Abstract
Purpose
To investigate the possible role of activating transcription factor 3 (ATF3) in retinal ganglion cell (RGC) neuroprotection and optic nerve regeneration after optic nerve crush (ONC).
Methods
Overexpression of proteins of interest (ATF3, phosphatase and tensin homolog [PTEN], placental alkaline phosphatase, green fluorescent protein) in the retina was achieved by intravitreal injections of recombinant adenovirus-associated viruses (rAAVs) expressing corresponding proteins. The number of RGCs and αRGCs was evaluated by immunostaining retinal sections and whole-mount retinas with antibodies against RNA binding protein with multiple splicing (RBPMS) and osteopontin, respectively. Axonal regeneration was assessed via fluorophore-coupled cholera toxin subunit B labeling. RGC function was evaluated by recording positive scotopic threshold response.
Results
The level of ATF3 is preferentially elevated in osteopontin+/RBPMS+ αRGCs following ONC. Overexpression of ATF3 by intravitreal injection of rAAV 2 weeks before ONC promoted RBPMS+ RGC survival and preserved RGC function as assessed by positive scotopic threshold response recordings 2 weeks after ONC. However, overexpression of ATF3 and simultaneous downregulation of PTEN, a negative regulator of the mTOR pathway, combined with ONC, only moderately promoted short distance RGC axon regeneration (200 μm from the lesion site) but did not provide additional RGC neuroprotection compared with PTEN downregulation alone.
Conclusions
These results reveal a neuroprotective effect of ATF3 in the retina following injury and identify ATF3 as a promising agent for potential treatments of optic neuropathies.
Keywords: ATF3, retinal ganglion cells, neuroprotection, PTEN, αRGC
Retinal ganglion cell (RGC) death is implicated in a number of eye disorders that are broadly defined as optic neuropathies.1 Glaucoma, the most common form of optic neuropathy, is characterized by a progressive loss of RGCs and their axons, and, if left untreated, leads to irreversible vision loss.2 Currently, there are no effective treatments for degenerative retinal diseases resulting from RGC loss. The only clinically proven treatment of glaucoma is a pharmacological or surgical reduction of intraocular pressure, one of the main risk factors in this devastating eye disease.3 However, the reduction of intraocular pressure is not always sufficient to stop RGC death and axon damage. There are growing efforts to develop strategies for effective reduction of RGC death such as suppression of pro-apoptotic signaling, modulation of the inflammatory response, and neurotrophic factor delivery.4–7
While lower vertebrates like zebrafish can functionally regenerate injured central nervous system (CNS) axons after injury such as optic nerve crush (ONC),8–10 CNS axon regeneration fails in mammals and adult birds. Adult RGCs are part of the CNS and are thus incapable of regenerating their axons beyond the injury site.11 As a result, many of the axotomized RGCs die. This inability of CNS axons to regenerate is mainly caused by the cell's intrinsic growth capacity failing to activate after injury12 as well as the presence of extrinsic inhibitory factors.13–15 Nevertheless, some RGCs survive the injury with αRGCs showing increased survival rate.16
Several proteins of different functional classes have been identified to promote survival of RGCs and/or regeneration of their axons.17–24 Some of them are transcription factors, such as Sox1125 and a number of the Kruppel-like factor family.26 Activating transcription factor 3 (ATF3), also known as LRF-1, LRG-21, CRG-5, and TI-241, belongs to the ATF/cyclic AMP responsive element binding family of proteins characterized by a basic region leucine zipper motif.27 ATF3 is described as stress inducible and an adaptive response gene.28,29 ATF3 is not expressed in most healthy intact neurons, however expression is induced after axonal injury.30 In the peripheral nervous system, increased and retained expression of ATF3 has been associated with both neuroprotective and regenerative effects.31–33 It has been demonstrated in several species, including mice22,34,35, rats,36,37 and zebrafish,38 that the level of Atf3 gene expression is low in RGCs but is upregulated after ONC.
In this study, we investigated the role of ATF3 in RGC survival and regeneration of their axon after ONC in mice. We demonstrated that overexpression of ATF3 in RGCs by intravitreal injection of recombinant adenovirus-associated virus (rAAV), delivered before the ONC, promoted survival of RGCs and partially preserved their function 2 weeks after ONC. In addition, overexpression of ATF3 together with downregulation of phosphatase and tensin homolog (PTEN) promoted RGC axon regeneration after ONC. These results identify ATF3 as a potential agent for the treatment of traumatic optic neuropathies.
Materials and Methods
Animals
Adult C57BL/6 mice were obtained from the Jackson Laboratory. Mice were 35 days old at the time of rAAV2 injections and 49 days old at the time of ONC. Animals were kept on a standard 12/12 hours light/dark cycle and all assessments of visual functions were conducted in the first 8 hours of the light phase. Adult floxed PTENf/f mice were maintained as previously reported.39 Mice were maintained and treated in accordance with guidelines set forth by the National Eye Institute Committee on the Use and Care of Animals and the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmic and Visual Research.
Plasmid and rAAV Production
Plasmid preparation and rAAV production were performed as previously described.40 Plasmids pAAVrep2cap2, pAAV-CMV-Cre, pAAV-CMV-LacZ, and pAAV-CMV-PLAP (placental alkaline phosphatase) have been previously described.39 Plasmid pAAV-CMV-ATF3 was generated from previously described plasmid pAAV2-CMV-Otx2 by subcloning and replacing the OTX2 sequence with the ATF3 sequence cloned from plasmid pCMV-SPORT6-mATF3 (Dharmacon, Lafayette, CO). The pAAV2 vectors with transgene cassettes encoding PLAP, GFP, Cre, or ATF3 under control of the CMV promoter were packaged into the AAV2 capsid as previously described.41 Briefly, HEK 293T cells were triple transfected with pHelper, pAAVrep2cap2, and pAAV2-CMV-(Transgene) plasmids using polyethylenimine (Sigma Aldrich, Allentown, PA ). Cells were harvested 48 hours after transfection. Viral particles were purified by centrifugation through iodixanol (Sigma Aldrich) gradient (15%, 25%, 40%, and 60%). The 40% fraction containing the rAAV viral particles was collected and passed through the column for desalting. Viral particles were suspended and stored in 1× phosphate-buffered saline (PBS), 0.001% Pluronic (Life Technologies, Foster City, CA). Titers (viral genomes per milliliter) were determined by real-time polymerase chain reaction using primers targeting the CMV promoter: 5′-ATGCGGTTTTGGCAGTACAT-3′ and 5′-GTCAATGGGGTGGAGACTTG-3′.
