ABSTRACT
Background
Glycemic load (GL) reflects the quantity and quality of carbohydrates in the diet; dietary fiber and added sugar are components of GL. Few epidemiologic studies have assessed the association between these dietary factors and fecundability.
Objective
We prospectively evaluated the associations of GL, total carbohydrates, dietary fiber, and added sugar with fecundability.
Methods
Snart Foraeldre (SF) and Pregnancy Study Online (PRESTO) are parallel web-based prospective preconception cohorts of couples attempting to conceive in Denmark and North America. At baseline, female participants completed a web-based questionnaire on demographic and lifestyle factors and a validated FFQ. We calculated GL, total carbohydrate intake, total dietary fiber, carbohydrate-to-fiber ratio, and added sugar based on reported frequencies for individual foods, standard recipes for mixed foods, and average serving sizes. The analysis included 2709 SF participants and 4268 PRESTO participants. We used proportional probabilities regression models to estimate fecundability ratios (FR) and 95% CIs.
Results
Compared with an average daily GL of ≤100, FRs for an average daily GL of ≥141 were 0.89 (95% CI: 0.73, 1.08) in SF and 0.87 (95% CI: 0.77, 0.98) in PRESTO participants. Compared with consuming ≤16 g/d of dietary fiber, FRs for consuming ≥25 g/d were 0.99 (95% CI: 0.81, 1.22) in SF and 1.06 (95% CI: 0.94, 1.20) in PRESTO. Compared with a carbohydrate-to-fiber ratio of ≤8, FRs for a ratio of ≥13 were 0.86 (95% CI: 0.73, 1.01) in SF and 0.87 (95% CI: 0.78, 0.98) in PRESTO. Compared with ≤27 g/d of added sugar, FRs for ≥72 g/d were 0.87 (95% CI: 0.68, 1.10) in SF and 0.86 (95% CI: 0.75, 0.99) in PRESTO participants.
Conclusions
Among women attempting to conceive in Denmark and North America, diets high in GL, carbohydrate-to-fiber ratio, and added sugar were associated with modestly reduced fecundability.
Keywords: time-to-pregnancy, fecundability, glycemic load, carbohydrate, dietary fiber, added sugar
Introduction
Approximately 10–15% of couples experience infertility, the inability to conceive within 12 mo of unprotected intercourse (1). Although infertility imposes a large financial and emotional burden for affected couples, few modifiable risk factors have been identified.
The glycemic index (GI) is the relative ranking of carbohydrates based on how a carbohydrate affects blood sugar. GI is the measure of the glycemic effect of a specific food's carbohydrate content compared with the equivalent carbohydrate quantity in standard glucose (2). High-fiber foods tend to lower blood glucose, resulting in lower GI, while simple carbohydrates tend to raise blood glucose, resulting in higher GI. Glycemic load (GL) is a more accurate way to assess the effect of diet on blood glucose concentration because it accounts for carbohydrate quality through the GI and carbohydrate quantity through portion size. Low carbohydrate quality and high GL have been linked via insulin resistance to increased risk of diabetes (3), cardiovascular disease (4), and polycystic ovary syndrome (PCOS) (5).
Insulin resistance may be an important determinant of ovulatory function, as high insulin concentration may upregulate free testosterone production, resulting in hyperandrogenism (6, 7). Improving insulin sensitivity through medications or dietary interventions has been shown to decrease circulating testosterone concentrations and improve hormonal function for individuals with obesity or PCOS (7). One cross-sectional study (8), 1 randomized control trial (9), and 2 case-control studies (10, 11) reported increased cycle regularity among women with PCOS who were treated with metformin, an insulin sensitivity–improving drug. In a randomized controlled trial that assigned overweight or obese women to a low-GI diet or a conventional healthy diet, insulin sensitivity was improved with the low GI diet (12).
While several studies have examined how dietary changes affect the hormonal milieu (8–11), few studies have assessed the influence of GL on fertility. One prospective analysis, conducted within the Nurses’ Health Study II, showed that individuals with the highest quintile of carbohydrate intake and the highest quintile of daily GL had nearly double the risk of ovulatory infertility compared with those in the lowest quintile of each respective factor (13).
We evaluated the extent to which GL and its major determinants, total carbohydrates, fiber, and added sugar, were associated with fecundability in 2 internet-based preconception cohort studies of women attempting to conceive in Denmark and North America.
Methods
Study population
Snart Foraeldre (SF) (“Soon Parents”) is an ongoing internet-based prospective preconception cohort study of couples attempting to conceive in Denmark. Launched in 2011, SF is an expansion of the Snart Gravid (“Soon Pregnant”) study, described in detail elsewhere (14). Eligible participants are women aged 18–45 y who are attempting pregnancy and not using fertility treatment. Participants were recruited primarily through advertising on a popular health-related website and social media (15, 16). Upon enrollment, female participants complete an online self-administered baseline questionnaire. Beginning in February 2013, participants were asked to complete a food-frequency questionnaire (SF-FFQ) that was designed for and validated in this population (17). Of the 6354 eligible women who completed the baseline questionnaire, we excluded 128 whose last menstrual period (LMP) was >6 mo before study entry and 140 women with missing or implausible LMP information. We excluded 1554 women attempting to achieve pregnancy for >6 mo at study entry, and 995 women who filled out the baseline questionnaire prior to SF-FFQ implementation. We further excluded 761 women who did not complete the SF-FFQ (response rate of 78%), 34 women with implausible total energy intake (<600 or >3800 kcal/d), and 33 who had >12 missing food items on the SF-FFQ, for a final analytic sample of 2709 women (Supplementary Figure 1).
The Pregnancy Study Online (PRESTO) is an ongoing internet-based prospective preconception cohort study of couples attempting to conceive in the United States and Canada. PRESTO was initiated in 2013 and modeled after SF. Study methods have been described in detail elsewhere (18). Women aged 21–45 y who are attempting pregnancy and not using fertility treatment are eligible for participation. Participants were recruited primarily through advertising on social media and pregnancy related websites (18). Female participants complete a baseline questionnaire and may complete the National Cancer Institute's Diet History Questionnaire II (DHQII) (19). A total of 8773 eligible women completed the baseline questionnaire. We excluded 102 women whose baseline LMP was >6 mo before study entry, 35 women with insufficient or missing LMP data, and 1762 women attempting to achieve pregnancy for >6 mo at study entry. We further excluded 2514 women who did not fill out the DHQII (response rate of 63%) and 92 women with total energy intake <600 or >3800 kcal/d, for a final analytic sample of 4268 women (Supplementary Figure 1).
In both cohorts, baseline questionnaires are used to ascertain information on demographic, lifestyle, reproductive, and medical histories. Female participants complete bimonthly follow-up questionnaires to ascertain self-reported pregnancy status, regardless of outcome, for up to 12 mo or reported conception. SF is registered at Aarhus University (2016-051-000001, number 431) and complies with Danish and European Union legislation on data protection. SF and PRESTO were approved by the Institutional Review Board at Boston Medical Center. Participants in both cohorts provided online informed consent.
Exposure assessment
We estimated food groups and macro- and micronutrient intakes using the nutrient composition of all food items in each cohort. In SF, we used the Danish Nutrient Database (20), and in PRESTO, we used the National Cancer Institute's Diet*Calc software (version 1.5.0). A priori, we evaluated the association between GL and its 2 primary components, dietary fiber and added sugar, and fecundability. In addition, we examined the association between other measures of carbohydrate quality, including total carbohydrate intake, GI, and carbohydrate-to-fiber ratio, for a more comprehensive analysis and to provide comparability with prior literature. We estimated GL, total carbohydrate intake, dietary fiber (including fruit, vegetable, and cereal fiber), and added sugar in both cohorts. In PRESTO, we estimated soluble fiber, insoluble fiber, sucrose, and fructose (data not available in SF). We derived an average daily GI by dividing average daily GL by the average daily carbohydrate intake (21). We used pure glucose as the scale for measuring GI and GL. We calculated carbohydrate-to-fiber ratios by dividing average daily carbohydrate intake by average daily dietary fiber intake. Nutrients, including total carbohydrates, fiber, and added sugar, were validated within each population (17–22). The SF-FFQ was validated against a 4-d food record in Denmark among 100 study participants, with deattenuated Pearson correlation coefficients for total carbohydrates, fiber, and added sugar of 0.70, 0.70, and 0.47, respectively (17). The DHQII was validated against repeated 24-h dietary recalls in the United States, with deattenuated Pearson correlation coefficients for total carbohydrates, fiber, and added sugar of 0.69, 0.77, and 0.79, respectively (19). In Denmark, GL was calculated using published GI values (23) for each SF-FFQ food item. For foods specific to the Danish diet, published values (24) were used. If published GI values did not exist, GI values for similar foods were chosen based on nutritional content. In PRESTO, GL was calculated using published GI values (23) for each DHQII food and, if published GI values did not exist, decision criteria (25) were used to assign GI values. Serving size–specific GL values were calculated for each food item (25). We adjusted nutrient intakes utilizing the nutrient residual method, standardizing to 2000 kcal in both cohorts (26).
