ABSTRACT
Background
Advanced glycation end products (AGEs) accumulate in tissues with age and in conditions such as diabetes mellitus and chronic kidney disease (CKD), and they may be involved in age-related diseases. Skin AGEs measured as skin autofluorescence (SAF) are a noninvasive reflection of long-term AGE accumulation in tissues. Whether AGEs present in the diet (dAGEs) contribute to tissue AGEs is unclear.
Objectives
Our aim was to investigate the association between dietary and skin AGEs in the Rotterdam Study, a population-based cohort of mainly European ancestry.
Methods
In 2515 participants, intake of 3 dAGEs [carboxymethyl-lysine (CML), N-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MGH1), and carboxyethyl-lysine (CEL)] was estimated using FFQs and the content of AGEs measured in commonly consumed foods. SAF was measured 5 y (median value) later using an AGE Reader. The association of dAGEs with SAF was analyzed in linear regression models and stratified for diabetes and chronic kidney disease (CKD, defined as estimated glomerular filtration rate ≤60 mL/min) status.
Results
Mean ± SD intake was 3.40 ±0.89 mg/d for CML, 28.98 ±7.87 mg/d for MGH1, and 3.11 ±0.89 mg/d for CEL. None of them was associated with SAF in the total study population. However, in stratified analyses, CML was positively associated with SAF after excluding both individuals with diabetes and individuals with CKD: 1 SD higher daily CML intake was associated with a 0.03 (95% CI: 0.009, 0.05) arbitrary units higher SAF. MGH1 and CEL intake were not significantly associated with SAF. Nevertheless, the associations were stronger when the time difference between dAGEs and SAF measurements was shorter.
Conclusions
Higher dietary CML intake was associated with higher SAF only among participants with neither diabetes nor CKD, which may be explained by high AGE formation in diabetes and decreased excretion in CKD or by dietary modifications in these disease groups. The dAGE–SAF associations were also modified by the time difference between measurements. Our results suggest that dAGEs can influence tissue AGE accumulation and possibly thereby age-related diseases. This trial was registered at the Netherlands National Trial Register as NTR6831 (http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6831) and at the WHO International Clinical Trials Registry Platform as NTR6831 (http://www.who.int/ictrp/network/primary/en/).
Keywords: advanced glycation end products (AGEs), diet, skin autofluorescence, carboxymethyl-lysine, N-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine, carboxyethyl-lysine
Introduction
Advanced glycation end products (AGEs) are a cluster of heterogeneous molecules that are formed endogenously through nonenzymatic glycation of proteins, lipids, or nucleic acids and subsequent chemical rearrangements (1). They accumulate widely as part of normal aging, especially in long-lived tissues, because of irreversible formation and limited clearance mostly depending on tissue turnover rates (2). Independent of chronological age, AGEs are involved in the pathophysiology of aging and age-related disorders such as diabetes, cardiovascular diseases, and dementia by forming cross-links, modifying structures of proteins, or triggering inflammatory pathways by binding to the AGEs receptor (3). The amount of AGEs in the skin has been used as a reflection of the long-term accumulation of AGEs because skin collagen on which the AGEs form has a long half-life of ∼15 y (2, 4). Skin AGE measured as skin autofluorescence (SAF) was recently found to be related to incident diabetes, cardiovascular events, and mortality among Dutch people (5).
In addition to endogenous formation, AGEs are largely ingested via diet (6). Both unprocessed and processed foods (e.g., sausage roll, beefsteak, and skimmed milk) can contain AGEs, which are similar chemical entities as AGEs in vivo. Certain cooking conditions, such as high temperatures and low moisture during frying, baking, and grilling, can produce more AGEs due to excessive Maillard reactions taking place (7).
Several human, animal, and in vitro studies have suggested the involvement of dietary AGEs (dAGEs) in, for example, metabolic diseases (8, 9), cardiovascular diseases (9, 10), diabetes (11), Alzheimer's disease (12), food allergy (13), and hampered skeletal muscle growth and muscle contractile function (14). They can also trigger pathways related to cancer risk (15). A large longitudinal population study showed that dietary intake of carboxymethyl-lysine (CML)—one of the most widely studied AGEs—was associated with modestly increased risk of pancreatic cancer in men (but not in women, with a smaller sample size) and might partially explain the positive association between red meat intake and pancreatic cancer (16). However, results on health outcomes are inconclusive because several studies did not observe a protective effect from a low-AGEs diet, whereas others observed a protective effect on, for example, inflammation and cardiovascular risk (17, 18, 19). The inconsistency in results might be explained by differences in study design, population, or study duration ranging from a few weeks to years (a short duration may not capture effects adequately) (20).
Dietary AGEs may affect health via an effect on tissue AGE accumulation (21). Although a link between dietary and serum AGEs has been reported by several studies with small sample sizes (22, 23) and previous studies estimated that 10–30% of AGEs are absorbed into the circulation after oral administration (24), whether excessive dAGE intake associates with more tissue accumulation is unknown from large human studies. Deciphering whether dAGEs influence tissue AGEs in an adequately sized population is important to untangle the link between dAGEs and disease risk.
To narrow the gap, we studied associations between daily intake of 3 dAGEs and skin AGEs in a population-based cohort—the Rotterdam Study. The primary outcome was the association of dAGEs with SAF in the total study population, and the secondary outcome was the association among participants without diabetes or chronic kidney disease (CKD).
Methods
Study population
Study participants were from the Rotterdam Study (RS), which was initiated in 1990 when all inhabitants of the suburb Ommoord in the city of Rotterdam aged ≥55 y were invited to participate. The first subcohort (RS-I) included 7983 participants aged ≥55 y and started in 1990. The second subcohort (RS-II) included 3011 new participants aged ≥55 y and started in 2000. The third subcohort (RS-III) included 3932 new participants aged ≥45 y and started in 2006. All participants were examined at baseline, and every 4–6 y follow-up examinations took place. The majority of the study population is of European ancestry. The design and objectives of the RS have been extensively described previously (25). The RS was approved by the institutional review board (Medical Ethics Committee) of Erasmus Medical Center and by the review board of The Netherlands Ministry of Health, Welfare and Sports. All participants in the current analysis provided written informed consent to participate.