Gene Transfer and Surgical Methods
For intravitreal injections and ONC, mice were anesthetized with a single intraperitoneal injection of ketamine/xylazine (100 and 10 mg/kg, respectively). An ophthalmic solution with phenylephrine hydrochloride (Paragon, Portland, OR), tropicamide (Akorn, Lake Forest, IL), and proparacaine hydrochloride (Sandoz, Princeton, NJ) was applied to the cornea. rAAV intravitreal injections were conducted using a 34-gauge Hamilton needle and a Hamilton syringe (Hamilton Company, Reno, NV). Approximately 3 μL (3 × 1010 rAAV2 viral particles) were injected into the vitreous chamber using UltraMicroPump (UMP3, World Precision Instruments, Sarasota, FL). ONC was performed 2 weeks after virus injection. The optic nerve was exposed intraorbitally and crushed with self-closing Dumont Tweezers #5 (World Precision Instruments) for 3 seconds approximately 0.5 mm behind the eyeball. Care was taken not to damage the underlying retinal artery. One microliter of Alexa Fluor 594-conjugated cholera toxin subunit B (CTB) (ThermoFisher Scientific, Marietta, OH) was injected intravitreally 2 to 3 days before euthanasia to label regenerating axons. Eye ointment containing neomycin (Akorn, Somerset, NJ) was applied to protect the cornea after CTB injection. Animals received buprenorphine as an analgesic after the operation.
Histology
Enucleated eyes and surgically removed optic nerve segments were fixed in 4% paraformaldehyde in PBS for 20 minutes and then washed three times for 20 minutes each in PBS. To prepare frozen sections (14 µm), tissues were immersed in 30% sucrose for 2 days and then embedded in OCT before sectioning using a cryostat. For immunofluorescent staining, sections were washed in PBS, incubated with 0.5% Triton X-100 for 20 minutes and then washed again with PBS for 10 minutes. Sections were incubated in 0.5% bovine serum albumin, 15% normal goat serum, and 0.3% Tween-20 for 10 minutes followed by the incubation with primary antibodies in a blocking buffer (0.5% BSA and 0.3% Tween-20 in PBS) at 4°C overnight, and then with secondary antibodies in a blocking buffer at room temperature for 2 hours. Whole-mount retinas were incubated with 0.5% Triton X-100 at -80°C for 10 minutes. Blocking was performed using 2% BSA and 2% Triton X-100 at 4° C for 4 hours followed by the incubation with primary antibodies in a blocking buffer overnight at 4°C and secondary antibodies in a blocking buffer for 2 hours at room temperature. After incubation with antibodies, frozen sections and whole-mount retinas were extensively washed with PBS at room temperature and mounted in prolong gold anti-fade mount (ThermoFisher Scientific). The following primary and secondary antibodies were used for staining: guinea pig anti-RBPMS (1:500, Millipore SIGMA, Burlington, MA), mouse anti-ATF3 (1:50, Abcam, Cambridge, MA), rabbit anti-phospho-S6 ribosomal protein (Ser235/236) (1:100, Cell Signaling, Danvers, MA) and goat anti-osteopontin (1:1,000, R&D Systems Inc., Minneapolis, MN), goat anti-guinea pig IgG (1:500, ThermoFisher Scientific), goat anti-mouse IgG (1:500, ThermoFisher Scientific), and goat anti-rabbit IgG (1:500, ThermoFisher Scientific).
Imaging and Quantification
Fluorescently stained cells were analyzed using a Zeiss LSM 700 confocal laser-scanning microscope (Carl Zeiss Inc, Thornwood, NY). Images were analyzed and organized using Fiji (National Institutes of Health, Bethesda, MD) and Photoshop (Adobe, San Jose, CA). Investigators were blinded to the identity of the samples at the time of image analysis. For RGC quantification (RGCs/mm2) in whole-mount retina samples, 12 fields (0.3 × 0.3 mm) positioned in the center, middle part, and periphery of the RGC layer were selected. Five retinas from five mice were used for the calculation of the mean number of RGCs/mm2 for rAAV-injected samples and three retinas from three mice were used for a control intact group. Regenerating RGC axons in injured optic nerves distal to the crush site were quantified as described.39,42 Harvested optic nerves were fixed in 4% paraformaldehyde, treated with 30% sucrose, and then frozen in OCT and stored at -80° degrees until they were sectioned. Ten-micrometer longitudinal sections of optic nerves were used to visualize CTB stained axons. The number of CTB-labeled axons was estimated by counting the number of CTB-labeled fibers extending different distances from the crush site. The cross-sectional width of the optic nerve was measured at the point at which the counts were taken and was used to calculate the number of axons per millimeter of nerve width. The number of axons per millimeter was then averaged over all sections. Σad, the total number of axons extending distance, d, in a nerve having a radius, r, was estimated by summing over all sections: Σad = πr2 × [average number of axons/mm width]/section thickness.
Electroretinography
An electroretinography (ERG) response was recorded using the Espion Ganzfeld full field system (Diagnosys LLC, Lowell, MA) before ONC (baseline) and 7 days post-ONC (dpc). Mice were dark adapted for 12 hours overnight and prepared for ERG recording under dim red light (>630 nm). Anesthesia was induced with intraperitoneal injection of ketamine/xylazine and eyes were dilated as described previously. A scotopic flash ERG response was recorded from 10−7 to 0.005 mcd/s units with respect to a standard flash in half log-unit steps. ERG traces were analyzed using in-built Espion software and the peak amplitude (with respect to baseline) was used as a measure of visual function. ERGs traces at the light intensity of 1 × 10−5 mcd/s were chosen for analysis as they gave a clean, unambiguous positive scotopic threshold response (pSTR) with a mean latency of 100 ms. The response of photoreceptors was assessed at 0.005 mcd/s.
