereas experts propose a higher intake for older adults (1.0–1.2 g/kg BW/d). It is unknown whether optimal protein intake differs by sex or race.
Objectives
We examined the shape of sex- and race-specific associations of dietary protein intake with 3- and 6-y changes in appendicular lean mass (aLM) and gait speed and also 6-y incidence of mobility limitation in community-dwelling older men and women.
Methods
We used data on men (n = 1163) and women (n = 1237) aged 70–81 y of the Health, Aging, and Body Composition Study. Protein intake was assessed using an FFQ (1998–1999). aLM and gait speed were measured at baseline and at 3 and 6 y. Difficulty walking one-quarter mile or climbing stairs was measured every 6 mo over 6 y. Prospective associations were evaluated with linear and Cox regression models, comparing fit of models with and without spline functions. All analyses were stratified by sex and additionally by race.
Results
Mean ± SD protein intake was 0.94 ± 0.36 g/kg adjusted body weight (aBW)/d in men and 0.95 ± 0.36 g/kg aBW/d in women. There were no strong indications of nonlinear associations. In women, higher protein intake was associated with less aLM loss over 3 y (adjusted B per 0.1 g/kg aBW/d: 39.4; 95% CI: 11.6, 67.2), specifically in black women, but not over 6 y or with gait speed decline. In men, protein intake was not associated with changes in aLM and gait speed. Higher protein intake was associated with a lower risk of mobility limitation in men (adjusted HR per 1.0 g/kg aBW/d: 0.55; 95% CI: 0.34, 0.91) and women (adjusted HR: 0.56; 95% CI: 0.33, 0.94), specifically white women.
Conclusions
Associations between protein intake and physical outcomes may vary by sex and race. Therefore, it is important to consider sex and race in future studies regarding protein needs in older adults.
Keywords: optimal intake, appendicular lean body mass, physical performance, gait speed, mobility limitation, community-dwelling, old age, spline functions
Introduction
Loss of muscle mass and loss of strength during aging contribute to an increased risk of frailty, disability, and mortality in old age (1). Dietary protein intake is a modifiable factor that may reduce these age-related processes (2). Short-term metabolic studies have shown that sufficient protein intake stimulates muscle protein synthesis (MPS) and suppresses breakdown of muscle protein (1, 3). Moreover, in observational studies, higher protein intake in old age has been associated with less decline in body weight (4), lean body mass (LM) (5–7), and muscle strength (8, 9); better physical function (9); and a lower risk of disabilities (10, 11). However, evidence from randomized controlled trials (RCTs) is less conclusive: a meta-analysis of 36 RCTs on protein supplementation showed no effects on musculoskeletal outcomes in nonfrail older adults (12), whereas some recent trials found positive effects on LM (13), appendicular LM (aLM) (14), and gait speed (14).
The current protein recommendation established by the Institute of Medicine is 0.8 g/kg body weight (BW)/d for adults, irrespective of age, sex, and race (15), but there is an ongoing debate (16, 17) whether or not healthy older people should be recommended 1.0–1.2 g/kg BW/d (18, 19). The recommendations are the same for older men and women, despite sex differences in body composition and hormonal milieu, which may influence protein needs (20, 21). Men have relatively more muscle mass and less fat mass compared with women (22). Although with aging muscle mass generally declines and fat mass increases (23), men retain their higher muscle mass per kilogram body weight, which suggests that the protein needs of men may exceed those of women. However, women may have higher MPS rates compared with men (24, 25), suggesting that women's protein needs may be higher. Differences in body composition also exist between white (Caucasian) and black (African American) people. Whites have more abdominal visceral fat and less bone mineral content (26–30) and lose less lean mass compared with blacks (31). Consequently, protein needs may differ between races.
The few previous studies that have studied sex-specific associations between protein intake and functional outcomes showed inconsistent findings. Some found associations in both men and women (32), whereas others found associations in women only (33, 34). Furthermore, to the best of our knowledge, none of these previous studies examined the shape of the associations—that is, whether a certain amount of protein intake is optimal.
In the Health, Aging, and Body Composition (Health ABC) Study, protein intake, muscle mass, and physical function have previously been studied. A higher protein intake was associated with smaller 3-y losses in LM and aLM (7), as well as a lower risk of mobility limitation over 6 y (11). To extend this previous work, we investigated comparable associations in a priori sex-stratified data and studied the shape of the associations to potentially detect optimal amounts of protein intake. Next, we stratified our analyses by race within sex in order to further increase homogeneity. Thus, this study aims to examine the shape of sex-specific associations of baseline dietary protein intake with 3- and 6-y changes in muscle mass and gait speed and also with 6-y incidence of mobility limitation in US older men and women. The secondary aim is to further examine whether race-specific optimal amounts of protein intake within sex can be found.
Methods
Study population
The Health ABC Study is a prospective cohort study in the United States that focuses on risk factors for functional decline and disability, including changes in body composition, in initially healthier community-dwelling older persons. At the start in 1997–1998, 3075 black and white men and women aged 70–79 y were recruited from a random sample of white Medicare-eligible residents and all black Medicare-eligible residents in the metropolitan areas of Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they were free of reported difficulty walking one-quarter mile and climbing up 10 steps. After the baseline measurements, follow-up data were collected annually during a clinic visit, followed by a telephone interview after 6 mo. Written informed consent was provided by all participants, and approval for the study was given by the institutional review boards of the University of Tennessee and the University of Pittsburgh.
