Abstract
BACKGROUND:
Severely injured patients often progress from early hypocoagulable to normal and eventually hypercoagulable states, developing increased risk for venous thromboembolism (VTE). Prophylactic anticoagulation can decrease this risk, but its initiation is frequently delayed for extended periods due to concerns for bleeding. To facilitate timely introduction of VTE chemoprophylaxis, we characterized the transition from hypo- to hypercoagulability and hypothesized that trauma-induced coagulopathy resolves within 24 hours after injury.
METHODS:
Serial blood samples were collected prospectively from critically injured patients for 120 hours after arrival at an urban Level I trauma center. Extrinsic thromboelastometry maximum clot firmness was used to classify patients as hypocoagulable (HYPO, <49 mm), normocoagulable (NORM, 49–71 mm), or hypercoagulable (HYPER, >71 mm) at each time point. Changes in coagulability over hospital course, VTE occurrence, and timing of prophylaxis initiation were analyzed.
RESULTS:
898 patients (median Injury Severity Score, 13; mortality, 12%; VTE, 8%) were enrolled. Upon arrival, 3% were HYPO (90% NORM, 7% HYPER), which increased to 9% at 6 hours before down-trending. Ninety-seven percent were NORM by 24 hours, and 53% were HYPER at 120 hours. Median maximum clot firmness began in the NORM range, up-trended gradually, and entered the HYPER range at 120 hours. Patients with traumatic brain injury (TBI) followed a similar course and were not more HYPO at any time point than those without TBI. Failure to initiate prophylaxis by 72 hours was predicted by TBI and associated with VTE development (27% vs 16%, p < 0.05).
CONCLUSIONS:
Regardless of injury pattern, trauma-induced coagulopathy largely resolves within 24 hours, after which hypercoagulability becomes increasingly more prevalent. Deferring initiation of chemoprophylaxis, which is often biased toward patients with intracranial injuries, is associated with VTE development.
Keywords: Trauma-induced coagulopathy, hypercoagulability, venous thromboembolism, chemoprophylaxis, thromboelastometry
Dysregulated coagulation, either hypo- or hypercoagulability, occurs in more than half of severely injured patients and is associated with increased morbidity and mortality.1 Although changes in coagulation are multifactorial and vary among patients, the early postinjury period is often characterized by a hypocoagulable state known as trauma-induced coagulopathy (TIC).2,3 Trauma-induced coagulopathy results directly from tissue injury and shock; can be exacerbated iatrogenically by hypothermia, acidosis, and hemodilution during resuscitation; and facilitates uncontrolled hemorrhage.2–4 Patients who survive can become hypercoagulable with increased risk for venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE).5–8 These complications lead to prolonged hospitalizations, excess costs, and greater mortality.9,10
The transition from hypo- to hypercoagulability after physiologic stress was first recognized in 1914 by Walter Cannon,11 but despite much work over the last century to characterize and manage these contradictory states,1–8,10,12–20 the timing of this change remains poorly understood. One reason is that conventional coagulation tests like international normalized ratio and partial thromboplastin time cannot detect hypercoagulability. Because it is unknown when patients progress to normal and then hypercoagulable states, surgeons face a dilemma when determining the time to initiate prophylactic anticoagulation, which can decrease the risk of VTE.18,19 Concerns for thrombosis must be weighed against those for bleeding; consequently, introduction of VTE chemoprophylaxis is often delayed.
Increased use of viscoelastic hemostatic assays like thromboelastography (TEG) and thromboelastometry (TEM) in trauma has furthered our understanding of changes in coagulation after injury. Unlike conventional, plasma-based tests, viscoelastic assays quantify whole-blood clot formation and degradation and can identify hypercoagulability.21 Given these advantages, we used serial TEMs in this prospective study to characterize the transition from hypo- to hypercoagulability and determine the time to resolution of TIC, which might facilitate initiation of VTE chemoprophylaxis. We hypothesized that coagulopathic patients progress to a normal or hypercoagulable state within 24 hours after injury.