Statistical Analysis
All statistic tests were performed using SPSS 17.0 (IBM SPSS, Inc., Chicago, IL), and data presented as mean ± standard error of the mean (SEM) with graphs constructed using GraphPad Prism (La Jolla, CA). A t-test or one-way or two-way ANOVA with Tukey or Sidak correction were used for multiple comparisons, as detailed in the corresponding figure legends. Statistical differences were considered significant at P values < 0.05.
Results
Elevation of ATF3 Level in αRGCs After Optic Nerve Crush
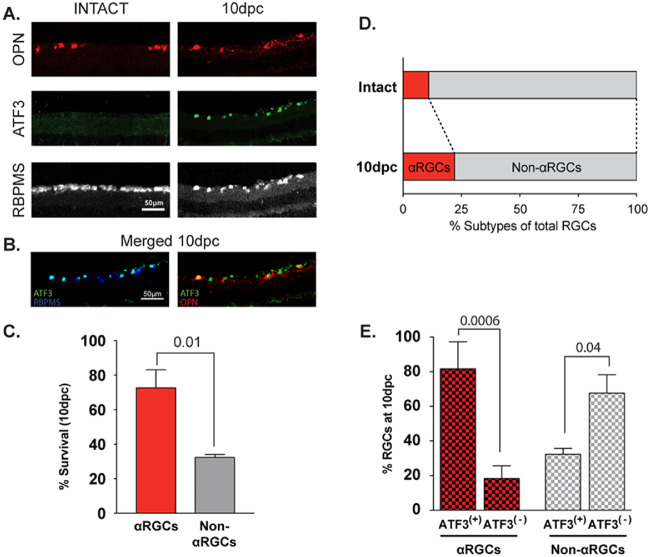
There are more than 40 different RGC types differing by their morphology, physiological properties, gene expression patterns, and reaction to different insults in mice.43–45 It is not known whether upregulation of ATF3 levels after ONC occurs in all RGC types or is associated with a particular RGC type(s). First, we checked whether ATF3 is upregulated in osteopontin+ (OPN+) αRGCs after ONC (in comparison to the total RBPMS+ RGC population). OPN is a marker for αRGCs,16 which survive much better than non-αRGCs after ONC16,39 and play an important role in visual processing. RBPMS is consistently expressed in all RGC types before and after optic nerve injury46,47 Uninjured retinal samples showed very low levels of ATF3 labeling, whereas the levels of ATF3 staining in RGCs was dramatically increased after ONC (Fig. 1A). Consistent with previous reports,16,39 about 36% of RGCs survived over the first 10 dpc (Fig. 1A). αRGCs survived much better than non-αRGCs (72.6% ± 10.4% vs. 32.3 ± 1.7%, respectively, P < 0.001, unpaired t-test) (Figs. 1A, C). As a result, the fraction of αRGCs raised from 8% in control uninjured retina to 22% in retina by 10 dpc (Fig. 1D). Most of the surviving OPN+ αRGCs were ATF3+ (81.7 ± 15.6%), whereas only 32.3 ± 3.4% of surviving non-αRGCs were ATF3+ (Figs. 1B, E).
Figure 1.
ONC leads to preferential elevation of ATF3 level in αRGC. (A) Transverse sections of intact uninjured retina and retina 10 dpc were stained for OPN (red), ATF3 (green) and RBPMS (gray scale). (B) Merged images for ATF3/RBPMS and ATF3/OPN staining at 10 dpc. (C) Percent of αRGCs and non-αRGCs that survived at 10 dpc. (D) Fraction of all RGCs comprised by αRGCs and non-αRGCs in intact retina and at 10 dpc. (E) Percent of ATF3 positive αRGCs and non-αRGCs at 10 dpc. Six and five biological replicates were used for 10 dpc and control, non-crushed eyes groups, respectively. Data are shown as mean ± SEM. Unpaired t-test was used for statistical analysis; P values are shown above corresponding columns.
ATF3 Overexpression Promotes RGC Survival Following ONC
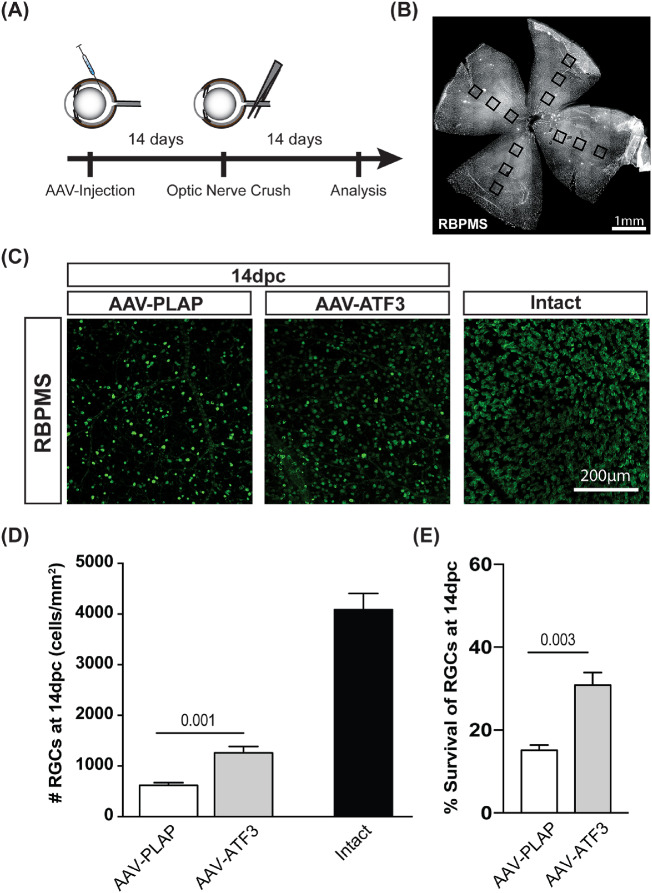
Since ATF3 is preferentially upregulated in αRGCs after ONC, we tested whether overexpression of ATF3 in different RGC types may improve their survival. Our preliminary experiments demonstrated that rAAV2 expressing EGFP (rAAV-EGFP) efficiently transduced RGCs. EGFP fluorescence was detected in more than 80% of RGCs 1 week after intravitreal viral injection in the conditions described in the Materials and Methods (data not shown). rAAV2s expressing ATF3 or PLAP were injected into the vitreous 14 days before ONC (Figs. 2A-D). rAAV-PLAP was used as a negative control because it has been previously demonstrated that overexpression of PLAP does not affect retinal morphology and RGC axon regeneration.48 The number of RBPMS+ RGCs in the intact retina was 4087 ± 319 cells/mm2. Overexpression of ATF3 led to a statistically significant increase in the number of surviving RGCs over PLAP-injected samples (1259 ± 127 cells/mm2 vs. 617 ± 53 cells/mm2, respectively; P = 0.001) after ONC (Figs. 2C, D). Compared with intact retina, 30.8 ± 3.1% RGCs survived after ATF3 overexpression, whereas only 15.1 ± 1.3% RGCs survived after PLAP overexpression (P = 0.003) (Fig. 2E). However, a single rAAV-ATF3 injection was not sufficient to provide RGC neuroprotection 6 weeks after ONC (Supplementary Fig. S1).