Fecundability assessment
At baseline, women reported their LMP and the number of cycles they attempted pregnancy at study entry. Women with regular cycles, defined as being able to “predict about when the next period would start” during times when they were not using hormonal contraception, were also asked about their usual cycle length. On each follow-up questionnaire, participants reported their LMP and whether they had conceived since the last questionnaire. For women with irregular cycles, we estimated cycle length based on reported LMP at baseline and over follow-up. Fecundability, the primary outcome for this research, was estimated based on total discrete menstrual cycles at risk, calculated as: cycles of attempt at study entry + [(LMP date from most recent follow-up questionnaire − date of baseline questionnaire completion)/usual cycle length] +1. Females contributed observed cycles from baseline until reported conception, initiation of fertility treatment, cessation of pregnancy attempt, withdrawal, loss to follow-up, or 12 cycles, whichever came first.
Covariate assessment
At baseline, participants reported their age, weight, height, race, ethnicity, marital status, education, income, smoking status, alcohol intake, physical activity, parity, gravidity, last form of contraception, intercourse frequency, use of any methods to time intercourse (e.g., ovulation testing, menstrual charting), and multivitamin use. BMI was calculated as kg/m2. In SF, total metabolic equivalents (METs) were calculated using the International Physical Activity Questionnaire short form by summing MET-hours from walking, moderate physical activity, and vigorous physical activity (27). In PRESTO, MET-hours were calculated by multiplying the average hours per week spent in various activities by metabolic equivalents estimated by the Compendium of Physical Activity (28). Based on the dietary questionnaires, we assessed diet quality [measured via the Nutrient Rich Diet Score (NRDS) in SF and the Healthy Eating Index 2010 (HEI) in PRESTO] and total energy intake (29, 30). To avoid overadjustment, we calculated an adjusted diet quality measure by removing the proportion of the diet quality score contributed by whole grains and added sugar for the GL–fecundability and carbohydrate–fecundability associations; the proportion of the score contributed by whole grains for the fiber–fecundability association; and the proportion of the score contributed by added sugar for the added sugar–fecundability association. All other potential confounders were identical, with the exception of race/ethnicity (not ascertained in SF) and education, income, and marital status, which were ascertained differently across studies.
Data analysis
We used proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% CIs for the association between selected dietary factors and fecundability. The FR is the ratio of the average per-cycle probability of conception comparing the exposed category with the unexposed (reference) category. An FR <1 indicates a longer time to pregnancy among exposed relative to unexposed women. The discrete-time proportional probabilities model in Weinberg et al. (31) includes indicator variables for each cycle at risk, thereby accounting for the decline in fecundability over time in the population still being followed. The Andersen–Gill data structure outputs a single menstrual cycle per observation and accounts for left truncation from delayed entry into the study.
We conducted parallel analyses across cohorts and then, as the cohorts were designed to have virtually identical data collection methods and instruments, harmonized the data to conduct a pooled analysis (32). We additionally conducted a fixed-effect meta-analysis to allow for heterogeneity in the exposure and covariates. To facilitate comparison, we used the same categories for GL, carbohydrate intake, dietary fiber, carbohydrate-to-fiber ratio, and added sugar within each cohort, based on daily recommended values for each nutrient (2, 33, 34). We used the same categories for GI, fruit fiber, and vegetable fiber in both cohorts, based on the distribution across cohorts. Due to nonoverlapping distributions, we categorized cereal fiber based on the cohort-specific distribution. We categorized soluble fiber, insoluble fiber, fructose (total, contribution from fruit, and contribution from other sources), and sucrose based on the distribution in PRESTO. We examined the associations of dietary factors in either 5-unit (total dietary fiber, carbohydrate-to-fiber ratio, and soluble, insoluble, cereal, fruit, and vegetable fiber) or 10-unit increments (GL, GI, carbohydrates, and added sugar) and fecundability. We additionally examined the associations of GL, dietary fiber, and added sugar as continuous variables by fitting restricted cubic splines to allow for nonlinear associations (35).
Final models were adjusted for age (<25, 25–29, 30–34, 35–39, or ≥40 y), BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), income (<25, 25–39, 40–64, or ≥65 k DKK/mo in SF and <50 , 50–99, 100–149, or ≥150 k US$/y in PRESTO), energy intake (kilocalories per day), smoking status (never, current, occasional, or past), parity (parous or nulliparous), alcohol intake (number of drinks per week), physical activity per week (<10, 10–19, 20–39, or ≥40 MET-h/wk), last form of birth control (natural, barrier, or hormonal), married or live together (yes or no), intercourse frequency (<1, 1, 2–3, or ≥4 times/wk), using method to improve chances of pregnancy (yes or no), daily use of prenatal or multivitamins (yes or no), education (≤12, 13–15, 16, or ≥17 y of education), and adjusted dietary quality (HEI or NRDS). PRESTO models were additionally adjusted for race/ethnicity (non-Hispanic white or other race/ethnicity). In a secondary analysis, multivariable models for carbohydrate intake were additionally adjusted for total protein and trans-fatty acid intake to simulate the substitution for carbohydrates at the expense of naturally occurring fats. In pooled analyses, we additionally adjusted for cohort.
We previously reported an association between sugar-sweetened soda intake (both partners) and fecundability in PRESTO (limited data on male diet were available in SF) (36); therefore, in the present analysis we conducted sensitivity analyses within PRESTO where we 1) removed the portion of added sugar contributed by sugar-sweetened soda and 2) restricted the cohort to women with complete partner data (n = 1380) and adjusted for male sugar-sweetened beverage (SSB) intake (36).
We assessed the extent to which the association between GL, carbohydrate intake, dietary fiber, carbohydrate-to-fiber ratio, and added sugar varied by BMI (<25 compared with ≥25 kg/m2), as adiposity may modify the GL–fecundability association (2). Because Chavarro et al. reported that parity modified the effect of GL on ovulatory infertility (13), we conducted analyses stratified by parity (parous compared with nulliparous). Finally, to assess the potential for reverse causation, we stratified by attempt time at study entry (<3 compared with 3–6 cycles).
We used multiple imputation to impute missing data on covariates and pregnancy outcomes (37). We generated 5 imputed datasets for SF and PRESTO, and combined coefficient and SE results across imputed datasets within each cohort (38). For women with no follow-up data (SF, n = 177; PRESTO, n = 460), we assigned them 1 cycle of follow-up and imputed their pregnancy status (38). Missingness for covariates ranged from <1.0% (prior pregnancy) to 10% (income) in SF and from <0.1% (prior pregnancy) to 4% (income) in PRESTO. There were no missing values for age or energy intake. All statistical analyses were performed using SAS version 9.4 (39). We interpreted results following the recommendations of the American Statistical Association regarding statistical significance testing (40). Based on these guidelines, we eschewed significance testing, instead interpreting our findings based on the magnitude, precision, and potential for bias in the estimates we report.
Results
During 2013 to 2018, 2709 SF participants contributed a total of 1818 pregnancies and 9609 cycles, and 4268 PRESTO participants contributed a total of 2652 pregnancies and 17,390 cycles. Average GL intake was similar across cohorts (SF: 120, IQR: 110–129; PRESTO: 121, IQR: 104–135), but average carbohydrate and dietary fiber intakes were slightly higher in SF (SF: 238 g/d; IQR: 221–256 g/d; PRESTO: 229 g/d; IQR: 204–254 g/d and SF: 25 g/d; IQR: 21–28 g/d; PRESTO: 21 g/d; IQR: 17–25 g/d, respectively) and average added sugar intake was higher in PRESTO (SF: 36 g/d; IQR: 23–42 g/d; PRESTO: 53 g/d; IQR: 33–63 g/d). Dietary fiber intake was higher in SF due to greater intake of cereal fiber; fiber intake from fruit and vegetables was comparable across cohorts.
In SF, the top food contributor to GL and dietary fiber was rye bread and the top contributor to added sugar was SSB; in PRESTO, the top food contributor to GL and added sugar was sugar-sweetened soda and the top food contributor to dietary fiber was nuts and seeds. In SF, high GL was positively associated with parity and PCOS diagnosis and inversely associated with alcohol intake, intercourse frequency, and education (Table 1). In PRESTO, high GL was positively associated with parity and current smoking, and inversely associated with education, income, and alcohol intake.
TABLE 1.
