ABSTRACT
Background
Adolescents with endometriosis are a particularly underserved population who struggle with chronic pain. Despite widespread use, there are no published trials examining the individual effects of vitamin D and omega-3 (n–3) fatty acid supplementation on endometriosis-associated pain in adolescents.
Objectives
We aimed to determine whether supplementation with vitamin D or ω-3 fatty acids remediates pain, changes frequency of pain medication usage, or affects quality of life in young women with endometriosis.
Methods
Women (aged 12–25 y) with surgically confirmed endometriosis and pelvic pain enrolled in a double-blind, randomized, placebo-controlled trial. The primary outcome was pain measured by the visual analog scale (VAS). Secondary outcomes were quality of life, pain catastrophizing, and pain medication usage. Participants were randomly assigned to receive 2000 IU vitamin D3, 1000 mg fish oil, or placebo daily for 6 mo.
Results
A total of 147 women were screened and 69 were randomly assigned as follows: 27 to vitamin D3; 20 to fish oil; and 22 to placebo. Participants in the vitamin D arm experienced significant improvement in VAS pain [mean (95% CI) worst pain in the past month, from baseline to 6 mo: 7.0 (6.2, 7.8) to 5.5 (4.2, 6.8), P = 0.02]; however, an improvement of nearly identical magnitude was observed in the placebo arm [6.0 (5.1, 6.9) to 4.4 (3.0, 5.8), P = 0.07]. A more modest improvement was observed in the fish oil arm [5.9 (4.8, 7.0) to 5.2 (3.7, 6.8), P = 0.39]. Neither of the intervention arms were statistically different from placebo.
Conclusions
In young women with endometriosis, supplementation with vitamin D led to significant changes in pelvic pain; however, these were similar in magnitude to placebo. Supplementation with fish oil resulted in about half of the VAS pain reduction of the other 2 arms. Studies are needed to better define the physiology underlying the observed reduction in pain score in the placebo arm that persisted across 6 mo.
This trial was registered at clinicaltrials.gov as NCT02387931.
Keywords: endometriosis, vitamin D, omega-3 fatty acids, pelvic pain, randomized controlled trial, adolescents
Introduction
Endometriosis is a gynecologic disorder defined by the presence of endometrial glands and stroma outside the uterus. This estrogen-dependent, chronic inflammatory condition is present in 6%–10% of women during the reproductive years (1, 2) and 25%–38% of adolescent girls with chronic pelvic pain (3).
Adolescents with endometriosis often struggle with chronic pain, missed school, poor social relationships, and concerns about future infertility (3). They may have significantly more pain than adults with endometriosis (4), and studies have shown a median delay in diagnosis of 7 y (5), and longer when symptoms of endometriosis begin before 19 y of age (6).
A multitude of articles and books exist in the lay press regarding the use of diet and supplementation to manage endometriosis (7–9). Providers are often hesitant to perform surgery or prescribe pain medications to young women with endometriosis, and many turn to nutritional supplementation for management of endometriosis symptoms. Despite these hypotheses and claims, there are no published data demonstrating an association between vitamin D or omega-3 supplementation and modification of endometriosis symptoms.
Endometriosis is a proinflammatory condition characterized by elevated concentrations of cytokines and growth factors (10–12), decreased cell apoptosis (13), and increased angiogenesis (14), all of which may be associated with greater pain (15). Vitamin D may decrease inflammatory factor proliferation (16, 17) and increase cell apoptosis (18). ω-3 Fatty acids may decrease growth factors (19) and limit cell survival (20), which may reduce progression of endometriosis.
Our hypothesis was that adjuvant nutritional supplementation with vitamin D or ω-3 fatty acids would result in clinically and statistically significant improvement in pain and quality of life, and reduce frequency of pain medication usage, in adolescent girls with endometriosis when compared with placebo.
Methods
Participants
We recruited nonpregnant females aged 12–25 y with a surgical diagnosis of endometriosis from the Pediatric Gynecology Clinic at Boston Children's Hospital (BCH) between October 2014 and November 2015. Patients with a history of kidney stones or concurrent chronic illnesses known to affect gastrointestinal absorption of nutrients were ineligible. Eligible participants were required to have a visual analog scale (VAS) score ≥ 3 out of 10 for their worst pain in the month preceding study enrollment. Participants with a baseline 25-hydroxyvitamin D [25(OH)D] concentration ≥ 100 ng/mL were excluded.
This nutrient trial (NCT02387931) tested adjuvant treatment in addition to participants’ current clinical standard of care. Therefore, participants were allowed to continue medical treatment of their endometriosis throughout the trial. Participants were required to discontinue all vitamins and nutritional supplements from the time of enrollment through the final 6-mo evaluation. Participation began ≥6 wk after surgery for endometriosis.
Study design
This was a single-site, double blind, randomized, placebo-controlled, multiarm parallel study performed in the United States.
