ABSTRACT
Background
Oligosaccharides are the third most abundant component in human milk. They are a potential protective agent against neonatal sepsis.
Objectives
We aimed to explore the association between human milk oligosaccharides (HMOs) and late-onset sepsis in very-low-birth-weight infants, and to describe the composition and characteristics of HMOs in Peruvian mothers of these infants.
Methods
This is a secondary data analysis of a randomized clinical trial. We conducted a retrospective cohort study of mothers and their very-low-birth-weight (<1500 g) infants with ≥1 milk sample and follow-up data for >30 d. HMOs were measured by high performance liquid chromatography (HPLC). We used factor analysis and the Mantel–Cox test to explore the association between HMOs and late-onset neonatal sepsis.
Results
We included 153 mother–infant pairs and 208 milk samples. Overall, the frequency of the secretor phenotype was 93%. Secretors and nonsecretors were defined by the presence and near-absence of α1-2-fucosylated HMOs, respectively. The most abundant oligosaccharides were 2'-fucosyllactose, lacto-N-fucopentaose (LNFP) I, and difucosyllacto-N-tetraose in secretors and lacto-N-tetraose and LNFP II in nonsecretors. Secretors had higher amounts of total oligosaccharides than nonsecretors (11.45 g/L; IQR: 0.773 g/L compared with 8.04 g/L; IQR: 0.449 g/L). Mature milk samples were more diverse in terms of HMOs than colostrum (Simpson's Reciprocal Diversity Index). We found an association of factor 3 in colostrum with a reduced risk of late-onset sepsis (HR: 0.63; 95% CI: 0.41, 0.97). Fucosyl-disialyllacto-N-hexose (FDSLNH) was the only oligosaccharide correlated to factor 3.
Conclusions
These findings suggest that concentrations of different HMOs vary from one individual to another according to their lactation period and secretor status. We also found that FDSLNH might protect infants with very low birth weight from late-onset neonatal sepsis. Confirming this association could prove 1 more mechanism by which human milk protects infants against infections and open the door to clinical applications of HMOs.
This trial was registered at clinicaltrials.gov as NCT01525316.
Keywords: human milk oligosaccharides, breast milk, neonatal sepsis, very-low-birth-weight infants, intensive care unit, breastfeeding
Introduction
Neonatal sepsis is a major global health problem that contributes significantly to infant morbidity and mortality. Late-onset sepsis, occurring after the first 72 h of life, is associated with nosocomial and community-acquired infections (1). Human milk has proven protective against neonatal sepsis and other infections, especially in preterm and very-low-birth-weight infants (2). Multiple components of human milk seem to be responsible for this protection, including immunoglobulins, cytokines, lactoferrin, and human milk oligosaccharides (HMOs) (3, 4).
HMOs are the third most abundant component of breast milk (ranging between 5 and 15 g/L in mature milk) and contribute to the many benefits of breastfeeding. Their most significant biological effects are 1) prebiotics, shaping the gut microbiome by serving as metabolic substrates for selected enteric microbes like Bifidobacterium and Lactobacillus (5, 6); 2) antiadhesives, blocking the adhesion of many bacterial, viral, or protozoan parasite pathogens or their toxins to epithelial cells based on structural homology between the milk glycan and the glycan on host cell surfaces—some of them include sepsis-related pathogens such as Streptococcus pneumoniae and Listeria monocytogenes (7) and enteric pathogens like rotavirus, norovirus, Campylobacter jejuni, enteropathogenic Escherichia coli, Entamoeba histolytica, and Clostridium perfringens (8, 9); 3) antimicrobials, by directly affecting proliferation of bacterial and fungal pathogens—HMOs also have a bacteriostatic effect on Group B Streptococcus and show a dose-dependent mitigation of Candida albicans invasion of human premature intestinal epithelial cells (4, 10); and 4) intracellular and extracellular immune modulators, because they alter host responses such as gene expressions that lead to changes in the cell surface, suppress apoptotic pathways, and reduce the expression of proinflammatory cytokines in macrophages (4, 11).
Previous studies have shown that composition of HMOs varies geographically (12). Moreover, there are different compositions even within similar demographic groups or within the same mother across the lactation period (13). Differences in the expression of the enzyme α1-2-fucosyltransferase (FUT2) seem to be accountable for part of this variation. Mothers with activity of this enzyme are known as secretors (14).
