Abstract
Nematodes are increasingly used as powerful bioindicators of soil food web composition and functioning in ecological studies. Todays’ ecological research aims to investigate not only local relationships but global patterns, which requires consistent methodology across locations. Thus, a common and easy extraction protocol of soil nematodes is needed. In this study, we present a detailed protocol of the Baermann-funnel method and highlight how different soil pre-treatments and equipment (soil type, soil height, sieving, and filter type) can affect extraction efficiency and community composition by using natural nematode communities. We found that highest nematode extraction efficiency was achieved using lowest soil height as indicated by the thickness of the soil sample in the extractor (1, 2, or 4 cm soil height) in combination with soil sieving (instead of no sieving), and by using milk filters (instead of paper towels). PCA at the family level revealed that different pre-treatments significantly affected nematode community composition. Increasing the height of the soil sample by adding more soil increased the proportion of larger-sized nematodes likely because those are able to overcome long distances but selected against small nematodes. Sieving is suggested to break up soil aggregates and, therefore, facilitate moving in general. Interestingly, sieving did not negatively affect larger nematodes that are supposed to have a higher probability of getting bruised during sieving but, even if not significant, tended to yield more extracted nematodes than no sieving. We therefore recommend to use small heights of sieved soil with milk filter to extract free-living soil nematodes with the Baermann-funnel method. The present study shows that variations in the extraction protocol can alter the total density and community composition of extracted nematodes and provides recommendations for an efficient and standardized approach in future studies. Having a simple, cheap, and standardized extraction protocol can facilitate the assessment of soil biodiversity in global contexts.
Keywords: soil organisms, comparability, reproducibility, extraction methods
1. Introduction
Nematodes appear in nearly any kind of soil from dry desert sand to the tundra (Meyl 1961). Their ubiquitousness, high species richness, and characteristic responses to environmental stressors make them unique biological indicators. Next to information about soil conditions, soil health, and soil processes (Bongers & Ferris 1999), nematodes and indices based on their community composition can be used to describe soil food web complexity, nutrient enrichment, and decomposition channels (Ferris 2001). These variables can provide important information in ecological research, e.g., exploring the consequences of environmental change and biodiversity loss (Cesarz et al. 2015, Gingold et al. 2013, Viketoft & Sohlenius 2011).
To study general patterns and ecological principles, global networks of ecological experiments have been set up, such as Nutrient Network (https://www.nutnet.umn.edu/), investigating the consequences of multiple nutrient additions in grasslands (Borer et al. 2014), or TreeDivNet (http://www.treedivnet.ugent.be/) comprising different tree diversity experiments across the globe (Verheyen et al. 2015). Thus far, soil nematodes have mostly been studied in single and local experiments, but global assessments are scare (but see Nielsen et al. 2014, Song et al. 2017, van den Hoogen et al. 2019) or non-existing for global analyses using soil nematodes as bioindicators to environmental change. Generally, the assessment of soil biodiversity is largely neglected, leading to a strong under-representation of soil biodiversity in ecological databases, especially at the global scale (Phillips et al. 2017).
The high trophic and functional diversity of nematodes comes along with a large number of extraction methods available (reviewed in PM7/119(1) 2013), highlighting that there may not be one ideal technique for all taxa, and different research questions can ask for different approaches (Viglierchio & Schmitt 1983). In addition, the diversity of extraction methods is usually accompanied with complex equipment like the Oostenbrink elutriator (Verschoor & De Goede 2000). However, labs agreeing to extract nematodes in the frame of global ecological networks need one extraction technique, which can be easily implemented and is at low cost.
In this study, we focus on the very common Baermann-funnel method (Baermann 1917) as a simple, fast, and cheap approach for nematode extraction in global assessments (Fig. 1). By the use of the Baermann-funnel method, hundreds of samples can be extracted in parallel, and it can be rebuild easily by laboratories without having experience in nematode extraction. The amount of soil needed is relatively small (under hundred grams) and only a low amount of water is required (ca. 200 ml by sample). Another advantage to other methods is the cleanliness of the final solution (less soil particles) making microscope work easier and faster. However, this method only selects active nematodes, thereby excluding cysts and inactive forms. The Baermann-funnel method has been used with different extraction times (24–72 h) and temperatures. Mostly, nematodes are extracted at 20°C (or room temperature), but modifications exist using a temperature gradient to accelerate the extraction by forcing nematodes to move from heated, rapidly dring upper soil parts downwards into cooler, moister regions, where nematodes can be collected (PM7/119(1) 2013).
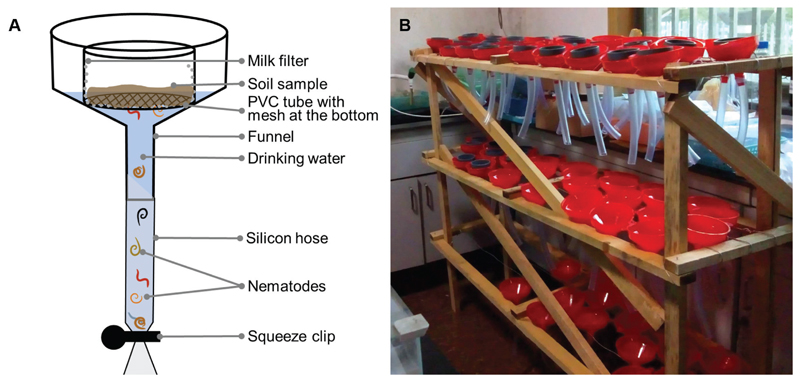
Figure 1.
(A) Schematic Baermann-funnel apparatus for nematode extraction from soil. (B) Wooden frame to carry single Baermann funnels build in China with simple materials (Photo credit: Rémy Beugnon).
Building on many previous studies testing nematode extraction efficiencies (Robinson & Heald 1989, Ruess 1995, Viglierchio & Schmitt, 1983), we evaluated main extraction treatments within the Baermann-funnel method to provide recommendations to extract soil nematodes (e.g., for global assessments), focusing on a consistent methodology and high extraction efficiency, which can be used by many laboratories worldwide. By doing so, we highlight how common soil pre-treatments and equipment (sieving, soil height, and filter type) can affect extraction efficiency and community composition and should therefore be considered in future studies.