METHODS
This study was a secondary analysis of a larger prospective cohort study. From October 2010 to May 2016, serial blood samples were collected prospectively from patients meeting criteria for highest-level trauma activation at Zuckerberg San Francisco General Hospital, the only Level I trauma center in San Francisco, California. Samples were obtained upon arrival and at 6, 12, 24, 48, 72, 96, and 120 hours thereafter until the patient was transferred from the intensive care unit (ICU) or died. Patients were excluded if they were younger than 18 years, pregnant, incarcerated, transferred from another hospital, or taking outpatient anticoagulant or antiplatelet medications. Informed consent was obtained for study enrollment under a protocol approved by the Committee on Human Research at the University of California San Francisco.
Our procedures for sample collection and laboratory analysis have previously been described.22 Extrinsic thromboelastometry (EXTEM) was performed on each sample to assess clot function following activation with tissue factor using a ROTEM delta hemostasis analyzer (Tem International GmbH; Munich, Germany). Parameters measured by EXTEM included clotting time (CT; the time from the start of the test to initial clot formation; normal range, 42–74 seconds), alpha angle (α; the slope of the tracing, which represents the rate of clot formation; normal range, 63–81 degrees), and maximum clot firmness (MCF; the greatest amplitude of the tracing, which illustrates clot strength; normal range, 49–71 mm).23 These results were obtained only for research and were not known by clinicians. Maximum clot firmness and its TEG equivalent, maximum amplitude (MA), reflect platelet-fibrin interactions, a component involved in both clot formation and degradation, and have been shown to correlate with hypercoagulability in trauma and other surgical populations.24–28 Therefore, in this study, MCF was used to evaluate overall coagulability and classify patients as hypocoagulable (HYPO, <49 mm), normocoagulable (NORM, 49–71 mm), or hypercoagulable (HYPER, >71 mm) at each time point. Maximum clot firmness greater than 71 mm has been associated with thromboembolic events in another series.25
Demographic, injury, resuscitation, and outcome data were also collected prospectively for all patients. Venous thromboembolisms consisted of symptomatic DVT or PE at any time during admission, which were confirmed by ultrasound or computed tomography angiogram, respectively. These diagnostic tests were ordered only if clinical suspicion warranted; routine surveillance was not conducted. The diagnosis of traumatic brain injury (TBI) required radiographic evidence of intracranial injury on computed tomography or magnetic resonance imaging and head Abbreviated Injury Scale (AIS) score greater than 3. Acute respiratory distress syndrome was determined using the Berlin Definition,29 which included blinded, two-physician adjudication of chest radiographs during the first 8 days of admission as previously described.30 Multiorgan failure was defined using the Denver Postinjury Multiple Organ Failure Score.31
Changes in coagulability over hospital course, VTE occurrence, and timing of chemoprophylaxis initiation were analyzed. Summary statistics are reported as median with interquartile range for continuous data and as percentage for binary data. Univariate differences between groups were evaluated with Wilcoxon rank-sum or Kruskal-Wallis tests for continuous data and the Fisher exact test for binary data. Multivariate logistic regression was performed to identify independent predictors of chemoprophylaxis initiation or VTE while controlling for age, sex, injury severity, and shock. A p < 0.05 was considered significant, except when adjusted for multiple comparisons using Bonferroni correction. Statistical analyses were performed with Stata/SE 15 (StataCorp, College Station, Texas).
RESULTS
Study Population
During the 6-year period, 898 patients were enrolled (Table 1). These patients were mostly male (83%) with a median age of 35 years, representing a typical trauma population. They had a median Injury Severity Score of 13 and median base deficit of 2.3. Fifty-seven percent sustained blunt trauma, and 31% experienced TBI. Seventy-one patients (8%) developed a symptomatic VTE at any point during admission. Overall mortality was 12%.
TABLE 1.