The aforementioned biological functions and their interaction with neonatal sepsis–related pathogens (7, 8) suggest that there may be an association between HMOs and late-onset neonatal sepsis. However, to the best of our knowledge, there are no studies evaluating HMOs' impact on severe clinical outcomes, especially in high-risk populations such as very-low-birth-weight infants.
Therefore, this study aims to explore the association between HMOs and the risk of developing late-onset sepsis (probable or confirmed) in very-low-birth-weight infants and describe the composition and characteristics of HMOs in Peruvian mothers of very-low-birth-weight infants.
Methods
The present study is designed as an observational retrospective cohort and is a secondary data analysis of a randomized placebo-controlled trial. The NEOLACTO study (NCT01525316), the parent trial, evaluated bovine lactoferrin supplementation for prevention of late-onset neonatal sepsis in infants <2000 g at birth.
The NEOLACTO study enrolled infants from May 2012 to September 2014 in the neonatal units of 3 tertiary care hospitals in Lima, Peru. As part of the clinical trial, milk samples (2–3 mL) were collected during the morning (no specific time) before feed or milk extraction in the first 7 d of life (colostrum) and at 1 mo ± 7 d (mature milk). Neonates were randomly assigned to receive either lactoferrin or placebo for 8 wk (200 mg · kg−1 · d−1). Infants were followed up daily, looking for local and systemic infections. All systemic episodes were analyzed to determine if they corresponded to possible, probable, or confirmed sepsis based on Haque criteria (1). Their milk consumption (breast milk doses in volume) was recorded daily during their hospitalization period.
The parent trial included newborns with birth weights <2000 g who were transferred in their first 72 h of life to the neonatal intermediate or intensive care units of any of the 3 participating hospitals, and excluded those with underlying gastrointestinal problems that prevented oral intake, those with existing conditions that profoundly affected growth and development, those with a family history of cow milk allergy, and those who lived outside Lima.
The present study included those very-low-birth-weight infants (≤1500 g) who had participated in the NEOLACTO study, had ≥1 milk samples with a minimum volume of 50 μL (sufficient to measure HMO concentrations), and had completed ≥30 d of follow-up to account for sepsis within the neonatal period. Because the clinical trial did not prove any significant difference between the intervention and placebo groups (15), the consumption of bovine lactoferrin was less likely to be a confounding factor. Therefore, participants of the parent trial were eligible for inclusion regardless of their lactoferrin consumption.
HMO composition was measured by high-performance liquid chromatography with fluorescence detection (HPLC-FL) using raffinose as an internal standard (16). This technique allows for the identification and absolute quantification of 19 different oligosaccharides listed in Supplemental Table 1. Secretor phenotype was defined based on the presence or near absence of the α1-2-fucosylated oligosaccharides 2'-fucosyllactose (2'FL) and lacto-N-fucopentaose (LNFP) I (17). HMO concentrations were examined in micrograms per milliliter (μg/mL) using medians, quartiles, and IQRs to describe their characteristics and composition. To assess differences in distributions between colostrum and mature milk, we analyzed gross differences in medians and quartiles using paired data of children who had both samples. Simpson's Reciprocal Diversity Index D was calculated as the reciprocal sum of the square of the relative abundance of each HMO to measure the HMO diversity in every milk sample.
A possible association of HMOs with serious infections was investigated by analyzing 2 outcomes: late-onset neonatal sepsis (probable or confirmed sepsis that occurred between the first 3 and 30 d of life); and a composite outcome consisting of late-onset neonatal sepsis, stage 2 or 3 necrotizing enterocolitis, and death by sepsis. The 19 HMOs were summarized through factor analysis to reduce the number of comparisons and, therefore, the chance of obtaining a false positive association. Factor analysis works by generating factors that represent the common variance of the observed variables (HMOs) and their underlying correlations, thus summarizing multiple observed variables into underlying unobserved factors (18). Colostrum and mature milk samples were analyzed separately. For this analysis, we excluded infants who did not consume milk on any day within the first 7 d of life from the colostrum cohort and those who did not consume milk on any day within the whole neonatal period from the mature milk cohort. We predetermined 3 factors with an eigenvalue >1 for each sample type. The eigenvalue represents the total of the variance explained by each factor. We did not rotate any of the factors. The association between the resulting factors and the outcomes was examined through survival analysis using the Mantel–Cox test in each sample type, yielding 12 comparisons. We performed all the statistical analyses using Stata 16 software by StataCorp LLC.
The NEOLACTO trial was approved by the Institutional Review Boards (IRBs) of the University of Texas Health Science Center at Houston, Universidad Peruana Cayetano Heredia (UPCH), and the 3 participating hospitals. Parents gave written consent to participate in the study and to use the biological samples for future studies related to protective factors of human milk. The present study was reviewed and approved by the IRB of the UPCH (inscription number: 101865).
