ABSTRACT
Background
We recently presented associations between serum-based biomarkers of carotenoid and tocopherol intake and chronic disease risk in a Women's Health Initiative (WHI) Measurement Precision subcohort (n = 5488). Questions remain as to whether self-reported dietary data can usefully augment such biomarkers or can be calibrated using biomarkers for reliable disease association estimation in larger WHI cohorts.
Objectives
The aims were to examine the potential of FFQ data to explain intake variation in a WHI Feeding Study and to compare association parameter estimates and their precision from studies based on biomarker-calibrated FFQ intake in larger WHI cohorts, with those previously presented.
Methods
Serum-based intake measures were augmented by using FFQ data in a WHI Feeding Study (n = 153). Corresponding calibration equations were generated, both in a companion Nutritional Biomarker Study (n = 436) and in the previously mentioned subcohort (n = 5488), by regressing these intake measures on dietary data and participant characteristics, for α- and β-carotene, lutein plus zeaxanthin, and α-tocopherol. The supplemental value of FFQ data was considered by examining the fraction of feeding study intake variation explained by these regression models. Calibrated intake and disease association analyses were evaluated by comparisons with previously reported subcohort results.
Results
The inclusion of FFQ data led to some increases in feeding study intake variation explained (total R2 of ∼50%). Calibrated intake estimates explained 25–75% of serum-based intake variation, whether developed using either of the 2 cohort subsamples. Related disease associations for micronutrients were precisely estimated in larger WHI cohorts (n = 76,691) but were often closer to the null compared with previously reported associations.
Conclusions
FFQ data may usefully augment blood concentrations in estimating the intake of carotenoids and tocopherols. Calibrated intake estimates using FFQ, dietary supplement, and participant characteristics only may require further justification to ensure reliable estimation of related disease associations.
Keywords: biomarker, cancer, cardiovascular disease, carotenoid, diabetes, diet, measurement error, tocopherol
Introduction
In a recent publication we presented biomarker equations for the intake of α-carotene, β-carotene, lutein plus zeaxanthin (L+Z), and α-tocopherol (1) based on blood concentration nutrient measures and participant characteristics, using a 153-person feeding study embedded within Women's Health Initiative (WHI) cohorts. Proposed biomarkers were required to explain (R2) ≥36% of feeding study estimated intake variation (correlation of ≥0.60). This criterion was motivated by a benchmark doubly-labeled water (DLW) energy intake biomarker (2) and a urinary nitrogen protein intake biomarker (3), which had R2 values of ∼50% and 40%, respectively, in this feeding study context. The feeding study aimed to approximate a participant's usual diet over a 2-wk feeding period, so that FFQ dietary data obtained at feeding study baseline can be considered toward explaining more of the feeding study intake variation for these nutritional variables, and thereby to obtain possibly strengthened intake assessments.
In a subsequent article (4), we augmented the biomarker equations in the previous article (1) to include potential confounding factors, separately for cardiovascular diseases (CVDs), cancers, and diabetes, and associated the resulting biomarker intake estimates with chronic disease risk in a WHI Measurement Precision subcohort of 5488 women, where baseline blood concentrations for the 4 micronutrients were measured routinely. There were nominally significant reductions in certain CVD, cancer, and diabetes outcomes at higher estimated carotenoid intake, with estimated HRsfor a doubling of intake in the 0.7–1.0 range, and certain CVD risk elevations at higher α-tocopherol intake. The possibility of strengthening these analyses by including FFQ dietary data, along with serum concentrations and covariates, in intake estimation is considered here.
Two possibilities were mentioned in Prentice et al. (4) for examining these same associations more precisely in the much larger WHI cohorts. The first involved using baseline blood specimens, stored for all WHI participants, for blood nutrient concentration determinations in a case-control mode. A second, less expensive, approach combines available blood concentrations with FFQ (and dietary supplement) data in WHI cohort subsets to produce calibration equations for intake estimation in larger WHI cohorts. These equations can be used for intake estimation in prospective disease association analyses. Resulting HR estimates from this second approach are presented here and compared, both in respect to HR agreement and precision, with HR estimates from the Measurement Precision subcohort.
Methods
Study cohorts
During 1993–1998, 48,835 participants were randomly assigned in the WHI Dietary Modification (DM) trial, with 29,294 assigned to the usual-diet comparison group (DM-C) and 93,676 participants enrolled in the prospective WHI Observational Study (OS) (5). All participants were postmenopausal and in the age range of 50–79 y at study entry. The WHI FFQ (6) targeted dietary (but not supplement) intake over the preceding 3-mo period and was administered at baseline and year 1 in the DM trial, and approximately every 3 y thereafter during the trial intervention period, and was administered at baseline and at year 3 in the OS. Here, self-reported intake estimates used FFQs collected at 1 y following randomization in the DM-C, rather than at baseline, to avoid assessment biases related to the use of the FFQ for trial eligibility screening. Baseline FFQs were used for self-reported intake estimation in the OS. Nutrient content estimates in WHI cohorts were derived using the University of Minnesota's Nutrition Data System for Research (NDS-R® version 2005). All women provided core questionnaires at enrollment, including medical history, reproductive history, family history, personal habits, medications, and dietary supplements, and provided a fasting blood sample (5). β-Carotene and α-tocopherol were used as single supplements by ∼3% and 30% of WHI participants, respectively, at baseline (4), but the use of α-carotene and L+Z supplements was rare.
Measurement Precision Study
Subsets of the WHI Clinical Trial and OS were selected for a baseline Measurement Precision Study. Approximately 5.8% of clinical trial participants and 1% of OS participants were enrolled, with oversampling of minority participants, resulting in a combined subcohort of 5488 participants, for whom baseline serum samples were analyzed routinely to estimate concentrations of the 4 micronutrients considered here, among other analytes.
Measurements from this subcohort are used here to develop calibration equations that yield micronutrient intake estimates from FFQs, dietary supplement reports, and participant characteristics only, for use in chronic disease association analyses in larger WHI cohorts. Baseline characteristics for this subcohort were given in our previous publication (4).
Nutrition and Physical Activity Assessment Study
The Nutrition and Physical Activity Assessment Study (NPAAS) Nutritional Biomarker Study (7, 8) was conducted in 2007–2009 among 450 OS participants. Its purpose was to examine the measurement properties of dietary self-report data for nutritional variables having an established biomarker and to use biomarker data to correct dietary self-report data for measurement error, for use in disease association analyses. Fourteen of the NPAAS participants subsequently enrolled in the NPAAS Feeding Study (FS) described below, and data from the remaining participants (n = 436) are used here for additional calibration equation development for the 4 nutritional variables. NPAAS recruited WHI participants at 9 clinical centers, with overrepresentation of minority women and of women having relatively high BMI. The study protocol (7, 8) required 2 clinic visits separated by 2 wk and included various at-home activities. A 20% reliability subsample repeated the protocol ∼6 mo after their initial study participation. The first NPAAS visit included eligibility confirmation; measured height and weight; DLW dosing; completion of FFQ, dietary supplement, and other questionnaires; and collection of a blood specimen, among other activities. At the second clinic visit, participants provided additional fasting blood samples, and engaged in various other activities.