Participants
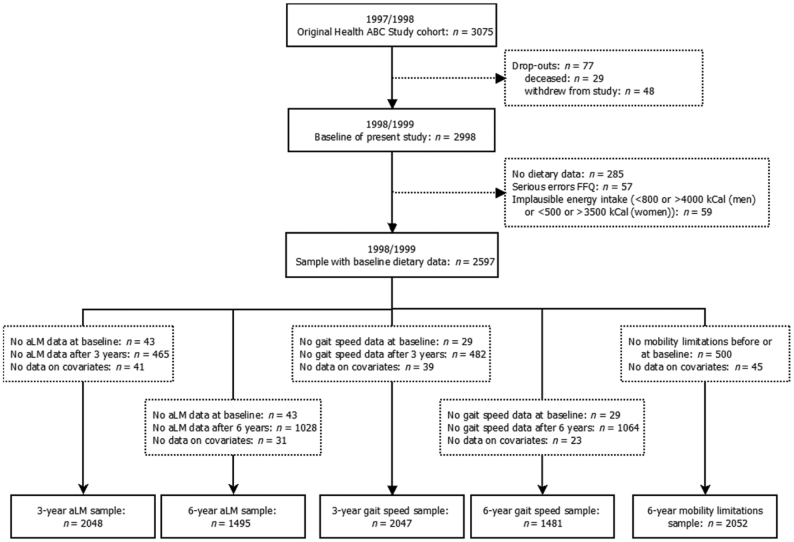
At the first 12-mo follow-up examination, dietary intake was assessed, which served as the baseline of the current study. Participants were excluded if they dropped out before the study baseline (n = 77), had no dietary data (n = 285), had serious errors on the FFQ (n = 57), or had implausible energy intake [<800 kcal or >4000 kcal for men and <500 kcal or >3500 kcal for women (35)] (n = 59), leaving 2597 participants. For each outcome measure and each follow-up period, additional exclusions were made, as shown in Figure 1. For the 3-y analyses of aLM, participants for whom there were missing data on aLM at baseline (n = 43) or after 3 y (n = 465) or on covariates (n = 41) were excluded, leaving 2048 participants for the analytical sample. For the 6-y aLM analyses, participants for whom there were missing data on aLM at baseline (n = 43) or after 6 y (n = 1028) or on covariates (n = 31) were excluded (analytical sample: n = 1495). The analytical sample for the 3-y analyses of gait speed consisted of 2047 participants, after exclusion of those with missing data on gait speed at baseline (n = 29) or after 3 y (n = 482) or on covariates (n = 39). For the 6-y gait speed analyses, participants with missing data on gait speed at baseline (n = 29) or after 6 y (n = 1064) or on covariates (n = 23) were excluded (analytical sample: n = 1481). For the 6-y analyses of mobility limitation, participants with difficulty walking one-quarter mile and/or climbing 10 steps before or at baseline (2 consecutive reports; n = 500) were excluded, as were those with missing data on covariates (n = 45), leaving 2052 participants for the analytical sample.
FIGURE 1.
Flowchart of participants included in the statistical analyses. aLM, appendicular lean mass; Health ABC, Health, Aging, and Body Composition.
Outcome measurements
Body composition was measured annually by DXA scans (Hologic 4500A, version 8.20a). aLM was used as an indicator of muscle mass and calculated as the sum of LM in arms and legs, where LM is total fat-free mass minus bone mineral content. Absolute change in aLM was calculated by subtracting aLM at baseline from the 3- or 6-y measurement.
Usual gait speed was used as an objective measure of physical function. Participants were asked to walk a 20-m course at their usual walking pace. Absolute 3- and 6-y changes in gait speed were calculated by subtracting baseline gait speed from the follow-up measurement.
Mobility limitation was used as a subjective indicator of physical function and defined as 2 consecutive reports of having any difficulty walking one-quarter mile or climbing 10 steps without resting due to a health or a physical problem. Occurrence of mobility limitation was asked annually during the clinic visits as well as semiannually during the telephone interviews. Incident cases of mobility limitation were ascertained over a period of 6 y.
Protein intake
Dietary intake during the past year was assessed at baseline (1998–1999) by a 108-item, interviewer-administered modified version of the Block FFQ (36). The list of foods was specifically developed for the Health ABC Study on the basis of 24-h recall data from the NHANES-III for older (>65 y) non-Hispanic white and black adults. Trained interviewers used wood blocks, food models, standard kitchen measures, and flash cards to assist participants in estimating their portion sizes. Intake of nutrients was determined by Block Dietary Data Systems.
Dietary protein intake was expressed in grams per kilogram adjusted body weight per day (g/kg aBW/d). Adjusted BW is the nearest BW that would put participants with underweight or overweight into a healthy BMI (in kg/m2) range: 18.5–25.0 for those aged ≤70 y and 22.0–27.0 for those aged >70 y (37). We chose to use aBW because underweight people require extra protein for building muscle tissue, whereas in overweight people, much of the excess BW consists of fat tissue requiring less protein.
Other variables
Demographic, lifestyle, and health-related factors were collected by an interviewer-administered questionnaire. Self-reported sex and age were used. Race was based on 3 sources: 1) self-report, 2) record by examiners of the physical measurements, and 3) administrative files of the Health Care Financing Administration (HCFA). If there were any discrepancies between these 3 sources, the final race variable (white/black) was based on the largest agreement between these 3 sources. For example, in case of agreement between examiners and HCFA but not with self-report, the category from HCFA was accepted. The highest attained level of education was categorized into low (less than high school), medium (high school graduation), and high (postsecondary education). Physical activity was based on the reported time spent walking in the past 7 days (minutes per week). Categories of smoking status were never, former, and current smoker. Current use of alcohol (yes/no), derived from the FFQ, was defined as the consumption of any alcoholic beverages in the past year. Total energy intake and the Healthy Eating Index (HEI) were also calculated from the FFQ. The HEI (38) is a diet quality index that reflects compliance with the 1995 Dietary Guidelines for Americans (39) and the Food Guide Pyramid of 1992 (40) and variety in the diet. Body height was measured with a Harpenden stadiometer (Holtain). The number of chronic diseases was derived from questions on a physician's diagnosis of arthritis, cardiac diseases, stroke, peripheral arterial disease, hypertension, pulmonary diseases, diabetes mellitus, and cancer. Use of oral steroids was determined from drug data coded by using the Iowa Drug Information System codes (41). Overnight hospitalizations in the year before the study baseline were dichotomized as none or ≥1 hospitalization. Serum creatinine was used to estimate glomerular filtration rate (eGFR), indicating kidney function, using the Modification of Diet in Renal Disease equation (42). Depressive symptoms were assessed with the 20-item Center for Epidemiological Studies Depression Scale (CES-D) (43), and cognitive function was measured with the Modified Mini-Mental State Examination (3MSE; only used in sensitivity analyses) (44). All covariates were measured at study baseline when diet was assessed (1998–1999), except for education, smoking status, height, number of chronic diseases, eGFR, depressive symptoms, and cognitive function, which were assessed 1 y earlier in 1997–1998. Last, absolute change in fat mass, measured by DXA, was calculated over 3 and 6 y.
Statistical analyses
Because our main interest was sex-specific associations, all analyses were a prioristratified by sex. For the secondary aim, we additionally stratified by race. Descriptive statistics (means and SDs or percentages) were used to summarize participants’ baseline characteristics. Absolute changes in aLM and gait speed over 3 and 6 y were checked for normality.
Prospective associations of protein intake (g/kg aBW/d, continuous) with change in aLM and change in gait speed (both continuous) were analyzed by multivariable linear regression analysis. The associations between protein intake and incidence of mobility limitation over 6 y were examined by estimating HRs using multivariable Cox proportional hazards models. The time to event was calculated as the time between study baseline (1998–1999) and 1 of the following: 1) participant's last examination (2004–2005) for those who survived without evidence of incident mobility limitation, 2) first occurrence of incident mobility limitation, 3) participant's last examination for those lost to follow-up, or 4) participant's date of death for those who died with no occurrence of incident mobility limitations, whichever occurred first. The proportional hazard assumption was tested using Schoenfeld residuals.