Results
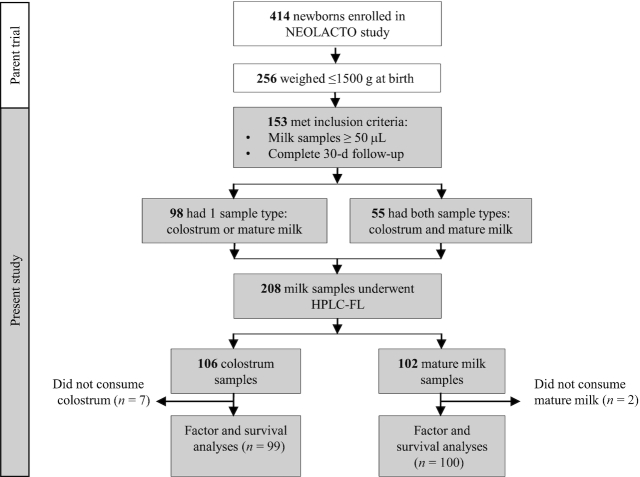
Among the 414 neonates enrolled in the parent trial, 256 weighed <1500 g at birth; 153 met the inclusion criteria, out of which 55 individuals had both colostrum and mature milk samples. We compared the HMO composition of colostrum with that of mature milk in these 55 individuals to avoid any potential bias introduced by different person-to-person enzymatic profiles, including scretor status. A total of 208 milk samples were analyzed by HPLC-FL. From these, 106 corresponded to colostrum and 102 to mature milk (Figure 1).
FIGURE 1.
Flow diagram of newborns and milk samples through the study. For factor analysis, we excluded infants who did not consume breast milk on any day within the first 7 d of life from the colostrum cohort and those who did not consume breast milk on any day within the entire neonatal period from the mature milk cohort. HPLC-FL, high-performance liquid chromatography with fluorescence detection.
Of the total participants (n = 153), 39 individuals (25%) had extremely low birth weight (<1000 g) and 43 (28%) developed late-onset neonatal sepsis. Most of the participants (83%) were born by caesarean delivery; the median birth gestational age was 29.4 ± 1.5 wk; 41 infants (27%) were born small for their gestational age. The characteristics of the 55 infants who had both milk samples were very similar to those of the total population of participants (Table 1).
TABLE 1.
Participants’ characteristics1
| Characteristics | All participants (n = 153) | Participants with both colostrum and mature milk samples (n = 55) |
|---|---|---|
| Sex | ||
| Male | 81 (53) | 28 (51) |
| Female | 72 (47) | 27 (49) |
| Birth weight, g | ||
| <1000 | 39 (25) | 14 (25) |
| 1000–1500 | 114 (75) | 41 (75) |
| Delivery mode | ||
| Vaginal | 26 (17) | 7 (13) |
| Cesarean | 127 (83) | 48 (87) |
| Adequacy of weight for gestational age | ||
| AGA | 106 (69) | 40 (73) |
| SGA | 41 (27) | 12 (22) |
| LGA | 6 (4) | 3 (5) |
| Gestational age, wk | ||
| ≥37 | 1 (1) | 1 (1) |
| 32–36 | 36 (23) | 13 (24) |
| 28–31 | 79 (52) | 26 (48) |
| <28 | 37 (24) | 15 (27) |
| Mean ± SD | 29.4 ± 1.5 | 29.4 ± 2.9 |
| Secretor phenotype | 143 (93) | 53 (96.4) |
| Lactoferrin2,3 | 83 (54.2) | |
| Outcomes3 | ||
| Late-onset sepsis | 43 (28.1) | |
| NEC | 10 (6.5) | |
| Sepsis-associated death | 8 (5.2) | |
Values are n (%) unless otherwise indicated. AGA, appropriate for gestational age; LGA, large for gestational age; NEC, necrotizing enterocolitis; SGA, small for gestational age.
Number of participants who received lactoferrin in the NEOLACTO trial (parent study). A potential, but unlikely, confounder.
Neither the proportion of participants who received lactoferrin nor the ones who developed the listed outcomes are reported for the cohort with both milk sample types (n = 55) because this cohort was solely used to compare concentrations of oligosaccharides between sample types (colostrum compared with mature milk), not for outcome assessment.