Figure 2.
Effects of rAAV-ATF3 overexpression on RGC survival. (A) Timeline of in vivo study. (B) Scheme of RGC counting; squares represent counting fields. (C) Representative images of regions within whole-mount retinas for different groups. Right image (intact) corresponds to noninjected/non-ONC retina. (D) Quantification of RGCs for different groups. (E) Percentage of surviving RGCs 14 dpc normalized to intact retinas (100%). Eight biological replicates were used for rAAV-ATF3 and rAAV-PLAP groups, three biological replicates were used for intact retina. Each biological replicate represents the average of 12 different fields in the retina. Data are shown as mean ± SEM. Two-way ANOVA with a Tukey post hoc test, was used for D, and unpaired t-test comparison for E. P values are shown above corresponding the graph.
To test whether overexpression of ATF3 affects RGC axon regeneration, optic nerves of rAAV-ATF3 or rAAV-PLAP injected eyes were analyzed 10 dpc. Overexpression of ATF3 increased the number of regenerating axons at 0.2 mm and 0.5 mm distances from the ONC site compared with PLAP overexpression (Figs. 3A, B), but the observed differences were not statistically significant (Figs. 3A, B).
Figure 3.
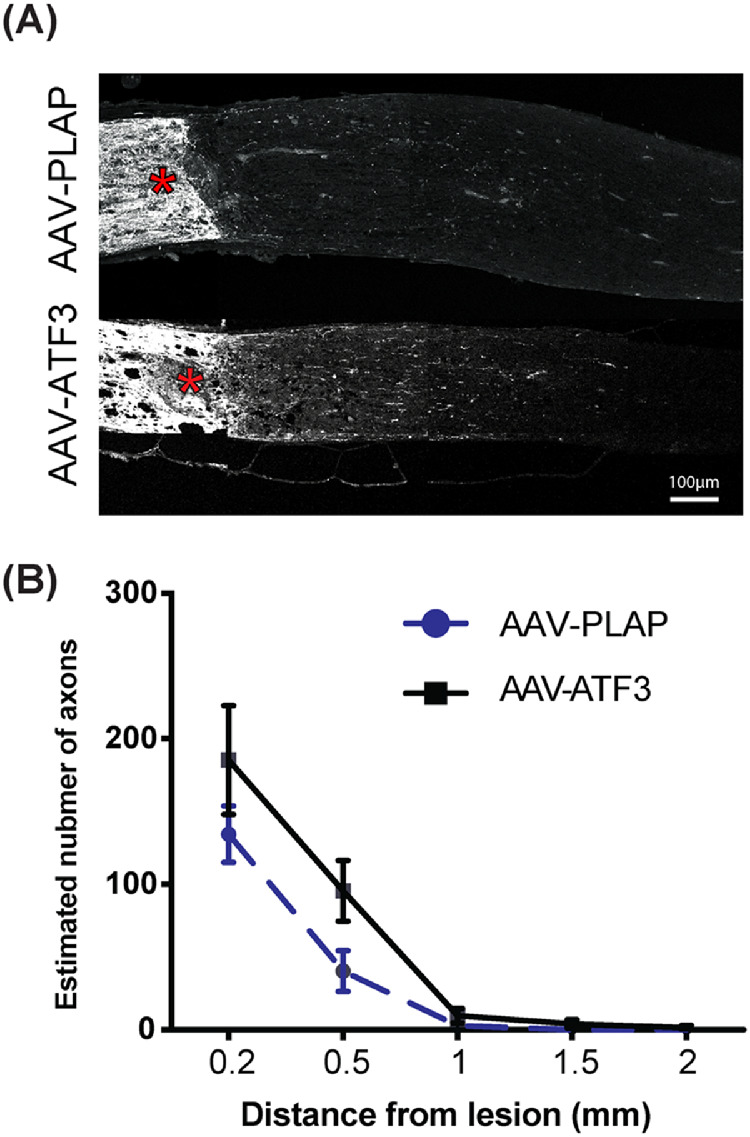
Effects of ATF3 overexpression on RGC axon regeneration after ONC. (A) Representative images of optic nerve sections from rAAV-ATF3- and rAAV-PLAP-treated eyes 10 dpc. CTB-labeled regenerating axons were visualized as described in Materials and Methods, crush sites are indicated by asterisks. (B) Quantification of regenerating axons as in A. N = 9 and 8 for ATF- and PLAP-injected samples, respectively. Data are shown as mean ± SEM. Two-way ANOVA test was used for B.