Homogenization of the soil by sieving has been shown to be handled differently. Van Bezooijen (2006) suggested to homogenize soil samples before extraction by sieving using mesh sizes from 1 mm up to 5 mm. In contrast, sieving was also mentioned to bruise nematodes (PM7/119(1) 2013). Mortality can occur when samples are handled roughly, and loss of species after sieving was shown to be species-specific (Viglierchio & Schmitt, 1983, Yeates 1999). On the other hand, homogenization breaks soil aggregates and is assumed to facilitate the movement of nematodes through the soil, likely resulting in higher nematode extraction efficiency. However, despite the inconsistency of mesh sizes used in different studies, the consequences for extraction efficiency and comparability of results have not been tested before.
In ecological long-term experiments or in pristine habitats, destructive samplings like taking soil cores often are strongly limited to prevent destruction of the plots, and only small amounts of soil may be available for nematode extraction. Using large amounts of soil, on the other hand, may also reduce extraction efficiency as less mobile nematodes are discriminated (PM7/119(1) 2013, Ruess 1995). Thus, exploring the role of the amount of extracted soil for nematode extraction efficiency is required to provide general recommendations.
Different permeable filters are used to separate nematodes from soil. Most often cotton-wool milk filters are used, but also cheesecloth, filter paper, or paper tissue are suggested. However, knowledge of the influence of different filters on extraction efficiency is missing. Regarding the availability of materials and costs, we tested milk filters and common paper towels in this study.
A well-chosen combination of the settings described above may help to increase nematode extraction efficiency and to avoid potential biases of different extraction protocols. In this study, we evaluated different settings of the Baermann-funnel method by varying 1) different sieving mesh sizes, 2) different amounts of soil by varying soil height, and 3) two different filter types to investigate the consequences for the total amount of extracted nematodes and for nematode community composition. In addition, three very different soil types, i.e., sandy, loamy, and silt loamy soil, were used to enable us to make general recommendations. The soil was derived from two different locations, i.e, Germany (loam and sand soil), and China (silt loam soil). A remote location was used for testing if our approach could be easily transferred to places without any existing extraction infrastructure.
2. Material and methods
We tested the effects of four factors in soil nematode extraction in a factorial design: three soil types (loam, silt loam, and sand), three soil sieving treatments (2 mm mesh size, 5 mm mesh size, and no sieving), three amounts of extracted soil reflecting different soil heights (1, 2, and 4 cm soil height, i.e., ca. 25 g, 50 g, and 100 g, respectively), and two permeable filters (milk filters and paper towels; only applied for loam and sand soil). All treatments were replicated five times resulting in 225 samples.
The different soils were taken at three different locations in Germany and China with different soil properties (Tab. 1) at a depth of 0–20 cm at three locations (1–5 m apart from each other, randomly selected) close to the experimental plots forming a composite sample per site. The sandy soil was taken from the Kreinitz Experiment, a tree biodiversity experiment in Zeithain, Saxony, Germany (Hantsch et al. 2014). The loamy soil was taken from the Jena Experiment, a grassland biodiversity experiment in Jena, Germany (Roscher et al. 2004). The silt loamy soil was taken from site A of the BEF-China tree diversity experiment (Bruelheide et al. 2014).
Table 1. Soil properties of the sites selected for testing soil nematode extraction use efficiency.
| Site | Country | Coordinates | soil pH | C (%) | N (%) | CN | Clay (%) | Silt (%) | Sand (%) | Textural class | Soil | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kreinitz | Germany | 51°23’N, 13°15‘E | 5.5 | 1.1 | 0.1 | 11.0 | 2 | 5 | 94 | Sand | Humic Cambisol | Hantsch et al. (2014) |
| Jena | Germany | 50°55‘N, 11°35‘E | 8.1 | 4.6 | 0.3 | 15.3 | 14 | 41 | 45 | Loam | Eutric Fluvisol | Roscher et al. 2004) |
| BEF China | China | 29°7‘ N, 117°54‘E | 4.8 | 1.6 | 0.16 | 10.0 | 26 | 50 | 24 | Silt loam | Endoleptic Cambisols | Pei et al. (2017) |
Before any treatments were applied, soil was gently mixed. Afterwards, a fraction of the soil was sieved with a mesh of 2 mm or 5 mm. One fraction of the soil was not sieved, but roots and stones were removed by hand to correctly evaluate soil weight (PM7/119(1) 2013). Three different soil heights of fresh soil were used for extraction representing a thickness of the soil during extraction of about 1, 2, and 4 cm, respectively. Finally, two different filter types were used: commonly used milk filters (Sana, type FT 25) and paper towels (ZVG Zellstoff-Vertriebs-GmbH & Co. KG, EAN: 4026899028532). We did not use paper towels in China, because first results of the other two soils already showed very low nematode extraction with paper towels.
2.1. Baermann-funnel apparatus
The Baermann-funnel apparatus consisted of a funnel with an inner diameter of 11 cm. Using a different diameter is possible but should be constant among and within studies. A 12 cm piece of silicone hose was attached to the funnel ending and fixed with hot glue to prevent water leakage. The tube was closed with a squeezer clip at the end of the silicon hose. The Baermann-funnel apparatus was installed in a horizontal position without buckling of the silicon hose. Soil of a certain height was filled into circular PVC tubes (7 cm in diameter) with a mesh of 250 μm at the bottom, allowing nematodes to traverse the mesh. The same PVC tubes were used at all three locations. The mesh was covered with a filter (milk filter or paper towel) to prevent soil particles to enter the nematode solution (Fig 1). To obtain clean samples, we used a large piece of the filter material to prevent soil particles to enter the nematode solution from the side. This, however, increases evaporation and water has to be added if necessary to prevent the soil from drying.