Exposure
Participants were randomly assigned to receive either 1000 mg fish oil [720 mg ω-3 fatty acids, including 488 mg EPA (20:5n–3) and 178 mg DHA (22:6n–3)] daily, 2000 IU vitamin D3 (cholecalciferol) daily, or a placebo tablet taken orally daily for 6 mo. The fish oil and vitamin D3 used in this trial were produced by Nature MadeTM and are commercially available. The concentration and purity of the pills purchased for this trial were not verified. To ensure blinding, the fish oil and vitamin D3 were encapsulated by the BCH Research Pharmacy in opaque white size 00 gelatin capsules, with identical appearance and feel. The placebo was produced by filling these same white gelatin capsules with inert lactose powder. Supplement dosing was chosen to be below tolerable upper limits for daily intake in 12- to 25-y-old females to minimize possible side effects (21, 22).
Outcome
The primary outcome measure was worst pain in the past month measured using the validated VAS, which quantifies pain on a scale of 0–10, with 0 representing no pain and 10 representing severe pain (23). In addition, a “clinically meaningful” change in VAS pain scores was defined as ≥1.6 based on previous studies (24). Secondary outcomes included quality of life, measured by the validated Short Form 12 (SF-12) questionnaire (25). The SF-12 has physical and mental components that are each scored on a scale of 0–100, with 0 indicating the lowest quality of life. Catastrophic thinking, a validated measure of pain sensitivity, was evaluated on a scale of 0–52, with higher scores indicating a greater amount of catastrophizing, such as “I think that the pain will never improve” (26). Participants also reported the mean number of nonnarcotic pain tablets taken per week. All measures were assessed at the baseline, 3-mo, and 6-mo study visits.
Sample size calculation
A priori, the study was powered to detect a minimum difference in change of 1.6 in VAS pain scores from baseline to 6 mo between each intervention study arm and placebo. This difference of 1.6 in VAS pain scores has been demonstrated to be the minimal change in pain that is clinically perceptible (24), that is, the minimal change in pain that can be perceived by the patient as an improvement or a worsening of pain. For comparing each intervention group with placebo we determined that it would require 19 participants per arm to detect a difference of ≥1.6 in VAS score, assuming an SD of 1.7, with 80% power (type II error = 0.20) and an α (type I) error = 0.05.
Randomization
An independent biostatistician developed a permuted blocks randomization schema to achieve groups of similar size (block size = 9, not varied randomly) utilizing Stata StataCorp version 12.1. This schema was sent by the biostatistician directly to the BCH Research Pharmacy and was not shared with any study team member. The BCH Research Pharmacy applied intervention arm assignment as dictated by the randomization schema on the day of participant enrollment and allocated the blinded study medications accordingly.
Data collection and participant interaction
Enrolled participants presented to the Clinical and Translational Study Unit at BCH for study visits at baseline, 3 mo, and 6 mo after enrollment. At baseline, participants were given a capsule to swallow in order to assess whether they could swallow the study medications. Participants were then randomly assigned, and given a bottle containing their study medication. Participants completed a baseline survey including demographic information, medical and surgical history, physical activity, and pain medication history that is an expanded version of the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project standard clinical questionnaire (27). Participants also completed a standardized 128-item FFQ at baseline to allow investigators to quantify their usual food and nutrient intake during the last year (28). At each study visit, participants completed a pain and quality of life survey (details as aforementioned) and provided information regarding pain medication usage, hormonal therapy, and perceived medication side effects.
Biochemical assessments
Laboratory measurements were obtained at the baseline (before study exposure) and 6-mo visits. Serum samples were analyzed in the BCH Core Laboratory for measurement of 25(OH)D concentrations (chemiluminescent assay: Liason®, Diasorin). Serum ω-3 fatty acid assessment was performed using LC-MS by the Harvard School of Public Health Biomarker Research Laboratory. The purpose of both the 25(OH)D and ω-3 fatty acid measurements was to evaluate compliance with taking the study nutrient to which the participant was randomly assigned, whereas the purpose of the baseline 25(OH)D measurements was to ensure that no trial participant had a serum 25(OH)D concentration > 100 ng/mL at study entry.
Ethics
The BCH Institutional Review Board approved this trial. Written informed consent was obtained, with parental consent and participant assent for patients younger than 18 y of age.
Statistical analysis
We employed intention-to-treat analysis under the assumption of missing at random for all statistical models. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.).
We used linear mixed models to compare change in pain severity, physical and mental quality of life, catastrophic thinking, and mean number of nonnarcotic pain pills taken per week over the course of the study period. Random intercepts were included to allow for different intercepts for each patient. Study arm, time, and study arm-by-time interaction terms were modeled as fixed effects. This method allowed for the inclusion of participants with missing data at various time points.
To examine a “clinically meaningful” change in VAS pain scores (as aforementioned) between baseline and 6 mo, we used a logistic regression model and dichotomized change in VAS score (<1.6 and ≥1.6). We conducted sensitivity analyses restricted to those taking contraceptives containing estrogen.
To examine adherence to assigned treatment arm we compared change in serum markers [25(OH)D, total ω-3 fatty acids, EPA, and DHA] at baseline and 6 mo within groups and between groups (placebo compared with each intervention arm) using linear mixed models.