ATF3 Overexpression Partially Preserves RGC Function
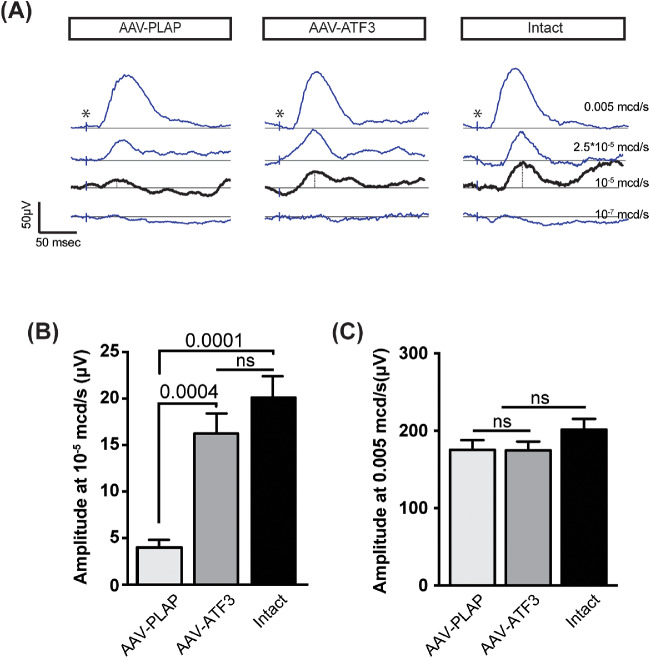
Changes in the functional properties of RGCs after ONC were tested by measuring the pSTR because the amplitude of the pSTR is a measure of RGC functions.47 pSTR recordings were performed 7 dpc (Fig. 4). Twelve different biological replicates were used for each group. In the intact retina, the pSTR amplitude recorded at a low flash intensity (10−5 mcd/s) was 20.1 ± 2.2 µV (Fig. 4B). ONC in the rAAV-PLAP injected control group led to a significant decrease in the pSTR amplitude compared with intact retina (3.95 ± 0.85 μV, P < 0.0001), while overexpression of ATF3 preserved the pSTR amplitude (16.3 ± 2.0 μV) (Figs. 4A, B). ONC in combination with injections of viral constructs did not lead to statistically significant changes in the scotopic photoreceptor response (a-wave) recorded at a higher flash intensity (0.005 mcd/s) 7 dpc (Figs. 4A, C), indicating that photoreceptor functions were not affected.
Figure 4.
Effects of ATF3 overexpression on the pSTR and scotopic responses 7 dpc. (A) Representative traces of the observable pSTR at different light intensities. *The time of flash-light stimulation. The pSTR was measured at the position marked by dash line. (B) Mean pSTR and (C) scotopic amplitudes in different experimental groups. Intact group corresponds to undamaged samples. Twelve different biological replicated were used for each group. P value are shown above the graph; ns, not statistically significant. Data are shown as mean ± SEM. One-way ANOVA test was used.
ATF3 Overexpression Works Synergistically with PTEN Knockdown to Induce RGC Axon Regeneration
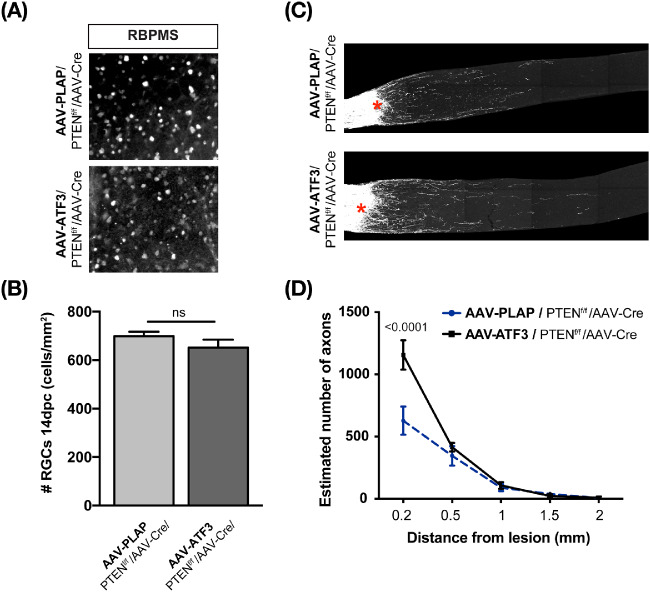
Previous studies demonstrated that deletion of PTEN, a negative regulator of the mTOR pathway, in RGCs promotes the survival of αRGCs and regeneration of their axons after ONC.16,39 To test whether ATF3, like some other factors,25 may act synergistically with activation of the mTOR pathway by downregulation of PTEN, we used PTENf/f mice.49 Activation of the mTOR pathway was achieved by downregulation of PTEN using intravitreal injection of rAAV-Cre as previously described.39 rAAV-Cre was injected into vitreous together with rAAV-ATF3 or rAAV-PLAP. ONC was performed 14 days later and retina/optic nerve samples were analyzed 14 dpc. Six different biological replicates were used for a PLAP/PTEN control group and four for an ATF3/PTEN group. Although ATF3 improved survival of RGCs at 14 dpc in wild-type mice (Fig. 2), no significant differences were detected between rAAV-ATF3 and control rAAV-PLAP when combined with PTEN deletion (652 ± 33 and 699 ± 12 RGCs/mm2, respectively), suggesting the neuroprotective effects between the two treatment conditions are not synergistic (Figs. 5A, B). At the same time, PTEN deletion combined with ATF3 overexpression produced more regenerating axons than PTEN deletion combined with PLAP overexpression at 0.2 mm distance from the ONC site (1156 ± 117 and 628 ± 114 axons, respectively, P < 0.0001). No statistically significant differences between these two conditions were observed at longer distances (>0.2 mm) from the ONC site (Figs. 5C, D).
Figure 5.
Effects of ATF3 overexpression and PTEN deletion on RGC survival and axon regeneration after ONC. (A) Representative images of areas of whole-mount retinas stained with antibodies against RBPMS at 14 dpc. (B) Quantification of RGCs. The number of RGCs/mm2 is shown. (C) Representative images of optic nerve sections at 14 dpc. CTB-labeled regenerating axons were visualized as described in Materials and Methods; crush sites are indicated with asterisks. (D) Quantification of regenerating axons as in C. Six and four different biological replicates were used for PLAP/PTEN control group and ATF3/PTEN, respectively. Data are shown as mean ± SEM. Unpaired t-test was used for B; ns, not statistically significant. Two-way ANOVA test was used for D. No statistically significant differences were detected at a distance >0.2 mm.