NPAAS FS
The NPAAS FS enrolled 153 WHI women in the Seattle area during 2010–2014. Participants were provided food and beverages over a 2-wk feeding period, with individualized diets that were intended to approximate their usual diets (1), so that blood and urine concentrations would stabilize quickly and intake variations in the study cohort would be substantially retained during the feeding period. Biomarkers developed for the micronutrient intakes studied here are based primarily on corresponding serum nutrient concentrations, with the inclusion of readily available study subject characteristic measures (1, 4). Because the concentrations of the lipophilic micronutrients studied are influenced by circulating lipoprotein, micronutrient concentrations were adjusted for serum cholesterol (9) in all analyses. Baseline demographic and lifestyle characteristics for participants in the NPAAS FS have been reported (4). FFQs covering the preceding 3-mo period were collected just prior to the beginning of the feeding period.
Serum micronutrient measures
Serum aliquots from fasting blood samples in the Measurement Precision Study (n = 5488), the NPAAS Nutritional Biomarker Study (n = 436), and the NPAAS FS were stored at –80°C until analysis. All serum micronutrient determinations were carried out within a few months of blood draw. Carotenoids and tocopherols were measured by HPLC, and interbatch CVs for laboratory quality-control samples were <6.0% for all analytes (1).
Analytic objectives
These cohort study resources provide the potential to address 2 questions: first, can FFQ dietary data explain additional variation in micronutrient intake in the NPAAS FS, beyond that explained by serum nutrient concentration and participant characteristics; second, using intake estimates from equations developed in NPAAS FS, can calibrated intake procedures be developed in the NPAAS Nutritional Biomarker Study (n = 436) and in the Measurement Precision subcohort (n = 5488) using FFQ data for α-carotene and L+Z, and FFQ plus dietary supplement intake data (FFQ+Supp) for β-carotene and α-tocopherol, along with participant characteristics, that lead to reliable disease association analyses in larger WHI cohorts? The NPAAS was conducted ∼10 y after the Measurement Precision subcohort data collection, so that comparison of disease association estimates between the 2 sets of calibrated intake analyses may provide insight into the preferred size and timing of nutritional biomarker substudies.
Outcome ascertainment, follow-up, and disease categories
Clinical outcomes were reported biannually in the DM trial and annually in the OS, by self-administered questionnaire (10) throughout the time period from enrollment in 1993–1998 to the end of the intervention period (31 March 2005), and annually thereafter in both cohorts. An initial report of CVD or invasive cancer during cohort follow-up was confirmed by review of medical records and pathology reports by physician-adjudicators. Additionally, coronary heart disease (CHD; defined as nonfatal myocardial infarction plus CHD death), stroke (ischemic plus hemorrhagic), and all deaths were centrally reviewed by expert physician investigator committees; and all cancers except for nonmelanoma skin cancer were centrally coded using the National Cancer Institute's Surveillance, Epidemiology and End Results procedures. Prevalent (treated) diabetes at baseline was self-reported during eligibility screening. Incident diabetes during follow-up was documented by self-report at each annual contact. These sources have been shown to be consistent with medication inventories of oral agents or insulin (11).
Following the intervention period, WHI participants had the opportunity to enroll in additional follow-up through 30 September 2010, and subsequently for additional open-ended follow-up, with >80% of women doing so on each occasion. Cancer, diabetes, and mortality (including National Death Index matching) outcomes through 31 December 2013 are included here. Follow-up for CVD incidence is included only through 30 September 2010, since self-reports for most WHI participants were not adjudicated after that date. The average follow-up duration here is 11.3 y for CVD incidence and 13.2 y for cancer, diabetes, and mortality. To facilitate comparisons with our recent report, disease outcome categories and follow-up periods are the same as in our previous Measurement Precision subcohort analyses (4).
For the present analyses, CHD and invasive breast cancer can be considered as co-primary outcomes. These were the primary outcomes in the WHI Hormone Therapy and DM trials, respectively. Other CVD categories, total invasive cancer, and diabetes can be considered as secondary outcomes.
Statistical methods
Micronutrient intake estimation in the NPAAS FS
Intake equations for the 4 nutritional variables were developed in the NPAAS FS as in Prentice et al. (4), and these developments were repeated with (natural) log-transformed FFQ intake also included in the regression model. Specifically, log-transformed feeding study intake, including self-reported intake from dietary supplements for β-carotene and α-tocopherol, was regressed linearly on log-serum concentration and disease-specific potential confounding factors as in Prentice et al. (4), but further augmented by including log(FFQ) intake (does not include dietary supplements) as collected just prior to the feeding study period. Regression R2 values were compared to examine the utility of the FFQ assessment for explaining additional feeding study intake variation. Participant characteristics were included that enhanced the fraction of log (feeding study intake) explained at a P < 0.10 level in a forward-selection procedure and were retained at each step using the same P < 0.10 criterion. Supplemental Table 1 shows the set of disease-specific potential confounding factors considered.
Micronutrient intake estimation in the DM-C and OS cohorts
Calibration equations for use in estimating micronutrient intake in the DM-C at year 1 following enrollment, and at enrollment in the OS, were developed in both the NPAAS Nutritional Biomarker Study (n = 436) and the Measurement Precision subcohort (n = 5488) by regressing log-intake estimates using the intake equations just described from the NPAAS FS on log(FFQ) intake for α-carotene and L+Z, and on log(FFQ+Supp) intake for β-carotene and α-tocopherol, and disease-specific participant characteristics (Supplemental Table 1). FFQ+Supp is defined as daily dietary intake in FFQ assessment plus daily intake of the micronutrient as a single supplement or as a component of a combination supplement. Demographic and lifestyle characteristics for the Measurement Precision subcohort were presented in Prentice et al. (4). Supplemental Table 2 shows corresponding characteristics for the NPAAS Nutritional Biomarker Study sample used here (n = 436).
Disease association analyses in the DM-C and OS
These calibration equations were used to calculate calibrated micronutrient intake estimates throughout the DM-C and OS cohorts. Table 1 presents baseline demographic and lifestyle characteristics for the 76,691 participants, 15,319 from the DM-C and 61,372 from the OS, considered for these analyses.
TABLE 1.