RS-I 5th, RS-II 3rd, and RS-III 1st visits constitute the baseline of our study. Dietary data were available for 5508 participants after excluding participants with implausible reported energy intake values (<500 or >5000 kcal/d). For 2523 of the 5508 participants, skin AGEs were measured as SAF on average 5 y later (RS-I 6th visit, RS-II 4th visit, and RS-III 2nd visit), from which we excluded 8 outliers (> ±6 SD), leaving 2515 participants included. The flowchart for participant inclusion and exclusion is shown in Figure 1.
FIGURE 1.
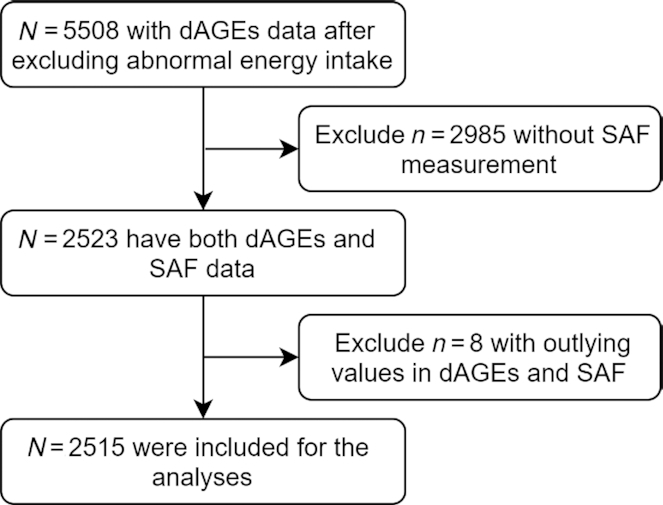
Participants inclusion and exclusion flowchart. dAGEs, dietary advanced glycation end products; SAF, skin autofluorescence.
Estimation of dietary AGE intake and other dietary characteristics using FFQ
Participants received a semiquantitative 389-item FFQ when they visited the RS research center (26). The FFQ collects information covering the past month on food types, frequencies, portion sizes, and some preparation methods. The FFQ has been validated to properly rank subjects for nutrient intake among Dutch adults (27, 28).
Referent AGE amounts in food items were derived from 2 published data sets: 1 primary Dutch database reporting CML, N-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MGH1), and carboxyethyl-lysine (CEL) contents of 190 food items from the contemporary Dutch population (29) and 1 complementary database from Northern Ireland containing CML contents of 257 of the most commonly consumed foods by the Northern Irish young adult population (30). For both databases, protein fractions of food items were extracted and subsequently hydrolyzed and then quantified by ultra-HPLC–tandem mass spectrometry (UPLC-MS/MS). Although information on food processing methods was lacking, it was assumed they were usual methods that the RS population also followed, but differences in cooking time and temperature cannot be taken into account.
Dietary intakes of 3 AGEs (CML, MGH1, and CEL) were estimated following 4 steps. First, FFQ food items were matched with items in the Dutch database. Second, food items not found in the Dutch database were matched with items in the Northern Ireland database. When multiple AGE values were provided in the reference database for a single food item, the mean of all the values was aligned. For example, for rye bread, the mean of “rye bread, dark, brand A,” “rye bread, dark, brand B,” and “rye bread, light” was used for the food item rye bread. For foods that only presented as groups in the FFQ, the available food item that represents the group or mean of the food items in the reference databases was used. For example, the mean of “small cookies and biscuits” and “spiced biscuit” was used to represent the food small biscuits. Third, for single food items from the FFQ that were not available in either of the databases, similar food items were used. As a check, macronutrient composition was compared between the missing food item and the replacement food item through the Dutch Food Composition Database (NEVO) online version 2016/5.0. For instance, the mean of available CML AGE values for fruit and vegetables was assigned to other FFQ fruit and vegetable food items. Last, for combination food items, individual food components of the dish were computed according to the contribution of ingredients in the dish based on information on the package or standard recipe online. A detailed workflow is shown in Figure 2.
FIGURE 2.
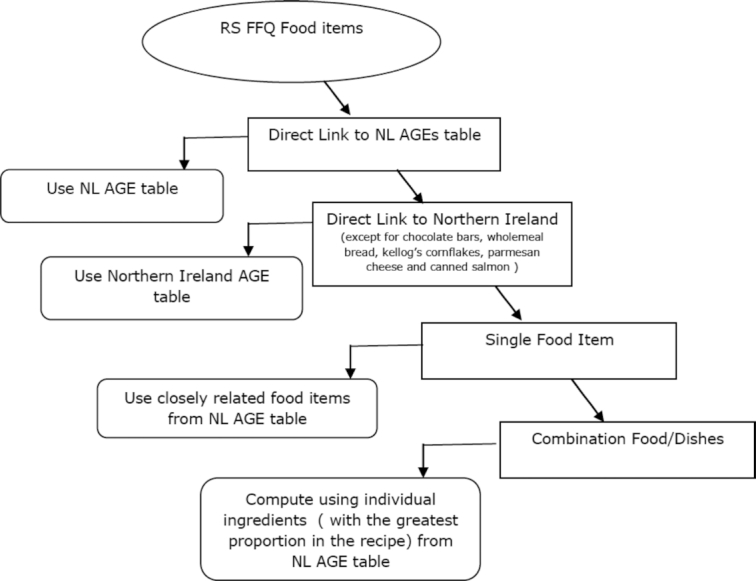
Overview of the procedure of linking of RS FFQ food items to AGEs database. AGE(s), advanced glycation end product(s); NL, The Netherlands; RS, Rotterdam Study.
The AGE intake of individual food items per day was calculated by multiplying the AGE reference content in the food item (mg/100 g) with the mean portion of the food item per day (g/day; portion of the food item × frequency) (31). The daily intake of 1 dAGE is the sum of its content in all food items. The calculation was done for CML, MGH1, and CEL, respectively.
Dietary energy and macronutrients intake were calculated using the NEVO. Overall diet quality was approximated in a diet quality score (range: 0–14) reflecting adherence to the Dutch dietary guidelines (26).