The samples were stratified according to their sSecretor status. Of the mothers, 93% were identified as secretors. In samples corresponding to secretors (n = 196 ; colostrum: 102, mature milk: 94), the 3 most abundant HMOs were 2'FL (median: 3282 μg/mL), LNFP I (2007 μg/mL), and difucosyllacto-N-tetraose (994 μg/mL). In samples corresponding to nonsecretors (n = 12; colostrum: 4, mature milk: 8), the 2 most abundant HMOs were lacto-N-tetraose (median: 1654 μg/mL) and LNFP II (1544 μg/mL) (Supplemental Table 1). There was a significant difference between secretors and nonsecretors in terms of the total amount of HMOs. Their distributions were compared by the Mann–Whitney U test (11.45 g/L; [IQR: 11.06–11.83 g/L] compared with 8.04 g/L; [IQR: 7.80–8.25 g/L] g/L; P < 0.0001, respectively). Medians of the total HMO concentrations were stratified according to the sample type. In colostrum (n = 106) the median was 11.47 g/L (IQR: 11,063–11,946 g/L), whereas in mature milk ( n = 102) the median was 11.34 g/L (IQR: 10,978–11,732 g/L).
The variation in the HMO concentrations (μg/mL) over the lactation period was examined in the 55 individuals who had both colostrum and mature milk samples. The oligosaccharides 2'FL and sialyllacto-N-tetraose c had higher concentrations in colostrum (3600 compared with 2777, and 662 compared with 304, respectively), whereas lacto-N-tetraose (LNT), sialyllacto-N-tetraose b, difucosyllacto-N-tetraose (DFLNT), and fucosyl-disialyllacto-N-hexose (FDSLNH) had higher concentrations in mature milk (Table 2). In addition, HMO diversity was examined in paired samples. The HMO diversity in mature milk was greater than in colostrum (median: 5.44; IQR: 4.05–6.79 compared with median: 4.25; IQR: 3.44–5.15; P = 0.0001 using the Wilcoxon matched-pairs test).
TABLE 2.
HMO concentrations in individuals with paired milk samples (μg/mL)1
| HMO | Colostrum (n = 55) | Mature milk (n = 55) |
|---|---|---|
| 2' FL | 3600 [2862–4339 ] | 2777 [1926 –3776] |
| 3FL | 211 [156–291] | 199 [121–251] |
| DFLac | 353 [256–618] | 349 [230– 524] |
| 3'SL | 342 [238–495] | 329 [213–506] |
| 6'SL | 460 [295–633] | 429 [309– 563] |
| LNT | 526 [353–793] | 891 [661–1491] |
| LNnT | 533 [444–631] | 496 [347 -–587] |
| LNH | 16 [10–31] | 29 [10–47] |
| LNFP I | 1947 [1358– 2525] | 1912 [1287–2663] |
| LNFP II | 429 [335 - 533] | 518 [389–784] |
| LNFP III | 92 [62–118] | 65 [47–94] |
| DFLNT | 819 [367–1390] | 1089 [523 –727] |
| FLNH | 61 [30–85] | 78 [38–123] |
| DFLNH | 137 [73 - 202] | 157 [9–25] |
| LSTb | 38 [26 -55] | 73 [56 - 137] |
| LSTc | 662 [496–816] | 304 [229–417] |
| DSLNT | 302 [207–394] | 337 [229–418] |
| DSLNH | 244 [203–348] | 253 [166– 327] |
| FDSLNH | 42 [31–77] | 79 [54–124] |
| Total | 11,482 [11,063–11,946] | 11,424 [10,978 –11,732] |
Values are medians [IQR]. DFLac, difucosyllactose; DFLNH, difucosyllacto-N-hexaose; DFLNT, difucosyllacto-N-tetraose; DSLNH, disialyllacto-N-hexaose; DSLNT, disialyllacto-N-tetraose; FDSLNH, fucosyl-disialyllacto-N-hexose; FLNH, fucosyllacto-N-hexaose; HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LSTb, sialyllacto-N-tetraose b; LSTc, sialyllacto-N-tetraose c; 2'FL, 2'-fucosyllactose; 3FL, 3-fucosyllactose; 3'SL, 3'-sialyllactose; 6'SL, 6'-sialyllactose.