Baseline demographic and lifestyle characteristics of the analytic sample composed of 15,319 women from the WHI DM-C and 61,372 from the OS, enrolled during 1993–1998 at 40 US clinical centers1
| OS (n = 61,372) | DM-C (n = 15,319) | |||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Age, y | ||||
| 50–54 | 8519 | 13.9 | 1351 | 8.8 |
| 55–59 | 11,778 | 19.2 | 3258 | 21.3 |
| 60–64 | 13,599 | 22.2 | 3885 | 25.4 |
| 65–69 | 13,483 | 22.0 | 3531 | 23.0 |
| 70–74 | 9735 | 15.9 | 2305 | 15.0 |
| ≥75 | 4258 | 6.9 | 989 | 6.5 |
| BMI, kg/m2 | ||||
| <25 | 25,448 | 41.5 | 4161 | 27.2 |
| 25 to <30 | 20,958 | 34.1 | 5430 | 35.4 |
| ≥30 | 14,966 | 24.4 | 5728 | 37.4 |
| Race/ethnicity | ||||
| White | 52,804 | 86.0 | 13,133 | 85.7 |
| Black | 3956 | 6.4 | 1130 | 7.4 |
| Hispanic | 1891 | 3.1 | 433 | 2.8 |
| American Indian | 213 | 0.3 | 37 | 0.2 |
| Asian/PI | 1728 | 2.8 | 393 | 2.6 |
| Unknown | 780 | 1.3 | 193 | 1.3 |
| Education | ||||
| Less than high school | 2321 | 3.8 | 545 | 3.6 |
| High school/GED | 9679 | 15.8 | 2620 | 17.1 |
| School after high school | 22,328 | 36.4 | 6031 | 39.4 |
| College degree or higher | 27,044 | 44.1 | 6123 | 40.0 |
| Family income (per year) | ||||
| <$20,000 | 8680 | 14.2 | 2024 | 13.2 |
| $20,000 to <$35,000 | 14,190 | 23.1 | 3720 | 24.3 |
| $35,000 to <$50,000 | 12,554 | 20.5 | 3306 | 21.6 |
| $50,000 to <$75,000 | 12,765 | 20.8 | 3330 | 21.7 |
| ≥$75,000 | 13,183 | 21.5 | 2939 | 19.2 |
| Season of FFQ completion | ||||
| Spring | 15,911 | 25.9 | 3977 | 26.0 |
| Summer | 17,122 | 27.9 | 3730 | 24.3 |
| Fall | 14,247 | 23.2 | 3824 | 25.0 |
| Winter | 14,092 | 23.0 | 3788 | 24.7 |
| Current smoker | ||||
| No | 57,674 | 94.0 | 14,397 | 94.0 |
| Yes | 3698 | 6.0 | 922 | 6.0 |
| Alcohol intake | ||||
| Non-/past drinker | 17,436 | 28.4 | 5241 | 34.2 |
| <1 drink/wk | 19,457 | 31.7 | 4446 | 29.0 |
| 1 to <7 drinks/wk | 16,413 | 26.7 | 4191 | 27.4 |
| ≥7 drinks/wk | 8066 | 13.1 | 1441 | 9.4 |
| Any supplement use | 34,314 | 55.9 | 7594 | 49.6 |
| Taking single supplement of β-carotene | 3221 | 5.2 | 437 | 2.9 |
| Taking single supplement of vitamin E (α-tocopherol) | 21,120 | 34.4 | 4774 | 31.2 |
| Taking single supplement of vitamin A | 4415 | 7.2 | 662 | 4.3 |
| Daily intake from multivitamins, other combination supplements, or single supplements | ||||
| β-Carotene, median (IQR), μg | 0 | (0, 4500) | 0 | (0, 4500) |
| Vitamin E (α-tocopherol),2 median (IQR), mg | 27 | (0, 364) | 27 | (0, 364) |
| Vitamin A, median (IQR), μg RE | 429 | (0, 1500) | 0 | (0, 1500) |
| Medication use | ||||
| Antihyperlipidemic medication | 5668 | 9.2 | 1432 | 9.3 |
| Antidiabetic medication | 1818 | 3.0 | 615 | 4.0 |
| Antihypertensive medication | 18,039 | 29.4 | 5063 | 33.1 |
| Postmenopausal hormone use | ||||
| Never | 23,994 | 39.1 | 6089 | 39.7 |
| Past | 9118 | 14.9 | 2994 | 19.5 |
| E-alone | 15,464 | 25.2 | 3614 | 23.6 |
| E+P | 12,796 | 20.8 | 2622 | 17.1 |
| Recreational physical activity, MET-h/wk | ||||
| None | 7868 | 12.8 | 2647 | 17.3 |
| >0 to ≤9.5 | 21,440 | 34.9 | 6241 | 40.7 |
| >9.5 to ≤20.5 | 16,967 | 27.6 | 3746 | 24.5 |
| >20.5 | 15,097 | 24.6 | 2685 | 17.5 |
| History of CVD3 | ||||
| No | 58,470 | 95.3 | 14,718 | 96.1 |
| Yes | 2902 | 4.7 | 601 | 3.9 |
| History of MI | 1334 | 2.2 | 298 | 1.9 |
| History of CABG/PCI | 1063 | 1.7 | 193 | 1.3 |
| History of heart failure | 607 | 1.0 | 121 | 0.8 |
| History of stroke | 777 | 1.3 | 160 | 1.0 |
| History of cancer | ||||
| No | 53,682 | 87.5 | 14,552 | 95.0 |
| Yes | 7690 | 12.5 | 767 | 5.0 |
| Breast | 3952 | 5.7 | 65 | 0.4 |
| Colorectal | 550 | 0.9 | 15 | 0.1 |
| Ovary | 398 | 0.7 | 66 | 0.4 |
| Endometrium | 1060 | 1.7 | 143 | 0.9 |
| Thyroid | 333 | 0.5 | 61 | 0.4 |
| Cervix | 749 | 1.2 | 195 | 1.3 |
| Melanoma | 829 | 1.4 | 107 | 0.7 |
| Liver | 23 | <0.1 | 1 | <0.1 |
| Lung | 140 | 0.2 | 15 | 0.1 |
| Brain | 30 | <0.1 | 6 | <0.1 |
| Bone | 40 | 0.1 | 8 | 0.1 |
| Stomach | 33 | 0.1 | 1 | <0.1 |
| Leukemia | 54 | 0.1 | 5 | <0.1 |
| Bladder | 116 | 0.2 | 10 | 0.1 |
| Non-Hodgkin lymphoma | 141 | 0.2 | 6 | <0.1 |
| Hodgkin lymphoma | 37 | 0.1 | 5 | <0.1 |
| History of treated hypertension | 15,069 | 24.6 | 4677 | 30.5 |
| History of treated diabetes | 2243 | 3.7 | 741 | 4.8 |
| Family history of MI | 31,859 | 51.9 | 7947 | 51.9 |
| Family history of stroke | 23,338 | 38.0 | 5832 | 38.1 |
| Family history of breast cancer | 9782 | 15.9 | 2213 | 14.4 |
| Family history of colorectal cancer | 10,020 | 16.3 | 2402 | 15.7 |
| Family history of diabetes | 19,755 | 32.2 | 5275 | 34.4 |
n = 76,691. CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CVD, cardiovascular disease; DM-C, Dietary Modification Trial comparison group; E-alone, estrogens alone; E+P, estrogens plus progestin; GED, General Educational Development; MET, metabolic equivalent unit; MI, myocardial infarction; OS, Observational Study; PI, Pacific Islander; RE, retinol equivalents; WHI, Women's Health Initiative.
Converted from IU/d to mg/d using a conversion factor of 0.67 for natural and 0.91 for synthetic ɑ-tocopherol.
Nonfatal MI, CABG/PCI, or stroke.
Calibrated intake values were entered into Cox regression models (12), along with disease-specific potential confounding factors. As in previous analyses (4), a linear modeling of logHRon log micronutrient intake is assumed, and this implies a fixed HR for a fractional increase in intake. As in Prentice et al. (4) we present HR estimates for a doubling in intake. For these micronutrients, a doubling of intake is well within the intake variation estimated in WHI cohorts. Baseline hazard rates in the Cox model analyses were stratified on “baseline” age (year 1 for participants in DM-C and enrollment for participants in OS) in 5-y categories, race/ethnicity, on cohort (DM-C or OS), and, in the DM-C, were further stratified on participation in the WHI Hormone Therapy trials (estrogen, estrogen placebo, estrogen plus progestin, estrogen plus progestin placebo; not randomized). The same set of disease-specific potential confounding factors as in earlier analyses (4) were considered for inclusion (Supplemental Table 1). Specifically, CVD outcome analyses included the following: age (linear); family income; education; cigarette smoking history; alcohol consumption; leisure physical activity; height; weight; any dietary supplement use; prior menopausal hormone use; hypertension; CVD in a first-degree relative; personal history of cancer; family history of myocardial infarction, stroke, or diabetes; use of medications to lower blood pressure, blood lipids, or blood glucose; and season in which the FFQ was completed. Invasive cancer analyses included these same variables, exclusive of prevalent CVD and of family history of CVD or diabetes, and inclusive of family history of breast cancer, family history of colorectal cancer, and personal history of colon polyp removal. Diabetes incidence analyses included the same variables as the CVD analyses except for family history of myocardial infarction or stroke. Missing data rates were generally low for specific covariates, but ≥20% of participants had missing data on ≥1 modeled covariates in some of our analyses. Participants were excluded from outcome-specific analyses if any modeled covariate was missing. Based on sensitivity analyses that excluded covariates having relatively high missingness rates, this exclusion is not expected to materially bias disease association HR estimates. Participants having CVD, invasive cancer, or treated diabetes prior to enrollment were excluded from respective CVD, cancer, or diabetes analyses.