2.2. Procedure
The first step was to check if the apparatus is tight by filling drinking water (room temperature or below) into the funnel with the closed silicone hose at the bottom until it reached the lower end of the funnel. Due to different tap water quality, we do not recommend using tap water, if drinking water quality cannot be guaranteed as it can vary widely from location to location (e.g. salinity, pH), thereby likely causing hypoosmotic stress during extraction, or tap water may even contain nematodes (Ainsworth & WHO 2004). In case tap water needs to be used and it cannot be guaranteed that tap water is free of nematodes, we suggest to filter the tap water with a 5 μm mesh. Ideally, in line with recent standardized approaches to estimate litter decomposition using a common substrate (Keuskamp et al. 2013), the possibility should be explored to use one brand of drinking water all over the world to guarantee a standardized procedure. However, it should be considered that chemical properties can vary greatly between such a common water and soil water at a given location. For this study, tap water with drinking water quality was used in Germany, and drinking water was used in China. The silicone hose had to be squeezed several times to remove air from the silicone hose. The weight of the empty PVC tube including the filter and a label to identify the sample was noted. Fresh soil was filled into the PVC tubes representing 1, 2, or 4 cm soil thickness. The exact weight was recorded to get soil water content and relate nematodes to g dry soil. Afterwards, the PVC tube with soil was inserted in the funnel.
Fresh drinking water was added from the side until the bottom of the mesh of the PVC tube touched the water to saturate the sample with water to increase nematode mobility. Soil samples were not submerged with water to prevent oxygen limitation. After 72 h (PM7/119(1) 2013), nematodes accumulating at the bottom of the tube were transferred into vials and fixed in 4 % hot formalin. Therefore, the silicone hose was opened and the water containing nematodes rinsed through a sieve with a 15 μm mesh to separate nematodes from water. Nematodes accumulating on the mesh were transferred into a vial by rinsing the mesh with hot formalin (4 %). Alternatively, hot water (60–80°C) can be used for heat killing, and formalin at room temperature can be used to fix nematodes. After extraction, the soil with the PVC tube were dried and weighed to obtain nematode densities per g dry soil.
2.3. Nematode counting and identification
All nematodes within one sample were counted with a microscope (Leica, DMI 3000 B) using 50 × magnification. To detect changes in nematode community composition due to the different treatments, a subset of treatments showing the strongest differences in the total number of extracted nematodes (see below) were identified to family level after Bongers (1994) and Andrássy (2005) using 1000 × magnification. We randomly identified 100 individuals per sample. The proportional value of each family was extrapolated to the total number of nematodes in the sample. Since the Chinese samples had very low densities, those samples were excluded from identification.
2.4. Statistical analysis
The factorial experiment was analyzed using a three-way (two way for the silt loam soil) Analysis of Variance (ANOVA) SS type 3, with the factors soil sieving (three levels: 5 mm mesh size, 2 mm mesh size, no sieving), soil height (three levels: 1 cm, 2 cm, and 4 cm sample thickness), filter type (two levels: milk filters and paper towels; paper towels were not used for the silt loamy soil in China), and all possible interactions. Including soil type to the ANOVA did not fulfill the statistical assumptions due to high differences in nematode densities among soil types (mean ± sd of nematodes extracted from 1 g of sandy soil, loamy soil, silt loamy soil, and sand were 1.45 ± 0.87, 17.54 ± 12.41, and 0.09 ± 0.07, respectively. Therefore, the dataset was separated by soil type. Log-transformation of the data was necessary to achieve homogenization of variances and normal distribution of the residuals. Analyses were performed using R (R i386 3.5.0; R Development Core Team, 2008).
We used principal component analysis (PCA) to detect if specific nematode families were selected by the different extraction treatments. Based on the strongest treatment effects on the total number of extracted nematodes, three treatments with n = 3 were chosen for more detailed identification and PCA analysis: i) 4 cm height of the extracted soil sample sieved at 5 mm (low extraction efficiency), ii) 1 cm height of the extracted soil sample without sieving (medium extraction efficiency), and iii) 1 cm height of the extracted soil sample sieved at 5 mm (high extraction efficiency). All samples used for the multivariate analysis were extracted with milk filters. As we assumed nematode body size to reflect different levels of mobility, three size classes were considered, i.e., small (up to 0.5 mm), intermediate (0.5 to 1.0 mm), and large (>1.0 mm) nematodes. Therefore, the mean size of all species/genera per family was calculated (Tab. 2) using values listed in Bongers (1994), and in Andrássy (2005) for the family Microlaimidae, which is not given in Bongers (1994). Those values are given for adult nematodes, thereby neglecting that juveniles dominated the samples. However, as this is likely to be true for all species, we assume that our size classification still reflects size differences of different families. In addition, nematode families were assigned to the five c-p classes according to Bongers (1990) and Bongers & Bongers (1998) reflecting life strategy histories with cp 1 and cp 2 indicate r-strategists and cp 3 to cp 5 indicate K-strategists. Furthermore, nematode families were classified according their occurrence to abundant (up to 5 %), medium (5–1 %), and rare (below 1 %) families using mean relative occurrence of nematode families in loamy and sandy soils. PCA was performed with R i386 3.5.0 (R Development Core Team 2008).
Table 2.
Nematode families extracted from loamy and sandy soil. List of nematode families extracted from loamy (L) and sandy (S) soil using the Baermann-funnel method with assigned c-p classes after Bongers (1990) and Bongers and Bongers (1998) and trophic groups after Yeates et al. (1993). In addition, assignment to r- and K-strategists. R-strategists were nematodes from c-p class 1 and 2, whereas c-p classes 3 to 5 are classified as K-strategists (Cesarz et al. 2017). Size classes (small, intermediate, and large) of all nematode families were calculated as the mean size of the minimum (min) and maximum (max) of all genera and species belonging to one family being listed in Bongers (1994), and in Andrássy (2005) for the family Microlaimidae. Occurrence describes in which soil types nematodes occurred. Using mean relative occurrence of nematode families in loamy and sandy soils were used to assign nematodes to abundant (up to 5%), medium (5-1%), and rare (below 1%) families. Means ± SD of nematode families are given for samples displayed in the PCA using different extraction treatments, i.e., using i) 4 cm of fresh soil sieved at 5 mm (low extraction efficiency; n = 3), ii) 1 cm of fresh soil without sieving (medium extraction efficiency; n=3), iii) 1 cm of fresh soil sieved at 5 mm (high extraction efficiency; n=3). Taxa were sorted by overall mean of extracted nematodes.