Factor analysis was applied in a parallel manner in all samples for both milk sample types. We predetermined 3 factors for each sample type. Table 3 shows the results of the log-rank test (Mantel–Cox test) for each factor. Factor 3 in colostrum showed a statistically significant association with a reduced risk of both late-onset neonatal sepsis (HR: 0.63; 95% CI: 0.41, 0.97; P < 0.05) and the composite outcome (HR: 0.67; 95% CI: 0.46, 0.96; P < 0.05). Moreover, Factor 3 in colostrum showed a correlation of 0.63 with the oligosaccharide FDSLNH. The correlations between this factor and the other oligosaccharides were <0.3 (Supplemental Table 2). No other statistically significant differences were observed.
TABLE 3.
Survival analysis using the Mantel–Cox test for the factors obtained from the individual concentrations of human milk oligosaccharides in colostrum and mature milk1
| Outcomes | |||||
|---|---|---|---|---|---|
| Late-onset sepsis | Composite outcome2 | ||||
| Sample type | Factors | HR (95% CI) | P | HR (95% CI) | P |
| Colostrum (n = 99) | |||||
| 1 | 1.00 (0.67, 1.49) | 0.98 | 1.18 (0.85, 1.63) | 0.31 | |
| 2 | 1.26 (0.87, 1.80) | 0.21 | 1.30 (0.92, 1.84) | 0.14 | |
| 3 | 0.63 (0.41, 0.97) | 0.04 | 0.67 (0.46, 0.96) | 0.03 | |
| Mature milk (n = 100) | |||||
| 1 | 0.74 (0.45, 1.20) | 0.22 | 0.71 (0.44, 1.14) | 0.15 | |
| 2 | 0.96 (0.65, 1.44) | 0.86 | 0.92 (0.62, 1.36) | 0.67 | |
| 3 | 1.24 (0.83, 1.84) | 0.29 | 1.24 (0.84, 1.82) | 0.28 | |
Proportional hazards were assumed after we tested for nonproportionality using the Schoenfeld residuals (P > 0.05). HRs were calculated using the Mantel–Cox test. Factors 1, 2, and 3 were generated independently for colostrum and mature milk through factor analysis. They summarize the concentrations of the 19 initial oligosaccharides. We predetermined 3 factors for each sample type with eigenvalues >1. No rotation was applied for those factors.
Includes late-onset neonatal sepsis, necrotizing enterocolitis, and death by sepsis.
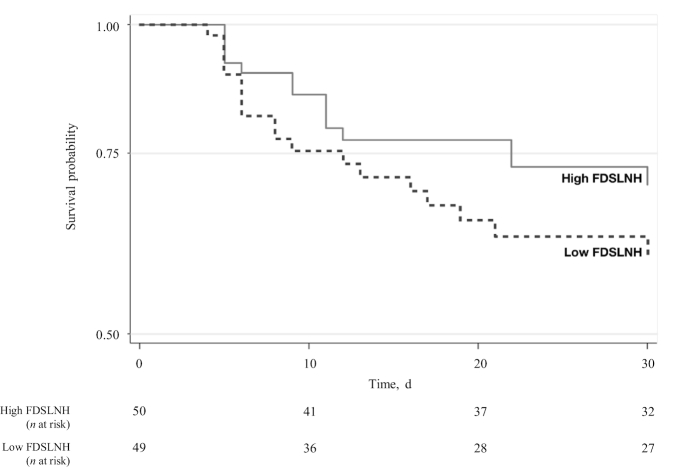
Post hoc analysis revealed that FDSLNH concentrations were lower in colostrum given to infants who had late-onset neonatal sepsis compared with control infants without late-onset neonatal sepsis (Figure 2). This was also observed in a Kaplan–Meier survival plot, where FDSLNH concentration was dichotomized using the median as the cutoff (Figure 3).
FIGURE 2.
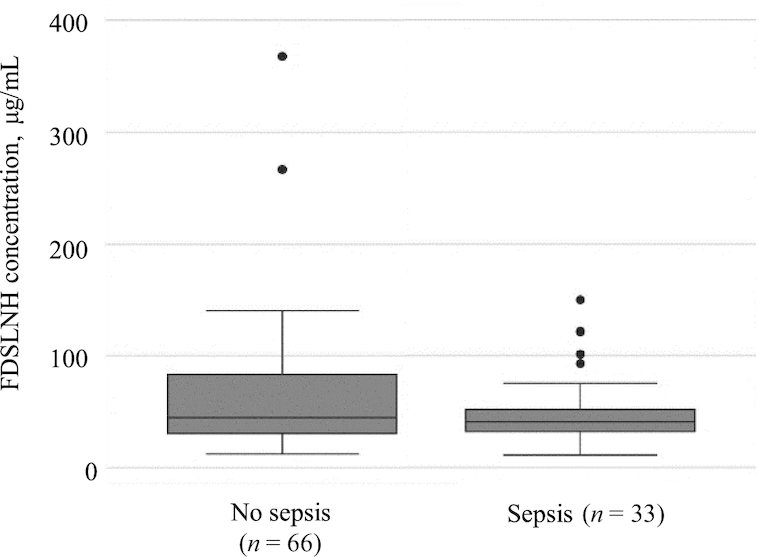
Concentrations of FDSLNH (μg/mL) in colostrum of mothers of infants with and without late-onset neonatal sepsis. Data are displayed in a box plot where the median is represented by a horizontal line within the box and the upper and lower borders of the box correspond to quartiles 3 and 1, respectively. FDSLNH, fucosyl-disialyllacto-N-hexose.