Disease occurrence time for a “case” developing a study outcome was days from “baseline” (year 1 in the DM-C and enrollment in the OS) to diagnosis, and censoring time for “noncases” was days from baseline to the earliest of date of death without the outcome under study, last contact, or either 30 September 2010 for CVD incidence outcomes or 31 December 2013 for cancer, diabetes, and mortality outcomes. Because of uncertainty in the coefficients in the intake estimating equations, a “sandwich-type” estimator was used to estimate the variance for the logHR parameter estimates (13–15), as in our previous analyses (4).
Disease association analyses in the Measurement Precision subcohort
Additional Cox regression analyses with the same definitions and procedures just described were conducted in the Measurement Precision subcohort (n = 5488) for each of the clinical outcomes and each of the 4 micronutrients. These are the same as those reported in Prentice et al. (4), except that log(FFQ) is included in the NPAAS FS regression equations used to estimate micronutrient intake in this subcohort. In fact, if the FFQ data lead to an increase in feeding study intake explained, this inclusion may be needed to avoid bias in corresponding calibrated intake disease association analyses.
All P values for calibration equations and for disease association analyses were 2-sided, with P < 0.05 considered as significant.
Additional methods
Participants provided written informed consent for their overall WHI, NPAAS, and NPAAS FS activities, and protocols were approved by the Institutional Review Boards at the Fred Hutchinson Cancer Research Center and at each participating clinical center.
Figure 1 shows cohorts and participant flow in the WHI DM-C and the OS, in the Measurement Precision subcohort, and in the NPAAS Nutritional Biomarker Study and FS subsamples, over the intervention and postintervention study phases.
FIGURE 1.
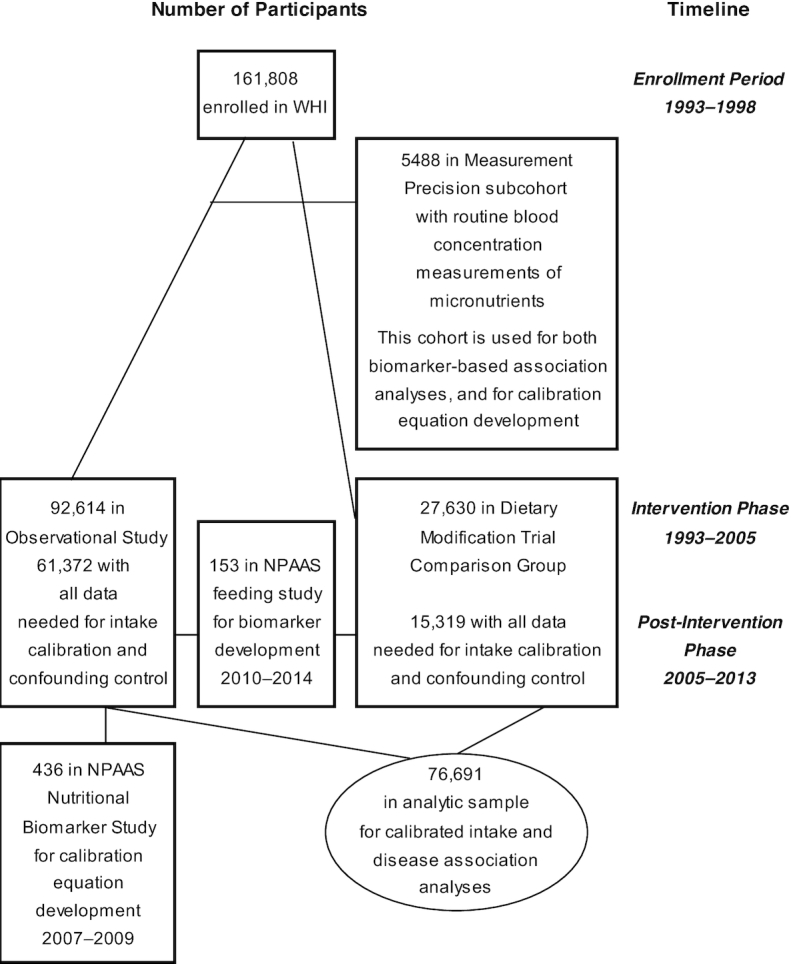
Study sample and flow in the WHI and in its Measurement Precision and NPAAS subsets. NPAAS, Nutrition and Physical Activity Assessment Study; WHI, Women's Health Initiative.
Results
Table 2 shows regression R2 values from NPAAS FS analyses based on serum measures and participant characteristics, and based on serum measures and FFQ and participant characteristics, for each of the 4 nutritional variables. Separate displays are provided for participant characteristics selected from those relevant to CVD, cancer, and diabetes. Note that the inclusion of FFQ micronutrient assessments tends to yield somewhat larger R2 values, especially for β-carotene and L+Z, comparing the 2 analyses of Table 2 in this NPAAS FS sample.
TABLE 2.
Fraction of WHI NPAAS Feeding Study (n = 153) intake variation explained (R2) by linear regression of log-intake on intake measures and covariates1
| Nutritional variable | Log(serum) concentration and covariates | Log-serum concentration, log(FFQ) intake, and covariates2 |
|---|---|---|
| CVD covariates | ||
| α-Carotene | 0.535 | 0.528 |
| β-Carotene | 0.415 | 0.462 |
| Lutein + zeaxanthin | 0.443 | 0.512 |
| α-Tocopherol | 0.547 | 0.572 |
| Cancer covariates | ||
| α-Carotene | 0.528 | 0.523 |
| β-Carotene | 0.346 | 0.411 |
| Lutein + zeaxanthin | 0.447 | 0.499 |
| α-Tocopherol | 0.530 | 0.552 |
| Diabetes covariates | ||
| α-Carotene | 0.520 | 0.515 |
| β-Carotene | 0.371 | 0.451 |
| Lutein + zeaxanthin | 0.432 | 0.490 |
| α-Tocopherol | 0.528 | 0.553 |
Covariates considered for inclusion in regression models for CVD, cancer, and diabetes analyses are listed in Supplemental Table 1. Covariates are included through forward selection with a P value threshold of 0.1 and are retained using the same P = 0.1 criterion. CVD, cardiovascular disease; NPAAS, Nutrition and Physical Activity Assessment Study; R2, % of variation in response variable explained by regression variable(s); WHI, Women's Health Initiative.
R 2 values may be less than that for the model without FFQ due to missing FFQ data.