Baseline demographics for the SF and PRESTO cohorts based on glycemic load1
| SF | PRESTO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average daily glycemic load | Average daily glycemic load | |||||||||
| ≤100 | 101–114 | 115–125 | 126–140 | ≥141 | ≤100 | 101–114 | 115–125 | 126–140 | ≥141 | |
| Women, n | 299 | 730 | 776 | 665 | 239 | 897 | 888 | 828 | 832 | 823 |
| Age, y | 29.3 ± 3.4 | 29.2 ± 3.7 | 28.9 ± 3.8 | 28.7 ± 3.4 | 28.6 ± 4.1 | 30.3 ± 3.7 | 30.1 ± 3.9 | 30.1 ± 3.8 | 30.0 ± 3.9 | 30.0 ± 4.3 |
| BMI, kg/m2 | 24.4 ± 4.6 | 24.2 ± 4.9 | 23.9 ± 4.6 | 23.9 ± 4.9 | 24.1 ± 5.5 | 26.1 ± 6.1 | 26.4 ± 6.6 | 26.9 ± 7.0 | 27.3 ± 7.1 | 29.0 ± 7.8 |
| HEI (PRESTO) | — | — | — | — | — | 69.4 ± 69.7 | 68.6 ± 10.0 | 68.1 ± 10.3 | 65.8 ± 10.0 | 59.8 ± 12.1 |
| NRDS (SF) | 1050 ± 74 | 1054 ± 56 | 1040 ± 60 | 1020 ± 72 | 944 ± 122 | — | — | — | — | — |
| Energy intake, kcal/d | 1820 ± 559 | 1923 ± 571 | 1892 ± 493 | 1831 ± 460 | 1727 ± 500 | 1579 ± 539 | 1593 ± 513 | 1580 ± 488 | 1577 ± 525 | 1578 ± 550 |
| Smoking status | ||||||||||
| Current | 8.5 | 5.6 | 4.0 | 5.3 | 5.5 | 2.7 | 2.8 | 3.1 | 4.1 | 10.2 |
| Never | 67.9 | 68.7 | 76.5 | 75.7 | 77.5 | 77.4 | 77.6 | 79.0 | 89.3 | 85.4 |
| Occasional | 7.2 | 8.0 | 4.5 | 5.5 | 4.2 | 3.3 | 3.2 | 3.7 | 3.4 | 3.0 |
| Past | 16.4 | 17.5 | 15.0 | 13.6 | 12.8 | 16.5 | 16.5 | 14.3 | 10.7 | 14.6 |
| Parous | 22.3 | 29.2 | 33.8 | 37.5 | 37.0 | 23.3 | 27.9 | 28.1 | 31.6 | 36.7 |
| Alcohol intake, drinks/wk | 3.4 ± 3.6 | 3.0 ± 3.3 | 2.8 ± 3.6 | 2.3 ± 3.3 | 1.9 ± 2.6 | 4.8 ± 7.1 | 3.7 ± 4.3 | 3.1 ± 3.9 | 2.4 ± 3.7 | 1.9 ± 3.0 |
| SSB intake, drinks/wk | 0.3 ± 0.8 | 0.5 ± 1.1 | 0.8 ± 1.6 | 1.1 ± 1.7 | 2.1 ± 2.9 | 0.8 ± 1.9 | 1.4 ± 2.5 | 1.9 ± 2.8 | 2.7 ± 4.6 | 5.1 ± 6.7 |
| Physical activity, MET-h/wk | ||||||||||
| <10 | 6.7 | 9.4 | 11.6 | 9.5 | 16.6 | 7.5 | 10.5 | 10.5 | 11.8 | 18.0 |
| 10–19 | 11.9 | 11.8 | 12.9 | 16.5 | 13.4 | 17.5 | 18.9 | 16.7 | 20.7 | 19.7 |
| 20–39 | 33.9 | 27.1 | 26.4 | 25.0 | 26.1 | 35.9 | 35.1 | 36.7 | 35.7 | 32.0 |
| ≥40 | 47.5 | 51.7 | 49.0 | 48.9 | 43.9 | 39.2 | 35.5 | 36.2 | 31.7 | 30.3 |
| Most recent birth control method | ||||||||||
| Natural | 2.7 | 3.3 | 4.4 | 3.2 | 5.5 | 22.5 | 22.6 | 18.3 | 18.6 | 19.5 |
| Hormonal | 60.0 | 57.3 | 56.0 | 60.5 | 57.7 | 32.9 | 35.3 | 38.9 | 43.0 | 45.1 |
| Barrier | 37.4 | 39.5 | 39.7 | 36.4 | 36.8 | 44.6 | 42.1 | 42.8 | 38.4 | 35.4 |
| Intercourse frequency, times/wk | ||||||||||
| <1 | 15.9 | 19.5 | 18.6 | 17.8 | 24.4 | 20.4 | 20.1 | 21.2 | 20.2 | 23.5 |
| 1 | 19.9 | 18.7 | 21.8 | 21.6 | 21.9 | 20.4 | 19.2 | 18.0 | 17.5 | 18.7 |
| 2–3 | 46.1 | 45.1 | 46.8 | 48.1 | 46.2 | 44.2 | 45.9 | 48.5 | 47.8 | 41.3 |
| ≥4 | 18.1 | 16.7 | 12.7 | 12.5 | 7.5 | 15.1 | 14.8 | 12.3 | 14.6 | 16.5 |
| Using method to improve pregnancy chances | 73.0 | 71.9 | 74.2 | 72.0 | 71.7 | 76.6 | 75.2 | 76.8 | 74.8 | 74.9 |
| Daily use of multivitamin | 73.7 | 68.9 | 71.9 | 70.1 | 65.3 | 87.2 | 85.8 | 83.7 | 84.1 | 78.8 |
| Non-Hispanic white | — | — | — | — | — | 86.9 | 87.6 | 87.5 | 87.9 | 82.7 |
| Married2 | 96.0 | 97.9 | 98.3 | 97.3 | 95.5 | 94.9 | 94.7 | 93.5 | 93.1 | 89.3 |
| Education, y | ||||||||||
| ≤12 | 2.4 | 2.4 | 2.6 | 5.2 | 6.3 | 1.9 | 1.4 | 1.8 | 2.7 | 6.7 |
| 13–15 | 15.3 | 14.0 | 14.0 | 16.2 | 21.1 | 13.2 | 15.4 | 17.1 | 19.0 | 26.5 |
| 16 | 37.3 | 37.7 | 36.9 | 39.0 | 36.5 | 34.5 | 37.3 | 34.8 | 33.2 | 30.9 |
| ≥17 | 45.0 | 46.0 | 46.4 | 39.6 | 36.2 | 50.5 | 45.9 | 46.3 | 45.1 | 36.0 |
| Income, DKK/mo or US$/y | ||||||||||
| <25/50 k | 14.6 | 12.8 | 12.6 | 12.6 | 11.4 | 9.7 | 12.9 | 14.7 | 16.4 | 28.0 |
| 25–39/50–99 k | 23.6 | 23.2 | 20.3 | 22.6 | 22.9 | 37.8 | 40.3 | 39.0 | 44.1 | 37.4 |
| 40–64/100–149 k | 38.4 | 40.8 | 43.8 | 45.3 | 46.9 | 29.0 | 26.2 | 29.6 | 25.3 | 23.5 |
| ≥65/150 k | 23.4 | 23.3 | 23.3 | 19.5 | 18.8 | 23.5 | 20.7 | 16.8 | 14.1 | 11.1 |
| History of diabetes diagnosis | 1.6 | 0.5 | 0.8 | 0.7 | 0.0 | 2.1 | 1.4 | 0.8 | 1.0 | 2.2 |
| History of PCOS diagnosis | 9.9 | 5.7 | 3.1 | 3.2 | 3.7 | 7.6 | 5.9 | 4.9 | 7.6 | 8.9 |
All covariates, except age, are age adjusted to cohort at baseline and values are means ± SEMs or percentages. HEI, Healthy Eating Index (range: 28–92); MET, metabolic equivalent; NRDS, Nutrient Rich Diet Score (range: 397–1231); PCOS, polycystic ovary syndrome; PRESTO, Pregnancy Study Online; SF, Snart Foraeldre; SSB, sugar-sweetened beverage.
For SF cohort, live together.
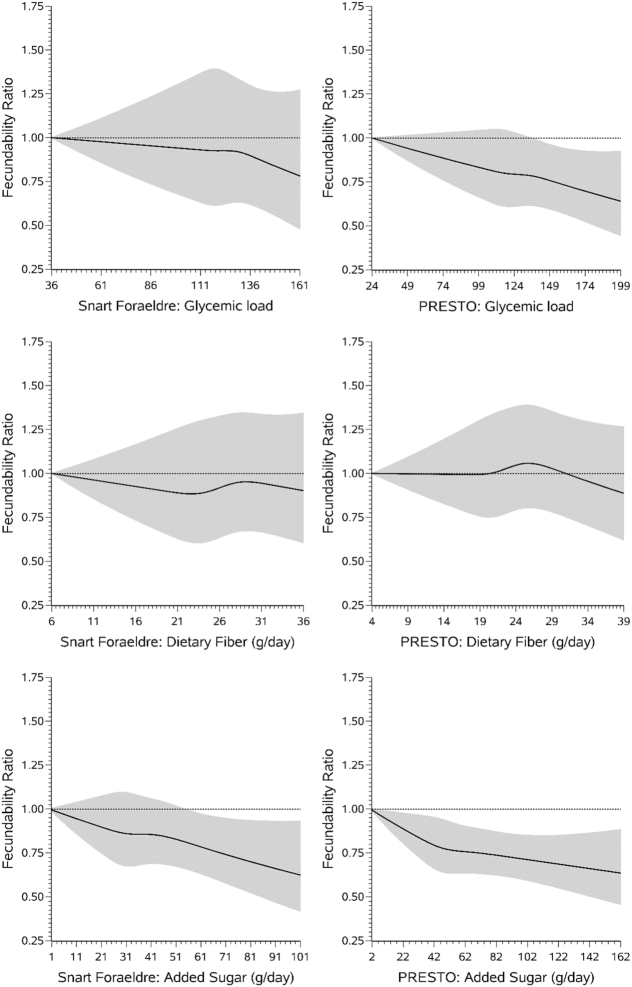
In both cohorts, relative to a GL of ≤100, a GL of ≥141 was associated with a slight reduction in fecundability (SF: FR: 0.89; 95% CI: 0.73, 1.08; PRESTO: FR: 0.87; 95% CI: 0.77, 0.98) (Table 2). Results were similar in the pooled analysis (FR: 0.86; 95% CI: 0.78, 0.95). Findings were consistent when displayed using restricted cubic splines, with a slight reduction in fecundability observed among consumers with the highest GL values in SF and a stronger dose–response reduction observed in PRESTO (Figure 1). Results were similar for the association between GI and fecundability across cohorts and in the pooled analysis.