FIGURE 3.
Kaplan–Meier survival graph of infants developing late-onset neonatal sepsis within the neonatal period (3–30 d). Having an FDSLNH concentration in colostrum above the median was considered high FDSLNH (n = 50). Having an FDSLNH concentration in colostrum below the median was considered low FDSLNH (n = 49). FDSLNH, fucosyl-disialyllacto-N-hexose.
Discussion
Previous studies have suggested that HMOs have a protective effect against some infectious agents; however, clinical studies to validate associations in human cohorts are often missing. To our knowledge, this is the first longitudinal study that explores the association between HMOs and late-onset neonatal sepsis. Through factor analysis and the Mantel–Cox test, we found an association of Factor 3 in colostrum with a reduced risk of late-onset sepsis (HR: 0.63; 95% CI: 0.41, 0.97), which correlated with the oligosaccharide FDSLNH. We further explored this association in the post hoc analysis where we found greater concentrations of FDSLNH in colostrum of infants who did not develop late-onset neonatal sepsis. Little is known about the biological effects of this HMO. In other studies, FDSLNH measured at 1 mo has been proven to have a linear positive association with the weight of infants at 6 mo of age (19). Also, a longitudinal study in a Canadian population showed that greater concentrations of FDSLNH are associated with a lower risk of food sensitization at 1 y (20). The association between this HMO and late-onset neonatal sepsis must be confirmed by future studies.
There is growing evidence that the biological effects of some HMOs are structure specific (21). One of the most comprehensive examples is the antimicrobial effect of α1-2-fucosylated HMOs over C. jejuni by specifically inhibiting its attachment to the epithelial surface and further colonization (9). In contrast, in order to block the attachment of E. histolytica, fucose has to be linked by both α1-4- and α1-3-linkages, and the α-1-2-linkage has to be removed. Some sepsis-related pathogens have also been studied. One study conducted by Andreas et al. (10) suggested that the oligosaccharide lacto-N-difucohexaose I could be associated with reduced colonization by one of the most common late-onset sepsis pathogens, Group B Streptococcus (GBS). They also observed in vitro a bacteriostatic effect over GBS in a dose-dependent manner (10). An animal model study conducted by Idänpään-Heikkilä et al. (22) reported that the administration of lacto-N-neotetraose and some of its sialylated derivatives can inhibit the colonization of Streptococcus pneumoniae in the oropharynx and even serve as a therapeutic agent once pneumonia or bacteremia have been installed. Despite the need for confirmation of the association observed in our cohort, our exploratory model supports future research into effects of FDSLNH on the pathogenesis of late-onset neonatal sepsis. In Peru, the most frequently isolated pathogens are coagulase-negative Staphylococcus, Staphylococcus aureus, and Gram-negative bacteria (23). Interestingly, a Finnish study found a strong correlation between greater concentrations of FDSLNH and bacterial counts of S. aureus in milk (24).
The frequency of the secretor phenotype in this study was 93%. Other studies have reported that the frequency of the secretor phenotype varies geographically, but it is more common than the nonsecretor phenotype (7, 17, 25, 26). The secretor phenotype is more frequent in Latin-American populations than it is in Caucasians (12). A study of geographic variation published in 2017 by McGuire et al. (12) reported that 98% of Peruvian mothers who lived in a peri-urban area in Lima were secretors (n = 43), compared with 68% in Ghana and 68% in Washington, USA. Moreover, Bode () reported that 70% of Caucasian women carry the secretor gene.