Measurement of SAF
During the visit to the RS research center, SAF of participants’ inner dominant forearm skin was measured using the AGE Reader device (DiagnOptics), and the value was expressed in arbitrary units (AU). Details of the measurement have been described previously (32). The device utilizes the fluorescent property of some AGEs (4) and has been validated previously against AGEs (pentosidine, CML, and CEL) measured in skin biopsies from the same site at which SAF was measured (4). Participants were asked not to use skin cream before the measurement. We measured each participant 3 times consecutively, and the mean was used for analyses. Extreme values in triple measurements were pre-excluded as detected by the Grubbs test and if the value exceeded the mean ± 4 SD scope. The device also measured skin UV reflectance reflecting the color of the skin. It automatically excluded participants with darker skin with a mean reflectance <6% and applied a correction on the SAF value if the reflectance was between 6% and 10%.
Assessment of covariates at baseline
Other lifestyle, clinical, and dietary characteristics were assessed at the time of FFQ collection. Physical activity, smoking, alcohol intake, education level, medication use, and medical history were measured during home interview and were categorized later. Weight and height were measured at the research center without shoes and with light clothes. Blood samples were collected, and total cholesterol, HDL cholesterol, triglycerides, creatinine, serum glucose, and insulin were measured by standard techniques. European ancestry was determined by genetic information. Briefly, the method utilized the genetic data of the participants obtained from the single nucleotide polymorphism array and the data of HapMap samples. The genotype data were pruned to end up with a variant in linkage equilibrium. The ADMIXTURE program was used to estimate ancestry. Finally, ancestral groups were created based on having ≥50% genetic material from that ancestral group.
Physical activity was assessed using the LASA Physical Activity Questionnaire, and it expressed in metabolic equivalents hours per week (33), covering activities of walking, cycling, sports, gardening, and housework. Smoking status was categorized as never, past, or current smoker based on habits of cigarette, cigar, and pipe smoking. Alcohol intake was harmonized to grams of alcohol per day. Acquired highest level of education was harmonized across all 3 RS subcohorts according to the United Nations Educational, Scientific and Cultural Organization classification (34).
BMI (in kg/m2) was calculated as weight divided by height2. LDL cholesterol (millimoles per liter) was calculated using the Friedewald formula. The estimated glomerular filtration rate (eGFR; milliliters per minute per 1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. CKD was defined as eGFR ≤60 mL/min per 1.73 m2. Diabetes was defined as fasting serum glucose concentration ≥7.0 mmol/L or the use of antidiabetic medication or self-reported as having diabetes during home interviews.
Statistical analysis
Statistical analyses were performed using SPSS 24 software (IBM). Descriptive statistics described the lifestyle, clinical, and dietary characteristics of the study population. Normality was determined based on histograms and quantile–quantile plots. Depending on the normal or nonnormal distribution, data were represented as respectively means ± SDs or medians (IQR). We divided the population into 3 equal frequency groups with low, medium, and high CML intake (adjusted for age and sex) to show the inequality in the mean or median values of covariates. Briefly, we calculated the residuals for every individual from the linear regression model in which CML was the dependent variable and age and sex were the independent variables. Participants were categorized into 3 groups by tertiles of residuals.
We used energy-adjusted intake of CML, MGH1, and CEL for analyses to reduce bias caused by self-reported FFQ information. They were calculated by the residual method, namely by adding the mean dAGE intake and the residuals of dAGE intake adjusted by energy intake in the linear regression (35). All dAGE intakes we referred to were energy-adjusted dAGE intakes unless specified. Contributions of individual food categories to AGE intake were the category-specific AGE amount divided by the total AGE intake.
The intake of CML, MGH1, and CEL was rescaled to z scores with mean = 0 and SD ± 1, respectively. Subsequently, multivariable linear regression models were constructed with SAF as the outcome and z scores of dAGE intake as exposures. Results of CML intake are discussed in the Results section, and the results of the other 2 dietary AGEs are presented in the supplemental materials. Potential confounders were identified based on a combination of literature review and biological reasoning, such as increased AGE accumulation under diabetic or CKD conditions (32). Then we restricted covariates to the ones that are (proxy of) shared causes (risk factors) of dAGEs and SAF to remove redundancy and overfitting problems in the models (36). We built 3 confounder models: model 1 was adjusted for age, sex, and RS subcohorts; model 2 was additionally adjusted for energy intake and physical activity; and model 3 was further adjusted for diet quality, smoking status, eGFR, and diabetes status.
Multicollinearity was assessed by tolerance tests, with a tolerance level of 0.40 considered threshold. Heteroscedasticity was determined by plotting the linear regression residuals against the predicted outcome value. P < 0.05 was regarded as statistically significant.
Participants with different age or sex may show differences in physiology and dietary pattern. Diabetes mellitus and CKD are well-established risk factors for excessive tissue AGE accumulation and higher SAF; on the other hand, people may receive dietary recommendations because of the diseases that may influence AGE accumulation. Thus, the potential effect modification of the association between dAGEs and SAF by age, sex, diabetes status, CKD status, and diet quality was checked by adding 2-way interaction terms between suspected effect modifiers and dAGEs into linear regression model 3. In the case of significant interaction (P < 0.1), stratified analyses were performed. Unless specified, the results shown in this article were obtained from model 3.
We observed a range from 0.4% to 5.8% of missing data for physical activity (n = 146, 5.8%), smoking status (n = 9, 0.4%), eGFR (n = 47, 1.9%), diabetes status (27, 1.1%), and BMI (n = 14, 0.6%). They were imputed by multiple imputation under the missing at random assumption using the predictive mean matching method (37). Ten imputation results were acquired through 10 iterations per imputation. The results from the 5th imputation were used to replace the missing value in covariates. We performed the analyses in both the population after missing data were imputed and the population with complete records.
In addition, several sensitivity analyses were performed (1). Because BMI and dietary macronutrients could be potential confounders as well as covariates on the pathway of the dAGEs–SAF link, as a sensitivity test, we further adjusted for BMI and macronutrient intake in addition to model 3 and the results were compared (2). All analyses were repeated in participants of European ancestry (3). A longer time interval between assessment of dAGEs and skin AGEs may in theory dilute the dAGEs–SAF association because of changes in dietary habits, health status, or metabolism. Therefore, we further explored the effect of time difference between dAGEs and SAF measurements on the dAGEs–SAF association by including an interaction term of time difference with dAGEs to linear regression model 3. Furthermore, we generated plots to show the change of association along incremental time difference between measurements (“interplot” package in R, version 3.6.1; R Foundation for Statistical Computing).