Results
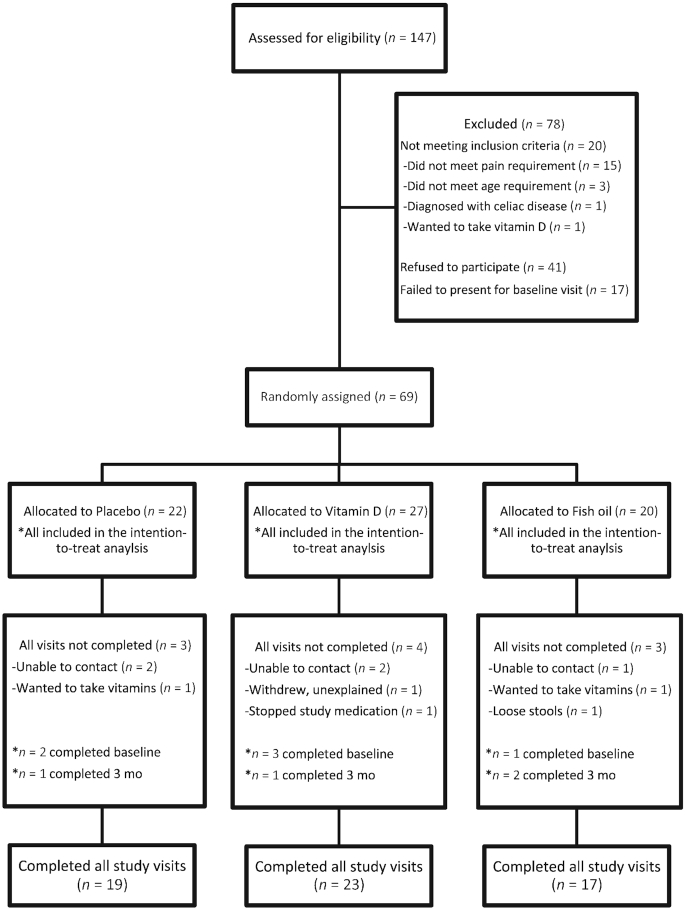
A total of 69 participants were randomly assigned, and data from all of these participants were included in the analyses (Figure 1). Ten participants did not complete all study visits. There were no differences between trial arms in terms of the number of participants who did not complete the study (Figure 1). Fifty-nine participants completed all study visits; there were no material differences in characteristics between completers and noncompleters (Supplemental Table 1). For example, the baseline mean ± SD worst pain in the previous month was 6.3 ± 2.0 for participants who did not complete the study and 6.4 ± 2.4 for those who completed the study.
FIGURE 1.
Flow diagram for participant recruitment and random assignment.
Study arms were similar in age, race, BMI, and other baseline characteristics (Table 1). Those women who declined to participate in the trial were similar in these characteristics to those from the eligible source population who chose to participate (29). The majority of the participants had stage 1 endometriosis at the time of surgical diagnosis. Most participants were normal weight, self-reported white race, and were using hormonal medications for treatment of endometriosis at baseline. Four study participants began the trial exposed to a method of hormonal suppression and then discontinued use during the 6-mo trial duration. Two participants were not using a method of hormonal suppression at the beginning of the trial and subsequently began hormonal suppression during the trial. Only 3 participants had no hormonal suppression exposure at any point during the trial.
TABLE 1.
Baseline characteristics of 69 randomly assigned participants with surgically confirmed endometriosis, by study arm1
| Patient characteristics | Placebo (n = 22) | Vitamin D (n = 27) | Fish oil (n = 20) |
|---|---|---|---|
| Age, y | 20.1 ± 3.5 | 20.0 ± 2.7 | 18.9 ± 3.1 |
| Race | |||
| White | 21 (95.5) | 24 (88.9) | 19 (95.0) |
| Other | 1 (4.5) | 3 (11.1) | 1 (5.0) |
| BMI | |||
| Kg/m2 | 25.6 ± 5.1 | 26.2 ± 5.7 | 26.4 ± 5.6 |
| Under- to normal weight | 13 (59.1) | 15 (55.8) | 8 (40.0) |
| Overweight | 3 (13.6) | 3 (11.1) | 7 (35.0) |
| Obese | 6 (27.3) | 9 (33.3) | 5 (25.0) |
| Currently in school | |||
| Yes | 19 (86.4) | 24 (88.9) | 17 (85.0) |
| No | 3 (13.6) | 3 (11.1) | 3 (15.0) |
| If in school, what grade? | |||
| Middle to high school | 8 (42.1) | 8 (33.3) | 10 (58.8) |
| College or grad school | 11 (57.9) | 17 (66.7) | 7 (41.2) |
| Baseline pain, visual analog scale score | |||
| Worst pain during the last week | 4.1 ± 2.4 | 5.4 ± 2.7 | 4.8 ± 2.6 |
| Worst pain during the last month | 6.0 ± 1.9 | 7.0 ± 2.2 | 5.9 ± 2.9 |
| Baseline nonnarcotic pain medications, tablets/wk | 2.5 ± 3.8 | 3.5 ± 4.8 | 2.9 ± 4.7 |
| Baseline nutrition | |||
| Total energy, kcal/d | 1813.1 ± 828.3 | 1762.1 ± 648.0 | 1865.3 ± 616.3 |
| Calories from fat, % | 33.0 ± 6.0 | 31.0 ± 4.0 | 33.0 ± 7.0 |
| Omega-3 fatty acids, g/d | 1.4 ± 0.9 | 1.2 ± 0.6 | 1.4 ± 0.6 |
| Vitamin D, IU/d | 260.1 ± 242.0 | 305.4 ± 348.9 | 322.4 ± 268.2 |
| Endometriosis rASRM stage | |||
| I | 17 (77.3) | 23 (85.2) | 13 (65.0) |
| II | 4 (18.2) | 3 (11.1) | 7 (35.0) |
| III | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IV | 1 (4.5) | 1 (3.7) | 0 (0.0) |