| Familiy | cp class | Trophic group | r/K strategy | Mean size | Size | Occurrence | Mean % occurrence loam | Mean % occurrence sand | Occurrence in loamy soil | Occurrence in sandy soil |
|---|---|---|---|---|---|---|---|---|---|---|
| Cephalobidae | 2 | Bacterial feeder | r | 0.65 | medium | L,S | 6.67 ± 2.00 | 25.57 ± 8.59 | abundant | abundant |
| Tylenchidae | 2 | Plant feeder + Fungal feeder | r | 0.6 | medium | L,S | 13.33 ± 4.66 | 9.18 ± 3.67 | abundant | abundant |
| Dolichodoridae | 3 | Plant feeder | K | 0.85 | medium | L,S | 18.44 ± 7.04 | 3.18 ± 3.12 | abundant | medium |
| Plectidae | 2 | Bacterial feeder | r | 0.8 | medium | L,S | 8.78 ± 4.24 | 8.86 ± 4.66 | abundant | abundant |
| Rhabditidae | 1 | Bacterial feeder | r | 1.35 | large | L,S | 5.22 ± 2.17 | 11.95 ± 5.24 | abundant | abundant |
| Hoplolaimidae | 3 | Plant feeder | K | 0.9 | medium | L | 12.56 ± 3.94 | 0.00 ± 0.00 | abundant | not occurring |
| Qudsianematidae | 4 | Omnivore | K | 1.45 | large | L,S | 4.22 ± 2.33 | 6.29 ± 4.24 | medium | abundant |
| Aphelenchoididae | 2 | Fungal feeder | r | 0.65 | medium | L,S | 1.44 ± 1.59 | 6.35 ± 3.31 | medium | abundant |
| Paratylenchidae | 2 | Plant feeder | r | 0.35 | small | L,S | 6.22 ± 2.68 | 1.21 ± 3.62 | abundant | medium |
| Alaimidae | 4 | Bacterial feeder | K | 1.55 | large | L,S | 2.33 ± 1.94 | 4.48 ± 3.87 | medium | medium |
| Monhysteridae | 1 | Bacterial feeder + Substrate ingestion | r | 0.75 | medium | L,S | 1.44 ± 1.01 | 5.02 ± 4.86 | medium | medium |
| Aporcelaimidae | 5 | Omnivore | K | 4.3 | large | L,S | 0.11 ± 0.33 | 5.23 ± 3.95 | rare | abundant |
| Diphtherophoridae | 3 | Fungal feeder | K | 0.7 | medium | L,S | 4.44 ± 1.94 | 0.54 ± 1.13 | medium | rare |
| Mononchidae | 4 | Predator | K | 1.5 | large | L,S | 2.11 ± 1.76 | 2.83 ± 2.64 | medium | medium |
| Criconematidae | 3 | Plant feeder | K | 0.45 | small | L,S | 3.33 ± 2.00 | 0.62 ± 1.85 | medium | rare |
| Pratylenchidae | 3 | Plant feeder | K | 0.75 | medium | L,S | 3.44 ± 2.51 | 0.48 ± 0.73 | medium | rare |
| Tripylidae | 3 | Predator | K | 1.4 | large | L,S | 3.11 ± 2.57 | 0.23 ± 0.46 | medium | rare |
| Trichodoridae | 4 | Plant feeder | K | 0.8 | medium | S | 0.00 ± 0.00 | 3.04 ± 3.44 | not occurring | medium |
| Prismatolaimidae | 3 | Bacterial feeder | K | 1.05 | large | L,S | 0.11 ± 0.33 | 1.57 ± 1.83 | rare | medium |
| Thornenematidae | 5 | Omnivore | K | 1.95 | large | L,S | 1.00 ± 1.32 | 0.11 ± 0.33 | medium | rare |
| Microlaimidae | 3 | Bacterial feeder | r | 0.55 | medium | L | 0.89 ± 1.05 | 0.00 ± 0.00 | rare | not occurring |
| Osstellidae | 2 | Bacterial feeder | r | 0.45 | small | S | 0.00 ± 0.00 | 0.87 ± 2.60 | not occurring | rare |
| Aulolaimidae | 3 | Bacterial feeder | K | 0.95 | medium | S | 0.00 ± 0.00 | 0.72 ± 0.88 | not occurring | rare |
| Anguinidae | 2 | Fungal feeder | r | 1.65 | large | S | 0.00 ± 0.00 | 0.67 ± 1.07 | not occurring | rare |