Results
Descriptives
In total, 2515 participants (aged 67.8 ± 9.6 y, 56.9% female) were included in the study. For CML intake calculation, 246 items of the FFQ had values matched from the Dutch database and 121 items had values matched from the Northern Ireland database. After energy adjustment, mean dietary intake per day was 3.40 ± 0.89 mg for CML, 28.98 ± 7.87 mg for MGH1, and 3.11 ± 0.89 mg for CEL (Table 1). The top 5 food categories (by mean values) contributing to the CML intake were milk, whole grain, unprocessed meat, refined grains, and pulses. Details on the contribution of food categories to 3 types of AGE intake are shown in Supplemental Table 1 and Supplemental Figure 1.
TABLE 1.
Characteristics of the study population by tertiles of dietary CML intake (age and sex adjusted)1
| Total | Low CML | Medium CML | High CML | |
|---|---|---|---|---|
| Characteristic | (n = 2515) | (n = 838) | (n = 838) | (n = 839) |
| CML, mg/d (energy-adjusted) | 3.40 ± 0.89 | 2.55 ± 0.48 | 3.30 ± 0.19 | 4.33 ± 0.72 |
| Age, y | 67.76 ± 9.59 | 66.90 ± 9.46 | 68.62 ± 9.03 | 67.75 ± 10.17 |
| Sex (women) | 1432 (57) | 474 (57) | 481 (57) | 477 (57) |
| Time between FFQ and SAF measurement | 5.00 (4.54, 5.57) | 5.13 (4.55, 5.58) | 4.82 (4.53, 5.54) | 5.12 (4.56, 5.57) |
| Education level (n = 2476) | ||||
| Primary, n (%) | 157 (6) | 54 (7) | 52 (6) | 51 (6) |
| Lower, n (%) | 963 (39) | 319 (39) | 338 (41) | 306 (37) |
| Intermediate, n (%) | 760 (31) | 254 (31) | 253 (31) | 253 (31) |
| Higher, n (%) | 596 (24) | 201 (24) | 180 (22) | 215 (26) |
| Physical activity, MET h/wk (n = 2369) | 42.50 (17.55, 83.86) | 42.07 (17.00, 86.13) | 44.54 (17.86, 84.22) | 41.50 (17.93, 81.15) |
| Alcohol intake, g/d (n = 2289) | 8.57 (1.61, 8.57) | 8.57 (6.43, 15.00) | 8.57 (1.61, 8.57) | 6.43 (1.52, 8.57) |
| Smoking status (n = 2506) | ||||
| Never, n (%) | 800 (32) | 227 (27) | 280 (34) | 293 (35) |
| Former, n (%) | 1347 (54) | 463 (55) | 437 (53) | 447 (53) |
| Current, n (%) | 359 (14) | 147 (18) | 115 (14) | 97 (12) |
| Coffee intake,2 g/d | 406 (174, 406) | 406 (174, 406) | 406 (174, 406) | 406 (174, 406) |
| BMI, kg/m2 (n = 2501) | 27.47 ± 4.25 | 27.21 ± 4.16 | 27.62 ± 4.24 | 27.59 ± 4.35 |
| Fasting serum glucose, mmol/L (n = 2472) | 5.40 (5.10, 5.90) | 5.40 (5.10, 5.90) | 5.50 (5.10, 6.00) | 5.40 (5.10, 5.90) |
| Total cholesterol, mmol/L (n = 2472) | 5.46 ± 1.07 | 5.52 ± 1.08 | 5.47 ± 1.06 | 5.39 ± 1.06 |
| HDL cholesterol, mmol/L (n = 2472) | 1.42 (1.19, 1.71) | 1.42 (1.22, 1.73) | 1.46 (1.21, 1.74) | 1.39 (1.14, 1.67) |
| Triglycerides, mmol/L (n = 2472) | 1.26 (0.96, 1.73) | 1.28 (0.94, 1.75) | 1.24 (0.97, 1.68) | 1.26 (0.96, 1.75) |
| eGFR, mL/min per 1.73 m2 (n = 2468) | 78.70 (68.12, 88.01) | 80.15 (69.71, 89.00) | 77.35 (67.64, 86.83) | 78.64 (67.17, 87.60) |
| Chronic kidney disease (n = 2468) | 296 (12.0) | 92 (11.2) | 97 (11.8) | 107 (13.0) |
| Diabetes (n = 2488) | 303 (12.2) | 82 (9.9) | 114 (13.8) | 107 (12.9) |
| Energy intake, kcal/d | 2140 ± 681 | 2212 ± 714 | 1984 ± 645 | 2224 ± 655 |
| Protein intake, g/d | 82.0 ± 25.4 | 78.8 ± 24.8 | 78.0 ± 23.8 | 89.4 ± 26.1 |
| Carbohydrate intake, g/d | 240.7 ± 85.0 | 246.3 ± 91.2 | 224.0 ± 80.7 | 251.7 ± 80.0 |
| Fat intake, g/d | 71.3 (54.9, 92.5) | 70.7 (54.3, 93.9) | 68.0 (51.2, 86.1) | 75.5 (58.9, 97.6) |
| Diet quality score | 7.0 ± 1.9 | 6.6 ± 1.9 | 7.0 ± 1.8 | 7.3 ± 1.8 |
| MGH1, mg/d (energy-adjusted) | 28.98 ± 7.87 | 24.36 ± 5.86 | 28.95 ± 5.10 | 33.62 ± 9.10 |
| CEL, mg/d (energy-adjusted) | 3.11 ± 0.89 | 2.55 ± 0.64 | 3.11 ± 0.60 | 3.66 ± 0.99 |
| SAF, AU | 2.40 ± 0.50 | 2.38 ± 0.50 | 2.43 ± 0.50 | 2.40 ± 0.51 |
Values are n (%) or means ± SDs if normally distributed and medians (IQRs) if nonnormally distributed, n = 2515 unless otherwise indicated. The low, medium, and high CML intake groups were determined by tertiles of energy-adjusted CML intake after adjusting for age and sex in linear regression. Energy-adjusted AGEs were calculated by adding the mean dAGEs intake and residuals of dAGEs adjusted by energy intake in the linear regression. AGEs, advanced glycation end products; AU, arbitrary units; CEL, carboxyethyl-lysine; CML, carboxymethyl-lysine; dAGEs, advanced glycation end products present in the diet; eGFR, estimated glomerular filtration rate; MET, metabolic equivalent of task, with 1 MET defined as 1 kcal/kg/h; MGH1, N-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; SAF, skin autofluorescence.