To potentially identify optimal cutoff amounts of protein intake, spline functions were added to the linear and Cox regression models to estimate the shape of the associations between protein intake and the outcomes. A spline function is a piecewise function that can describe the association for multiple intervals of a continuous determinant, without the assumption of linear associations. The points at which 2 intervals smoothly join are called “knots”. Spline functions were added to the univariable regression models (crude model) and the models adjusted for only baseline outcome (model 1). Next to a model without a spline (no spline), we applied linear and restricted cubic splines, both with 3, 4, and 5 knots. Linear splines estimate linear functions between the knots, whereas restricted cubic splines estimate cubic functions. The latter are restricted to be linear in the end regions to provide more conservative estimates of the association where data are often sparse (45, 46). The fit of the models was tested with the likelihood-ratio test, which compares the model fit of models with and without a specific spline function. If improvement in fit was not statistically significant (P < 0.05) and visual inspection of the plots confirmed this, the model without spline was chosen and vice versa. We used the following consecutive steps: 1) no spline versus spline; 2) 3, 4, or 5 knots; and 3) optimal position of the inner knot(s). If a model with a spline function fitted best, the protein intake amount associated with the least decline was identified by visual inspection of the plot.
After choosing the best-fitting model per sex, outcome, and time period, potential confounders that appeared to be important based on the literature were added to the crude model, resulting in 4 additional models. Model 1 was adjusted for the baseline value of the outcome (except for incident mobility limitation because persons with mobility limitation at baseline were excluded). Model 2 was additionally adjusted for age, race, study site, educational level, walking activity, smoking, alcohol use, body height, number of chronic diseases, use of oral steroids, overnight hospitalizations, eGFR, and depressive symptoms. Model 3 was additionally adjusted for energy intake and the HEI score (fully adjusted model). To examine the influence of changes in fat mass, model 4 was additionally adjusted for 3- or 6-y change in fat mass.
For our secondary aim, all analyses were additionally stratified by race—that is, performed in the 4 sex–race groups. Similar to the procedures per sex as described previously, regression models with and without spline functions were compared, after which the best-fitting model was chosen and adjustment for confounders was performed.
Sensitivity analyses were conducted by repeating the analyses of the fully adjusted model (model 3) in different samples. First, we excluded persons with a poor cognitive performance (3MSE < 80) 1 y before baseline because they might have problems recalling their dietary intake. Furthermore, we repeated the analyses for aLM and gait speed in an analytical sample of participants with outcome data at baseline and after both 3 and 6 y to fairly compare the 3-y and 6-y results.
A P value <0.05 (2-sided) was considered statistically significant. Descriptive analyses were performed in SPSS version 26 software (SPSS) and the spline regression models in R version 3.6.1 software (R Foundation for Statistical Computing) (47).
Results
Characteristics
At baseline, participants included in any of the analytical samples (n = 2400) had a mean ± SD age of 74.6 ± 2.9 y, 51.5% were women, and 36.2% were black. Baseline characteristics for men and women as well as per sex–race group are shown in Table 1. Protein intake was 71.3 ± 26.6 g/d in men and 60.7 ± 22.3 g/d in women. Protein intake below the current recommendation (0.8 g/kg aBW/d) was observed in 39.7% of men and 37.6% of women. Protein intake was 0.93, 0.94, 0.95, and 0.95 g/kg aBW/d in white men, black men, white women, and black women, respectively, and a low protein intake was also comparable between the 4 groups.
TABLE 1.
Baseline characteristics of older participants of the Health ABC Study, according to sex and sex–race group, 1998–19991
| Men | White men | Black men | Women | White women | Black women | |
|---|---|---|---|---|---|---|
| Participants, n (%) | 1163 (48.5) | 800 (33.3) | 363 (15.1) | 1237 (51.5) | 731 (30.5) | 506 (21.1) |
| Characteristics | ||||||
| Age, y | 74.8 ± 2.9 | 74.9 ± 2.9 | 74.6 ± 2.8 | 74.4 ± 2.8 | 74.6 ± 2.8 | 74.3 ± 2.9 |
| White race, % | 68.8 | 59.1 | ||||
| Memphis study site, % | 47.8 | 48.8 | 45.7 | 48.8 | 52.0 | 44.3 |
| Educational level2 | ||||||
| Less than high school | 22.5 | 13.1 | 43.3 | 20.4 | 9.6 | 36.0 |
| High school graduation | 25.9 | 25.5 | 26.7 | 39.1 | 42.1 | 34.8 |
| Postsecondary education | 51.6 | 61.4 | 30.0 | 40.5 | 48.3 | 29.2 |
| Walking, min/wk | 165 ± 295 | 176 ± 308 | 140 ± 264 | 116 ± 228 | 135 ± 252 | 89 ± 184 |
| Smoking,2 % | ||||||
| Never | 30.6 | 29.8 | 32.5 | 58.2 | 58.8 | 57.3 |
| Former | 60.4 | 65.6 | 48.8 | 34.0 | 34.6 | 33.0 |
| Current | 9.0 | 4.6 | 18.7 | 7.8 | 6.6 | 9.7 |
| Current alcohol user, % | 45.5 | 52.0 | 31.1 | 30.5 | 38.7 | 18.6 |
| Height,2 cm | 173.5 ± 64.5 | 173.6 ± 62.3 | 173.3 ± 69.2 | 159.6 ± 60.8 | 159.5 ± 60.6 | 159.7 ± 61.2 |
| Weight, kg | 81.1 ± 12.8 | 80.9 ± 12.2 | 81.5 ± 13.9 | 69.8 ± 14.5 | 65.7 ± 11.8 | 75.7 ± 15.9 |
| BMI, kg/m2 | 26.9 ± 3.8 | 26.8 ± 3.6 | 27.1 ± 4.2 | 27.4 ± 5.4 | 25.8 ± 4.4 | 29.7 ± 5.9 |
| No. of chronic diseases2 | ||||||
| 0 diseases | 14.8 | 15.0 | 14.3 | 11.2 | 13.8 | 7.5 |
| 1 disease | 27.4 | 27.8 | 26.7 | 28.7 | 31.2 | 25.1 |
| ≥2 diseases | 57.8 | 57.3 | 59.0 | 60.1 | 55.0 | 67.4 |
| Oral steroid use, % | 2.1 | 2.8 | 0.8 | 3.2 | 4.0 | 2.0 |
| Hospitalization in past year, % | 15.2 | 16.1 | 13.2 | 11.7 | 11.1 | 12.6 |
| eGFR2 | 73.8 ± 16.3 | 72.2 ± 14.5 | 77.4 ± 19.2 | 71.7 ± 14.9 | 68.6 ± 12.7 | 76.3 ± 16.6 |
| Depressive symptoms,2 CES-D score | 4.0 ± 4.8 | 3.9 ± 4.7 | 4.3 ± 4.8 | 5.0 ± 5.5 | 5.2 ± 5.8 | 4.7 ± 5.0 |
| Cognitive function,2 3MSE score | 89.5 ± 8.7 | 92.5 ± 5.8 | 85.8 ± 9.5 | 90.6 ± 8.1 | 94.0 ± 5.1 | 88.2 ± 8.4 |
| Dietary intake | ||||||
| Total energy intake, kcal | 2003 ± 658 | 1974 ± 633 | 2067 ± 707 | 1688 ± 565 | 1629 ± 513 | 1773 ± 624 |
| HEI score | 69.0 ± 11.8 | 71.0 ± 11.3 | 64.5 ± 11.8 | 71.1 ± 11.8 | 72.6 ± 11.7 | 68.9 ± 11.6 |
| Protein intake, g/d | 71.3 ± 26.6 | 71.3 ± 25.6 | 71.2 ± 28.7 | 60.7 ± 22.3 | 59.5 ± 20.7 | 62.6 ± 24.4 |
| Protein intake, g/kg aBW/d | 0.94 ± 0.36 | 0.93 ± 0.35 | 0.94 ± 0.40 | 0.95 ± 0.36 | 0.95 ± 0.34 | 0.95 ± 0.38 |
| <0.8 g/kg aBW/d | 39.7 | 39.1 | 41.0 | 37.6 | 36.5 | 39.1 |
| <1.0 g/kg aBW/d | 63 | 63.6 | 61.7 | 60.7 | 61.6 | 59.5 |
Values are means ± SDs or %, n = 2400: persons included the analytical sample of ≥1 outcome measure. aBW, adjusted body weight; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; Health ABC, Health, Aging, and Body Composition; HEI, Healthy Eating Index; 3MSE, Modified Mini-Mental State Examination.