Supplemental Table 3 shows details of the regression analyses underlying Table 2. These analyses show significant (P < 0.05) associations between feeding study intake and FFQ dietary intake just prior to the feeding period for β-carotene and L+Z, but no such association is evident for α-carotene or α-tocopherol intake.
These regression models were used to calculate log intake estimates for the 4 nutritional variables in the NPAAS Nutritional Biomarker Study (n = 436) and the Measurement Precision Study (n = 5488). Supplemental Table 4 gives geometric means and 95% confidence ranges for serum concentrations, FFQ intake, and FFQ+Supp intake for β-carotene and α-tocopherol in these cohorts. These log-intake estimates were regressed on log(FFQ) for α-carotene and L+Z, and log(FFQ+Supp) for β-carotene and α-tocopherol, along with disease-specific covariates in each context to produce calibration equations for intake estimation throughout the larger DM-C and OS cohorts.
Table 3 gives corresponding calibration equation R2 values, separately according to which of the 3 disease-oriented covariate sets were used. R2 values were in the 25–75% range, and somewhat larger for the NPAAS Nutritional Biomarker Study analyses than for the Measurement Precision subcohort analyses.
TABLE 3.
Fraction of biomarker intake variation explained by linear regression of log-biomarker intake on log(FFQ) or log(FFQ+Supp) intake and covariates in an NPAAS Nutritional Biomarker Study (n = 436) and in a Measurement Precision subcohort (n = 5488)1
| Nutritional variable | CVD (n = 436) | Covariates (n = 5488) | Cancer (n = 436) | Covariates (n = 5488) | Diabetes (n = 436) | Covariates (n = 5488) |
|---|---|---|---|---|---|---|
| α-Carotene | 0.401 | 0.255 | 0.384 | 0.250 | 0.388 | 0.249 |
| β-Carotene | 0.606 | 0.509 | 0.682 | 0.618 | 0.587 | 0.499 |
| Lutein + zeaxanthin | 0.573 | 0.521 | 0.546 | 0.516 | 0.563 | 0.516 |
| α-Tocopherol | 0.762 | 0.669 | 0.662 | 0.604 | 0.673 | 0.614 |
Listed numbers are the fraction of log-biomarker intake explained (R2) by log(FFQ) intake and covariates for α-carotene and lutein + zeaxanthin, and by log(FFQ+Supp) and covariates for β-carotene and α-tocopherol. Covariates considered for inclusion in regression models for CVD, cancer, and diabetes analyses are listed in Supplemental Table 1. Covariates are included through forward selection with a P value threshold of 0.1, and at each step are retained using the same criterion. CVD, cardiovascular disease; FFQ+Supp, food-frequency questionnaire plus dietary supplement; NPAAS, Nutrition and Physical Activity Assessment Study; R2, % of variation in response variable explained by regression variable(s).
Supplemental Tables 5 and 6provide detail on the respective sets of calibration equations. The log(FFQ) or log(FFQ+Supp) intake values contributed substantially to the Table 3R2 values for each of the micronutrients.
Table 4 provides information on the distribution of calibrated intake estimates for each of the 3 outcome categories, using the NPAAS Nutritional Biomarker Study for calibration equation development.
TABLE 4.
Baseline estimated micronutrient intake from FFQs and dietary supplement questionnaires in the WHI analytic sample (n = 76,691) composed of 15,319 women from the DM-C and 61,372 from the OS, enrolled during 1993–98 at 40 US clinical centers1
| OS (n = 61,372) | DM-C (n = 15,319) | |||
|---|---|---|---|---|
| Nutritional intake measures | Geometric mean | 95% CR | Geometric mean | 95% CR |
| Self-reported FFQ | ||||
| α-Carotene, μg/d | 562.1 | (102.2, 2241.8) | 508.4 | (98.6, 1874.1) |
| β-Carotene, μg/d | 2801.7 | (719.5, 9212.8) | 2559.8 | (698.2, 7868.5) |
| Lutein + zeaxanthin, μg/d | 1465.2 | (434.8, 6085.4) | 1360.1 | (420.1, 5438.3) |
| α-Tocopherol,2 mg/d | 3.49 | (1.45, 9.50) | 3.65 | (1.58, 9.40) |
| Self-reported FFQ + supplements | ||||
| β-Carotene, μg/d | 4687.7 | (866.0, 21,797.2) | 4153.3 | (835.7, 18,019.5) |
| α-Tocopherol,2 mg/d | 39.18 | (2.57, 904.2) | 34.01 | (2.71, 813.9) |
| Calibrated3 for CVD outcomes | ||||
| α-Carotene, μg/d | 556.4 | (133.0, 1980.1) | 589.7 | (141.3, 2059.3) |
| β-Carotene, μg/d | 12,661.0 | (5243.1, 28,385.2) | 12,845.5 | (5437.7, 28,277.6) |
| Lutein + zeaxanthin, μg/d | 3796.0 | (1594.5, 9028.3) | 3592.1 | (1563.7, 8192.8) |
| α-Tocopherol, mg/d | 40.8 | (6.02, 44.5) | 36.1 | (5.42, 40.7) |
| Calibrated3 for cancer outcomes | ||||
| α-Carotene, μg/d | 458.3 | (58.7, 1771.7) | 507.4 | (70.7, 1879.8) |
| β-Carotene, μg/d | 4748.8 | (2115.7, 10,187.7) | 4825.6 | (2180.8, 10,253.4) |
| Lutein + zeaxanthin, μg/d | 4005.0 | (1948.5, 8448.0) | 3892.9 | (1965.0, 8007.1) |
| α-Tocopherol, mg/d | 40.9 | (9.6, 225.6) | 36.3 | (8.8, 218.4) |
| Calibrated3 for diabetes outcome | ||||
| α-Carotene, μg/d | 555.4 | (139.0, 1846.7) | 579.7 | (148.5, 1889.2) |
| β-Carotene, μg/d | 4500.0 | (1859.0, 10,019.4) | 4519.8 | (1910.1, 9871.1) |
| Lutein + zeaxanthin, μg/d | 3986.8 | (1766.6, 9044.2) | 3809.8 | (1746.0, 8351.6) |
| α-Tocopherol, mg/d | 41.6 | (9.2, 254.0) | 36.9 | (8.6, 246.2) |
CR, confidence range (2.5 percentile, 97.5 percentile); CVD, cardiovascular disease; DM-C, Dietary Modification Trial comparison group; NPAAS, Nutrition and Physical Activity Assessment Study; OS, Observational Study.
Converted from IU/d to mg/day using a conversion factor of 0.67 for natural and 0.91 for synthetic ɑ-tocopherol.
Calibrated intake calculated using calibration equations developed in the NPAAS Nutritional Biomarker Study (n = 436) using the regression model in the right column of Table 2, and based on FFQ intake assessments, supplement intake for β-carotene and α-tocopherol, and participant covariates.
Table 5 shows HR estimates and 95% CIs for a doubling of micronutrient intake, based on 3 sets of analyses. The first “biomarker” analyses derive from the Measurement Precision subcohort with intakes estimated from the Table 1/Supplemental Table 3 regression equations that include both serum concentrations and FFQ measures. The other 2 sets of analyses take place in the combined DM-C and OS cohorts (n = 76,691), with calibrated intakes derived from regression equations in the NPAAS Nutritional Biomarker Study and Measurement Precision subcohort, respectively. Corresponding to Table 5, Supplemental Table 7 shows the number of disease events and crude disease rates in these analyses.