Identical values in coffee intake by coincidence.
In the total study population, mean SAF value was 2.40 ± 0.50 AU. A total of 553 individuals had either diabetes (303, 12.2%) or CKD (296, 12.0%) at baseline, 1914 individuals had neither diabetes nor CKD, and 48 individuals had undetermined diabetes and CKD status because of missing information. In low, medium, and high CML tertiles, energy-adjusted CML intake was 2.55 ± 0.48, 3.30 ± 0.19, and 4.33 ± 0.72 mg/d, respectively. The low CML intake group had the lowest SAF, and the medium CML intake group had the highest SAF. Compared with the low and high CML tertiles, the medium CML tertile was characterized by older age; lower eGFR; lower total energy intake; lower carbohydrate, fat, and protein intake; higher serum HDL cholesterol; and higher prevalence of diabetes. Across CML tertiles, higher CML intake was accompanied by a higher prevalence of CKD, a lower percentage of current smokers, and a higher adherence to the Dutch Dietary Guidelines (Table 1).
The association between dietary AGE intake and SAF
In multiple linear regression analyses, we did not observe any association between CML and SAF after adjustment for age, sex, and RS subcohorts (adjusted mean difference per SD higher CML = 0.009; 95% CI: −0.01, 0.027) or further adjustment for energy intake, physical activity, dietary quality, smoking status, diabetes status, and eGFR (adjusted mean difference per SD higher CML = 0.011; 95% CI: −0.007, 0.029) (Table 2). The linearity of the linear regression models is met as the residuals against the fitted value of the linear regression model were symmetrically distributed with constant variance, and adding a squared term of CML intake did not improve the model fit.
TABLE 2.
The association between CML intake and SAF in the total study population and in subgroups based on disease status1
| Coefficient (95% CI) | |||||
|---|---|---|---|---|---|
| Group | n | Model 1 | Model 2 | Model 3 | P-interaction |
| Total | 2515 | 0.009 [−0.01, 0.027] | 0.008 [−0.011, 0.026] | 0.011 [−0.007, 0.029] | |
| Nondiabetes | 2185 | 0.012 [−0.007, 0.032] | 0.012 [−0.008, 0.031] | 0.019 [−0.001, 0.038] | 0.062 |
| Diabetes | 303 | −0.036 [−0.093, 0.02] | −0.037 [−0.094, 0.019] | −0.036 [−0.092, 0.021] | |
| Non-CKD | 2172 | 0.015 [−0.005, 0.034] | 0.014 [−0.005, 0.034] | 0.018 [−0.001, 0.038] | 0.033 |
| CKD | 296 | −0.044 [−0.105, 0.017] | −0.044 [−0.105, 0.017] | −0.04 [−0.101, 0.021] | |
| With neither diabetes nor CKD | 1914 | 0.021 [0.001, 0.042] | 0.021 [0, 0.042] | 0.03 [0.009, 0.05] | |
Among the 2515 participants, 553 individuals had either diabetes or CKD or both diseases (n = 303 diabetes, n = 296 CKD), 1914 individuals did not have either diabetes or CKD, and for 48 individuals the diabetes and CKD status could not be determined due to missing information. Regression coefficients [95% CIs] of CML z score were estimated from the linear regression models and represent the adjusted difference of SAF (AU) associated with one SD difference of dietary CML intake. Model 1 adjusted for age, sex, and RS subcohorts; model 2 further adjusted for energy intake and physical activity; model 3 adjusted for diet quality score, smoking status, eGFR, and diabetes status (except for subgroups categorized by diabetes status) in addition to model 2. AU, arbitrary units; CKD, chronic kidney disease; CML, carboxymethyl-lysine; eGFR, estimated glomerular filtration rate; RS, Rotterdam Study; SAF, skin autofluorescence.
Model: SAF ∼ CML + age + sex + RS subcohorts + energy intake + physical activity + diet quality score + smoking status + eGFR + diabetes status + CML × diabetes status.
Model: SAF ∼ CML + age + sex + RS subcohorts + energy intake + physical activity + diet quality score + smoking status + CKD status + diabetes status + CML × CKD status.
We did not observe significant effect modification by age, sex, and dietary quality score on the dAGEs–SAF association. Potential effect modification for the association was observed for diabetes status (P value for interaction: Pdiabetes status × CML = 0.06) and for CKD status (P value for interaction: PCKD status × CML = 0.03 if the main effect of eGFR in model 3 was replaced by CKD). A detailed comparison of demographic, lifestyle, and clinical characteristics of participants by diabetes and CKD status is shown in Supplemental Table 2. The energy-adjusted CML intake was 3.42 ± 0.86 mg/d for diabetics, 3.35 ± 0.90 mg/d for CKD participants, and 3.39 ± 0.88 mg/d for participants with neither diabetes nor CKD. The age of these groups was 70.9 ± 7.8, 75.1 ± 6.9, and 66.4 ± 9.5 y, respectively.
Stratified analysis of model 3 by diabetes and CKD showed that higher dietary CML intake was not significantly associated with higher SAF in the nondiabetes group nor in the non-CKD group. Because renal insufficiency is one of the major diabetes complications, we checked for overlap between CKD and diabetes, which was not very large (16.6% of the diabetic participants had CKD compared with 11.4% of nondiabetics). The positive association between CML intake and SAF was present after excluding both individuals with diabetes and individuals with CKD (adjusted mean difference per SD higher CML = 0.03; 95% CI: 0.009, 0.05). The results of stratified analyses are shown in Table 2. Due to the age differences in the subgroups of participants by diabetes and CKD status, we examined the dAGEs–SAF association among different age groups. Stratifying the total population by age ≥70 y (the median age) or age <70 y did not exhibit substantial difference in effect size in results of model 3 [0.017 (95% CI: −0.013, 0.046) compared with 0.009 (95% CI: −0.014, 0.032)].