Assessed 12 mo before the study baseline (i.e., in 1997–1998).
Compared with the included participants (n = 2400), the excluded participants (n = 598) were more likely to be older, black, less educated, and a current smoker. They also walked less; had more depressive symptoms, chronic diseases, and overnight hospitalizations; used alcohol less often; had a poorer cognitive performance; and had a higher energy and protein intake. Baseline aLM was higher and baseline gait speed lower in those excluded, but changes in both outcome measures were similar (data not shown).
Outcome measurements
Measures of body composition, gait speed, and mobility limitation per sex and sex–race group are presented in Table 2. At baseline, mean aLM was 23.7 ± 3.5 kg and 16.5 ± 3.1 kg in men and women, respectively. The mean loss of aLM over 3 y (611 ± 1164 g in men and 349 ± 943 g in women) was doubled to, respectively, 1169 ± 1480 g (−4.9%) and 705 ± 1117 g (−4.3%) over 6 y. Men and women had comparable mean gait speed values at baseline (1.21 ± 0.20 m/s and 1.12 ± 0.20 m/s, respectively) and similar declines over time (6 y: −0.15 ± 0.17 m/s and −0.13 ± 0.17 m/s, respectively). Over 6 y, 405/1052 (38.5%) men and 430/1000 (43.0%) women developed mobility limitation.
TABLE 2.
Relevant body composition measures, gait speed, and mobility limitation of older participants of the Health ABC Study, according to sex and sex–race group, 1998–19991
| Men | White men | Black men | Women | White women | Black women | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| valid n | 1163 (48.5%) | valid n | 800 (33.3%) | valid n | 363 (15.1%) | valid n | 1237 (51.5%) | valid n | 731 (30.5%) | valid n | 506 (21.1%) | |
| Baseline aLM, kg | 962 | 23.7 ± 3.5 | 672 | 23.1 ± 3.2 | 290 | 25.1 ± 3.7 | 1086 | 16.5 ± 3.1 | 655 | 15.2 ± 2.4 | 431 | 18.4 ± 3.1 |
| 3-y change in aLM,2 g | 962 | −611 ± 1164 | 672 | −552 ± 1072 | 290 | −750 ± 1345 | 1086 | −349 ± 943 | 655 | −256 ± 821 | 431 | −490 ± 1090 |
| 6-y change in aLM,2 g | 688 | −1169 ± 1480 | 498 | −1094 ± 1446 | 190 | −1363 ± 1550 | 807 | −705 ± 1117 | 509 | −588 ± 994 | 298 | −904 ± 1278 |
| Baseline fat mass, kg | 962 | 24.4 ± 6.9 | 672 | 24.7 ± 6.8 | 290 | 24.7 ± 7.3 | 1086 | 28.9 ± 9.2 | 655 | 26.9 ± 7.8 | 431 | 31.8 ± 10.2 |
| 3-y change in fat mass, g | 962 | 349 ± 2789 | 672 | 344 ± 2635 | 290 | 361 ± 3121 | 1086 | −67 ± 3309 | 655 | 57 ± 2883 | 431 | −256 ± 3863 |
| 6-y change in fat mass, g | 688 | −281 ± 3832 | 498 | −355 ± 3772 | 190 | −88 ± 3987 | 807 | −1363 ± 3976 | 509 | −1118 ± 3699 | 298 | −1782 ± 4385 |
| Baseline gait speed, m/s | 969 | 1.21 ± 0.20 | 678 | 1.3 ± 0.19 | 291 | 1.1 ± 0.20 | 1078 | 1.12 ± 0.20 | 647 | 1.2 ± 0.19 | 431 | 1.0 ± 0.19 |
| 3-y change in gait speed,2 m/s | 969 | −0.052 ± 0.16 | 678 | −0.060 ± 0.15 | 291 | −0.031 ± 0.18 | 1078 | −0.053 ± 0.16 | 647 | −0.059 ± 0.16 | 431 | −0.043 ± 0.16 |
| 6-y change in gait speed,2 m/s | 693 | −0.15 ± 0.17 | 503 | −0.16 ± 0.16 | 190 | −0.13 ± 0.19 | 788 | −0.13 ± 0.17 | 495 | −0.13 ± 0.17 | 293 | −0.13 ± 0.17 |
| Event rate mobility limitation2,3 | 1052 | 83.3 | 731 | 73.8 | 321 | 107.4 | 1000 | 96.2 | 630 | 85.3 | 370 | 116.8 |
Values are means ± SDs or otherwise specified, n = 2400: persons included the analytical sample of ≥1 outcome. aLM, appendicular lean mass; Health ABC, Health, Aging, and Body Composition.
Used as outcome measure.
Event rate = number of events/1000 person-years.