| Leptonchidae | 4 | Fungal feeder + | K | 1 | medium | S | 0.00 ± 0.00 | 0.46 ± 0.92 | not occurring | rare |
| Panagrolaimidae | 1 | Bacterial feeder | r | 1.1 | large | L | 0.44 ± 0.53 | 0.00 ± 0.00 | rare | not occurring |
| Discolaimidae | 5 | Predator | K | 1.4 | large | L,S | 0.22 ± 0.44 | 0.19 ± 0.57 | rare | rare |
| Diplopeltidae | 3 | Bacterial feeder | K | 0.95 | medium | S | 0.00 ± 0.00 | 0.17 ± 0.52 | not occurring | rare |
| Bastianidae | 3 | Bacterial feeder | K | 1.5 | large | S | 0.00 ± 0.00 | 0.17 ± 0.52 | not occurring | rare |
| Anatonchidae | 4 | Predator | K | 2.5 | large | L | 0.11 ± 0.33 | 0.00 ± 0.00 | rare | not occurring |
3. Results
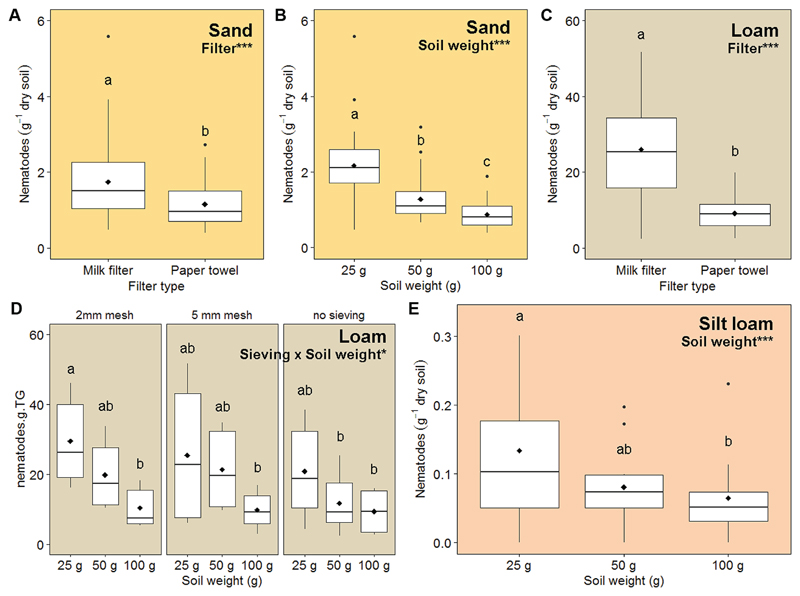
Generally, using paper towels and increasing soil height significantly decreased nematode extraction efficiency for each soil type (Fig. 2, Table 3, for the detailed set of treatments see Fig. S1; the supplement is available in the online version: DOI 10.25674/so91201). In sandy and silt loamy soil, sieving was not of significant importance (Tab. 3, Fig. S1a,c; the supplement is available in the online version: DOI 10.25674/so91201). In loamy soil, however, a significant interaction of sieving and soil height occurred: nematode densities were significantly higher in samples having a small soil height (1 cm) when sieved at 2 mm, compared to greater soil heights (4 cm) (Fig. 2D). Although the different sieving treatments were not significantly different from each other in respect to the low soil height (1 cm), extraction efficiency tended to decrease from 2 mm to 5 mm by -14 %, and from 2 mm to no sieving by -29 %. In the silty loam soil, using 1 cm soil height resulted in +40 % and +51 % more nematodes g-1 dry soil compared to 2 cm and 4 cm soil height, respectively (Fig. 2E).
Figure 2.
Significant treatment effects on nematode extraction efficiency using the Baermann-funnel method for three different soil types (sand, loam, and silt loam; highlighted with different background colors) after application of different pre-treatments (filter type, sieving mesh size, and soil height) in three different soil types. ANOVA (SS type 3) is displayed for log-transformed abundances but figures show untransformed values. Different letters indicate significant difference (Tukey’s HSD; α=0.05). Diamonds on boxplots give the mean.
Table 3.
Treatment effects on nematode extraction. ANOVA (SS type 3) table of F and P values of the effect of soil height (1 cm, 2 cm, and 4 cm of fresh soil), type of filter (milk filter and paper towel), and sieving (2 mm, 5mm, no sieving), and all possible interactions on nematode extraction efficiency (total nematode densities expressed as individuals g-1 dry soil) in three different soil types (sandy, loamy and silt loamy soil) using the Baermann-funnel method. Note: Only milk filters were used in the loamy silt soil. Df: degrees of freedom. Significant results are marked in bold.