| Baseline hormonal therapies | |||
| Combination hormone | 12 (54.5) | 14 (51.9) | 14 (70.0) |
| Progestin-only hormone | 8 (36.4) | 11 (40.7) | 4 (20.0) |
| Leuprolide acetate | 1 (4.5) | 0 (0.0) | 0 (0.0) |
| None | 1 (4.5) | 2 (7.4) | 2 (10.0) |
| Baseline 25(OH)D serum by season of enrollment, ng/mL | |||
| Winter: January–March | 9 (40.9); 27.5 ± 6.7 | 8 (29.6); 35.3 ± 13.9 | 10 (50.0); 33.0 ± 9.8 |
| Spring: April–June | 2 (9.1); 36.3 ± 3.9 | 3 (11.1); 23.1 ± 5.8 | 3 (15.0); 31.7 ± 7.1 |
| Summer: July–September | 2 (9.1); 39.4 ± 17.2 | 4 (14.8); 41.2 ± 15.9 | 2 (10.0); 35.5 ± 5.1 |
| Fall: October–December | 9 (40.9); 31.9 ± 8.8 | 12 (44.4); 33.0 ± 13.5 | 5 (25.0); 38.5 ± 9.5 |
| Time from surgery to study enrollment | |||
| 6 wk to <6 mo | 6 (27.3) | 7 (25.9) | 2 (10.0) |
| 6 mo to <1 y | 4 (18.2) | 4 (14.8) | 3 (15.0) |
| 1 y to <2 y | 7 (31.8) | 9 (33.3) | 10 (50.0) |
| ≥2 y | 5 (22.7) | 7 (25.9) | 5 (25.0) |
Values are means ± SDs or n (%). rASRM, revised American Society for Reproductive Medicine; 25(OH)D, 25-hydroxyvitamin D.
Participants were similar in baseline VAS pain scores, with mean ± SD worst pain in the previous month recorded as 6.0 ± 1.9 in the placebo arm, 7.0 ± 2.2 in the vitamin D arm, and 5.9 ± 2.9 in the fish oil arm (Table 1). Participants in all study arms also reported similar use of nonnarcotic pain pills for control of endometriosis at baseline. Participants in all study arms reported similar baseline consumption of vitamin D, ω-3 fatty acids, and other nutritional components (Table 1). The mean serum 25(OH)D concentration among participants in all groups suggested vitamin D sufficiency [25(OH)D > 30 ng/mL] at baseline (Table 1).
All 3 study arms demonstrated improvements in pain severity, with “worst pain in the last month” mean VAS scores improving from baseline to 6 mo in the placebo (6.0 to 4.4, P value = 0.07), vitamin D (7.0 to 5.5, P value = 0.02), and fish oil (5.9 to 5.2, P value = 0.39) arms. However, a statistically significant change applying a P value < 0.05 dichotomization was noted only in the vitamin D arm. Further, there were no statistically significant differences in change in VAS pain scores when comparing either vitamin D or fish oil with placebo (Table 2). When “clinically meaningful” change in VAS (dichotomized at a change ≥1.6) score for “worst pain in the last month” was examined, the odds of a clinically meaningful change in pain score for those randomly assigned to vitamin D supplementation between the baseline and 6-mo time points were 2 times greater than for those randomly assigned to placebo and to fish oil. However, the CI was wide, and therefore a statistically significant difference between exposure arms in the percentage of participants who experienced this level of change was not identified (Table 2). In sensitivity results restricted to women taking contraceptives containing estrogen, no material differences in results were observed. For example, in this restricted population, VAS pain scores improved from baseline to 6 mo in the placebo (n = 12, 5.5 to 4.6, P value = 0.32), vitamin D (n = 14, 6.3 to 5.3, P value = 0.15), and fish oil (n = 14, 5.6 to 5.1, P value = 0.67) arms, with no significant differences observed when comparing either vitamin D or fish oil with placebo.
TABLE 2.