Protein intake and change in appendicular lean mass
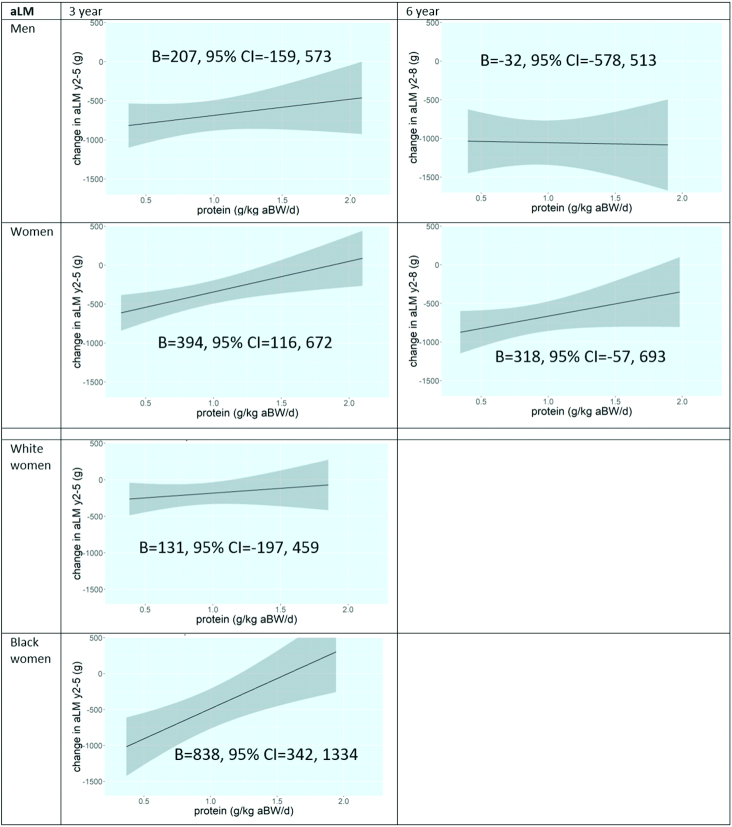
For the association between protein intake and change in aLM, linear or restricted cubic spline functions did not improve the regression models in men or women based on likelihood-ratio tests and visual inspection, so linear models are presented. Figure 2 shows plots of the fully adjusted models (model 3) by sex and time period. We did not observe significant associations in men over 3 and 6 y. For women, a higher protein intake at baseline was associated with less loss of aLM over 3 y (B, model 3: 394 g; 95% CI: 116, 672): a 0.1 g/kg aBW/d higher protein intake was associated with an increase in aLM of 39.4 g over 3 y (i.e., less aLM loss). The association over 6 y was similar but not significant (B, model 3: 318 g; 95% CI: −57.2, 693). Additional adjustment for change in fat mass attenuated the 3-y association in women somewhat (B, model 4: 277 g; 95% CI: 25.8, 528). Results of the different models are presented in Supplemental Table 1. Additional stratification by race did not reveal significant associations in white or black men. However, the 3-y association in women was significant in black women (B, model 3: 838 g; 95% CI: 342, 1334) but not in white women (B, model 3: 131 g; 95% CI: −197, 459) (Figure 2). Over 6 y, the associations were similar between white and black women (Supplemental Table 2).
FIGURE 2.
Plots of associations of protein intake with 3- and 6-y change in aLM in older participants of the Health, Aging, and Body Composition Study, according to sex and sex–race group (if applicable). Model 3 is shown: adjusted for baseline aLM, age, (race,) study site, educational level, walking, smoking, alcohol use, height, number of chronic diseases, oral steroid use, hospitalizations, eGFR, CES-D score, energy intake, and HEI score. aBW, adjusted body weight; aLM, appendicular lean mass; B, unstandardized regression coefficient; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; HEI, Healthy Eating Index; y2, study baseline; y5, 3-y follow-up examination; y8, 6-y follow-up examination.
Protein intake and change in gait speed
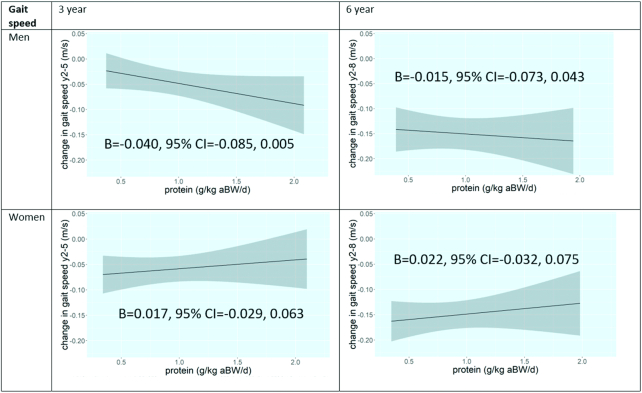
Linear models were also found to be appropriate for change in gait speed. Although the regression coefficients were positive as well as negative in all models, no significant associations were found in men or women (Figure 3, Supplemental Table 1). The associations remained not significant when further stratified by race (Supplemental Table 2).
FIGURE 3.
Plots of associations of protein intake with 3- and 6-y change in gait speed in older participants of the Health, Aging, and Body Composition Study, according to sex. Model 3 is shown: adjusted for baseline gait speed, age, race, study site, educational level, walking, smoking, alcohol use, height, number of chronic diseases, oral steroid use, hospitalizations, eGFR, CES-D score, energy intake, and HEI score. aBW, adjusted body weight; B, unstandardized regression coefficient; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; HEI, Healthy Eating Index; y2, study baseline; y5, 3-y follow-up examination; y8, 6-y follow-up examination.
Protein intake and incidence of mobility limitation
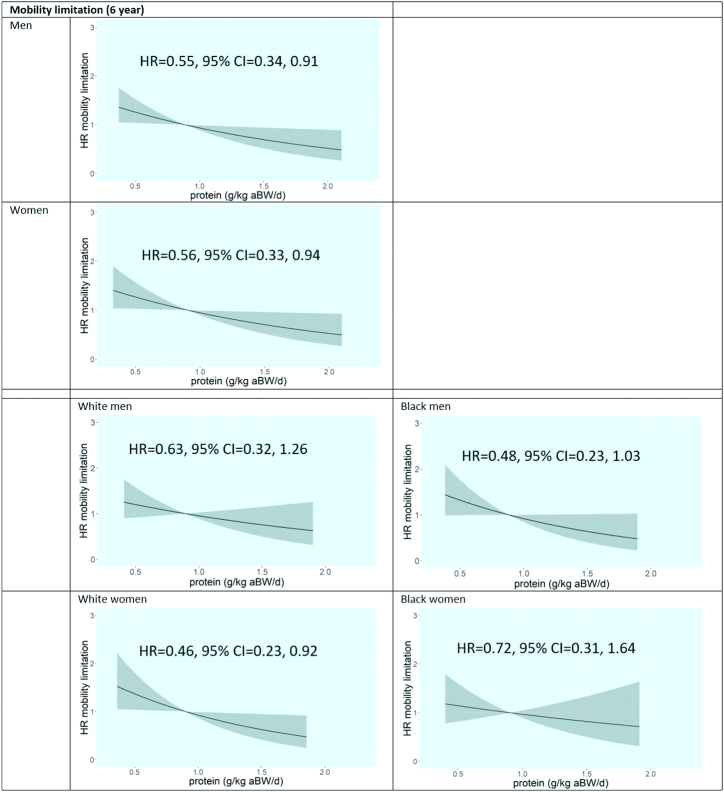
For incident mobility limitation, again no strong indication for nonlinear associations was found. Also, in none of the analyses was the proportional hazard assumption violated. A higher protein intake was associated with a lower 6-y incidence of mobility limitations in men (HR, model 3: 0.55; 95% CI: 0.34, 0.91) and women (HR, model 3: 0.56; 95% CI: 0.33, 0.94) (Figure 4, Supplemental Table 1). Additional stratification by race showed that the magnitude of the association was comparable between black and white men. The association was no longer significant in black women (HR, model 3: 0.72; 95% CI: 0.31, 1.64) but became stronger and remained significant in white women (HR, model 3: 0.46; 95% CI: 0.23, 0.92) (Figure 4, Supplemental Table 2).