All 3 dietary AGEs were correlated [Pearson correlation coefficient (r) between MGH1 and CEL was 0.810, that between CEL and CML was 0.582, and that between MGH1 and CML was 0.545; P < 0.0001 for all]. The associations between other types of AGEs and SAF were less strong than that of CML because none of them was significant in the total population and in stratified analysis, although the coefficients were similar (Supplemental Table 3, Supplemental Figure 2).
Using the unimputed complete record data set exhibited similar results (Supplemental Table 4). Additional adjustments in model 3 for BMI and dietary protein and fat intake did not change the results substantially (Supplemental Table 5). We repeated the analysis of the association between CML intake and SAF in participants of European ancestry and the results were similar (Supplemental Table 6).
Furthermore, time difference between dAGEs and SAF measurements modified the dAGEs–SAF association, indicated by the interaction terms included additionally in model 3 in 2515 participants (P value for interaction: PCML × time = 0.06, PMGH1 × time = 0.15, and PCEL × time = 0.06). Because we observed the association among participants with neither diabetes nor CKD, Supplemental Figure 3 shows how coefficients of dAGEs changed along the time difference between measurements in this subpopulation. We observed a stronger positive association between dAGEs and SAF for a shorter time difference, and the point estimate of coefficient decreased as the time difference increased, although the 95% CI overlapped. For longer time differences, the point estimates of the coefficient became negative; however, there were sparse participants with a very large time difference between dAGEs and SAF measurements.
Discussion
Interested in the association between dietary and tissue AGEs, we calculated the dietary AGEs from the FFQ and assessed skin AGEs as SAF in a large population-based cohort. The distributions of dAGEs are comparable to those of another study using the same reference database (38). No association was observed in the total population, but higher CML–AGE intake was weakly but significantly associated with higher SAF among participants with neither diabetes nor CKD.
One can assume that dietary AGEs will go through a complex cascade of transportations and metabolism from ingestion to skin deposition and are influenced by complex dietary, metabolic, and physiological conditions. However, after adjusting for several influencing factors, 1 SD (0.89 mg) difference in daily CML intake was still associated with 0.030 (95% CI: 0.009, 0.050) AU difference in SAF in people with neither diabetes nor CKD, which is considerable compared with an approximate 0.024 AU natural yearly increase reported for healthy people <70 y (39). Diabetes and CKD are well-known risk factors for AGE accumulation due to hyperglycemia that results in high formation or reduced AGE elimination through kidney (3, 40). For participants with diabetes or CKD, a small increase in skin AGEs due to dietary AGEs may go undetected because of the predisposed large amount of AGEs in the skin. We also cannot rule out the possibility of dietary alterations because of the diseases. Nonetheless, the association among diabetic and CKD participants remains inconclusive due to inadequate sample size.
The dAGEs–SAF association was further strengthened by the results showing the time difference between dAGEs and SAF measurements did modify the associations of all 3 types of dAGEs with SAF. One of the explanations for a stronger association for smaller time differences between measurements is that the larger time difference could lead to changes in diet, health status, or metabolism. In addition, the association may fade with time as more clearance of previously formed skin AGEs occurs despite the continuing exposure of dAGEs. Future studies may explore the range of time interval fits for best capturing the association.
The other 2 dAGEs, MGH1 and CEL, were more correlated with each other than with CML, which could be partly due to the fact that CML has a richer reference source with additionally the Northern Ireland database. The 3 dAGEs we studied constitute only part of a variety of dAGEs, let alone the AGE precursors, but their contents in the Dutch diet are not yet available (1, 41). However, the high correlations among intakes of the 3 different types of dAGEs implied their rankings are likely representative of other dAGEs.
Direct evidence for deposition of dietary CML in multiple tissues was observed in mice after 30 d of oral administration of 13C-labeled CML (42). However, only a few human studies are available focusing on circulating or urinary AGEs (43) instead of tissue AGEs. For example, in 172 healthy individuals, dietary CML from 3-d food records was positively correlated with circulating CML (23). Our results are in line with this in the sense that high dAGEs increase the circulating load of AGEs, and thus a long-term AGE-rich diet may contribute to a higher load of tissue AGEs (23, 43). Results from the CODAM Study (n = 450) using the same Dutch AGE reference database (38) further support our observation: in that study, dAGEs were nonsignificantly positively associated with their corresponding protein-bound AGEs in plasma, with the exception of a positive association with free AGEs in plasma and urine. Although circulating protein-bound AGEs do not have as low a turnover as tissue AGEs, their half-life is longer than that of free forms in the circulation, and they may thus be more relevant to the cumulative AGE load. Nonetheless, unlike our study, their population had a high risk of cardiovascular disease, including a larger proportion of diabetes, but had fewer participants with CKD. In 73 elderly Dutch people with an increased risk of cardiovascular disease, dietary AGE was not related to SAF, but this study lacked statistical power and used a foreign AGE reference database (44). Other dietary factors have also been shown to contribute to AGE accumulation, including the Mediterranean diet and coffee intake (45, 46). Adjusting for dietary quality score as a proxy for dietary quality did not modify the association in our analysis, nor did adjusting for coffee.
The disposition and metabolic transition of dAGEs to endogenous AGEs remain unclear. We estimated the protein-bound AGEs in the diet, but AGEs can present both in free forms and in protein- and lipid-bound forms. Protein-bound AGEs can be digested into free AGE molecules or continue to bind to peptides and protein fractions. Free CML can enter the circulation by passive penetration (47, 48), whereas dipeptide-bound CML is likely to be transported actively by the peptide transporter 1 into intestinal epithelium cells and may enter the circulation as AGE-modified amino acids after peptidolysis (49). Notably, transportability can depend on the type of AGE (48, 50). In addition, dietary AGEs might provoke endogenous AGE formation by, for example, increasing inflammation (51).
The strengths of our study include a well-phenotyped and intensively followed population with adequate sample size. We were able to control for confounding by adjusting for the dietary quality of each individual using a validated index. Moreover, we provided a reliable and representative estimation of habitual intake of 3 AGEs by using a well-validated FFQ covering most of the daily-consumed foods. A domestic contemporary AGE database was chosen as our main reference to allow for a maximum match with food items in our FFQ. AGEs were quantified by UPLC-MS/MS technology in this database, outperforming the immunological methods in veracity and precision, and were expressed in easy-to-interpret units of milligrams per 100 grams rather than international units.