mTOR Activation Inhibits Endogenous ATF3 Expression After ONC
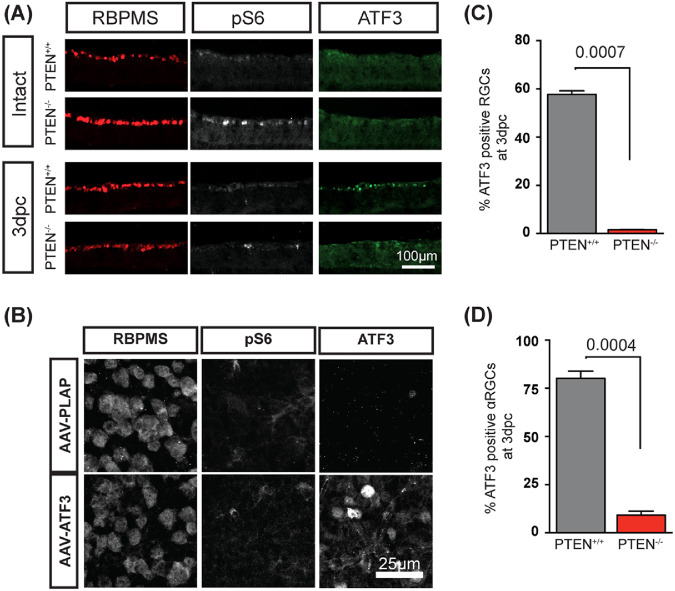
A phosphorylated form (Ser235/236) of ribosome protein S6 (pS6) is a reliable marker of mTOR activity.39,50 It has been shown that the level of pS6 is reduced in RGCs of wild-type retina after ONC while PTEN suppression restores pS6 level.16,39 The pS6 level is more dramatically increased in αRGCs in comparison with non-αRGCs when PTEN deletion was combined with ONC.16 Since ATF3 level is increased in αRGCs after ONC (Fig. 1), we tested whether mTOR activity and ATF3 expression were correlated at the molecular level. The activation of mTOR signaling in non-injured retinas by PTEN deletion (PTEN−/−) did not induce ATF3 expression (Fig. 6A). As expected, ONC induced expression of ATF3 in the RGCs of control PTEN+/+ mice (Fig. 6A). However, upregulation of ATF3 expression in RGCs after ONC was suppressed when PTEN was deleted (Fig. 6A), suggesting that ONC-induced expression of ATF3 is PTEN-dependent. At 3 dpc, a significant decrease in the percentage of ATF3-positive RGCs in PTEN−/− was observed compared with PTEN+/+ retinas, (1.56% ± 0.03 and 57.7% ± 1.5, respectively, P = 0.0007) (Fig. 6C). Similarly, a significant decrease in the percentage of ATF3 positive αRGCs in PTEN−/− was found compared to PTEN+/+ samples (9.23% ± 2.05 and 80.13% ± 9.23, respectively, P = 0.0004) (Fig. 6D).
Figure 6.
mTOR signaling inhibits endogenous expression of ATF3. (A) Transverse sections of control retina (intact) and retina 3 dpc with or without PTEN deletion. Sections were stained with indicated antibodies. (B) Representative images of whole-mount, nonoptic nerve crush retinas immunohistochemically stained for ATF3, RBPMS, and pS6 7 days after intravitreal injections of corresponding rAAV viruses. (C) Percentage of ATF3 positive RGCs in PTEN+/+ and PTEN−/− retinas at 3 dpc. (D) Percentage of ATF3 positive αRGCs in PTEN+/+ and PTEN−/− retinas at 3 dpc. Data are shown as mean ± SEM. Unpaired t-test was used for C and D. N = 3 for each group in A-D.
Next, we tested whether overexpression of ATF3 may modulate the mTOR pathway. In a noninjured retina, overexpression of ATF3 by intravitreal injection of rAAV-ATF3 had no detectable effect at the level of pS6 expression compared to control retina from eyes injected with rAAV2-PLAP 1 week after viral injection (Fig. 6B).
Discussion
Only a few RGCs survive after ONC and even fewer can extend their axons through the injury site,51 with different subtypes showing dramatic differences in the survival rate and ability to regenerate their axons.16 αRGCs have been previously shown to survive better than many other RGC types and account for nearly all the regeneration seen following downregulation of PTEN and subsequent activation of the mTOR pathway.16 Furthermore, αRGCs preferentially express OPN and receptors for insulin-like growth factor 1. Overexpression of these proteins promotes regeneration of αRGC axons.16 Other factors and signaling pathways might be also involved in RGC neuroprotection and stimulation of axon regeneration.10 One of these factors is the transcription factor ATF3, known to be inducible by stress and axonal injury in both the peripheral nervous system and CNS.27,30 In the adult intact retina, expression of ATF3 is not detected in RGCs; however, a fraction of RGCs were shown to upregulate ATF3 after ONC.22 In this paper, we demonstrated that more than 80% of αRGCs surviving after ONC were positive for ATF3 expression while only 30% of non-αRGCs were ATF3+ (Fig. 1D). Although we did not analyze the nature of surviving ATF3+ non-αRGCs, we assumed that some of these cells could be melanopsin expressing M1-RGCs because these cells, similar to αRGCs, preferentially survive after ONC.16 Indeed, it has been recently demonstrated that, similar to αRGCs, the level of Atf3 gene expression is very low in melanopsin expressing RGCs in a control undamaged mouse retina and is dramatically upregulated in these cells after ONC.52 We showed that overexpression of ATF3 by intravitreal injection of rAAV-ATF3 before ONC promoted survival of RGCs for at least 2 weeks (Fig. 2). The neuroprotective effect of ATF3 overexpression was similar to that of neuritin, a known RGC neuroprotective factor.7,53 However, a single rAAV-ATF3 injection was not sufficient for RGC neuroprotection over 6 weeks. This indicates that ATF3 effects on injured RGCs may be transient or that multiple rAAV-ATF3 injections are needed, similar to a rat glaucoma model where RGC neuroprotection by intravitreal injections of small extracellular vesicles required monthly injections while a single injection was not effective over 2 months.54 Stimulation of RGC axon regeneration by ATF3 after ONC was minor with no statistically significant differences between control and ATF3 samples detected (Fig. 3). Although abilities of a particular RGC type to survive and regenerate axons after ONC very often correlate, this is not always the case. For example, melanopsin-expressing M1-RGCs and αRGCs survive preferentially after ONC but only the αRGCs, not M1-RGCs, regenerate their axons.16 Overexpression of Sox11 kills αRGCs yet promotes the regeneration of non-αRGC axons after ONC.25 It appears that overexpression of ATF3 is not sufficient to activate a set of genes essential for RGC axon growth.