FIGURE 4.
Plots of associations of protein intake with 6-y incidence of mobility limitation in older participants of the Health, Aging, and Body Composition Study, according to sex and sex–race group (if applicable). Model 3 is shown: adjusted for age, (race,) study site, educational level, walking, smoking, alcohol use, height, number of chronic diseases, oral steroid use, hospitalizations, eGFR, CES-D score, energy intake, and HEI score. aBW, adjusted body weight; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; HEI, Healthy Eating Index.
Sensitivity analyses
Findings of the associations of protein intake with any outcome measure by sex were similar when participants with a low cognitive status (3MSE < 80) were excluded (n = 54–92 in men; n = 31–57 in women). The findings were also similar in the 4 sex–race groups. When the analyses were repeated in smaller samples of participants with complete aLM data after both 3 and 6 y (n = 676 men; n = 777 women), the 3-y association in women was attenuated and lost significance (B, model 3: 174 g; 95% CI: −138, 486), whereas the 6-y association did not markedly change (B, model 3: 286 g; 95% CI: −96, 668). In black women, the 3-y association was attenuated but remained significant (B, model 3: 637 g; 95% CI: 31.9, 1242). In men, the aLM findings did not change. The 3- and 6-y gait speed findings by sex remained similar in smaller samples of participants with complete gait speed data after 3 and 6 y (n = 675 men; n = 758 women); findings in the 4 sex–race groups remained similar as well.
Discussion
This prospective study in US older black and white men and women showed some associations between protein intake and physical outcomes, dependent on sex and race. A higher baseline protein intake was associated with less loss of muscle mass over 3 y in women, specifically black women, whereas no associations were observed over 6 y in women or in men. Protein intake was not associated with gait speed decline over 3 and 6 y in either men or women. However, higher protein intake was associated with a lower risk of mobility limitation over 6 y in both sexes and specifically white women. For all outcome measures, we did not find indications of nonlinear associations, so we could not detect an optimal amount of protein intake for any of the outcomes.
We a priori stratified the analyses and found sex-specific associations only for the outcome measure aLM. We observed an association for change in aLM solely in women. A higher protein intake may thus reduce muscle mass loss in women, potentially indicating higher protein needs compared with those of men. This might be caused by older women's higher MPS rates compared with those of older men found in some (24, 25) but not all (48–50) studies. A study in obese, older adults (aged 65–80 y) showed that the fractional synthesis rate of muscle protein was ∼30% higher in women than in men (24). Moreover, a study in healthy persons observed that muscle protein fractional synthesis rate as well as whole-body protein synthesis were higher in women than in men, at both young and old age (25). The higher MPS coincides with an accelerated loss of muscle in older women, which may imply that they also have an increased rate of protein breakdown. An upregulation of stimulatory and inhibitory muscle growth-regulatory genes was reported in postmenopausal women (51). Our observed association between protein intake and aLM in older women adds to these studies that found sex differences in MPS rates and suggests a higher dependency of dietary protein in older women compared with men. However, further study is needed to unravel these sex differences.
The race-specific analyses additionally showed that the aLM association remained only significant in black women. A potential explanation is that mean aLM loss in black women was much larger than in white women (see Table 2); the larger range of aLM change may have increased our ability to pick up an association. A higher MPS rate in black women might also explain our race-specific finding, although to our knowledge, no studies are available on race differences in MPS. Further research on the race difference of this association is needed. Another race-specific association was found for mobility limitation; only in white women was a higher protein intake significantly associated with a lower risk of mobility limitation, which is in contrast to our aLM association specifically in black women.
The effect sizes of our associations were moderate. A daily 0.2 g/kg aBW higher protein intake, for example from 0.8 to 1.0/kg aBW/d, was associated with 78.8 g (2 × 39.4) less loss of aLM in women during a 3-y period, which is 23% of the mean 3-y aLM loss (78.8/349 × 100). For mobility limitation, such increment was associated with an 11% lower risk [exp(−0.59/10 × 2) = 0.89] of mobility limitation in both sexes. So our findings need to be interpreted in this light. Because we also did not adjust for multiple testing, our findings need to be interpreted with caution.
We hypothesized similar findings for gait speed and mobility limitation because of the relation between these 2 measures of physical function (52). However, although we found associations between protein intake and mobility limitation, we did not find any association for gait speed, our objective outcome measure. Because the variation in both protein intake and gait speed change was substantial, our null associations probably cannot be explained by a lack of variation. One of the few previous studies on protein intake and gait speed showed a cross-sectional association but—similar to our study—no prospective association in older women (9). Because the Health ABC cohort was well-functioning at recruitment, results may not be generalizable to populations with lower physical function. More studies on the protein intake and gait speed are warranted.
The 2 previous articles on the association of protein intake with aLM and mobility limitation in the Health ABC Study showed findings comparable with those of the current study. Houston et al. (7) found a significant association between energy-adjusted protein intake (i.e., residuals, continuous, and in quintiles) and 3-y aLM loss. Interactions with sex and race were tested but not significant (P > 0.15). To our knowledge, this is the first study that a prioristratified the analyses by sex (and race), and we observed a significant association with aLM only in women, specifically black women. We examined aLM also over 6 y, showing an attenuated association but of similar effect size in women. When we restricted our sample to those with complete longitudinal aLM data, the 3-y association disappeared, possibly indicating that the association is driven by more unhealthy persons (who were lost to follow-up). Houston et al.’s (11) article on mobility limitation, with almost the same confounders as in this study, also did not find significant interactions with sex or race (P > 0.20) but showed associations stratified by sex and race (whites compared with blacks). In men, women, whites, and blacks, associations between protein intake and incident mobility limitation were observed, and after adjustment for baseline lean mass, only the association in women and whites remained significant. Comparison with our study is difficult because Houston et al. used categories of protein intake (<0.7, 0.7–1.0, and ≥1.0 g/kg BW/d), whereas we used protein intake continuously and used adjusted body weight (g/kg aBW/d) in order to correct the protein intake of underweight and overweight participants. We also stratified by race within sex, which showed that the associations remained only significant in white women. Last, we studied the association shape by using spline functions, but this did not reveal optimal amounts of protein intake.