TABLE 2.
Association between average daily glycemic load, total carbohydrates, dietary fiber intake, and added sugar intake and fecundability1
| SF | PRESTO | Pooled | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted2 | Unadjusted | Adjusted2 | Adjusted2 | ||||||||||
| Pregnancies, n | Cycles, n | FR | 95% CI | FR | 95% CI | Pregnancies, n | Cycles, n | FR | 95% CI | FR | 95% CI | FR | 95% CI | |
| Average daily GL | ||||||||||||||
| ≤100 | 185 | 997 | 1.00 | Ref | 1.00 | Ref | 578 | 3491 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 101–1143 | 478 | 2558 | 1.01 | 0.87, 1.18 | 0.99 | 0.85, 1.15 | 583 | 3458 | 0.99 | 0.89, 1.10 | 0.99 | 0.89, 1.10 | 0.98 | 0.90, 1.07 |
| 115–125 | 542 | 2790 | 1.01 | 0.86, 1.19 | 0.96 | 0.82, 1.13 | 531 | 3470 | 0.96 | 0.86, 1.07 | 0.96 | 0.86, 1.07 | 0.95 | 0.87, 1.04 |
| 126–140 | 464 | 2386 | 1.04 | 0.90, 1.22 | 0.97 | 0.83, 1.14 | 522 | 3389 | 0.95 | 0.85, 1.05 | 0.95 | 0.85, 1.06 | 0.96 | 0.88, 1.04 |
| ≥141 | 149 | 878 | 0.92 | 0.76, 1.12 | 0.89 | 0.73, 1.08 | 438 | 3582 | 0.80 | 0.71, 0.89 | 0.87 | 0.77, 0.98 | 0.86 | 0.78, 0.95 |
| Per 10-unit increase | 1.00 | 0.97, 1.02 | 0.99 | 0.96, 1.01 | 0.97 | 0.96, 0.98 | 0.98 | 0.97, 0.99 | 0.98 | 0.97, 0.99 | ||||
| Average daily GI | ||||||||||||||
| ≤47 | 399 | 1933 | 1.00 | Ref | 1.00 | Ref | 478 | 2722 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 48–49 | 534 | 2817 | 0.92 | 0.82, 1.04 | 0.91 | 0.81, 1.02 | 394 | 2353 | 0.96 | 0.85, 1.08 | 0.97 | 0.86, 1.09 | 0.94 | 0.86, 1.02 |
| 50–51 | 475 | 2581 | 0.91 | 0.80, 1.02 | 0.88 | 0.78, 1.00 | 460 | 2809 | 0.95 | 0.84, 1.06 | 1.00 | 0.89, 1.12 | 0.94 | 0.86, 1.02 |
| 52–54 | 339 | 1886 | 0.89 | 0.78, 1.01 | 0.89 | 0.78, 1.02 | 661 | 4242 | 0.90 | 0.81, 1.00 | 0.93 | 0.83, 1.04 | 0.91 | 0.83, 0.99 |
| ≥55 | 71 | 392 | 0.85 | 0.67, 1.09 | 0.90 | 0.69, 1.18 | 659 | 5264 | 0.76 | 0.69, 0.85 | 0.87 | 0.77, 0.98 | 0.83 | 0.75, 0.91 |
| Per 10-unit increase | 0.94 | 0.86, 1.02 | 0.94 | 0.86, 1.02 | 0.87 | 0.82, 0.93 | 0.95 | 0.89, 1.01 | 0.93 | 0.88, 0.98 | ||||
| Total carbohydrates, g/d | ||||||||||||||
| ≤119 | 121 | 684 | 1.00 | Ref | 1.00 | Ref | 575 | 3506 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 200–224 | 382 | 2056 | 1.02 | 0.84, 1.24 | 0.99 | 0.82, 1.20 | 694 | 4301 | 0.97 | 0.88, 1.07 | 0.94 | 0.85, 1.04 | 0.96 | 0.88, 1.05 |
| 225–238 | 387 | 2046 | 1.03 | 0.85, 1.24 | 0.97 | 0.80, 1.18 | 402 | 2735 | 0.91 | 0.81, 1.02 | 0.87 | 0.78, 0.98 | 0.91 | 0.83, 1.00 |
| 239–261 | 578 | 2972 | 1.05 | 0.88, 1.26 | 0.96 | 0.80, 1.16 | 512 | 3320 | 0.97 | 0.87, 1.08 | 0.94 | 0.84, 1.05 | 0.94 | 0.86, 1.03 |
| ≥261 | 350 | 1851 | 1.04 | 0.86, 1.25 | 0.96 | 0.79, 1.16 | 469 | 3528 | 0.87 | 0.77, 0.97 | 0.87 | 0.77, 0.97 | 0.90 | 0.82, 0.98 |
| Per 10-unit increase | 1.01 | 0.99, 1.02 | 1.00 | 0.98, 1.01 | 0.99 | 0.98, 1.00 | 0.99 | 0.98, 1.00 | 0.99 | 0.98, 1.00 | ||||
| Fiber, g/d | ||||||||||||||
| ≤16 | 132 | 736 | 1.00 | Ref | 1.00 | Ref | 602 | 4718 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 17–20 | 360 | 2020 | 1.01 | 0.84, 1.21 | 0.93 | 0.77, 1.14 | 677 | 4454 | 1.15 | 1.04, 1.28 | 1.03 | 0.92, 1.14 | 1.05 | 0.96, 1.14 |
| 21–24 | 498 | 2779 | 1.04 | 0.87, 1.24 | 0.93 | 0.76, 1.13 | 649 | 3824 | 1.27 | 1.15, 1.40 | 1.12 | 1.00, 1.26 | 1.11 | 1.02, 1.22 |
| ≥253 | 828 | 4074 | 1.13 | 0.95, 1.34 | 0.99 | 0.81, 1.22 | 724 | 4394 | 1.25 | 1.13, 1.38 | 1.06 | 0.94, 1.20 | 1.13 | 1.02, 1.23 |
| Per 5-unit increase | 1.04 | 1.01, 1.08 | 1.02 | 0.98, 1.07 | 1.05 | 1.02, 1.07 | 1.01 | 0.98, 1.04 | 1.02 | 1.00, 1.05 | ||||
| Soluble fiber, g/d4 | ||||||||||||||
| ≤4 | — | — | 594 | 4435 | 1.00 | Ref | 1.00 | Ref | ||||||
| 5–6 | — | — | 808 | 5364 | 1.10 | 1.00, 1.21 | 0.99 | 0.89, 1.09 | ||||||
| 7–8 | — | — | 613 | 3639 | 1.22 | 1.10, 1.35 | 1.07 | 0.96, 1.20 | ||||||
| ≥9 | — | — | 637 | 3952 | 1.16 | 1.05, 1.29 | 1.02 | 0.91, 1.14 | ||||||
| Per 5-unit increase | 1.06 | 1.01, 1.11 | 1.00 | 0.94, 1.05 | ||||||||||
| Insoluble fiber, g/d4 | ||||||||||||||
| ≤10 | — | — | 578 | 4544 | 1.00 | Ref | 1.00 | Ref | ||||||
| 11–13 | — | — | 719 | 4751 | 1.16 | 1.05, 1.28 | 1.03 | 0.92, 1.15 | ||||||
| 14–17 | — | — | 645 | 3866 | 1.26 | 1.14, 1.40 | 1.07 | 0.94, 1.21 | ||||||
| ≥18 | — | — | 710 | 4229 | 1.28 | 1.16, 1.42 | 1.05 | 0.91, 1.21 | ||||||
| Per 5-unit increase | 1.08 | 1.04, 1.11 | 1.01 | 0.96, 1.05 | ||||||||||
| Cereal fiber, g/d (PRESTO/SF) | ||||||||||||||
| ≤1/≤8 | 519 | 2753 | 1.00 | Ref | 1.00 | Ref | 885 | 6196 | 1.00 | Ref | 1.00 | Ref | — | |
| 2/9–11 | 497 | 2701 | 0.99 | 0.89, 1.11 | 0.93 | 0.84, 1.04 | 616 | 3985 | 1.05 | 0.96, 1.16 | 1.06 | 0.97, 1.17 | — | |
| 3–4/12–14 | 378 | 2035 | 1.00 | 0.88, 1.12 | 0.91 | 0.81, 1.03 | 750 | 4780 | 1.07 | 0.98, 1.17 | 1.02 | 0.93, 1.11 | — | |
| ≥5/≥15 | 424 | 2120 | 1.04 | 0.92, 1.16 | 0.97 | 0.87, 1.09 | 401 | 2429 | 1.12 | 1.01, 1.25 | 1.07 | 0.96, 1.19 | — | |
| Per 5-unit increase | 1.01 | 0.96, 1.06 | 0.99 | 0.94, 1.04 | 1.06 | 0.98, 1.16 | 1.03 | 0.94, 1.13 | ||||||
| Fruit fiber, g/d | ||||||||||||||
| ≤1 | 444 | 2513 | 1.00 | Ref | 1.00 | Ref | 667 | 5029 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 2–3 | 413 | 2236 | 1.07 | 0.95, 1.21 | 1.04 | 0.92, 1.17 | 437 | 2835 | 1.15 | 1.03, 1.28 | 1.00 | 0.90, 1.13 | 1.04 | 0.96, 1.13 |
| 4–5 | 550 | 2830 | 1.11 | 0.98, 1.24 | 1.03 | 0.91, 1.16 | 704 | 4423 | 1.16 | 1.06, 1.28 | 1.00 | 0.89, 1.12 | 1.06 | 0.98, 1.15 |
| ≥6 | 411 | 2030 | 1.12 | 0.99, 1.27 | 1.05 | 0.92, 1.19 | 844 | 5103 | 1.22 | 1.11, 1.34 | 1.00 | 0.88, 1.13 | 1.09 | 1.00, 1.18 |
| Per 5-unit increase | 1.07 | 0.97, 1.17 | 1.03 | 0.93, 1.14 | 1.07 | 1.01, 1.14 | 1.01 | 0.95, 1.08 | 1.03 | 0.98, 1.09 | ||||
| Vegetable fiber, g/d | ||||||||||||||
| ≤3 | 320 | 1916 | 1.00 | Ref | 1.00 | Ref | 922 | 6659 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 4–6 | 508 | 2699 | 1.13 | 0.99, 1.28 | 1.12 | 0.97, 1.28 | 853 | 5322 | 1.13 | 1.04, 1.23 | 1.07 | 0.98, 1.17 | 1.11 | 1.03, 1.19 |
| 7–8 | 551 | 2690 | 1.19 | 1.05, 1.35 | 1.18 | 1.02, 1.37 | 627 | 3858 | 1.16 | 1.06, 1.27 | 1.07 | 0.97, 1.18 | 1.15 | 1.06, 1.24 |
| ≥9 | 439 | 2304 | 1.16 | 1.01, 1.33 | 1.16 | 0.99, 1.35 | 250 | 1551 | 1.17 | 1.03, 1.33 | 1.08 | 0.94, 1.23 | 1.13 | 1.03, 1.24 |