However, several limitations are also present. The results are restricted to the elderly Dutch population, although the diet shares similarities with those of other Western countries. The results should be interpreted with caution because we did not correct for multiple comparisons, which may give rise to false-positive findings. However, the subgroups were predefined based on disturbed regulation of AGEs in patients with diabetes and CKD. The fact that we observed that the time difference between dAGEs and SAF measurements modified the associations, with the strongest effects in the group with shorter time difference, suggests that the associations between dAGEs and skin AGEs are real and possibly underestimated in our study. Furthermore, detailed information on food processing methods was lacking, and intake of some nonperfect matched food items was replaced by mean values because the FFQ contained more items. Therefore, the variation in dAGE data is likely to be underrepresented, and this could have weakened any association. Last, we cannot rule out residual confounding mainly from complex dietary factors, recoding bias, and healthy survival effects, nor can we demonstrate whether relations are causal.
Future research is warranted on the influence of dAGEs on tissue deposition and ultimately on health outcomes, preferably in large cohorts with repeated measurements of SAF and dAGEs. Unified quantification methodology will reduce heterogeneity and facilitate comparison among studies. Domestic data sources on more AGE-related entities will also be helpful.
In conclusion, we found that there is a weak positive relation between dietary CML intake and AGE accumulation in the skin in persons with neither diabetes nor CKD, but not in those with diabetes or CKD. This observation suggests that dietary intake of AGEs may increase the AGE burden in tissues. The findings require replication in longitudinal studies or in randomized controlled trials.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists. We acknowledge Dr. Josje Schoufour for the guidance, data acquisition, discussion, and input during research planning, data analysis, and interpretation.
The authors’ responsibilities were as follows—JC and MCZ: study guarantors and take responsibility for the integrity of the data and the accuracy of the data analysis; JC and MCZ: designed the research; JC, RCT, and MCZ: conducted the research; TV, MAI, TECN, AGU, and MCZ: provided the essential databases; JC, KW, RCT, and MCZ: performed statistical analysis; JC and MCZ: wrote the manuscript; JC, KW, RCT, TV, MAI, LCPGMdG, AGU, and MCZ: critically reviewed the manuscript for important intellectual content; and all authors: read and approved the final manuscript. The authors declare no conflicts of interest.
Notes
The Rotterdam Study is supported by Erasmus Medical Center and Erasmus University, Rotterdam; the Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative; the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. JC is supported by the China Scholarship Council for PhD fellowship (no. 201606170110). Jaap Schouten Foundation, Rotterdam, Netherlands, kindly provided funding for the analyses of advanced glycation end products related to musculoskeletal health in the Rotterdam Study.
The funders played no role in the study design or in data collection and analysis.
Supplemental Tables 1–6 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the manuscript, code book, and analytic code will be made available upon request (for more information, see http://www.ergo-onderzoek.nl/wp/contact).
Abbreviations used: AGE, advanced glycation end product; AU, arbitrary unit; CEL, carboxyethyl-lysine; CKD, chronic kidney disease; CML, carboxymethyl-lysine; eGFR, estimated glomerular filtration rate; MGH1, N-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; NEVO, Dutch food composition table; RS, Rotterdam Study; SAF, skin autofluorescence; UPLC-MS/MS, ultra-HPLC– tandem mass spectrometry.
Contributor Information
Jinluan Chen, Department of Internal Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands; Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
Komal Waqas, Department of Internal Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
Robby Carlo Tan, Department of Internal Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands; Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands; Food and Nutrition Research Institute, Department of Science and Technology, The Philippines.
Trudy Voortman, Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
M Arfan Ikram, Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
Tamar E C Nijsten, Department of Dermatology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
Lisette C P G M de Groot, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
André G Uitterlinden, Department of Internal Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands; Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
M Carola Zillikens, Department of Internal Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
References
- 1. Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Suppl 1):3–27. [DOI] [PubMed] [Google Scholar]
- 2. Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–31. [DOI] [PubMed] [Google Scholar]
- 3. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46. [DOI] [PubMed] [Google Scholar]
- 4. Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, Gans R, Smit A. Simple noninvasive measurement of skin autofluorescence. Ann NY Acad Sci. 2005;1043:290–8. [DOI] [PubMed] [Google Scholar]
- 5. van Waateringe RP, Fokkens BT, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, Paterson AD, Smit AJ, Lutgers HL, Wolffenbuttel BHR. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia. 2019;62(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uribarri J, Cai WJ, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci. 2005;1043:461–6. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg T, Cai WJ, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104(8):1287–91. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28(3):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep. 2017;7(1):2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Pino A, Currenti W, Urbano F, Scicali R, Piro S, Purrello F, Rabuazzo AM. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27(11):978–84. [DOI] [PubMed] [Google Scholar]
- 11. Luevano-Contreras C, Gomez-Ojeda A, Macias-Cervantes MH, Garay-Sevilla ME. Dietary advanced glycation end products and cardiometabolic risk. Curr Diab Rep. 2017;17(8):6. [DOI] [PubMed] [Google Scholar]
- 12. Perrone L, Grant WB. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer's disease incidence and prevalence. J Alzheimers Dis. 2015;45(3):965–79. [DOI] [PubMed] [Google Scholar]
- 13. Smith PK, Masilamani M, Li XM, Sampson HA. The false alarm hypothesis: food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J Allergy Clin Immunol. 2017;139(2):429–37. [DOI] [PubMed] [Google Scholar]