TABLE 5.
HRs (95% CIs) for a doubling of nutritional variable in relation to the incidence of CVD, cancer, or diabetes, both in a WHI Measurement Precision subcohort (n = 5488) using “biomarker” intake and in a complementary larger WHI cohort (n = 76,691) using calibrated FFQ (plus dietary supplements for β-carotene and α-tocopherol) intake1
| α-Carotene | β-Carotene | Lutein + zeaxanthin | α-Tocopherol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intake measure | Outcome (cases) | HR | 95% CI | LogHR SE | HR | 95% CI | LogHR SE | HR | 95% CI | LogHR SE | HR | 95% CI | LogHR SE |
| CVD | |||||||||||||
| Biomarker | CHD (154) | 0.97 | 0.86, 1.09 | 0.063 | 1.00 | 0.76, 1.33 | 0.145 | 0.93 | 0.74, 1.16 | 0.115 | 0.97 | 0.27, 2.31 | 0.063 |
| Calibrated2 | CHD (2711) | 0.95 | 0.89, 1.02 | 0.034 | 0.91 | 0.85, 0.99 | 0.040 | 0.95 | 0.86, 1.05 | 0.049 | 0.94 | 0.87, 1.01 | 0.037 |
| Calibrated3 | CHD (2711) | 0.93 | 0.84, 1.02 | 0.051 | 0.90 | 0.82, 0.99 | 0.046 | 0.95 | 0.85, 1.05 | 0.055 | 0.95 | 0.89, 1.01 | 0.032 |
| Biomarker | CABG/PCI (172) | 0.86 | 0.77, 0.96 | 0.055 | 0.77 | 0.60, 0.98 | 0.125 | 0.88 | 0.81, 1.33 | 0.103 | 1.16 | 1.04, 1.29 | 0.056 |
| Calibrated2 | CABG/PCI (2933) | 0.99 | 0.93, 1.06 | 0.033 | 0.94 | 0.87, 1.01 | 0.039 | 1.01 | 0.92, 1.10 | 0.047 | 0.97 | 0.90, 1.04 | 0.036 |
| Calibrated3 | CABG/PCI (2933) | 0.99 | 0.90, 1.09 | 0.050 | 0.93 | 0.85, 1.02 | 0.045 | 1.01 | 0.92, 1.12 | 0.052 | 0.97 | 0.91, 1.03 | 0.031 |
| Biomarker | Stroke (124) | 0.96 | 0.83, 1.10 | 0.071 | 0.96 | 0.72, 1.27 | 0.144 | 0.77 | 0.60, 0.99 | 0.130 | 0.94 | 0.80, 1.10 | 0.081 |
| Calibrated2 | Stroke (2274) | 0.93 | 0.86, 1.00 | 0.038 | 1.01 | 0.92, 1.10 | 0.045 | 0.96 | 0.87, 1.07 | 0.053 | 1.00 | 0.92, 1.08 | 0.041 |
| Calibrated3 | Stroke (2274) | 0.90 | 0.80, 1.00 | 0.058 | 1.01 | 0.91, 1.11 | 0.052 | 0.96 | 0.85, 1.07 | 0.059 | 1.00 | 0.93, 1.07 | 0.035 |
| Biomarker | Total CVD (370) | 0.92 | 0.85, 1.00 | 0.040 | 0.89 | 0.76, 1.06 | 0.088 | 0.83 | 0.72, 0.95 | 0.073 | 1.07 | 0.99, 1.16 | 0.042 |
| Calibrated2 | Total CVD (6545) | 0.95 | 0.91, 0.99 | 0.022 | 0.96 | 0.91, 1.01 | 0.026 | 0.98 | 0.92, 1.04 | 0.032 | 0.97 | 0.93, 1.02 | 0.024 |
| Calibrated3 | Total CVD (6545) | 0.93 | 0.87, 0.99 | 0.033 | 0.91 | 0.85, 0.98 | 0.030 | 0.98 | 0.92, 1.05 | 0.035 | 0.97 | 0.94, 1.01 | 0.021 |
| Cancer | |||||||||||||
| Biomarker | Breast (176) | 0.86 | 0.75, 0.99 | 0.071 | 0.65 | 0.49, 0.86 | 0.146 | 0.89 | 0.71, 1.12 | 0.116 | 1.00 | 0.81, 1.13 | 0.062 |
| Calibrated2 | Breast (3908) | 0.95 | 0.90, 1.01 | 0.029 | 0.98 | 0.91, 1.05 | 0.035 | 0.98 | 0.90, 1.07 | 0.045 | 0.96 | 0.90, 1.03 | 0.034 |
| Calibrated3 | Breast (3908) | 0.94 | 0.86, 1.02 | 0.044 | 0.97 | 0.90, 1.05 | 0.039 | 0.99 | 0.90, 1.08 | 0.045 | 0.97 | 0.92, 1.03 | 0.029 |
| Biomarker | Total invasive (473) | 0.95 | 0.87, 1.03 | 0.041 | 0.88 | 0.74, 1.05 | 0.089 | 1.04 | 0.91, 1.19 | 0.070 | 1.05 | 0.97, 1.13 | 0.039 |
| Calibrated2 | Total invasive (9557) | 0.95 | 0.92, 0.99 | 0.019 | 1.01 | 0.97, 1.06 | 0.023 | 0.99 | 0.94, 1.05 | 0.029 | 1.00 | 0.96, 1.05 | 0.022 |
| Calibrated3 | Total invasive (9557) | 0.93 | 0.88, 0.99 | 0.028 | 1.01 | 0.96, 1.06 | 0.025 | 0.99 | 0.94, 1.05 | 0.029 | 1.00 | 0.97, 1.04 | 0.018 |
| Diabetes mellitus | |||||||||||||
| Biomarker | Treated (644) | 0.91 | 0.85, 0.97 | 0.033 | 0.76 | 0.67, 0.87 | 0.067 | 0.85 | 0.75, 0.97 | 0.063 | 1.04 | 0.97, 1.12 | 0.036 |
| Calibrated2 | Treated (8504) | 0.98 | 0.94, 1.02 | 0.019 | 1.01 | 0.97, 1.06 | 0.022 | 1.02 | 0.77, 1.08 | 0.029 | 1.04 | 0.99, 1.09 | 0.025 |
| Calibrated3 | Treated (8504) | 0.97 | 0.91, 1.02 | 0.050 | 1.02 | 0.97, 1.07 | 0.026 | 1.02 | 0.96, 1.09 | 0.031 | 1.03 | 0.99, 1.07 | 0.021 |
Biomarker intake in this Measurement Precision cohort of 5488 women, for whom concentrations of the micronutrients considered here and total cholesterol were routinely measured in blood collected at baseline, using the regression model on the right side of Table 2. FFQs were measured at 1 y postenrollment in the Dietary Modification clinical trial comparison group, and at enrollment in the Observational Study. Following exclusion of participants with prevalent disease at baseline or having missing covariate data, there were 3780, 3686, and 3693 participants included in CVD, cancer, and diabetes analyses, respectively. Follow-up from enrollment in 1993–1998 to 30 September 2010 for CVD incidence, and to 31 December 2013 for cancer diabetes and mortality outcomes. HRs were calculated using Cox regression with detailed baseline stratification of hazard rates, and with modeled regression variables composed of calibrated log-intake and potential confounding factors. CIs are calculated using a sandwich procedure that acknowledges random error in regression equation coefficients. Participants having a history of CVD, invasive cancer, or diabetes, or having missing covariates used to control confounding were excluded from respective CVD, cancer, and diabetes analyses. CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; NPAAS, Nutrition and Physical Activity Assessment Study; SE, estimated SE (for estimated logHR); WHI, Women's Health Initiative.