| Per 5-unit increase | 1.04 | 0.99, 1.10 | 1.04 | 0.98, 1.10 | 1.08 | 1.03, 1.14 | 1.05 | 1.00, 1.11 | 1.05 | 1.01, 1.09 | ||||
| Carbohydrate-to-fiber ratio | ||||||||||||||
| ≤8 | 607 | 3118 | 1.00 | Ref | 1.00 | Ref | 798 | 4683 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 9–103 | 716 | 3596 | 1.01 | 0.92, 1.11 | 1.00 | 0.91, 1.11 | 694 | 4135 | 0.99 | 0.90, 1.08 | 0.99 | 0.91, 1.09 | 1.00 | 0.94, 1.07 |
| 11–12 | 313 | 1786 | 0.90 | 0.80, 1.02 | 0.91 | 0.80, 1.03 | 506 | 3354 | 0.91 | 0.82, 1.01 | 0.92 | 0.83, 1.03 | 0.92 | 0.85, 1.00 |
| ≥13 | 182 | 1109 | 0.85 | 0.73, 0.98 | 0.86 | 0.73, 1.01 | 654 | 5218 | 0.78 | 0.71, 0.86 | 0.87 | 0.78, 0.98 | 0.85 | 0.78, 0.92 |
| Per 5-unit increase | 0.92 | 0.85, 0.98 | 0.93 | 0.86, 1.00 | 0.92 | 0.89, 0.96 | 0.98 | 0.94, 1.02 | 0.95 | 0.92, 0.99 | ||||
| Added sugar, g/d | ||||||||||||||
| ≤273 | 807 | 4100 | 1.00 | Ref | 1.00 | Ref | 405 | 2328 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 28–39 | 571 | 2951 | 0.99 | 0.89, 1.09 | 0.98 | 0.89, 1.08 | 691 | 4003 | 1.00 | 0.90, 1.12 | 0.97 | 0.87, 1.08 | 0.99 | 0.92, 1.06 |
| 40–51 | 224 | 1256 | 0.92 | 0.80, 1.05 | 0.90 | 0.78, 1.03 | 628 | 4175 | 0.91 | 0.81, 1.02 | 0.88 | 0.78, 0.98 | 0.89 | 0.82, 0.96 |
| 52–71 | 140 | 829 | 0.89 | 0.75, 1.05 | 0.91 | 0.77, 1.08 | 522 | 3502 | 0.87 | 0.77, 0.98 | 0.89 | 0.79, 1.01 | 0.90 | 0.82, 0.98 |
| ≥72 | 76 | 473 | 0.84 | 0.67, 1.05 | 0.87 | 0.68, 1.10 | 406 | 3382 | 0.76 | 0.67, 0.86 | 0.86 | 0.75, 0.99 | 0.83 | 0.75, 0.92 |
| Per 10-unit increase | 0.98 | 0.96, 1.00 | 0.98 | 0.96, 1.01 | 0.97 | 0.95, 0.98 | 0.98 | 0.97, 0.99 | 0.98 | 0.97, 0.99 | ||||
GI, glycemic index; GL, glycemic load; HEI, Healthy Eating Index 2010; MET, metabolic equivalent; NRDS, Nutrient Rich Diet Score; PRESTO, Pregnancy Study Online; Ref, reference; SF, Snart Foraeldre; SSB, sugar-sweetened beverage.
Adjusted for age (<25, 25–29, 30–34, 35–39, ≥40 y), BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), energy intake (kcal/d), smoking status (never, current, occasional, past), prior birth, alcohol intake (ml/d), physical activity per week (<10, 10–19, 20–39, ≥40 MET-h/wk), last form of birth control (natural, barrier, hormonal), married or live together, intercourse frequency (<1, 1, 2–3, ≥4 times/wk), using method to improve pregnancy chances, daily use of prenatal or multivitamin, race/ethnicity (non-Hispanic white or other race/ethnicity), education (high school or less, some college, college graduate, graduate school), income (<25, 25–39, 40–64, ≥65 k DKK/mo in SF and <50 , 50–99, 100–149, ≥150 k US$/y in PRESTO), altered HEI or NRDS score.
Daily recommended values.
Data not available in SF.
FIGURE 1.
Association between glycemic load, dietary fiber, and added sugar and fecundability among 2709 female SF participants (left) and 4268 female PRESTO participants (right), fitted by restricted cubic splines. The reference level for the fecundability ratio is the lowest value in the data. The splines are trimmed at the 99th percentile and have 4 knot points at the 25th, 50th, 75th, and 90th percentiles. Adjusted for female sex, age, BMI, energy intake, smoking status, parity, alcohol intake, physical activity, last form of birth control, marital status, intercourse frequency, using method to improve pregnancy chances, daily use of prenatal or multivitamin, education, race/ethnicity, income, and diet quality. For glycemic load, for SF, knot points are at 110, 120, 129, and 140, and for PRESTO, knot points are at 104, 120, 135, and 153. For dietary fiber, for SF, knot points are at 20, 24, 28, and 32 g/d, and for PRESTO, knot points are at 17, 21, 25, and 30 g/d. For added sugar, for SF, knot points are at 22, 31, 41, and 59 g/d, and for PRESTO, knot points are at 34, 46, 65, and 95 g/d. PRESTO, Pregnancy Study Online; SF, Snart Foraeldre.
In SF, we observed no appreciable association between total carbohydrate intake and fecundability (Table 2). We observed a slight inverse association between intake of total carbohydrates and fecundability within PRESTO (FR: 0.87; 95% CI: 0.77, 0.97). When examining the role of carbohydrate intake at the expense of intake of naturally occurring fats (Supplementary Table 1), we observed that results were similar to the analysis examining the association between total carbohydrate intake and fecundability. Within both cohorts, total fiber intake was not appreciably associated with fecundability. FRs for fiber intake of ≥25 g/d compared with ≤16 g/d were 0.99 (95% CI: 0.81, 1.22) in SF and 1.06 (95% CI: 0.94, 1.20) in PRESTO. In the pooled analysis, we observed a slight increase in fecundability among women with fiber intakes of 21–24 g/d (FR: 1.11; 95% CI: 1.02, 1.22) and ≥25 g/d (FR: 1.13; 95% CI: 1.02, 1.23) compared with those with fiber intakes of ≤16 g/d. In PRESTO, little association was observed between soluble or insoluble fiber and fecundability. Results were similar when separating total fiber into cereal, fruit, and vegetable fiber within both cohorts (Table 2). When data were displayed using restricted cubic splines, there was little association between fecundability and total dietary fiber (Figure 1). In both cohorts and in the pooled analysis, fecundability declined with increasing carbohydrate-to-fiber ratio: relative to carbohydrate-to-fiber ratios of ≤8, FRs for carbohydrate-to-fiber ratios of ≥13 were 0.86 (95% CI: 0.73, 1.01) in SF, 0.87 (95% CI: 0.78, 0.98) in PRESTO, and 0.85 (95% CI: 0.78, 0.92) in both cohorts combined.
In both cohorts, fecundability declined with increasing consumption of added sugar (Figure 1). In comparison with consuming ≤27 g/d, FRs for consuming ≥72 g/d in SF were 0.87 (95% CI: 0.68, 1.10) and in PRESTO were 0.86 (95% CI: 0.75, 0.99) (Table 2). In the pooled analysis, the inverse association between added sugar intake and fecundability persisted (FR: 0.83; 95% CI: 0.75, 0.92). When the portion of sugar contributed by soda intake in PRESTO was removed, the monotonic association was still evident, although slightly attenuated (Supplementary Table 2). Among PRESTO participants with partner-level data, additional adjustment for male SSB intake did not appreciably change the FR (Supplementary Table 3).