The oligosaccharides with the highest concentrations in this study were 2'FL, LNFP I, DFLNT, LNT, and LNFP II among secretors and nonsecretors, similar to other studies (27). The quantity of these oligosaccharides is also similar to those found by other studies, even in different populations. Kunz et al. (27) reported that LNFP I ranges between 1.2 and 1.7 g/L and LNT between 0.5 and 1.5 g/L in milk samples from German mothers of term infants (28). In our cohort, LNFP I showed a median concentration of 2 g/L in secretors, whereas LNT was present at 0.7 g/L in secretors and 1.7 g/L in nonsecretors. In 2015, Alderete et al. (19) reported that in American mothers of term neonates, the most abundant oligosaccharide was 2'FL, with a median concentration of 2.8 g/L in the first month; the second most abundant oligosaccharide was LNT with 1.4 g/L, followed by LNFP II with 1.3 g/L.
In the present study, which includes almost entirely preterm neonates, the total amount of HMO was similar in colostrum and mature milk, with values ∼11 g/L. This quantity seems low when compared with other studies that report 20–23 g/L in colostrum and 12–15 g/L in mature milk of preterm infants (14, 29, 30). Furthermore, these studies suggest a tendency toward higher concentrations in preterm infants than in term infants.
There was a significant difference in the total amount of HMOs between secretors and nonsecretors. This difference could be explained given that nonsecretors lack the enzyme FUT2, and therefore lack 2 of the most abundant HMOs: 2'FL and LNFP I (11).
This study had some limitations including the retrospective design and the limited quantity of milk samples per individual. In addition, the large number of HMOs analyzed did not allow us to establish individual direct associations because of the increased risk of obtaining a false positive association. To account for this problem we used factor analysis, a useful method for exploratory analysis. A potential limitation of this method is that factor analysis grouped HMOs according to the greater linear variation and not necessarily according to a metabolic or biological relation, thus potentially missing a true individual association.
In conclusion, these findings suggest that concentrations of different HMOs vary from one individual to another according to their lactation period and secretor status. We also found that FDSLNH might protect infants with very low birth weight from late-onset neonatal sepsis. If true, this association could prove one more mechanism by which human milk protects infants against infections. In addition, it would open the door to a wide range of clinical applications of HMOs: from testing adequate concentrations of protective agents to enhancing supplementation of infant formulas. Therefore, we recommend a prospective study testing FDSLNH with a larger population, broader geographic scope, and rigorous microbiological detection of the most frequent causal agents of late-onset neonatal sepsis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the NEOLACTO study team; especially Zegarra, Bellomo, Castaneda, and Cam, for allowing us to use the data and milk samples for this study. The support of the Family Larsson-Rosenquist Foundation (to LB) is gratefully acknowledged. Also, we thank our coworkers Nataly Espinoza and Ian Hargraves from the Knowledge and Evaluation Research Unit, Mayo Clinic, Rochester, MN, whose final edits improved our work.
The authors’ responsibilities were as follows—TJO: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; VDTR, MUS, TJO, CPC, and LB: designed the research; JG, CY, and VDTR: conducted the research; VDTR, MUS, CPC, LB, and TJO: analyzed and interpreted the data; VDTR, MUS, LB, and TJO: wrote the paper; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The data of this article were presented in abstract form at the 19th International Society for Research in Human Milk and Lactation Conference, Shonan Village, Japan, 6–11 October 2018.
Supported by National Institute of Child Health and Human Development grant R01-HD067694 (to TJO and the NEOLACTO study team).
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: DFLNT, difucosyllacto-N-tetraose; FDSLNH, fucosyl-disialyllacto-N-hexose; FUT2, α1-2-fucosyltransferase; GBS, group B streptococcus; HMO, human milk oligosaccharide; HPLC-FL, high-performance liquid chromatography with fluorescence detection; IRB, Institutional Review Board; LNFP, lacto-N-fucopentaose; LNT, lacto-N-tetraose; UPCH, Universidad Peruana Cayetano Heredia; 2'FL, 2'-fucosyllactose.
Contributor Information
Victor D Torres Roldan, Alberto Hurtado Faculty of Medicine, Cayetano Heredia Peruvian University, Lima, Peru.
Meritxell Urtecho S, Alberto Hurtado Faculty of Medicine, Cayetano Heredia Peruvian University, Lima, Peru.
Julia Gupta, Department of Pediatrics, and Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence, University of California San Diego, La Jolla, CA, USA.
Chloe Yonemitsu, Department of Pediatrics, and Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence, University of California San Diego, La Jolla, CA, USA.
Cesar P Cárcamo, School of Public Health and Administration, Cayetano Heredia Peruvian University, Lima, Peru.
Lars Bode, Department of Pediatrics, and Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence, University of California San Diego, La Jolla, CA, USA.