Calibrated intake calculated using calibration equations developed in the NPAAS Nutritional Biomarker Study (n = 436) using the regression model on the right side of Table 2, and based on FFQ (plus supplement intake for β-carotene and α-tocopherol) intake assessments and participant covariates. Following exclusion of participants with prevalent disease at baseline or having missing covariate data, there were 73,188, 68,234, and 73,707 participants included in CVD, cancer, and diabetes analyses, respectively.
Calibrated intake calculated using calibration equations developed in the WHI Measurement Precision subcohort (n = 5488), using the regression model on the right side of Table 2, and based on FFQ (plus supplements) intake estimates and participant covariates. Following exclusion of participants with prevalent disease at baseline or having missing covariate data, there were 73,188, 68,234, and 73,707 participants included in CVD, cancer, and diabetes analyses, respectively.
The serum concentration-based (biomarker) analyses in Table 5 show reductions in coronary artery bypass graft or percutaneous coronary intervention (CABG/PCI), invasive breast cancer, and diabetes with α-and β-carotene, reductions in stroke and diabetes with L+Z, and an increase in CABG/PCI with α-tocopherol. In fact, 10 of 28 associations are significant in these analyses, despite limited precision. These are the same 10 associations that were significant in Prentice et al. (4). In fact, HRs, 95% CIs, and logHR SE estimates are very similar in the 2 sets of analyses.
The 2 calibrated intake association analyses in Table 5 agree substantially with each other. Doubling of α-carotene was associated with reduction in the risk of stroke, total CVD, and total invasive cancer. Doubling of β-carotene was associated with reduced CHD incidence in both sets of analyses, while doubling of β-carotene was also associated with reduced total CVD in the analyses using the Measurement Precision Study for calibration. Despite greater precision in these analyses, the rather few significant associations reflect HR estimates that are often closer to the null compared with the biomarker-based analyses.
Table 5 also provides estimates of the SEs for the logHR estimates. These are mostly smaller by a factor of 1.5–3.0 for the calibrated intake HRs compared with the biomarker-based HRs, corresponding to the ∼14-fold greater cohort size for the calibrated intake analyses.
Analyses like those shown in Table 5 were also carried out after excluding the ∼50% of participants who were using some form of dietary supplement at baseline. These analyses (Supplemental Table 8) show similar trends to Table 5, but calibrated HR estimates are estimated with reduced precision. This is especially the case for α-tocopherol for which most intake arose from supplementation.
Analyses corresponding to those shown in Table 5 were also carried out using self-reported intake without calibration (Supplemental Table 9). Analyses tended to be consistent in direction with the calibrated intake analyses shown in Table 5, although HR estimates are mostly even closer to the null and nonsignificant.
Association analyses with BMI added to the disease risk model were also carried out, and mostly differed little from those in Table 5, although some logHR SE estimates were larger.
Discussion
There is a substantial epidemiologic literature relating self-reported intake of the dietary variables considered here to chronic disease risk, but few, if any, reported associations were regarded as convincing in expert reviews (16–18). There is also some literature relating serum concentrations of these dietary variables to chronic disease risk (e.g., 19), but only recently (1) has the utility of these concentrations for quantitative intake estimation been considered. This recent work indicates that serum concentrations for these nutritional variables need to be rescaled to reflect intake, and typically, the needed rescaling depends on study subject characteristics (1). Using this recent work we found that carotenoid intake assessments based on serum concentrations and pertinent participant characteristic were modestly but significantly associated with reduced CVD, cancer, and diabetes incidence; and α-tocopherol intake assessments were associated with increased risk for certain CVD outcomes in a WHI Measurement Precision subcohort where serum concentrations for these micronutrients at study enrollment were routinely assessed (4).
The present contribution addresses questions concerning the potential value of FFQ intake assessments in these association analyses, either in conjunction with the serum concentration measures in a study cohort or with FFQs, dietary supplement data, and participant characteristics only in a study cohort, while the serum nutrient concentration measures are available only in a cohort subsample.
For the first question, we observed that FFQ data can usefully augment serum concentration measures and participant characteristics for β-carotene and L+Z in explaining feeding study intake variation in these nutrients. However, corresponding disease association analyses in the 5488 Measurement Precision subcohort (Table 5) did not differ in any material way from those reported previously (4), which did not use FFQ data for intake assessments. Hence, if intake measures without the self-reported dietary data meet suitable biomarker criteria and are available throughout the study cohort, it may be desirable to use the (objective) intake measure, without the inclusion of FFQ data, in disease association analyses.
The answer to the second question is more nuanced. Here, calibration equations based on serum concentrations, FFQ, and dietary supplement data and covariates are developed in cohort subsets, either the NPAAS Nutritional Biomarker Study or the Measurement Precision subcohort. If the FFQ data help explain feeding study actual intake variation beyond that explained by serum concentrations and covariates, then the FFQ data are needed in the intake assessment to avoid bias in related calibrated intake disease association analyses. However, the reliability of the calibrated intake and disease association analyses depends on both the quality of the “biomarker” assessment in the cohort subsample and the quality of the associated calibration equation yielding intake estimates from FFQ, or FFQ+Supp data, and participant characteristics only. One might consider simply multiplying the R2 values from Tables 2 and 3 to examine this reliability, but doing so would not acknowledge the fact that the disease association analyses typically relate daily intake over some preceding months or years relevant to disease risk, rather than intake in the 2-wk feeding study period.
Nevertheless, unless the biomarker intake assessment is strong, and the calibration equations explain a large fraction of the biomarker variation, the reliability of the associated disease association analyses may be questionable. In fact, with either the biomarker-based intake assessments or the calibrated intake assessments, the reliability of the corresponding disease association analyses derives from an assumption that actual intake can be written as estimated intake plus random error that is independent of estimated intake. This so-called Berkson error assumption fits with the regression model development of the intake estimators considered here, provided the modeled regression variables yield an explanation for much of the actual intake variations among cohort participants. This seems more likely when intake is based on serum nutrient concentrations and participant characteristics, with or without FFQ assessments, than when an attempt is made in a 2-step development to explain actual intake variation in terms of FFQs, dietary supplements, and participant characteristics only. Hence, one expects the biomarker-based HRs in Table 5 to have reliability exceeding that for the calibrated intake association analyses because of the richer data sources for intake assessment, even though precision of these biomarker-based analyses is limited by a relatively small cohort size. If the modeled “predictor” variables are unable to substantially explain actual intake variations in the Table 5 analyses, then associated HR estimates may be biased toward the null as the HR association with actual intake is typically shared between the estimated intake and the corresponding error variable in the calibration equation, as is presumably the case for the calibrated intake-related HRs in Table 5. Hence, more precise estimation in WHI cohorts than is provided by the Measurement Precision subcohort alone may require using the biomarker-based intake in a case-control mode in the larger WHI cohorts. Calibration approaches may provide reliable disease association studies in some contexts, depending on the quality of the biomarker and the quality of the dietary self-report and covariate data used to develop calibration equations, but typically, careful justification of support for related measurement error assumptions will be needed. [See, for example, Zheng et al. (20) for an application of the calibrated intake approach to analyses of total energy intake and activity-related energy expenditure, with BMI as an important mediator, in relation to chronic disease risk in WHI cohorts.]