- 14. Egawa T, Tsuda S, Goto A, Ohno Y, Yokoyama S, Goto K, Hayashi T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Br J Nutr. 2017;117(1):21–9. [DOI] [PubMed] [Google Scholar]
- 15. Serban AI, Stanca L, Geicu OI, Dinischiotu A. Dietary advanced glycation end-products activate NF-κB, AKT1 and ERK1/2 linking cancer risk and diabetes. Diabetologia. 2017;60:S567–S8. [Google Scholar]
- 16. Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan ZG, Chen L, Kahle L, Risch A, Subar AF, Cross AJ, Hollenbeck A, et al.. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am J Clin Nutr. 2015;101(1):126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baye E, de Courten MPJ, Walker K, Ranasinha S, Earnest A, Forbes JM, de Courten B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: a randomised crossover trial. Sci Rep. 2017;7;4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Semba RD, Gebauer SK, Baer DJ, Sun K, Turner R, Silber HA, Talegawkar S, Ferrucci L, Novotny JA. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144(7):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guilbaud A, Niquet-Leridon C, Boulanger E, Tessier FJ. How can diet affect the accumulation of advanced glycation end-products in the human body?. Foods. 2016;5(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macias-Cervantes MH, Rodriguez-Soto JMD, Uribarri J, Diaz-Cisneros FJ, Cai WJ, Garay-Sevilla ME. Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition. 2015;31(3):446–51. [DOI] [PubMed] [Google Scholar]
- 23. Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol Ser A Biol Sci Med Sci. 2007;62(4):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94(12):6474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, Klaver CCW, Nijsten TEC, Peeters RP, Stricker BH et al.. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32(9):807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJA, Lahousse L, Tiemeier H, Brusselle GG, Franco OH, Schoufour JD. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32(11):993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feunekes GIJ, Vanstaveren WA, Devries JHM, Burema J, Hautvast JGAJ. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58(4):489–96. [DOI] [PubMed] [Google Scholar]
- 28. Goldbohm RA, Vandenbrandt PA, Brants HAM, Vantveer P, Al M, Sturmans F, Hermus RJ. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48(4):253–65. [PubMed] [Google Scholar]
- 29. Scheijen JLJM, Clevers E, Engelen L, Dagnelie PC, Brouns F, Stehouwer CDA, Schalkwijk CG. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: presentation of a dietary AGE database. Food Chem. 2016;190:1145–50. [DOI] [PubMed] [Google Scholar]
- 30. Hull GLJ, Woodside JV, Ames JM, Cuskelly GJ. N-ε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012;131(1):170–4. [Google Scholar]
- 31. van der Heijden L. Maten, gewichten en codenummer 2003. In: Informatorium Voeding en Diëtetiek - Voedingsleer, ed. Houten: Bohn Stafleu van Loghum; 2013, 732–5. [Google Scholar]
- 32. Chen JL, van der Duin D, Campos-Obando N, Ikram MA, Nijsten TEC, Uitterlinden AG, Zillikens MC. Serum 25-hydroxyvitamin D-3 is associated with advanced glycation end products (AGEs) measured as skin autofluorescence: the Rotterdam Study. Eur J Epidemiol. 2019;34(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57(3):252–8. [DOI] [PubMed] [Google Scholar]
- 34. United Nations Educational, Scientific and Cultural Organization (UNESCO) International Standard Classification of Education (ISCED). Paris: UNESCO; 1976. [Google Scholar]
- 35. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 36. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Br Med J. 2009;339:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheijen JLJM, Hanssen NMJ, van Greevenbroek MM, van der Kallen CJ, Feskens EJM, Stehouwer CDA, Schalkwijk CG. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin Nutr. 2018;37(3):919–25. [DOI] [PubMed] [Google Scholar]
- 39. Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12(5):399–403. [DOI] [PubMed] [Google Scholar]
- 40. Raj DS, Choudhury D, Welbourne TC, Levi M. Advanced glycation end products: a nephrologist's perspective. Am J Kidney Dis. 2000;35(3):365–80. [DOI] [PubMed] [Google Scholar]
- 41. Degen J, Hellwig M, Henle T. 1,2-Dicarbonyl compounds in commonly consumed foods. J Agric Food Chem. 2012;60(28):7071–9. [DOI] [PubMed] [Google Scholar]
- 42. Tessier FJ, Niquet-Leridon C, Jacolot P, Jouquand C, Genin M, Schmidt AM, Grossin N, Boulanger E. Quantitative assessment of organ distribution of dietary protein-bound C-13-labeled N-ε-carboxymethyllysine after a chronic oral exposure in mice. Mol Nutr Food Res. 2016;60(11):2446–56. [DOI] [PubMed] [Google Scholar]
- 43. Foerster A, Henle T.. Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): studies on the urinary excretion of pyrraline. Biochem Soc Trans. 2003;31:1383–5. [DOI] [PubMed] [Google Scholar]
- 44. Jochemsen BM, Mulder DJ, Van Doormaal JJ, Mulder G, Volmer M, Graaff R, Smit AJ, Mulder DJ. Relation between food and drinking habits, and skin autofluorescence and intima media thickness in subjects at high cardiovascular risk. J Food Nutr Res. 2009;48(1):51–8. [Google Scholar]
- 45. Sanchez E, Betriu A, Salas-Salvado J, Pamplona R, Barbe F, Purroy F, Farràs C, Fernández E, López-Cano C, Mizab C, et al.. Mediterranean diet, physical activity and subcutaneous advanced glycation end-products’ accumulation: a cross-sectional analysis in the ILERVAS project. Eur J Nutr. 2019;59(3):1233–42. [DOI] [PubMed] [Google Scholar]
- 46. Botros N, Sluik D, van Waateringe RP, de Vries JHM, Geelen A, Feskens EJM. Advanced glycation end-products (AGEs) and associations with cardio-metabolic, lifestyle, and dietary factors in a general population: the NQplus study. Diabetes Metab Res Rev. 2017;33(5):e2892. [DOI] [PubMed] [Google Scholar]
- 47. Grunwald S, Krause R, Bruch M, Henle T, Brandsch M. Transepithelial flux of early and advanced glycation compounds across Caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. Br J Nutr. 2006;95(6):1221–8. [DOI] [PubMed] [Google Scholar]
- 48. Snelson M, Coughlan MT. Dietary advanced glycation end products: digestion, metabolism and modulation of gut microbial ecology. Nutrients. 2019;11(2):E215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hellwig M, Geissler S, Matthes R, Peto A, Silow C, Brandsch M, Henle T. Transport of free and peptide-bound glycated amino acids: synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem. 2011;12(8):1270–9. [DOI] [PubMed] [Google Scholar]
- 50. Hellwig M, Matthes R, Peto A, Lobner J, Henle T. N-ε-fructosyllysine and N-ε-carboxymethyllysine, but not lysinoalanine, are available for absorption after simulated gastrointestinal digestion. Amino Acids. 2014;46(2):289–99. [DOI] [PubMed] [Google Scholar]
- 51. Kellow NJ, Coughlan MT.. Effect of diet-derived advanced glycation end products on inflammation. Nutr Rev. 2015;73(11):737–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