Overexpression of ATF3 also provided preservation of RGC functions as judged by measuring the pSTR amplitude (Fig. 4). Surprisingly, the preservation of RGC functions was more pronounced than the preservation of RGC numbers. It has been previously shown that ATF3 not only protects hippocampal neurons against neuronal death but also enhances their ability to recover synaptic connections and reestablish functional networks after excitotoxic insults.55 It is thus feasible that surviving RGCs overexpressing ATF3 may have enhanced ability to reestablish synaptic connections with neurons in the inner nuclear layer, counteracting the cell loss and preserving visual function and RGCs response to light stimuli. Another possibility is that overexpression of ATF3 may enhance the excitation of individual RGCs in response to ONC. It has been reported that sodium channel NaV1.6 was transiently increased in RGC axons in mice 2 weeks after intraocular pressure elevation, leading to enhanced axon firing in response to light stimulus.56 Further studies are necessary to find out whether any of these possibilities may contribute to the preservation of RGC functions after ATF overexpression.
It is now well established that deletion of PTEN, a negative regulator of the mTOR pathway, in RGCs promotes their survival and the regeneration of αRGC axons after ONC.16,39,57 PTEN deletion in combination with overexpression of Sox11 may further enhance axon regeneration from non-αRGCs.25 At the same time, overexpression of Sox11 enhanced αRGC death after ONC and, as a result of this, overexpression of Sox11 in combination with PTEN deletion decreased the survival of total population of RGCs.25 Unlike Sox11, overexpression of ATF3 in combination with PTEN deletion did not decrease the survival of total population of RGCs, but similar to Sox11, enhanced axon regeneration after PTEN deletion, although we do not know at present whether this occurs in all RGCs or just in some RGC subtypes.
In the undamaged retina, overexpression of ATF3 did not activate the mTOR pathway (Fig. 6D) and vice versa, mTOR activation did not induce ATF3 expression. This contradicts previous studies demonstrating the upregulation of ATF3 after PTEN deletion in lung epithelial and prostatic epithelial cells.58,59 This is likely however because of the differences between epithelial cells and RGCs and indeed, PTEN deletion in these cells, unlike in RGCs, led to tumorigenesis. PTEN deletion significantly decreased the number of ATF3-positive RGCs after ONC (Figs. 6A-C). At present, it is not entirely clear why overexpression of ATF3 alone promoted RGC survival but not RGC axon regeneration following ONC, whereas in combination with PTEN deletion, overexpression of ATF3 did not improve RGC survival but stimulated limited RGC axon regeneration. We can hypothesize that other players could be also involved. For example, it has been shown that optic nerve injury triggers rapid upregulation of the stress-induced proteins REDD1 and REDD2 (regulated in development and DNA damage responses 1 and 2, respectively), potent inhibitors of mTOR.53,54 REDD2 upregulation and mTOR inhibition led to RGC dendrite pathology causing neuronal dysfunction and subsequent RGC death,60 whereas suppression of REDD1 expression promoted RGC survival and axon elongation after ONC.61 Combinatorial action of REDD1/2, PTEN, ATF3, and some other factors may lead to the observed effects. The elucidation of molecular processes involved in neuroprotective mechanisms of ATF3 action in the retina and CNS in general represents an important avenue of further research.
Supplementary Material
Acknowledgments
The authors thank Haohua Qian, PhD, for his help with ERG; Ben Mead, PhD; and Alicia Kerr, PhD, for critical reading of the manuscript.
Supported by the Intramural Research Programs of the National Eye Institute, National Institutes of Health (CK, NN, MS, LB-P, TZ, WL, ST) and by the National Institutes of Health (Grant R01EY026939, ZH).
Disclosure: C. Kole, None; B. Brommer, None; N. Nakaya, None; M. Sengupta, None; L. Bonet-Ponce, None; T. Zhao, None; C. Wang, None; W. Li, None; Z. He, None; S. Tomarev, None
References
- 1. Ghaffarieh A, Levin LA. Optic nerve disease and axon pathophysiology. Int Rev Neurobiol. 2012; 105: 1–17. [DOI] [PubMed] [Google Scholar]
- 2. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017; 390: 2183–2193. [DOI] [PubMed] [Google Scholar]
- 3. Quigley HA. Glaucoma. Lancet. 2011; 377: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 4. Bull ND, Johnson TV, Welsapar G, DeKorver NW, Tomarev SI, Martin KR. Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies. Invest Ophthalmol Vis Sci. 2011; 52: 3309–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012; 119: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, et al.. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014; 137: 503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma TP, Liu Y, Wordinger RJ, Pang IH, Clark AF. Neuritin 1 promotes retinal ganglion cell survival and axonal regeneration following optic nerve crush. Cell Death Disease. 2015; 6: e1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vajn K, Plunkett JA, Tapanes-Castillo A, Oudega M. Axonal regeneration after spinal cord injury in zebrafish and mammals: differences, similarities, translation. Neurosci Bull. 2013; 29: 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becker T, Becker CG. Axonal regeneration in zebrafish. Curr Opin Neurobiol. 2014; 27: 186–191. [DOI] [PubMed] [Google Scholar]
- 10. He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016; 90: 437–451. [DOI] [PubMed] [Google Scholar]
- 11. Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994; 91: 1632–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giger RJ, Hollis ER 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harbor Perspectives Biol. 2010; 2: a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002; 296: 1860–1864. [DOI] [PubMed] [Google Scholar]
- 14. Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006; 361: 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nature Rev. 2006; 7: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mtor signaling. Neuron. 2015; 85: 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer D, Harvey AR, Pernet V, Lemmon VP, Park KK. Optic nerve regeneration in mammals: regenerated or spared axons? Exp Neurol. 2017; 296: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017; 356: 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li HJ, Sun ZL, Yang XT, Zhu L, Feng DF. Exploring optic nerve axon regeneration. Curr Neuropharmacol. 2017; 15: 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu QL, Li X, Yip HK, Shao Z, Wu W, Mi S, et al.. Combined effect of brain-derived neurotrophic factor and LINGO-1 fusion protein on long-term survival of retinal ganglion cells in chronic glaucoma. Neurosci. 2009; 162: 375–382. [DOI] [PubMed] [Google Scholar]