Change in pain and quality of life by study arm1
| Placebo | Vitamin D | Fish oil | Vitamin D vs. placebo | Fish oil vs. placebo | ||||
|---|---|---|---|---|---|---|---|---|
| n | n | n | P value | |||||
| VAS pain score for worst pain in the past month | ||||||||
| Baseline | 22 | 6.0 (5.1, 6.9) | 27 | 7.0 (6.2, 7.8) | 20 | 5.9 (4.8, 7.0) | ||
| 3 mo | 20 | 4.9 (3.7, 6.1) | 24 | 6.2 (5.2, 7.3) | 19 | 5.9 (4.7, 7.1) | 0.672 | 0.192 |
| 6 mo | 19 | 4.4 (3.0, 5.8) | 23 | 5.5 (4.2, 6.8) | 17 | 5.2 (3.7, 6.8) | 0.972 | 0.422 |
| P value | 0.073 | 0.023 | 0.393 | 0.914 | 0.394 | |||
| Clinically meaningful improvement (>1.6) in monthly VAS pain score from baseline to 6 mo, OR (95% CI) | 7 | 1.00 (reference) | 12 | 1.9 (0.5, 6.5) | 6 | 0.9 (0.2, 3.7) | 0.32 | 0.92 |
| SF-12 physical component | ||||||||
| Baseline | 20 | 46.8 (43.3, 50.2) | 26 | 41.6 (38.6, 44.7) | 20 | 45.7 (42.0, 49.5) | ||
| 3 mo | 17 | 46.0 (42.1, 50.0) | 24 | 40.7 (37.4, 44.2) | 19 | 49.9 (46.6, 53.2) | 0.982 | 0.052 |
| 6 mo | 18 | 49.1 (44.4, 53.8) | 22 | 44.7 (40.4, 49.0) | 16 | 50.4 (46.3, 54.6) | 0.722 | 0.392 |
| P value | 0.223 | 0.233 | 0.123 | 0.964 | 0.124 | |||
| SF-12 mental component | ||||||||
| Baseline | 20 | 41.2 (36.3, 46.0) | 26 | 44.5 (40.1, 48.8) | 20 | 44.1 (39.0, 49.2) | ||
| 3 mo | 17 | 42.5 (37.9, 47.1) | 24 | 47.4 (43.4, 51.4) | 19 | 45.7 (40.6, 50.9) | 0.692 | 0.982 |
| 6 mo | 18 | 41.8 (36.7, 46.8) | 22 | 43.6 (39.0, 48.2) | 16 | 46.9 (41.5, 52.4) | 0.702 | 0.472 |
| P value | 0.843 | 0.193 | 0.313 | 0.714 | 0.804 | |||
| Catastrophic thinking score | ||||||||
| Baseline | 22 | 23.1 (19.0, 27.1) | 27 | 25.3 (21.6, 28.9) | 20 | 21.0 (17.3, 24.8) | ||
| 3 mo | 20 | 20.0 (16.6, 23.5) | 24 | 21.9 (18.7, 25.0) | 19 | 18.6 (15.7, 21.6) | 0.852 | 0.762 |
| 6 mo | 19 | 19.6 (15.4, 23.8) | 23 | 20.8 (16.9, 24.6) | 17 | 20.3 (16.0, 24.7) | 0.672 | 0.522 |
| P value | 0.113 | 0.043 | 0.063 | 0.914 | 0.494 | |||
| Nonnarcotic tablets, n/wk | ||||||||
| Baseline | 22 | 2.5 (0.6, 4.3) | 27 | 3.5 (1.8, 5.2) | 20 | 2.9 (1.0, 4.8) | ||
| 3 mo | 20 | 2.2 (0.1, 4.4) | 24 | 4.8 (2.8, 6.7) | 19 | 1.7 (0.1, 3.3) | 0.372 | 0.542 |
| 6 mo | 19 | 3.3 (0.8, 5.9) | 23 | 3.2 (0.9, 5.6) | 16 | 1.3 (−1.2, 3.7) | 0.572 | 0.802 |
| P value | 0.283 | 0.413 | 0.143 | 0.184 | 0.284 | |||
Values are means (95% CIs) unless otherwise indicated. SF-12, Short Form 12; VAS, visual analog scale.
Test for difference between groups (group-by-time interaction) from baseline to 3 mo and from baseline to 6 mo. Comparing vitamin D or fish oil with placebo.
Test for change over the study period (baseline, 3 mo, and 6 mo) within each arm (i.e., placebo, vitamin D, and fish oil).
Test for change over the study period (group-by-time interaction) from baseline to 3 mo to 6 mo between each intervention arm (vitamin D or fish oil) and placebo using linear mixed models (fixed effects = study arm, time, and study arm-by-time interaction term; random effect = subject).
No consistent patterns in changes in physical or mental quality of life were observed within groups and there was no significant difference in the changes reported in these quality of life measures when comparing either vitamin D or fish oil with placebo over the 6-mo intervention (Table 2). Participants in all 3 study arms demonstrated improvement in catastrophic thinking score, with a statistically significant mean score improvement from baseline to 6 mo only in the vitamin D (25.3 to 20.8, P = 0.04) arm (Table 2). No significant differences between groups or changes over time were observed in the mean number of nonnarcotic pain tablets taken per week (Table 2).