| a) Sand | ||||
|---|---|---|---|---|
| Factor | Df | F | P | |
| Filter (F) | 1 | 24.21 | <.0001 | *** |
| Sieving (S) | 2 | 0.31 | 0.7381 | |
| Soil height (H) | 2 | 42.36 | <.0001 | *** |
| F × S | 2 | 2.69 | 0.0749 | . |
| F × H | 2 | 0.17 | 0.8458 | |
| S × H | 4 | 1.00 | 0.4135 | |
| F × S × H | 4 | 1.37 | 0.2539 | |
| Residuals | 71 | |||
| b) Loam | ||||
| Factor | Df | F | P | |
| Filter (F) | 1 | 197.77 | <.0001 | *** |
| Sieving (S) | 2 | 13.79 | <.0001 | *** |
| Soil height (H) | 2 | 49.33 | <.0001 | *** |
| F × S | 2 | 1.93 | 0.1523 | |
| F × H | 2 | 1.75 | 0.1803 | |
| S × H | 4 | 3.07 | 0.0214 | * |
| F ×S × H | 4 | 2.08 | 0.0922 | . |
| Residuals | 72 | |||
| c) Silt loam | ||||
| Factor | Df | F | P | |
| Sieving (S) | 2 | 1.39 | 0.2642 | |
| Soil weight (H) | 2 | 5.56 | 0.0083 | ** |
| S × H | 4 | 0.53 | 0.7173 | |
| Residuals | 33 | |||
***, P < 0.001; **, P < 0.01; *, P < 0.05; ., P < 0.1.
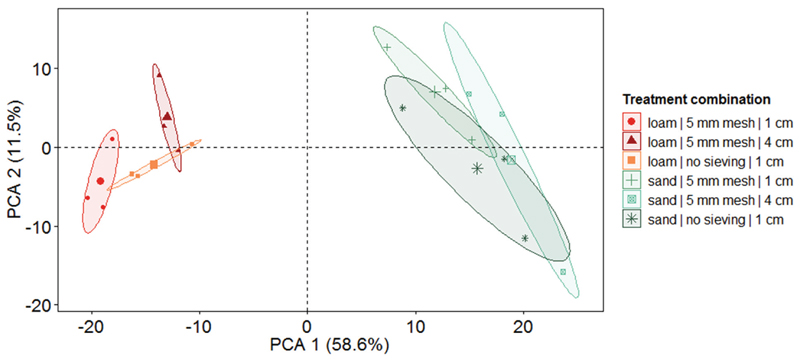
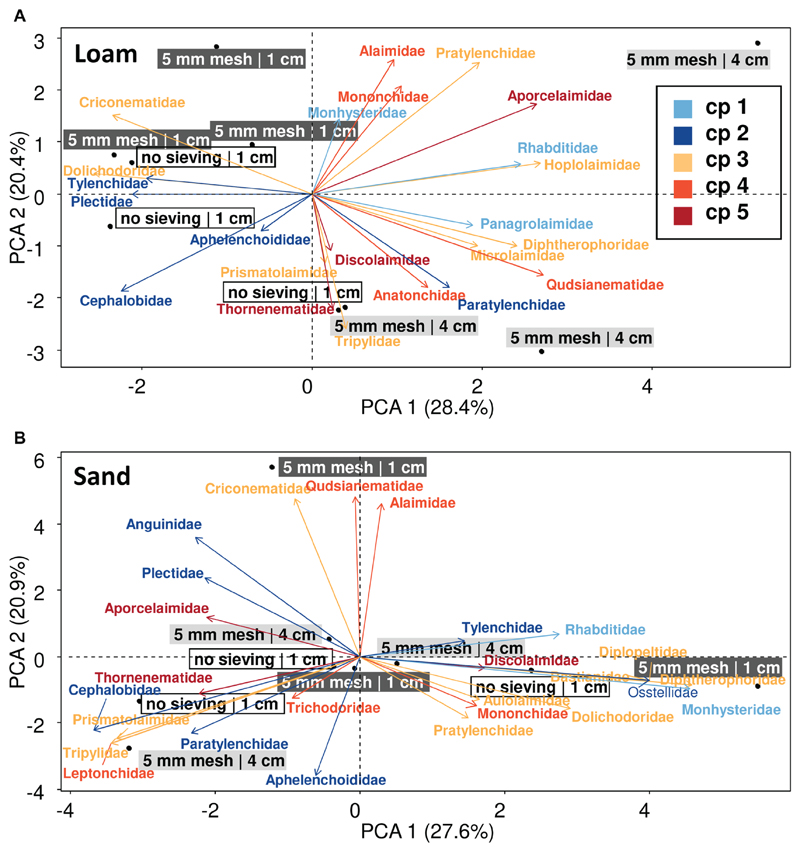
To assess the consequences of sieving and soil height on nematode community composition, we used a selection of three specific treatment combinations representing a low, medium, and high nematode extraction efficiency. Generally, nematode family composition differed strongly between soil types (explained 58.6 % of the variation, first axis; Fig. 3). In the loamy soil, extraction treatments had a stronger effect on the family composition as indicated by less overlapping ellipses, whereas in sandy soil family composition was more homogenous and sieving and soil height were of lower importance as indicated by more overlapping ellipses. In loamy soil, family composition in samples extracted from 4 cm fresh soil was more different from samples using 1 cm of fresh soil as indicated by the separation along the first axis explaining 28.4 % of the variation (Fig. 4A). In samples from loamy soil using 4 cm height, cp 4 and 5 nematodes (K-strategists) were found in higher frequencies (Fig. 4A). Those nematodes belong to medium and large size nematodes, and many of those nematodes were rare (Figs S2a, S3a; the supplements are available in the online version: DOI 10.25674/so91201). The second axis indicated a different nematode composition of the 5 mm sieving and no sieving samples of the 1 cm height samples. No sieving did not increase the amount of K-strategists (cp classes 3 to 5), i.e., larger organisms that are supposed to be more likely to be damaged by sieving (Fig. 4). However, selection of specific families due to treatments was stronger for 1 cm soil height samples than the pure association to specific cp classes, size classes, or occurrence (Figs S2a, S3a; the supplements are available in the online version: DOI 10.25674/so91201). In the sandy soil, nematode families from different c-p classes distributed more homogenously among the treatments, as no clear separation of treatments was found along the first and second axis indicating a less strong effect of sieving and soil weight in sandy soils (Figs 4B, S2b, S3b; the supplements are available in the online version: DOI 10.25674/so91201).
Figure 3.
Effect of soil type and soil pre-treatments on nematode community composition after Baermann-funnel extraction. Principal component analysis (PCA) of the nematode community (family level) as affected by soil type (sand and loam) and different treatments (sieving with 5 mm mesh size and no sieving, and different amounts of soil [1 cm and 4 cm of fresh soil]) prior to extraction reflecting treatment combinations with high (sieving with 5 mm and 1 cm soil), medium (no sieving and 1 cm soil), and lowest (5 mm sieving and 4 cm soil) nematode extraction efficiency in loamy soil. Symbols represent the specific treatment combinations with larger symbols display centroids. Numbers in brackets are variation explained by the first (PCA 1) and second (PCA 2) PCA axis, respectively.
Figure 4.
Distribution of nematode c-p classes after extracting nematode with different pre-treatments with the Baermann-funnel method. Principal component analysis (PCA) of the nematode community (family level) as affected by different treatments (sieving with 5 mm mesh size and no sieving, and different amounts of soil [1 cm and 4 cm fresh soil]) prior to extraction reflecting treatment combinations with high (sieving with 5 mm and 1 cm soil), medium (no sieving and 1 cm soil), and lowest (5 mm sieving and 4 cm soil) nematode extraction efficiency in a a) loamy and b) sandy soil. Nematode families were assigned to the five c-p classes according to Bongers (1990) and Bongers & Bongers (1998) with blueish colors indicate r-strategists and reddish colors K-strategists. Numbers in brackets are variation explained by the first (PCA 1) and second (PCA 2) PCA axis, respectively.
4. Discussion
In the present study, we found that the combination of different extraction treatments significantly affected nematode extraction efficiency. Although treatment combinations were of different importance in loamy, sandy, and silt loamy soil, overall highest numbers of extracted nematodes were observed when using milk filters and the lowest soil height, i.e., 1 cm height of fresh soil and sieving. The three different sieving treatments were not significantly different for samples of 1 cm soil height; however, extraction efficiency was 14 % higher when sieving with 2 mm as compared to 5 mm, and 29 % higher compared to no sieving. Therefore, to achieve high nematode extraction efficiency in different types of soil, it is recommended to use low amounts of soil in terms of thickness of the soil sample in combination with sieving and using milk filters.