It is noteworthy that no effect modification by sex or race seems to exist based on the nonsignificant interaction terms from the previous articles (7, 11), whereas the current study clearly did show differential associations between men and women (aLM) and between blacks and whites (aLM and mobility limitation) in stratified analyses. We performed post hoc tests of interaction terms, showing that—similar to previous articles—all had a P value >0.15, except for the protein–race interaction in the 3-y aLM analyses in women: P = 0.093 (crude model) and P = 0.063 (model 3). Because sex is increasingly recognized as an important factor in health-related research, it can be advised to report data separately for men and women (53), especially if sex differences have consistently been shown for measures of interest, such as body composition and physical function (20–22). This can also be considered for other individual (demographic) factors, including race.
Only a few observational studies have investigated protein intake and physical outcomes separately for men and women. A study in older adults did not find prospective associations of energy-adjusted protein intake with change in LM or aLM in both sexes (54). This contradicts our association between protein intake and aLM change observed only in women. Moreover, 2 longitudinal studies showed that a higher protein intake was associated with hand grip strength and physical performance (33) as well as with maintenance of subjective physical function (34) in women but not in men. However, we found associations for a subjective physical function measure in both men and women. These discrepancies in the sparse literature on sex-specific associations of protein intake, muscle mass, and physical function might be explained by differences in study design, length of follow-up, study population (including race), used measures, and adjustment for confounders.
Strengths of this study include the long follow-up of 3 and 6 y and the large sample of community-dwelling older adults allowing stratification by sex and in 4 race–sex groups. In addition, we used adjusted or “healthy” body weight for the determinant protein intake, studied objective (aLM and gait speed) as well as subjective (mobility limitation) outcome measures, and were able to adjust for various potential confounders. Last, the use of spline functions to examine the shape of associations using all available data is a strength; nonetheless, strong indications for nonlinear associations were not observed for any outcome, so spline functions were not included in the final models. Some limitations also have to be considered. First, protein intake was assessed by an FFQ, a method that provides inaccurate estimates of absolute dietary intake (55). Potential memory problems of older subjects may also have led to misreporting (56). Indeed, in the Health ABC Study, a lower cognitive function was associated with more FFQ errors, which differed by race (57). However, underreporting did not differ between sex and race groups (58). Second, the assessment of dietary intake only at baseline may limit the validity of our results because changes in diet over time were not captured. Finally, the Health ABC cohort was well-functioning and free of mobility limitation at recruitment; thus, the generalizability of our findings to the general older population is hampered.
In conclusion, our prospective study in a US older population showed that a higher protein intake at baseline was associated with less loss of muscle mass over 3 y in women, particularly black women, but not over 6 y. In men, no associations with muscle mass were observed. In both sexes, a higher protein intake was not associated with gait speed decline over 3 and 6 y but was associated with a lower 6-y risk of mobility limitation, particularly in white women. More prospective studies with a sex- and race-specific focus are needed to elucidate whether associations between protein intake, muscle mass, and physical function differ by sex and race. RCTs that examine the effects of increasing protein intake on age-related declines should preferably enroll sufficient numbers of men and women, whites and blacks, to be able to perform analyses by sex and race.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LEME, LAS, and HAHW: designed the research; LEME: performed the statistical analyses and wrote the initial manuscript; MWH: provided statistical advice; LEME, LAS, LMH, MV, and HAHW: contributed to the interpretation of the data and critically revised the manuscript; MWH, EN, DKH, SBK, EMS, ABN, and SF: contributed to the interpretation of the data and helped edit the manuscript; LEME: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Funding for this article was provided by the Netherlands Organization for Health Research and Development (ZonMw), programme Gender and Health, project 849500003. Funding for this article was also provided by the European Horizon 2020 PROMISS Project “PRevention Of Malnutrition In Senior Subjects in the EU,” grant agreement 678732. The content only reflects the authors’ view and the European Commission is not responsible for any use that may be made of the information it contains.This research was supported by National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval and signed agreements.
Abbreviations used: aBW, adjusted body weight; aLM, appendicular lean mass; BW, body weight; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; HCFA, Health Care Financing Administration; Health ABC, Health, Aging, and Body Composition; HEI, Healthy Eating Index; MPS, muscle protein synthesis; RCT, randomized controlled trial; 3MSE, Modified Mini-Mental State Examination.
Contributor Information
Liset E M Elstgeest, Department of Health Sciences, Faculty of Science, Vrije University Amsterdam, Amsterdam Public Health research institute, Amsterdam, the Netherlands.
Laura A Schaap, Department of Health Sciences, Faculty of Science, Vrije University Amsterdam, Amsterdam Public Health research institute, Amsterdam, the Netherlands.
Martijn W Heymans, Department of Epidemiology and Biostatistics, Amsterdam Public Health research institute, Amsterdam UMC – Location VU University Medical Center, Amsterdam, the Netherlands.
Linda M Hengeveld, Department of Health Sciences, Faculty of Science, Vrije University Amsterdam, Amsterdam Public Health research institute, Amsterdam, the Netherlands.
Elke Naumann, Department of Nutrition and Health, HAN University of Applied Sciences, Nijmegen, the Netherlands; European Federation of the Associations of Dietitians (EFAD), The Hague, the Netherlands.
Denise K Houston, Sticht Center for Healthy Aging and Alzheimer's Prevention and Department of Internal Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Stephen B Kritchevsky, Sticht Center for Healthy Aging and Alzheimer's Prevention and Department of Internal Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Eleanor M Simonsick, Intramural Research Program, National Institute on Aging, Baltimore, MD, USA.
Anne B Newman, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Samaneh Farsijani, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Marjolein Visser, Department of Health Sciences, Faculty of Science, Vrije University Amsterdam, Amsterdam Public Health research institute, Amsterdam, the Netherlands.
Hanneke A H Wijnhoven, Department of Health Sciences, Faculty of Science, Vrije University Amsterdam, Amsterdam Public Health research institute, Amsterdam, the Netherlands.