Theresa J Ochoa, Alberto Hurtado Faculty of Medicine, Cayetano Heredia Peruvian University, Lima, Peru; Institute of Tropical Medicine “Alexander von Humboldt,” Cayetano Heredia Peruvian University, Lima, Peru; Center for Infectious Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA.
References
- 1. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156(2 Suppl):S3–7. [DOI] [PubMed] [Google Scholar]
- 4. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91(11):619–22. [DOI] [PubMed] [Google Scholar]
- 5. Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58(9):5334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol. 2004;38(6 Suppl):S80–3. [DOI] [PubMed] [Google Scholar]
- 7. De Leoz ML, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, Tancredi DJ, Smilowitz JT, Kalanetra KM, Mills DA, German JB et al.. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res. 2012;11(9):4662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mielcarek C, Romond PC, Romond MB, Bezirtzoglou E. Anaerobe modulation of bacterial translocation in mice mediated through lactose and human milk oligosaccharides. Anaerobe. 2011;17(6):361–6. [DOI] [PubMed] [Google Scholar]
- 9. Miñana IV. Oligosacáridos en la leche humana. Acta Pediatr Esp. 2007;65(3):129–33. [Google Scholar]
- 10. Andreas NJ, Al-Khalidi A, Jaiteh M, Clarke E, Hyde MJ, Modi N, Holmes E, Kampmann B, Mehring Le Doare K. Role of human milk oligosaccharides in Group B Streptococcus colonisation. Clin Transl Immunology. 2016;5(8):e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Y, Lawlor NT, Newburg DS. Human milk components modulate toll-like receptor–mediated inflammation. Adv Nutr. 2016;7(1):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE et al.. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017;105(5):1086–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22(9):1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104(9):1261–71. [DOI] [PubMed] [Google Scholar]
- 15. Ochoa TJ, Zegarra J, Bellomo S, Carcamo CP, Cam L, Castaneda A, Villavicencio A, Gonzales J, Rueda MS, Turin CG et al.. Randomized controlled trial of bovine lactoferrin for prevention of sepsis and neurodevelopment impairment in infants weighing less than 2000 grams. J Pediatr. 2020;219:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, Patel AL, Hou J, Lewis NE, Bode L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2017;67(6):1064–70. [DOI] [PubMed] [Google Scholar]
- 17. Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012;11(12):6124–33. [DOI] [PubMed] [Google Scholar]
- 18. Ismail K. Unravelling factor analysis. Evid Based Ment Health. 2008;11(4):99. [DOI] [PubMed] [Google Scholar]
- 19. Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, Fields DA. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102(6):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miliku K, Robertson B, Sharma AK, Subbarao P, Becker AB, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, Bode L et al.. Human milk oligosaccharide profiles and food sensitization among infants in the CHILD Study. Allergy. 2018;73(10):2070–3. [DOI] [PubMed] [Google Scholar]
- 21. Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 2012;3(3):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Idänpään-Heikkilä I, Simon PM, Zopf D, Vullo T, Cahill P, Sokol K, Tuomanen E. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis. 1997;176(3):704–12. [DOI] [PubMed] [Google Scholar]
- 23. Alvarado-Gamarra G, Alcalá-Marcos KM, Abarca-Alfaro DM, Bao-Castro V. Características microbiológicas y terapéuticas de la sepsis neonatal confirmada en un hospital de Lima, Perú. Rev Peru Med Exp Salud Publica. 2016;33(1):74. [PubMed] [Google Scholar]
- 24. Aakko J. New insights into human gut microbiota development in early infancy: influence of diet, environment and mother's microbiota [thesis]. Turku: University of Turku; 2016. [Google Scholar]
- 25. Morrow A, Meinzen-Derr J, Huang P, Schibler K, Cahill T, Keddache M, Kallapur SG, Newburg D, Tabangin M, Warner B et al.. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr. 2012;158(5):745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunz C, Meyer C, Collado MC, Geiger L, García-Mantrana I, Bertua-Ríos B, Martínez-Costa C, Borsch C, Rudloff S. Influence of gestational age, secretor, and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr. 2017;64(5):789–98. [DOI] [PubMed] [Google Scholar]
- 27. Kunz C, Rudloff S. Biological functions of oligosaccharides in human milk. Acta Pediatr. 1993;82(11):903–12. [DOI] [PubMed] [Google Scholar]
- 28. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61(10):1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128(6):e1520–31. [DOI] [PubMed] [Google Scholar]
- 30. Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88(430):89–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.