- 21. Apara A, Galvao J, Wang Y, Blackmore M, Trillo A, Iwao K, et al.. KLF9 and JNK3 interact to suppress axon regeneration in the adult CNS. J Neurosci. 2017; 37: 9632–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaneko A, Kiryu-Seo S, Matsumoto S, Kiyama H. Damage-induced neuronal endopeptidase (DINE) enhances axonal regeneration potential of retinal ganglion cells after optic nerve injury. Cell Death Disease. 2017; 8: e2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li S, Yang C, Zhang L, Gao X, Wang X, Liu W, et al.. Promoting axon regeneration in the adult CNS by modulation of the melanopsin/GPRC signaling. Proc Natl Acad Sci U S A. 2016; 113: 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, et al.. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomic. Neuron. 2015; 86: 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, et al.. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron. 2017; 94: 1112–1120.e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore DL, Apara A, Goldberg JL. Kruppel-like transcription factors in the nervous system: novel players in neurite outgrowth and axon regeneration. Mol Cell Neurosci. 2011; 47: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunt D, Raivich G, Anderson P. Activating transcription factor 3 and the nervous system. Front Mol Neurosci. 2012; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson MR, Xu D, Williams BR. Atf3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009; 87: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hai T, Wolford CC, Chang YS. Atf3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, et al.. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000; 15: 170–182. [DOI] [PubMed] [Google Scholar]
- 31. Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006; 32: 143–154. [DOI] [PubMed] [Google Scholar]
- 32. Fagoe ND, Attwell CL, Kouwenhoven D, Verhaagen J, Mason MR. Overexpression of ATF3 or the combination of ATF3, c-Jun, STAT3 and Smad1 promotes regeneration of the central axon branch of sensory neurons but without synergistic effects. Human Mol Genet. 2015; 24: 6788–6800. [DOI] [PubMed] [Google Scholar]
- 33. Gey M, Wanner R, Schilling C, Pedro MT, Sinske D, Knoll B. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. 2016; 6: 160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satoru Ueno, Azusa Yoneshige, Yoshiki Koriyama, Man Hagiyama, Yoshikazu Shimomura, Ito A. Early gene expression profile in retinal ganglion cell layer after optic nerve crush in mice. Glaucoma. 2018; 59: 370–380. [DOI] [PubMed] [Google Scholar]
- 35. Ueno S, Yoneshige A, Koriyama Y, Hagiyama M, Shimomura Y, Ito A. Early gene expression profile in retinal ganglion cell layer after optic nerve crush in mice. Invest Ophthalmol Vis Sci. 2018; 59: 370–380. [DOI] [PubMed] [Google Scholar]
- 36. Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with Rhoa inactivation. J Neurosci. 2004; 24: 8726–8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, et al.. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007; 48: 5539–5548. [DOI] [PubMed] [Google Scholar]
- 38. Saul KE, Koke JR, Garcia DM. Activating transcription factor 3 (ATF3) expression in the neural retina and optic nerve of zebrafish during optic nerve regeneration. Comp Biochem Physiol A Mol Integr Physiol. 2010; 155: 172–182. [DOI] [PubMed] [Google Scholar]
- 39. Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al.. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008; 322: 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kole C, Klipfel L, Yang Y, Ferracane V, Blond F, Reichman S, et al.. Otx2-genetically modified retinal pigment epithelial cells rescue photoreceptors after transplantation. Mol Ther. 2018; 26: 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kole C, Berdugo N, Da Silva C, Ait-Ali N, Millet-Puel G, Pagan D, et al.. Identification of an alternative splicing product of the Otx2 gene expressed in the neural retina and retinal pigmented epithelial cells. PLoS ONE. 2016; 11: e0150758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000; 20: 4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, et al.. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018; 9: 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanes JR, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci. 2015; 38: 221–246. [DOI] [PubMed] [Google Scholar]
- 45. Baden T, Berens P, Franke K, Roman Roson M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016; 529: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez AR, de Sevilla Muller LP, Brecha NC. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014; 522: 1411–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mead B, Tomarev S. Evaluating retinal ganglion cell loss and dysfunction. Exp Eye Res. 2016; 151: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremoliere A, et al.. Doublecortin-like kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron. 2015; 88: 704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al.. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001; 294: 2186–2189. [DOI] [PubMed] [Google Scholar]
- 50. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Pérez MP, et al.. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Phil Transac Royal Soc London. Series B, Biol Sci. 1991; 331: 337–343. [DOI] [PubMed] [Google Scholar]
- 52. Bray ER, Yungher BJ, Levay K, Ribeiro M, Dvoryanchikov G, Ayupe AC, et al.. Thrombospondin-1 mediates axon regeneration in retinal ganglion cells. Neuron. 2019; 103: 642–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yao JJ, Zhao QR, Lu JM, Mei YA. Functions and the related signaling pathways of the neurotrophic factor neuritin. Acta Pharmacol Sin. 2018; 39: 1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mead B, Amaral J, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Ophthalmol Vis Sci. 2018; 59: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ahlgren H, Bas-Orth C, Freitag HE, Hellwig A, Ottersen OP, Bading H. The nuclear calcium signaling target, activating transcription factor 3 (ATF3), protects against dendrotoxicity and facilitates the recovery of synaptic transmission after an excitotoxic insult. J Biol Chem. 2014; 289: 9970–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Risner ML, Pasini S, Cooper ML, Lambert WS, Calkins DJ. Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc Natl Acad Sci U S A. 2018; 115: E2393–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, et al.. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011; 480: 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Z, Xu D, Ding HF, Kim J, Zhang J, Hai T, et al.. Loss of ATF3 promotes Akt activation and prostate cancer development in a Pten knockout mouse model. Oncogene. 2015; 34: 4975–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Marco C, Laudanna C, Rinaldo N, Oliveira DM, Ravo M, Weisz A, et al.. Specific gene expression signatures induced by the multiple oncogenic alterations that occur within the PTEN/PI3K/AKT pathway in lung cancer. PLoS ONE. 2017; 12: e0178865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morquette B, Morquette P, Agostinone J, Feinstein E, McKinney RA, Kolta A, et al.. REDD2-mediated inhibition of mTOR promotes dendrite retraction induced by axonal injury. Cell Death Differ. 2015; 22: 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morgan-Warren PJ, O'Neill J, de Cogan F, Spivak I, Ashush H, Kalinski H, et al.. siRNA-mediated knockdown of the mTOR inhibitor RTP801 promotes retinal ganglion cell survival and axon elongation by direct and indirect mechanisms. Invest Ophthalmol Vis Sci. 2016; 57: 429–443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.