References
- 1. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18(3):248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strasser B, Volaklis K, Fuchs D, Burtscher M. Role of dietary protein and muscular fitness on longevity and aging. Aging Dis. 2018;9(1):119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, D'Angelo E, Sisto A, Marzetti E. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients. 2016;8(5):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gray-Donald K, St-Arnaud-McKenzie D, Gaudreau P, Morais JA, Shatenstein B, Payette H. Protein intake protects against weight loss in healthy community-dwelling older adults. J Nutr. 2014;144(3):321–6. [DOI] [PubMed] [Google Scholar]
- 5. Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc. 2010;58(11):2129–34. [DOI] [PubMed] [Google Scholar]
- 6. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Association of protein intake with the change of lean mass among elderly women: the Osteoporosis Risk Factor and Prevention–Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci. 2015;4:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 8. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol Ser A. 2015;71(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr. 2016;115(7):1281–91. [DOI] [PubMed] [Google Scholar]
- 10. Mendonça N, Granic A, Hill TR, Siervo M, Mathers JC, Kingston A, Jagger C. Protein intake and disability trajectories in very old adults: the Newcastle 85+ Study. J Am Geriatr Soc. 2019;67(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, Newman AB, Kritchevsky SB. Protein intake and mobility limitation in community-dwelling older adults: the Health ABC Study. J Am Geriatr Soc. 2017;65(8):1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ten Haaf DSM, Nuijten MAH, Maessen MFH, Horstman AMH, Eijsvogels TMH, Hopman MTE. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108(5):1043–59. [DOI] [PubMed] [Google Scholar]
- 13. Ten Haaf DSM, Eijsvogels TMH, Bongers C, Horstman AMH, Timmers S, de Groot L, Hopman MTE. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park Y, Choi JE, Hwang HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2018;108(5):1026–33. [DOI] [PubMed] [Google Scholar]
- 15. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. [DOI] [PubMed] [Google Scholar]
- 16. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance?. J Gerontol A Biol Sci Med Sci. 2013;68(6):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Traylor DA, Gorissen SHM, Phillips SM. Perspective: protein requirements and optimal intakes in aging: are we ready to recommend more than the Recommended Daily Allowance?. Adv Nutr. 2018;9(3):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D et al.. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 19. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS et al.. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson LJ, Liu H, Garcia JM. Sex differences in muscle wasting. Adv Exp Med Biol. 2017;1043:153–97. [DOI] [PubMed] [Google Scholar]
- 21. Markofski MM, Volpi E. Protein metabolism in women and men: similarities and disparities. Curr Opin Clin Nutr Metab Care. 2011;14(1):93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bredella MA. Sex differences in body composition. Adv Exp Med Biol. 2017;1043:9–27. [DOI] [PubMed] [Google Scholar]
- 23. Buffa R, Floris GU, Putzu PF, Marini E. Body composition variations in ageing. Coll Antropol. 2011;35(1):259–65. [PubMed] [Google Scholar]
- 24. Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One. 2008;3(3):e1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23(2):631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stanforth PR, Jackson AS, Green JS, Gagnon J, Rankinen T, Desprès JP, Bouchard C, Leon AS, Rao DC, Skinner JS et al.. Generalized abdominal visceral fat prediction models for black and white adults aged 17–65 y: the HERITAGE Family Study. Int J Obes. 2004;28(7):925–32. [DOI] [PubMed] [Google Scholar]
- 27. Stults-Kolehmainen MA, Stanforth PR, Bartholomew JB, Lu T, Abolt CJ, Sinha R. DXA estimates of fat in abdominal, trunk and hip regions varies by ethnicity in men. Nutr Diabetes. 2013;3(3):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69(3):381–7. [DOI] [PubMed] [Google Scholar]
- 29. Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71(6):1392–402. [DOI] [PubMed] [Google Scholar]
- 30. Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M et al.. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring). 2009;17(5):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, Blunt BA, Harris TB. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94(6):2368–74. [DOI] [PubMed] [Google Scholar]
- 32. Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr. 2015;145(7):1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Adamson A, Siervo M, Mathers JC, Jagger C. Low protein intake, muscle strength and physical performance in the very old: The Newcastle 85+ Study. Clin Nutr. 2018;37(6 Pt A):2260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hruby A, Sahni S, Bolster D, Jacques PF. Protein intake and functional integrity in aging: the Framingham Heart Study Offspring. J Gerontol A Biol Sci Med Sci. 2020; ;75(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett WC. Issues in analysis and presentation of dietary data. In: Willett WC.ed. Nutritional epidemiology. New York: Oxford University Press; 1998. p. 321–46. [Google Scholar]
- 36. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 37. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113(6):809–15. [DOI] [PubMed] [Google Scholar]
- 38. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- 39. US Department of Agriculture , US Department of Health and Human Services. Nutrition and your health: dietary guidelines for Americans. 4th ed Washington (DC): National Technical Information Service; 1995. [Google Scholar]
- 40. USDA Center for Nutrition Policy and Promotion The Food Guide Pyramid. [Internet].1992. Available from: https://www.fns.usda.gov/FGP.
- 41. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–11. [DOI] [PubMed] [Google Scholar]
- 42. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 43. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 44. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–18. [PubMed] [Google Scholar]
- 45. Greenland S. Dose–response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–65. [DOI] [PubMed] [Google Scholar]
- 46. Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed Heidelberg (Germany):Springer; 2015. [Google Scholar]
- 47. R Core Team R: a language and environment for statistical computing[Internet]. Vienna (Austria): R Foundation for Statistical Computing; 2019. Available from: http://www.R-project.org. [Google Scholar]
- 48. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84(3):1007–10. [DOI] [PubMed] [Google Scholar]
- 49. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292(1):E77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91(3):1041–7. [DOI] [PubMed] [Google Scholar]
- 51. Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, Moseley AC, Mittendorfer B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab. 2014;99(1):256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Ser A Biol Sci Med Sci. 2000;55(4):M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research?. JAMA. 2016;316(18):1863–4. [DOI] [PubMed] [Google Scholar]
- 54. Farsijani S, Morais JA, Payette H, Gaudreau P, Shatenstein B, Gray-Donald K, Chevalier S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr. 2016;104(3):694–703. [DOI] [PubMed] [Google Scholar]
- 55. Molag ML, de Vries JHM, Ocké MC, Dagnelie PC, van den Brandt PA, Jansen MCJF, van Staveren WA, van't Veer P. Design characteristics of food frequency questionnaires in relation to their validity. Am J Epidemiol. 2007;166(12):1468–78. [DOI] [PubMed] [Google Scholar]
- 56. McNeill G, Winter J, Jia X. Diet and cognitive function in later life: a challenge for nutrition epidemiology. Eur J Clin Nutr. 2009;63:S33–37. [DOI] [PubMed] [Google Scholar]
- 57. Pope SK, Kritchevsky SB, Morris MC, Block G, Tylavsky FA, Lee JS, Stewart S, Harris T, Rubin SM, Simonsick EM. Cognitive ability is associated with suspected reporting errors on food frequency questionnaires. J Nutr Health Aging. 2007;11(1):55–8. [PubMed] [Google Scholar]
- 58. Shahar DR, Yu B, Houston DK, Kritchevsky SB, Newman AB, Sellmeyer DE, Tylavsky FA, Lee JS, Harris TB. Misreporting of energy intake in the elderly using doubly labeled water to measure total energy expenditure and weight change. J Am Coll Nutr. 2010;29(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.