Results for all exposures were generally consistent across the pooled analysis and fixed-effect meta-analysis (Supplementary Table 4).
In PRESTO, when examining the association between added sugar intake and fecundability, little association was observed between fructose from fruit and fecundability (Supplementary Table 5). Higher intake of fructose from other sources was associated with decreased fecundability. No consistent association was observed between sucrose and fecundability.
When stratified by BMI (Table 3), the direction and magnitude of FRs were similar across cohorts for GL, carbohydrate intake, and added sugar, although FRs for GL in PRESTO were slightly lower among women with BMI ≥25 kg/m2. Results for fiber intake were consistent across BMI strata for PRESTO. For SF, increased fiber intake was associated with improved fecundability for women with BMI ≥25 kg/m2. Within both cohorts, increasing carbohydrate-to-fiber ratio was associated with decreased fecundability for women with BMI ≤25 kg/m2. Results were attenuated for women with BMI ≥25 kg/m2. FRs for added sugar intake were similar across BMI strata in both cohorts.
TABLE 3.
Association between glycemic load, total carbohydrates, dietary fiber, and added sugar and fecundability, stratified by BMI1
| BMI <25 kg/m2 | BMI ≥25 kg/m2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SF | PRESTO | SF | PRESTO | |||||||||||||
| Adjusted2 | Adjusted2 | Adjusted2 | Adjusted2 | |||||||||||||
| Pregnancies, n | Cycles, n | FR | 95% CI | Pregnancies, n | Cycles, n | FR | 95% CI | Pregnancies, n | Cycles, n | FR | 95% CI | Pregnancies, n | Cycles, n | FR | 95% CI | |
| Average daily GL | ||||||||||||||||
| ≤100 | 118 | 631 | 1.00 | Ref | 318 | 1775 | 1.00 | Ref | 67 | 366 | 1.00 | Ref | 260 | 1716 | 1.00 | Ref |
| 101–1143 | 331 | 1759 | 1.04 | 0.86, 1.26 | 332 | 1752 | 1.02 | 0.89, 1.17 | 147 | 799 | 0.95 | 0.72, 1.26 | 251 | 1706 | 0.99 | 0.84, 1.16 |
| 115–125 | 398 | 1992 | 1.00 | 0.83, 1.20 | 290 | 1722 | 0.97 | 0.84, 1.11 | 144 | 798 | 0.92 | 0.68, 1.25 | 241 | 1748 | 0.97 | 0.83, 1.14 |
| 126–140 | 339 | 1634 | 1.03 | 0.85, 1.25 | 279 | 1536 | 0.97 | 0.84, 1.13 | 125 | 752 | 0.86 | 0.66, 1.12 | 243 | 1853 | 0.95 | 0.80, 1.12 |
| ≥141 | 100 | 614 | 0.86 | 0.67, 1.10 | 214 | 1342 | 0.94 | 0.79, 1.10 | 49 | 264 | 1.02 | 0.72, 1.44 | 224 | 2240 | 0.83 | 0.70, 0.99 |
| Total carbohydrates, g/d | ||||||||||||||||
| ≤119 | 72 | 391 | 1.00 | Ref | 305 | 1617 | 1.00 | Ref | 49 | 293 | 1.00 | Ref | 270 | 1889 | 1.00 | Ref |
| 200–224 | 249 | 1363 | 0.98 | 0.77, 1.24 | 376 | 2159 | 0.90 | 0.78, 1.02 | 133 | 693 | 1.09 | 0.80, 1.50 | 318 | 2142 | 1.02 | 0.88, 1.18 |
| 225–238 | 273 | 1419 | 0.98 | 0.78, 1.25 | 221 | 1294 | 0.89 | 0.76, 1.04 | 114 | 627 | 0.99 | 0.72, 1.37 | 181 | 1441 | 0.87 | 0.73, 1.04 |
| 239–261 | 437 | 2168 | 0.97 | 0.77, 1.22 | 272 | 1486 | 0.95 | 0.82, 1.10 | 141 | 804 | 0.93 | 0.69, 1.27 | 240 | 1834 | 0.96 | 0.81, 1.14 |
| ≥261 | 255 | 1289 | 0.96 | 0.76, 1.22 | 259 | 1571 | 0.86 | 0.73, 1.00 | 95 | 562 | 1.02 | 0.74, 1.41 | 210 | 1957 | 0.89 | 0.75, 1.06 |
| Fiber, g/d | ||||||||||||||||
| ≤16 | 80 | 392 | 1.00 | Ref | 245 | 1567 | 1.00 | Ref | 52 | 344 | 1.00 | Ref | 357 | 3151 | 1.00 | Ref |
| 17–20 | 229 | 1313 | 0.78 | 0.61, 1.00 | 348 | 2122 | 0.99 | 0.85, 1.16 | 131 | 707 | 1.23 | 0.89, 1.71 | 329 | 2332 | 1.06 | 0.91, 1.23 |
| 21–24 | 342 | 1857 | 0.80 | 0.63, 1.03 | 388 | 1879 | 1.20 | 1.03, 1.41 | 156 | 922 | 1.15 | 0.83, 1.60 | 261 | 1945 | 1.02 | 0.86, 1.21 |
| ≥253 | 635 | 3068 | 0.86 | 0.67, 1.10 | 452 | 2559 | 1.07 | 0.90, 1.27 | 193 | 1006 | 1.22 | 0.86, 1.73 | 272 | 1835 | 1.06 | 0.89, 1.28 |
| Cereal fiber, g/d (PRESTO/SF) | ||||||||||||||||
| ≤8/≤1 | 324 | 1718 | 1.00 | Ref | 418 | 2577 | 1.00 | Ref | 195 | 1035 | 1.00 | Ref | 467 | 3619 | 1.00 | Ref |
| 9–11/2 | 351 | 1899 | 0.94 | 0.82, 1.07 | 320 | 1754 | 1.10 | 0.96, 1.25 | 146 | 802 | 0.93 | 0.77, 1.13 | 296 | 2231 | 1.05 | 0.92, 1.20 |
| 12–14/3–4 | 279 | 1412 | 0.95 | 0.82, 1.10 | 438 | 2442 | 1.06 | 0.93, 1.19 | 99 | 623 | 0.85 | 0.67, 1.06 | 312 | 2338 | 1.00 | 0.88, 1.15 |
| ≥15/≥5 | 332 | 1601 | 1.00 | 0.87, 1.15 | 257 | 1354 | 1.12 | 0.97, 1.29 | 92 | 519 | 0.91 | 0.73, 1.15 | 144 | 1075 | 1.00 | 0.84, 1.20 |
| Fruit fiber, g/d | ||||||||||||||||
| ≤1 | 287 | 1600 | 1.00 | Ref | 282 | 1862 | 1.00 | Ref | 157 | 913 | 1.00 | Ref | 385 | 3167 | 1.00 | Ref |
| 2–3 | 291 | 1404 | 1.08 | 0.93, 1.25 | 224 | 1261 | 1.00 | 0.85, 1.19 | 122 | 832 | 0.95 | 0.75, 1.20 | 213 | 1574 | 1.02 | 0.87, 1.20 |
| 4–5 | 398 | 2025 | 1.03 | 0.89, 1.19 | 411 | 2157 | 1.10 | 0.93, 1.29 | 152 | 805 | 0.98 | 0.78, 1.24 | 293 | 2266 | 0.89 | 0.75, 1.05 |
| ≥6 | 310 | 1601 | 0.94 | 0.80, 1.11 | 516 | 2847 | 1.03 | 0.86, 1.23 | 101 | 429 | 1.39 | 1.07, 1.79 | 328 | 2256 | 0.96 | 0.81, 1.16 |
| Vegetable fiber, g/d | ||||||||||||||||
| ≤3 | 220 | 1273 | 1.00 | Ref | 473 | 2900 | 1.00 | Ref | 100 | 643 | 1.00 | Ref | 449 | 3759 | 1.00 | Ref |
| 4–6 | 352 | 1811 | 1.08 | 0.92, 1.27 | 464 | 2515 | 1.05 | 0.93, 1.18 | 156 | 888 | 1.14 | 0.88, 1.47 | 389 | 2807 | 1.09 | 0.95, 1.25 |
| 7–8 | 393 | 1882 | 1.15 | 0.97, 1.37 | 362 | 1914 | 1.06 | 0.93, 1.21 | 158 | 808 | 1.21 | 0.93, 1.58 | 265 | 1944 | 1.08 | 0.92, 1.26 |
| ≥9 | 321 | 1664 | 1.13 | 0.94, 1.35 | 134 | 798 | 0.99 | 0.83, 1.19 | 118 | 640 | 1.17 | 0.86, 1.59 | 116 | 753 | 1.22 | 1.00, 1.49 |
| Carbohydrate-to-fiber ratio | ||||||||||||||||
| ≤8 | 458 | 2275 | 1.00 | Ref | 501 | 2701 | 1.00 | Ref | 149 | 843 | 1.00 | Ref | 297 | 1982 | 1.00 | Ref |
| 9–103 | 510 | 2589 | 0.95 | 0.85, 1.07 | 380 | 1927 | 0.99 | 0.88, 1.12 | 206 | 1007 | 1.16 | 0.96, 1.41 | 314 | 2208 | 1.02 | 0.88, 1.18 |
| 11–12 | 202 | 1061 | 0.91 | 0.78, 1.07 | 263 | 1607 | 0.84 | 0.73, 0.97 | 111 | 725 | 0.92 | 0.73, 1.17 | 243 | 1747 | 1.05 | 0.90, 1.24 |
| ≥13 | 116 | 705 | 0.82 | 0.67, 1.00 | 289 | 1892 | 0.85 | 0.73, 1.00 | 66 | 404 | 0.96 | 0.73, 1.28 | 365 | 3326 | 0.92 | 0.77, 1.08 |
| Added sugar, g/d | ||||||||||||||||
| ≤273 | 601 | 3055 | 1.00 | Ref | 245 | 1268 | 1.00 | Ref | 206 | 1045 | 1.00 | Ref | 160 | 1060 | 1.00 | Ref |
| 28–39 | 397 | 1982 | 1.03 | 0.91, 1.15 | 403 | 2021 | 0.95 | 0.82, 1.09 | 174 | 969 | 0.87 | 0.71, 1.06 | 288 | 1982 | 0.99 | 0.83, 1.19 |
| 40–51 | 161 | 814 | 0.96 | 0.81, 1.12 | 363 | 2193 | 0.82 | 0.71, 0.95 | 63 | 442 | 0.72 | 0.56, 0.94 | 265 | 1982 | 0.97 | 0.81, 1.16 |
| 52–71 | 87 | 509 | 0.92 | 0.74, 1.14 | 253 | 1539 | 0.81 | 0.69, 0.95 | 53 | 320 | 0.84 | 0.63, 1.13 | 269 | 1963 | 0.99 | 0.82, 1.18 |
| ≥72 | 40 | 270 | 0.80 | 0.58, 1.10 | 169 | 1106 | 0.84 | 0.70, 1.00 | 36 | 203 | 0.91 | 0.64, 1.29 | 237 | 2276 | 0.88 | 0.72, 1.07 |
1GI, glycemic index; HEI, Healthy Eating Index 2010; MET, metabolic equivalent; NRDS, Nutrient Rich Diet Score; PRESTO, Pregnancy Study Online; Ref, reference; SF, Snart Foraeldre.