As expected, only participants randomly assigned to vitamin D supplementation demonstrated a significant change in mean serum 25(OH)D concentrations from baseline to 6 mo. Similarly, only participants randomly assigned to fish oil demonstrated a significant increase in total ω-3 fatty acids, EPA, and DHA from baseline to 6 mo (Table 3).
TABLE 3.
Serum 25(OH)D and fatty acid concentrations at baseline and 6 mo by study arm1
| Placebo | Vitamin D | Fish oil | Vitamin D vs. placebo | Fish oil vs. placebo | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | P values | ||
| 25(OH)D serum, ng/mL | ||||||||
| Baseline | 22 | 31.2 (26.2, 36.2) | 27 | 33.8 (29.3, 38.3) | 19 | 34.7 (30.7, 38.8) | 0.422 | 0.232 |
| 6 mo | 19 | 33.5 (28.2, 38.8) | 23 | 42.5 (37.7, 47.3) | 17 | 33.5 (28.2, 38.7) | 0.093 | 0.323 |
| P value4 | 0.32 | 0.003 | 0.48 | |||||
| Total omega-3 fatty acids, % of total fatty acids | ||||||||
| Baseline | 22 | 3.2 (2.9, 3.5) | 27 | 2.7 (2.4, 2.9) | 20 | 2.7 (2.3, 3.0) | 0.022 | 0.032 |
| 6 mo | 18 | 3.1 (2.8, 3.4) | 23 | 2.5 (2.2, 2.7) | 17 | 4.0 (3.6, 4.4) | 0.693 | <0.00013 |
| P value4 | 0.55 | 0.11 | <0.0001 | |||||
| EPA, % of total fatty acids, geometric mean (95% CI) | ||||||||
| Baseline | 22 | 0.5 (0.4, 0.6) | 27 | 0.3 (0.2, 0.4) | 20 | 0.3 (0.2, 0.4) | 0.032 | 0.022 |
| 6 mo | 18 | 0.5 (0.4, 0.6) | 23 | 0.3 (0.2, 0.4) | 17 | 1.0 (0.9, 1.2) | 0.753 | <0.00013 |
| P value4 | 0.60 | 0.67 | <0.0001 | |||||
| DHA, % of total fatty acids | ||||||||
| Baseline | 22 | 1.7 (1.5, 1.9) | 27 | 1.4 (1.2, 1.6) | 20 | 1.5 (1.2, 1.7) | 0.032 | 0.112 |
| 6 mo | 18 | 1.6 (1.5, 1.8) | 23 | 1.2 (1.1, 1.4) | 17 | 1.9 (1.6, 2.1) | 0.383 | 0.00023 |
| P value4 | 0.47 | 0.02 | <0.0001 | |||||
25(OH)D, 25-hydroxyvitamin D.
t Test comparing baseline mean scores between intervention arm (vitamin D or fish oil) and placebo.
Test for change from baseline to 6 mo (group-by-time interaction) between each intervention arm (vitamin D or fish oil) and placebo using linear mixed models (fixed effects = study arm, time, and study arm-by-time interaction term; random effect = subject).
Test for change from baseline to 6 mo within each arm (i.e., placebo, vitamin D, and fish oil).
Discussion
In this study, we have demonstrated that 6 mo of supplementation with either vitamin D or fish oil does not lead to a clinically or statistically significant change in pain in adolescent girls and young women with endometriosis when compared with placebo. However, a trend toward improvement in pelvic pain severity scores and in the amount of catastrophic thinking was demonstrated in all 3 study arms over the 6-mo intervention. This similar improvement among participants in all study arms, including those who took placebo, points to the effect of enhanced clinical interaction and trial participation on psychological and physical well-being. This finding not only reinforces the importance of a placebo arm in trials testing pain and quality of life, it also suggests that enhanced clinical interaction and focus on exposure to a new treatment may confer benefits in and of themselves.
To our knowledge, this is the first published randomized trial examining the association between nutritional supplementation with ω-3 fatty acids and modification of endometriosis symptoms in humans, and the first randomized trial examining vitamin D supplements in adolescents. Previous intervention trials in adult women with primary dysmenorrhea have demonstrated a significant reduction in pain after vitamin D (30) or ω-3 fatty acid (31) supplementation. In observational studies, adult diets high in vitamin D (32) and ω-3 fatty acids (33) have been associated with lower rates of endometriosis diagnosis. In animal studies, supplementation with vitamin D (34, 35) or ω-3 fatty acids (36) suggested significant reductions in the size of surgically implanted endometriosis lesions. Our results are consistent with the 1 previous randomized trial examining vitamin D supplementation and endometriosis-related pain among women ages 15–40 y (mean age: 29.9 y). Almassinokiani et al. (37) observed no difference in mean pelvic pain between the vitamin D and placebo groups, with both groups reporting a similar reduction in VAS pain score across the study period, with VAS decreasing from 3.8 to 2.0 in the vitamin D group and from 4.4 to 2.7 in the placebo group.