Sieving was of higher importance in the loamy soil, whereas in the sandy and silt loamy soil it was not significant at all. Loamy soil has more stable soil aggregates than sandy and silt soil, which is why we suggest that breaking up soil aggregates by sieving increases nematode mobility in loamy soil, as nematodes may be less limited by soil structure and pore space (Wallace 1968). We were not able to disentangle the specific effects of sieving treatments on community composition. However, 5 mm and no sieving of 1 cm soil did not indicate strong functional differences of the nematode community. In contrast, soil height may be of greater importance as indicated by the first axis which separated samples with a soil height (1 cm) from samples with a high soil height (4 cm). The higher proportion of large nematodes when extracting 4 cm high loamy soil samples suggests that small nematodes may not have been able to pass and/or exit thick soil volumes during the common extraction time of 72 h. MacMillan et al. (2009 observed an) entomopathogenic nematode to overcome maximally 80 mm in 14 days, indicating that nematodes may be rather slow, which is why the standard (PM7/119(1), 2013) even suggests to use a soil volume of only a few millimeters in height. In addition, a thick soil layer can reduce oxygen supply (Van Voorhies & Ward, 2000), which may decrease nematode survival in the soil sample during wet extraction. PCA revealed that using 4 cm of soil increased the number of identified nematodes of rare families. This may be the result of a higher probability that 4 cm of soil contains more rare species. Moreover, rare species often are large in body size, which is why they may have a higher chance to be extracted from larger amounts of soil, as mentioned above. However, the soil volume of 4 cm soil height samples also selected against the majority of other nematode families. In summary, using the combination of treatments that resulted in highest nematode extraction efficiency may select against some rare species but may better reflect total densities. To overcome this tradeoff, reducing the thickness of the soil layer by increasing the diameter of the funnel/PVC tube-system may help to increase the amount of soil used and the surface area, allowing nematodes to exit the soil for improved qualitative and quantitative nematode community assessments.
Using milk filters resulted in a significantly higher number of extracted nematodes than using paper towels. Paper towels are supposed to adsorb water, whereas milk filters are supposed to filter a solution. The fabric of paper towels is probably chosen such that the fibers will take up water, and this paper structure may hamper nematodes to pass the paper towel. Instead of using paper towels as an alternative for milk filters for biodiversity assessments, they may be used to artificially reduce nematode densities according to morphological traits and alter community composition for targeted nematode inoculation experiments.
Although we analyzed only a small fraction of possible treatment combinations on the family level, we were able to show that pretreating the soil can change the community composition of extracted nematodes. These results highlight the need to standardize nematode extraction protocols and to account for potential differences when comparing data from multiple sites and studies in syntheses and meta-analyses. The present study may guide the implementation of common nematode extraction protocols for future research. We note that further treatments can be taken into account, such as extraction time and temperature. Including a temperature gradient may fasten the extraction procedure, but comes at the cost of adding more technical equipment, which may not be possible for some locations.
Nematodes are a powerful indicator taxon, and global assessments of soil nematode communities could increase our understanding of global distribution patterns. Generally, only few datasets of global belowground biodiversity exist (Delgado-Baquerizo et al. 2018, Orgiazzi et al. 2016, Ramirez et al. 2018, Tedersoo et al. 2014), but these still have insufficient data coverage. The very recent study by van den Hoogen et al. (2019) shows the opportunities such global nematode datasets can provide. This study, however, was only able to use information on trophic groups. A more detailed look into the functional composition of the nematode community can deepen our understanding of global soil biodiversity and processes, and how these may change under future conditions (Eisenhauer & Guerra 2019). This requires identification at least to the genus level and the involvement of expert knowledge as well as a standardized extraction procedure. In this study, we present a rather cheap and simple method, i.e. the Baermann-funnel method, to extract nematodes from different soils. The simplicity of the method and the suggested standardized approach allows also non-experts to extract nematodes to participate in global soil biodiversity assessments.
Supplementary Material
5. Acknowledgements
We thank UFZ to access the Kreinitz tree diversity platform and two anonymous reviewers for previous comments, which improved the manuscript. In addition, we thank Anja Zeuner for extracting and counting nematodes from the silt loamy soil. Rémy Beugnon is funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) - 319936945/GRK2324. The Jena Experiment is funded by the German Research Foundation (DFG, FOR 1451). This project received additional support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 677232 to NE) and the German Centre for Integrative Biodiversity Research Halle–Jena–Leipzig, funded by the German Research Foundation (FZT 118).
References
- Ainsworth R, World Health Organization . Safe Piped Water: Managing Microbial Water Quality in Piped Distribution Systems. IWA Publishing; London: 2004. p. 168. [Google Scholar]
- Andrássy I. Free-living nematodes of Hungary, I. 3rd ed. István Matskási; Budapest: 2005. [Google Scholar]
- Baermann G. Eine einfache Methode zur Auffindung von Anklostomum (Nematoden) Larven in Erdproben. Tijdschr Diergeneeskd. 1917;57:131–137. [Google Scholar]
- Bongers T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- Bongers T. De nematoden van Nederland. 2nd ed. KNNV; Utrecht: 1994. [Google Scholar]
- Bongers T, Bongers M. Functional diversity of nematodes. Applied Soil Ecology. 1998;10:239–251. [Google Scholar]
- Bongers T, Ferris H. Nematode community structure as a bioindicator in environmental monitoring. Trends in Ecology & Evolution. 1999;14:224–228. doi: 10.1016/s0169-5347(98)01583-3. [DOI] [PubMed] [Google Scholar]
- Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD. Finding generality in ecology: A model for globally distributed experiments. Methods in Ecology and Evolution. 2014;5:65–73. [Google Scholar]