Adjusted for age (<25, 25–29, 30–34, 35–39, ≥40 y), BMI (continuous), energy intake (kcal/d), smoking status (never, current, occasional, past), prior birth, alcohol intake (ml/d), physical activity per week (<10, 10–19, 20–39, ≥40 MET-h/wk), last form of birth control (natural, barrier, hormonal), married, intercourse frequency (<1, 1, 2–3, ≥4 times/wk), using method to improve pregnancy chances, daily use of prenatal or multivitamin, race/ethnicity (non-Hispanic white or other race/ethnicity), education (high school or less, some college, college graduate, graduate school), income (<25, 25–39, 40–64, ≥65 k DKK/mo in SF and <50 , 50–99, 100–149, ≥150 k US$/y in PRESTO), altered HEI or NRDS score.
Daily recommended values.
When stratifying by parity (Supplementary Table 6), FRs were similar across cohorts for GL, carbohydrate intake, and added sugar. Across cohorts, results for GL were weaker among nulliparous women. FRs were stronger among parous women for carbohydrate-to-fiber ratio and, in PRESTO, for added sugar intake. For fiber, we observed no appreciable association when stratified by parity.
FRs across cohorts were similar for GL, carbohydrate intake, dietary fiber, and added sugar when stratifying by attempt time at study entry (Supplementary Table 7). FRs for GL, total carbohydrates, carbohydrate-to-fiber ratio, and added sugar were slightly stronger among those attempting pregnancy for <3 cycles at study entry. Higher fiber intake was associated with a slight increase in fecundability in women trying for <3 cycles at study entry, although results were attenuated when adjusting for the modified diet quality score.
Discussion
In 2 preconception cohorts of women attempting to conceive in Denmark and North America, increasing carbohydrate-to-fiber ratio and higher intake of added sugar were associated with reduced fecundability in a dose–response pattern. In PRESTO, higher GL was associated with reduced fecundability in a dose–response pattern. Results persisted for added sugar when removing the proportion of sugar from sugar-sweetened soda, and was strongest for nonfruit sources of fructose. We observed a slight decrease in fecundability with increasing carbohydrate intake in PRESTO and in the pooled analysis. While we observed slightly improved fecundability with increased intake of fiber in the pooled analysis, primarily driven by vegetable fiber intake, we observed no appreciable association for dietary fiber within each cohort. There was little evidence of effect measure modification by BMI for any of the associations of interest.
Our findings for GL agree with those from the Nurses’ Health Study II, a prospective cohort study of 18,555 women, which found that those in the highest quintile of GL consumption had a 90% increased risk of ovulatory infertility compared with those in the lowest quintile (13). While Chavarro et al. observed no appreciable association between carbohydrate intake and ovulatory infertility when carbohydrates were substituted for the average intake of other energy sources (i.e., fats and proteins), they did report an increase in ovulatory infertility when carbohydrate intake was increased at the expense of naturally occurring fats (13). In the present analysis, we observed little change in the measure of association between total carbohydrates and fecundability when we substituted carbohydrates for naturally occurring fats and fecundability. While we observed a slight decrease in fecundability with increasing carbohydrate intake in PRESTO, we observed little association in Denmark. Unlike total GL intake, which accounts for quality, total carbohydrate intake does not account for carbohydrate quality and the overall quality of carbohydrates consumed in Denmark likely differs from that in North America. The Nurses’ Health Study II additionally concluded that increased intake of dietary fiber was not associated with increased risk of ovulatory infertility. In the BioCycle Study, a prospective cohort that followed women through 2 menstrual cycles, dietary fiber intake was inversely associated with concentrations of hormones, including estradiol, progesterone, luteinizing hormone, and follicle stimulating hormone, and was associated with increased risk of anovulation (41), although the associations were imprecise. Within the pooled analyses, we observed that increased dietary fiber intake was associated with higher fecundability. We additionally observed that a higher carbohydrate-to-fiber ratio, another measure of carbohydrate quality, was associated with reduced fecundability. The American Heart Association recommends consuming meals with a total carbohydrate-to-fiber ratio of ≤10:1, a measure to evaluate the balance of whole grains compared with added sugars and refined grains in a product (42). One study conducted among couples undergoing infertility treatment examined the association between maternal whole-grain intake and outcomes of in vitro fertilization (IVF) (43). The authors observed that higher preconception whole-grain intake was associated with increased probability of implantation. When intermediate IVF endpoints were examined to understand potential mechanisms for higher implantation rates, the authors found that whole-grain intake was associated with endometrial thickness, an indicator of endometrial receptivity. Although we were unable to evaluate the cause of subfertility in our cohorts, endometrial receptivity is a key mechanism by which carbohydrate quality, including GL and carbohydrate-to-fiber ratio, could influence fecundity (44).
Diets high in sugar are associated with increased risks for insulin resistance and dyslipidemia, established risk factors for ovulatory disorders (7). Four studies have evaluated the association between female soda intake, a major contributor to added sugar intake in North America, and fecundability (36, 45–47). These 4 studies reported reduced fecundability or increased risk of ovulatory infertility. The BioCycle study found that women with above average consumption of added sugar (73.2 g/d) had 9% higher mean free estradiol and increased odds of anovulation (OR: 0.57; 95% CI: 0.31, 1.06) relative to women with below average intake (48). A study conducted among women undergoing IVF observed that higher intake of sugar-sweetened sodas was associated with fewer oocytes retrieved and fertilized and a lower proportion of clinical pregnancies and live births (49). A previous analysis conducted in PRESTO examined the association between SSB and fecundability and observed that both female and male intake of SSB were associated with reduced fecundability (36). In the present analysis, we found reduced fecundability for added sugar intake even after removing the portion contributed by sugar-sweetened soda. Additionally, when we adjusted for male SSB intake in the subset with both male and female participation in PRESTO, the association between female intake of added sugars and lower fecundability persisted. To our knowledge, the present study is the first to prospectively examine the association between total dietary added sugar and fecundability, even when taking into account sugar intake from sugar-sweetened soda and male intake of SSB.
Although the food frequency questionnaire is a validated instrument well suited to collecting long-term dietary data (50), misclassification of diet intake is expected. Validation studies have raised questions about the appropriateness of using GI to estimate glycemic response after mixed meals (51, 52). Errors in measuring GI introduce error into GL, but the magnitude of these errors is likely similar to measurement error in other standard nutrient values (25). Because diet was evaluated prospectively, misclassification is likely nondifferential, attenuating associations for extreme exposure categories. Some women, entering later in their pregnancy attempt, may have changed their diet in response to subfertility. When we stratified by attempt time at study entry, findings were stronger among women trying <3 cycles, indicating that reverse causation is an unlikely explanation of our findings. Our results may be affected by unmeasured confounding, such as male dietary factors. When controlling for male SSB intake in PRESTO, we saw no appreciable difference in our main associations (36). Another limitation was the inability to assess all fiber components within SF, although no appreciable effect was observed when examining individual components (e.g., soluble and insoluble fiber) in PRESTO. Additionally, the greater consumption of cereal fiber in SF necessitated the use of different categories for cereal fiber. These factors limit comparability of total and cereal fiber across cohorts. While all pregnancies included in the present analysis were self-reported, we expect misclassification to be minimal as 96% of participants in SF and PRESTO reported using home pregnancy tests on follow-up questionnaires to confirm their pregnancy status. Additionally, in a prior analysis using self-reported PRESTO data compared with daily diary–recorded data on the app FertilityFriend.com (FF), >97% of FF users who conceived reported their LMP on the PRESTO questionnaire within 1 d of the LMP recorded via FF (18). Lastly, we did not collect data on the cause of subfertility, and as dietary factors may have different mechanisms in specific etiologies of subfertility (e.g., anovulation, uterine factors, tubal factors), we are limited in our ability to compare our results to those reported in prior literature (13).