A potential weakness of the present trial is the relatively small sample size. It is possible that a larger trial with more participants could demonstrate a statistically significant improvement in clinically meaningful pain for users of vitamin D or fish oil when compared with placebo. In this trial, the similar improvements in pain severity scores and catastrophic thinking among participants in all 3 arms including placebo all point to a strong placebo effect. It is true that given this observation, a much larger sample size would be needed to detect statistically significant differences between the 2 treatment arms and the placebo group. However, even then this statistical significance would not be clinically translatable because the magnitude of the difference, for example in pain scores, would be too small (≤1.6 in VAS pain scores) to perceive a clinically superior improvement in 1 group over another.
An additional limitation of the small sample size is that we were not able to examine how polymorphisms in vitamin D–related genes (e.g., VDR) might interact with supplementation. Further, given that our participants were not low in vitamin D (<20 ng/mL) at baseline, there is the possibility that a stronger effect would have been observed for vitamin D supplementation among a low-vitamin-D or vitamin-D-deficient population. Another weakness was that the concentration and purity of the pills purchased for this trial designed to reflect real-world supplements to which participants would have had access were not verified, which could have resulted in an underestimation of the effect of the supplements.
A major strength of this study was the randomized, double-blind, placebo-controlled design. The placebo control made it possible for us to correctly attribute the improvement in pain seen among participants in this trial to the effect of trial participation, and not to the individual supplements themselves. Also, we utilized multiple components of response, such as VAS pain, quality of life, catastrophic thinking, and pain medication usage, which allowed us to more accurately gauge the effectiveness of the interventions. Another strength of this trial was the use of serum 25(OH)D and ω-3 fatty acids to measure compliance with study medications. Compliance is always a large concern in the design of trials of months-long duration, and this study had the additional challenge that participants were all adolescents. Differentiating endometriosis from primary dysmenorrhea via surgical confirmation was central to this study, because previous trials have already demonstrated symptomatic improvement in women with primary dysmenorrhea after supplementation with vitamin D and ω-3 fatty acids. The only way to definitively diagnose endometriosis is by surgical confirmation (38); however, it can be difficult to find an adequate number of participants, especially in the adolescent age range, that have undergone surgery.
In conclusion, supplementation with vitamin D in adolescents with surgically confirmed endometriosis led to statistically significant improvements in pelvic pain and catastrophic thinking; however, they did not differ in magnitude from the effect observed among those who received placebo. Improvement in VAS pain was observed among those exposed to fish oil, but it was of about half the magnitude of those in the other 2 arms and not a statistically significant trend across the 6 mo of follow-up. Change over 6 mo did not differ significantly between participants in either supplement arm and those in the placebo arm. Caution must be applied given the widespread direct marketing of these supplements to girls and women with endometriosis implying a beneficial impact on symptoms. The strong placebo effect was evident in multiple outcome measures, suggesting that participation itself, and not the supplements, conferred improvement even at 6 mo.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Katharine Correia for providing statistical review. We also thank the faculty and staff of the Boston Center for Endometriosis.
The authors’ responsibilities were as follows—JLN, ADD, MM, and SAM: designed the research; JLN, SK, and MM: conducted the research; SK and MM: collected the data; JLN and SAM: analyzed the data, wrote the paper, and had primary responsibility for the final content; ADD, AFV, and HRH: interpreted the results and contributed to manuscript revision; AFV, VS, AF, and HRH: performed the statistical analysis; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by a New England Fertility Society/Ferring REI Fellow research grant (to JLN); a Department of Obstetrics, Gynecology, and Reproductive Biology, Brigham and Women's Hospital, Expanding the Boundaries research grant (to JLN); a McCarthy Family Foundation research grant (to ADD); the J Willard and Alice S Marriott Foundation (to SAM); NIH research grants HD48544 and HD52473 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to SAM); Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI) grant CA50385 (to SAM); and NCI grant K22 CA193860 (to HRH).
Data collection was facilitated by and conducted in compliance with the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article will be made available upon request.
HRH and SAM contributed equally to this work.
Abbreviations used: BCH, Boston Children's Hospital; SF-12, Short Form 12; VAS, visual analog scale; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
James L Nodler, Department of Obstetrics and Gynecology, Colorado Center for Reproductive Medicine-Houston, Houston, TX, USA; Department of Obstetrics, Gynecology, and Reproductive Biology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Amy D DiVasta, Boston Center for Endometriosis, Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA, USA; Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA; Division of Gynecology, Department of Surgery, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
Allison F Vitonis, Department of Obstetrics, Gynecology, and Reproductive Biology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Boston Center for Endometriosis, Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA, USA.
Sarah Karevicius, Boston Center for Endometriosis, Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA, USA.
Maggie Malsch, Institutional Centers for Clinical and Translational Research, Boston Children's Hospital, Boston, MA, USA.
Vishnudas Sarda, Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
Ayotunde Fadayomi, Department of Obstetrics, Gynecology, and Reproductive Biology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Boston Center for Endometriosis, Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA, USA.