- Bruelheide H, Nadrowski K, Assmann T, Bauhus J, Both S, Buscot F, Chen X-Y, Ding B, Durka W, Erfmeier A, Gutknecht JLM, et al. Designing forest biodiversity experiments: general considerations illustrated by a new large experiment in subtropical China. Methods in Ecology and Evolution. 2014;5:74–89. [Google Scholar]
- Cesarz S, Ciobanu M, Wright AJ, Ebeling A, Vogel A, Weisser WW, Eisenhauer N. Plant species richness sustains higher trophic levels of soil nematode communities after consecutive environmental perturbations. Oecologia. 2017;184:715–728. doi: 10.1007/s00442-017-3893-5. [DOI] [PubMed] [Google Scholar]
- Cesarz S, Reich PB, Scheu S, Ruess L, Schaefer M, Eisenhauer N. Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors. Pedobiologia. 2015;58:23–32. [Google Scholar]
- Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N. A global atlas of the dominant bacteria found in soil. Science. 2018;359:320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- Eisenhauer N, Guerra CA. Global maps of soil-dwelling nematode worms. Nature. 2019 doi: 10.1038/d41586-019-02197-0. [DOI] [PubMed] [Google Scholar]
- Ferris H, Bongers T, de Goede R. A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Gingold R, Moens T, Rocha-Olivares A. Assessing the Response of Nematode Communities to Climate Change-Driven Warming: A Microcosm Experiment. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0066653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsch L, Bien S, Radatz S, Braun U, Auge H, Bruelheide H. Tree diversity and the role of non-host neighbour tree species in reducing fungal pathogen infestation. The Journal of Ecology. 2014;102:1673–1687. doi: 10.1111/1365-2745.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp JA, Dingemans BJJ, Lehtinen T, Sarneel JM, Hefting MM. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods in Ecology and Evolution. 2013;4:1070–1075. [Google Scholar]
- MacMillan K, Haukeland S, Rae R, Young I, Crawford J, Hapca S, Wilson M. Dispersal patterns and behaviour of the nematode Phasmarhabditis hermaphrodita in mineral soils and organic media. Soil Biology and Biochemistry. 2009;41:1483–1490. [Google Scholar]
- Meyl A. Fadenwürmer (Nematoden) Franckh’sche Verlagshandlung; Stuttgart: 1961. [Google Scholar]
- Nielsen UN, Ayres E, Wall DH, Li G, Bardgett RD, Wu T, Garey JR. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Global Ecology and Biogeography. 2014;23:968–978. [Google Scholar]
- Orgiazzi A, Bardgett RD, Barrios E, Behan-Pelletier V, Briones MJI, Chotte J-L, De Deyn GB, Eggleton P, Fierer N, Fraser T, Hedlund K, et al. Global soil biodiversity atlas. Publications Office of the European Union; Luxembourg: 2016. [Google Scholar]
- Pei ZQ, Leppert KN, Eichenberg D, Bruelheide H, Niklaus PA, Buscot F, Gutknecht JLM. Leaf litter diversity alters microbial community structure and nutrient cycling in a subtropical forest ecosystem. Biogeochemistry. 2017;134:163–181. [Google Scholar]
- Phillips HRP, Cameron EK, Ferlian O, Türke M, Winter M, Eisenhauer N. Red list of a black box. Nature Ecology & Evolution. 2017;1 doi: 10.1038/s41559-017-0103. 0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PM7/119(1) Nematode extraction. Vol. 43. EPPO Bulletin; 2013. pp. 471–495. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2008. R: A language and environment for statistical computing. [Google Scholar]
- Ramirez KS, Knight CG, de Hollander M, Brearley FQ, Constantinides B, Cotton A, Creer S, Crowther TW, Davison J, Delgado-Baquerizo M, Dorrepaal E, et al. Detecting macroecological patterns in bacterial communities across independent studies of global soils. Nature Microbiology. 2018;3:189–196. doi: 10.1038/s41564-017-0062-x. [DOI] [PubMed] [Google Scholar]
- Robinson AF, Heald CM. Accelerated movement of nematodes from soil in baermann funnels with temperature gradients. Journal of Nematology. 1989;21:370–378. [PMC free article] [PubMed] [Google Scholar]
- Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze E, Ökologie I, Jena F, Geographie I. The role of biodiversity for element cycling and trophic interactions : an experimental approach in a grassland community. Basic and Applied Ecology. 2004;121:107–121. [Google Scholar]
- Ruess L. Studies on the nematode fauna of an acid forest soil: spatial disturbance and extraction. Nematologica. 1995;41:229–239. [Google Scholar]
- Song D, Pan K, Tariq A, Sun F, Li Z, Sun X. Large-scale patterns of distribution and diversity of terrestrial nematodes. Applied Soil Ecology. 2017 [Google Scholar]
- Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, et al. Global diversity and geography of soil fungi. Science. 2014;346 doi: 10.1126/science.1256688. 1256688. [DOI] [PubMed] [Google Scholar]
- van Bezooijen J. Methods and techniques for nematology. Wageningen; 2006. [Google Scholar]
- van den Hoogen J, Geisen S, Routh D, Ferris H, Traunspurger W, Wardle DA, de Goede RGM, Adams BJ, Ahmad W, Andriuzzi WS, Bardgett RD, et al. Soil nematode abundance and functional group composition at a global scale. Nature. 2019 doi: 10.1038/s41586-019-1418-6. [DOI] [PubMed] [Google Scholar]
- Van Voorhies WAS, Ward S. Broad oxygen tolerance in the nematode Caenorhabditis elegans. The Journal of Experimental Biologyxperimental Biology. 2000;203:2467–2478. doi: 10.1242/jeb.203.16.2467. [DOI] [PubMed] [Google Scholar]
- Verheyen K, Vanhellemont M, Auge H, Baeten L, Baraloto C, Barsoum N, Bilodeau-Gauthier S, Bruelheide H, Castagneyrol B, Godbold D, Haase J, et al. Contributions of a global network of tree diversity experiments to sustainable forest plantations. Ambio. 2015 doi: 10.1007/s13280-015-0685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor B, De Goede RGM. The nematode extraction efficiency of the Oostenbrink elutriator-cottonwool filter method with special reference to nematode body size and life strategy. Nematology. 2000;2:325–342. [Google Scholar]
- Viglierchio DR, Schmitt RV. On the methodology of nematode extraction from field samples: comparison of methods for soil extraction. Journal of Nematology. 1983;15:450–454. [PMC free article] [PubMed] [Google Scholar]
- Viketoft M, Sohlenius B. Soil nematode populations in a grassland plant diversity experiment run for seven years. Applied Soil Ecology. 2011;48:174–184. [Google Scholar]
- Wallace HR. The dynamics of nematode movement. Annual Review of Phytopathology. 1968;6:91–114. Yeates, G. W. (1999): Effects of plants on nematode community structure. – Annual Review of Phytopathology 37: 127–149. [Google Scholar]
- Yeates GW, Bongers T, de Goede RGM, Freckman DW, Georgieva SS. Feeding habits in soil nematode families and genera - an outline for soil ecologist. Journal of Nematology. 1993;25:315–331. [PMC free article] [PubMed] [Google Scholar]
- Yeates GW. Effects of plants on nematode community structure. Annual Review of Phytopathology. 1999;37:127–149. doi: 10.1146/annurev.phyto.37.1.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.