In conclusion, diets high in GL, carbohydrate-to-fiber ratio, and added sugar were associated with modestly reduced fecundability among women attempting to conceive in Denmark and North America. These findings are consistent with existing literature on GL and added sugar intake and fertility. Given that diet is a modifiable risk factor for infertility, our findings may have important public health implications.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the in-kind donation of premium app subscriptions from FertilityFriend.com. We thank Mr. Michael Bairos for technical support in developing the study's web-based infrastructure.
The authors’ responsibilities were as follows—EEH, KJR, EMM, KLT, LAW: designed the research; SKW, EEH, AKW, KJR, EMM, LAW: conducted the research; SKW: analyzed the data; SKW, AKW, ET, LAW: coded the outcome and covariate data; SKW: took the lead in writing the manuscript; SKW: has primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the National Institute of Child Health and Human Development (grant numbers R21-HD072326 and R01-HD086742).
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
Supplementary Tables 1–7 and Supplementary Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AMH, anti-Müllerian hormone; DHQII, National Cancer Institute's Diet History Questionnaire II; FF, Fertility Friend; FR, fecundability ratio; GI, glycemic index; GL, glycemic load; HEI, Healthy Eating Index 2010; IVF, in vitro fertilization; LMP, last menstrual period; MET, metabolic equivalent; NRDS, Nutrient Rich Diet Score; PCOS, polycystic ovary syndrome; PRESTO, Pregnancy Study Online; SF, Snart Foraeldre; SF-FFQ, Snart Foraeldre food-frequency questionnaire; SSB, sugar-sweetened beverage.
Contributor Information
Sydney K Willis, Boston University School of Public Health, Department of Epidemiology, Boston, MA, USA.
Lauren A Wise, Boston University School of Public Health, Department of Epidemiology, Boston, MA, USA.
Amelia K Wesselink, Boston University School of Public Health, Department of Epidemiology, Boston, MA, USA.
Kenneth J Rothman, Boston University School of Public Health, Department of Epidemiology, Boston, MA, USA; RTI International, Research Triangle Park, NC, USA.
Ellen M Mikkelsen, Department of Clinical Epidemiology, Aarhus University, Aarhus, Denmark.
Katherine L Tucker, Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Ellen Trolle, Division of Risk Assessment and Nutrition, National Food Institute, Technical University of Denmark, Søborg, Denmark.
Elizabeth E Hatch, Boston University School of Public Health, Department of Epidemiology, Boston, MA, USA.
References
- 1. Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–31. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Björck I, Brand-Miller JC, Brighenti F, Buyken AE et al.. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. [DOI] [PubMed] [Google Scholar]
- 3. Brand-Miller J, Dickinson S, Barclay A, Celermajer D. The glycemic index and cardiovascular disease risk. Curr Atheroscler Rep. 2007;9(6):479–85. [DOI] [PubMed] [Google Scholar]
- 4. Greenwood DC, Threapleton DE, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Burley VJ. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. 2013;36(12):4166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85(3):679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36(1):105–11. [DOI] [PubMed] [Google Scholar]
- 7. Fica S, Albu A, Constantin M, Dobri GA. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1(4):415–22. [PMC free article] [PubMed] [Google Scholar]
- 8. Saleh BO, Ibraheem WF, Ameen NS. The role of anti-Mullerian hormone and inhibin B in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med J. 2015;36(5):562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chhabra N, Malik S.. Effect of insulin sensitizers on raised serum anti-Mullerian hormone levels in infertile women with polycystic ovarian syndrome. J Hum Reprod Sci. 2018;11(4):348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nascimento AD, Silva Lara LA, Japur de Sá Rosa-e-Silva AC, Ferriani RA, Reis RM. Effects of metformin on serum insulin and anti-Mullerian hormone levels and on hyperandrogenism in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2013;29(3):246–9. [DOI] [PubMed] [Google Scholar]
- 11. Tomova A, Deepinder F, Robeva R, Kirilov G, Mechandjiev Z, Kumanov P. Anti-Müllerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm Metab Res. 2011;43(10):723–7. [DOI] [PubMed] [Google Scholar]
- 12. Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83–92. [DOI] [PubMed] [Google Scholar]
- 13. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr. 2009;63(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, Sørensen HT, Rothman KJ. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid’. Eur J Epidemiol. 2010;25(5):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sørensen HT. Cohort profile: the Danish web-based pregnancy planning study–‘Snart-Gravid’. Int J Epidemiol. 2009;38(4):938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christensen T, Riis AH, Hatch EE, Wise LA, Nielsen MG, Sørensen HT, Mikkelsen EM. Costs and efficiency of online and offline recruitment methods: a web-based cohort study. J Med Internet Res. 2017;19(3):e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knudsen VK, Hatch EE, Cueto H, Tucker KL, Wise L, Christensen T, Mikkelsen EM. Relative validity of a semi-quantitative, web-based FFQ used in the ‘Snart Forældre’ cohort—a Danish study of diet and fertility. Public Health Nutr. 2016;19(6):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al.. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015;29(4):360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 20. Saxholt E, Christensen AT, Møller A, Hartkopp HB, Hess Ygil K, Hels OH. Danish Food Composition Databank, revision 7. Department of Nutrition, National Food Institute, Technical University of Denmark; 2008. [Google Scholar]
- 21. Salmerón J, Manson JE, Stamfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277(6):472–7. [DOI] [PubMed] [Google Scholar]
- 22. Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The National Cancer Institute diet history questionnaire: validation of pyramid food servings. Am J Epidemiol. 2006;163(3):279–88. [DOI] [PubMed] [Google Scholar]
- 23. Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76(1):5–56. [DOI] [PubMed] [Google Scholar]
- 24. Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. J Am Diet Assoc. 2006;106(3):393–402. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 27. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al.. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 28. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr., Schmitz KH, Emplaincourt PO et al.. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 29. Drewnowski A. Defining nutrient density: development and validation of the nutrient rich foods index. J Am Coll Nutr. 2009;28(4):421S–6S. [DOI] [PubMed] [Google Scholar]
- 30. Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129(5):1072–8. [DOI] [PubMed] [Google Scholar]
- 32. Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28(1):1–9. [DOI] [PubMed] [Google Scholar]
- 33. King DE, Mainous AG, Lambourne CA. Trends in dietary fiber intake in the United States, 1999–2008. J Acad Nutr Diet. 2012;112(5):642–8. [DOI] [PubMed] [Google Scholar]
- 34. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. [DOI] [PubMed] [Google Scholar]
- 35. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 36. Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sørensen HT, Rothman KJ, Wise LA. Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology. 2018;29(3):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9-10):1541–9. [DOI] [PubMed] [Google Scholar]
- 38. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. SAS, SAS Institute Inc. SAS/STAT® 9.4 User's Guide. 2014:Cary, NC: SAS Institute; 2014. [Google Scholar]
- 40. Wasserstein RL, Lazar NA.. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;129–33. [Google Scholar]
- 41. Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF et al.. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90(4):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 43. Gaskins AJ, Chiu YH, Williams PL, Keller MG, Toth TL, Hauser R, Chavarro JE; EARTH Study Team. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil Steril. 2016;105(6):1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heger A, Sator M, Pietrowski D. Endometrial receptivity and its predictive value for IVF/ICSI-outcome. Geburtshilfe Frauenheilkd. 2012;72(8):710–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology. 2009;20(3):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilcox AJ, Weinberg CR. Tea and fertility. Lancet. 1991;337(8750):1159–60. [DOI] [PubMed] [Google Scholar]
- 47. Wesselink AK, Wise LA, Rothman KJ, Hahn KA, Mikkelsen EM, Mahalingaiah S, Hatch EE. Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Reprod Toxicol. 2016;62:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schliep KC, Schisterman EF, Mumford SL, Pollack AZ, Perkins NJ, Ye A, Zhang CJ, Stanford JB, Porucznik CA, Hammoud AO et al.. Energy-containing beverages: reproductive hormones and ovarian function in the BioCycle Study. Am J Clin Nutr. 2013;97(3):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Machtinger R, Gaskins AJ, Mansur A, Adir M, Racowsky C, Baccarelli AA, Hauer R, Chavarro JE. Association between preconception maternal beverage intake and in vitro fertilization outcomes. Fertil Steril. 2017;108(6):1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Willett W. Nutritional Epidemiology. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 51. Flint A, Møller BK, Raben A, Pedersen D, Tetens I, Holst JJ, Astrup A. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr. 2004;91(6):979–89. [DOI] [PubMed] [Google Scholar]
- 52. Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61 Suppl 1:S122–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.