Holly R Harris, Program in Epidemiology, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
Stacey A Missmer, Boston Center for Endometriosis, Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA, USA; Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Obstetrics, Gynecology, and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, MI, USA.
References
- 1. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–96. [DOI] [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 310, April 2005. Endometriosis in adolescents. Obstet Gynecol. 2005;105(4):921–7. [DOI] [PubMed] [Google Scholar]
- 4. Lee D-Y, Kim HJ, Yoon B-K, Choi D. Clinical characteristics of adolescent endometrioma. J Pediatr Adolesc Gynecol. 2013;26(2):117–19. [DOI] [PubMed] [Google Scholar]
- 5. Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT; World Endometriosis Research Foundation Global Study of Women's Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–73..e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756–9. [DOI] [PubMed] [Google Scholar]
- 7. Case HS. The vitamin cure for women's health problems. Laguna Beach, CA: Basic Health Publications, Inc; 2012. [Google Scholar]
- 8. Goldfarb S, Waddell RW. Relieving pain naturally. Garden City Park, NY: Square One Publishers; 2005. [Google Scholar]
- 9. Mears J. Endometriosis: a natural approach. Berkeley, CA: Ulysses Press; 1998. [Google Scholar]
- 10. Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56(1):45–51. [DOI] [PubMed] [Google Scholar]
- 11. Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-β activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83(2):287–92. [PubMed] [Google Scholar]
- 12. Badaway SZ, Cuenca V, Freliech H, Stefanu C. Endometrial antibodies in serum and peritoneal fluid of infertile patients with and without endometriosis. Fertil Steril. 1990;53(5):930–2. [DOI] [PubMed] [Google Scholar]
- 13. Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod. 1998;13(12):3496–502. [DOI] [PubMed] [Google Scholar]
- 14. Laschke MW, Giebels C, Menger MD. Vasculogenesis: a new piece of the endometriosis puzzle. Hum Reprod Update. 2011;17(5):628–36. [DOI] [PubMed] [Google Scholar]
- 15. Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–82. [DOI] [PubMed] [Google Scholar]
- 16. Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, Bostick RM. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res. 2011;4(10):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20(2):R31–47. [DOI] [PubMed] [Google Scholar]
- 19. Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–73. [DOI] [PubMed] [Google Scholar]
- 20. Gazvani MR, Smith L, Haggarty P, Fowler PA, Templeton A. High ω-3:ω-6 fatty acid ratios in culture medium reduce endometrial-cell survival in combined endometrial gland and stromal cell cultures from women with and without endometriosis. Fertil Steril. 2001;76(4):717–22. [DOI] [PubMed] [Google Scholar]
- 21. Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14(5):938–9. [DOI] [PubMed] [Google Scholar]
- 22. Trumbo P, Schlicker S, Yates AA, Poos M, Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. [DOI] [PubMed] [Google Scholar]
- 23. Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–8. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20(4):287–90. [DOI] [PubMed] [Google Scholar]
- 25. Ware J Jr, Kosinski M, Keller SD. A 12-item Short-Form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan M. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- 27. Vitonis AF, Vincent K, Rahmioglu N, Fassbender A, Buck Louis GM, Hummelshoj L, Giudice LC, Stratton P, Adamson GD, Becker CM et al.. World Endometriosis Research Foundation Endometriosis Phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril. 2014;102(5):1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 29. Gallagher JS, DiVasta AD, Vitonis AF, Sarda V, Laufer MR, Missmer SA. The impact of endometriosis on quality of life in adolescents. J Adolesc Health. 2018;63(6):766–72. [DOI] [PubMed] [Google Scholar]
- 30. Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012;172(4):366–7. [DOI] [PubMed] [Google Scholar]
- 31. Rahbar N, Asgharzadeh N, Ghorbani R. Effect of omega-3 fatty acids on intensity of primary dysmenorrhea. Int J Gynaecol Obstet. 2012;117(1):45–7. [DOI] [PubMed] [Google Scholar]
- 32. Harris HR, Chavarro JE, Malspeis S, Willett WC, Missmer SA. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol. 2013;177(5):420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25(6):1528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abbas MA, Taha MO, Disi AM, Shomaf M. Regression of endometrial implants treated with vitamin D3 in a rat model of endometriosis. Eur J Pharmacol. 2013;715(1–3):72–5. [DOI] [PubMed] [Google Scholar]
- 35. Mariani M, Vigano P, Gentilini D, Camisa B, Caporizzo E, Di Lucia P, Monno A, Candiani M, Somigliana E, Panina-Bordignon P. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum Reprod. 2012;27(7):2010–19. [DOI] [PubMed] [Google Scholar]
- 36. Covens AL, Christopher P, Casper RF. The effect of dietary supplementation with fish oil fatty acids on surgically induced endometriosis in the rabbit. Fertil Steril. 1988;49(4):698–703. [DOI] [PubMed] [Google Scholar]
- 37. Almassinokiani F, Khodaverdi S, Solaymani-Dodaran M, Akbari P, Pazouki A. Effects of vitamin D on endometriosis-related pain: a double-blind clinical trial. Med Sci Monit. 2016;22:4960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.