Our analyses do not show major differences between use of the larger subcohort (n = 5488) as compared with the moderate-sized NPAAS Nutritional Biomarker Study (n = 436) for calibration equation development and disease association analyses. In fact, logHR SE estimates are mostly larger for the subcohort (n = 5488) analyses in Table 5. This difference may derive in part from the ∼10-y earlier ascertainment of FFQ data and serum for the Measurement Precision subcohort compared with the Nutritional Biomarker Study. On the other hand, calibrated intake association analyses using the Measurement Precision subcohort avoid certain potential biases that may attend analyses using the Nutritional Biomarker Study related to the exclusion of participants who died or discontinued WHI participation prior to the NPAAS study conduct. The estimated precision of logHR estimates was smaller in the calibrated intake analyses only by a factor of ∼1.5–3 compared with the biomarker-based analyses, a rather modest reduction given the ∼14-fold larger cohort size for the calibrated intake analyses.
Given the fundamental role of feeding studies for biomarker development and for biomarker-based intake estimation, an important question emerges concerning the transferability of biomarker-based intake estimators to other cohorts. The fact that biomarker equations depend on various measured participant characteristics, as well as micronutrient serum concentrations, may enhance the likelihood of useful transferability. However, this topic merits specific study using ≥2 cohorts, each with a suitable embedded feeding study, especially since food composition and preparation practices may be influential and may differ between populations of interest.
Our analyses reinforce the importance of continuing critical evaluation of research methods for intake assessment in the important, but challenging, nutritional epidemiology research area. Intake biomarkers have an important role to play in strengthening research findings, and human feeding studies are indispensable for intake biomarker development. Feeding studies in additional cohort study contexts, perhaps using a design similar to that for the NPAAS FS, are strongly encouraged.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—RLP, MLN, LFT, JEM, YM-R, GLA, and JWL: designed the research; RLP, MP, MLN, LFT, YH, CZ, and JLW: conducted the research, analyzed the data, and drafted the manuscript; RLP had primary responsibility for the final content; and all authors: participated actively in critical evaluation of the manuscript and read and approved the final manuscript. The authors report no conflicts of interest.
The authors acknowledge the following investigators in the WHI Program—Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD): Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: Brigham and Women's Hospital, Harvard Medical School, Boston, MA: JoAnn E Manson; MedStar Health Research Institute/Howard University, Washington, DC: Barbara V Howard; Stanford Prevention Research Center, Stanford, CA: Marcia L Stefanick; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ: Cynthia A Thomson; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Iowa, Iowa City/Davenport, IA: Jennifer Robinson; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker; University of Nevada, Reno, NV: Robert Brunner; Women's Health Initiative Memory Study (Wake Forest University School of Medicine, Winston-Salem, NC): Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Notes
This work was supported by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services (contracts HHSN268201100046C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN271201600004C), and National Cancer Institute grants R01 CA119171 and R01 CA210921. The views expressed are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI); the NIH; or the US Department of Health and Human Services. Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, and the decision to submit the manuscript for publication resided with committees composed of Women's Health Initiative investigators that included NHLBI representatives. The contents of the paper are solely the responsibility of the authors.
Supplemental Tables 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The data, codebook, and analytic code used in this report may be accessed in a collaborative mode as described on the Women's Health Initiative website (www.whi.org).
Abbreviations used: CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; DLW, doubly-labeled water; DM, Dietary Modification; DM-C, Dietary Modification comparison group; FFQ+Supp, food-frequency questionnaire plus dietary supplement; FS, Feeding Study; L+Z, lutein plus zeaxanthin; NPAAS, Nutrition and Physical Activity Assessment Study; OS, Observational Study; WHI, Women's Health Initiative.
Contributor Information
Ross L Prentice, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Mary Pettinger, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Marian L Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Lesley F Tinker, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Ying Huang, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Cheng Zheng, Joseph J Zilber School of Public Health, University of Wisconsin, Milwaukee, WI, USA.
JoAnn E Manson, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Yasmin Mossavar-Rahmani, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Garnet L Anderson, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Johanna W Lampe, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
References
- 1. Lampe JW, Huang Y, Neuhouser ML, Tinker LF, Song X, Schoeller DA, Kim S, Raftery D, Di C, Zheng C et al.. Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am J Clin Nutr. 2017;105:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schoeller DA. Recent advances from application of doubly-labeled water to measurement of human energy expenditure. J Nutr. 1999;129:1765–8. [DOI] [PubMed] [Google Scholar]
- 3. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(Suppl 3):921S–4S. [DOI] [PubMed] [Google Scholar]
- 4. Prentice RL, Pettinger M, Neuhouser ML, Tinker LF, Huang Y, Zheng C, Manson JE, Mossavar-Rahmani Y, Anderson GL, Lampe JW. Application of blood concentration biomarkers in nutritional epidemiology: example of carotenoid and tocopherol intake in relation to chronic disease risk. Am J Clin Nutr. 2019;109:1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 6. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 7. Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, Tinker L, Schoeller D, Bingham S, Eaton CB et al.. Evaluation and comparison of food records, recalls and frequencies for energy and protein assessment using recovery biomarkers. Am J Epidemiol. 2011;174:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neuhouser ML, Di C, Tinker LF, Thomson C, Sternfeld B, Mossavar-Rahmani Y, Stefanick ML, Sims S, Curb JD, Lamonte M et al.. Physical activity assessment: biomarkers and self-report of activity-related energy expenditure in the WHI. Am J Epidemiol. 2013;177:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gross M, Yu X, Hannan P, Prouty C, Jacobs DR Jr. Lipid standardization of serum fat-soluble antioxidant concentrations: the YALTA study. Am J Clin Nutr. 2003;77(2):458–66. [DOI] [PubMed] [Google Scholar]
- 10. Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M et al.. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–8. [DOI] [PubMed] [Google Scholar]
- 11. Margolis KL, Qi L, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LF et al.. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. J Soc Clin Trials. 2008;5:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox DR. Regression models and life tables [with discussion]. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 13. Prentice RL. Covariate measurement errors and parameter estimation in a failure time regression model. Biometrika. 1982;69:331–42. [Google Scholar]
- 14. Wang CY, Hsu L, Feng ZD, Prentice RL. Regression calibration in failure time regression. Biometrics. 1997;53:131–45. [PubMed] [Google Scholar]
- 15. Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement error in nonlinear models, a modern perspective. 2nd ed Boca Raton (FL): Chapman and Hall/CRC; 2006. [Google Scholar]
- 16. World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition and the prevention of cancer: a global perspective. Washington (DC): American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 17. World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): American Institute for Cancer Research; 2007. [Google Scholar]
- 18. World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation.World Health Organ Tech Rep Ser. 2003;916:i–viii, 1–149. [PubMed] [Google Scholar]
- 19. Tamimi RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, Willett WC, Hunter DJ. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. 2005;161:153–60. [DOI] [PubMed] [Google Scholar]
- 20. Zheng C, Beresford SA, Horn LV, Tinker LF, Thomson CA, Neuhouser ML, Di C, Manson JE, Mossavar-Rahmani Y, Sequin R et al.. Simultaneous association of total energy consumption and activity-related energy expenditure with risks of cardiovascular disease, cancer, and diabetes among postmenopausal women. Am J Epidemiol. 2014;180(5):526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