Characteristics of Study Population
| n = 898 | |
|---|---|
| Demographics | |
| Age, y | 35 (25, 50) |
| Male | 83% |
| BMI, kg/m2 | 25.9 (23.2, 29.4) |
| Injury | |
| Blunt mechanism | 57% |
| ISS | 13 (4, 26) |
| Head AIS score | 0 (0, 4) |
| TBI | 31% |
| Orthopedic injury | 33% |
| GCS score | 14(8, 15) |
| Coagulopathy and resuscitation | |
| INR | 1.1 (1.0, 1.2) |
| PTT, s | 27.6 (25.5, 30.4) |
| Platelet count, × 109/L | 272 (225, 322) |
| Fibrinogen, mg/dL | 212 (165, 276) |
| D-dimer, μg/mL | 1.3 (0.4, 6.3) |
| Base deficit | 2.3 (0.7, 6.4) |
| EXTEM CT, s | 58 (49, 70) |
| EXTEM α, degrees | 73 (69, 76) |
| EXTEM MCF, mm | 63 (60, 67) |
| EXTEM ML, % | 14(10, 18) |
| Crystalloid, first 24 h, mL | 3,750 (2,000, 5,798) |
| RBC, first 24 h, units | 0 (0, 2) |
| Plasma, first 24 h, units | 0 (0, 1) |
| Platelets, first 24 h, units | 0 (0, 0) |
| VTE prophylaxis | 54% |
| Outcomes | |
| Hospital LOS, d | 6 (2, 16) |
| ICU LOS, d | 2 (0, 6) |
| Ventilator-free days, first 28 d | 27 (22, 28) |
| ARDS | 15% |
| VTE | 8% |
| MOF | 10% |
| Mortality | 12% |
α, alpha angle; ARDS, acute respiratory distress syndrome; BMI, body mass index; GCS, Glasgow Coma Scale; INR, international normalized ratio; ISS, Injury Severity Score; LOS, length of stay; ML, maximum lysis; MOF, multiorgan failure; PTT, partial thromboplastin time; RBC, red blood cells.
Initial Coagulability
Upon arrival, 3% of patients were HYPO, 90% NORM, and 7% HYPER (Table 2). Accordingly, HYPO patients were coagulopathic by other conventional and viscoelastic parameters with elevated international normalized ratio (1.4 vs 1.1 vs 1.1), prolonged CT (101 vs 59 vs 52 seconds), and reduced α (54 vs 72 vs 79 degrees) and had lowest platelet count and fibrinogen compared with NORM and HYPER patients (all p < 0.02 for multiple comparisons). HYPO patients had lowest Glasgow Coma Scale score (9 vs 14 vs 15), greatest degree of shock (base deficit, 6.0 vs 1.6 vs 3.8), and highest mortality (47% vs 11% vs 2%; all p < 0.02 for multiple comparisons). The percentage of female patients was highest in the HYPER cohort. Initial hypercoagulability was not associated with VTE (4% vs HYPO 0% vs NORM 6%; p > 0.02 for multiple comparisons).
TABLE 2.
Comparison of Patients by Initial Coagulability
| HYPO (3%) | NORM (90%) | HYPER (7%) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 40 (23, 64) | 33 (24, 48) | 41 (32, 57) | 0.007 |
| Male | 89% | 86% | 60% | <0.001 |
| BMI, kg/m2 | 28.7 (25.2–31.3) | 25.9 (23.2–29.3) | 28.9 (24.1–32.8) | 0.012 |
| Injury | ||||
| Blunt mechanism | 53% | 52% | 52% | 1.000 |
| ISS | 13(1,35) | 10 (1, 26) | 8(1,11) | 0.051 |
| Head AIS score | 0 (0, 4) | 0 (0, 3) | 0 (0, 3) | 0.278 |
| TBI | 32% | 26% | 23% | 0.752 |
| Orthopedic injury | 16% | 29% | 25% | 0.432 |
| GCS score | 9 (3, 14) | 14 (9, 15) | 15 (10, 15) | 0.012 |
| Coagulopathy and resuscitation | ||||
| INR | 1.4 (1.1, 1.8) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.1) | <0.001 |
| PTT, s | 29.9 (26.3, 50.3) | 27.6 (25.5, 30.2) | 27.4 (25.2, 30.8) | 0.050 |
| Platelet count, × 109/L | 202(151,279) | 270 (225,315) | 334 (302, 434) | <0.001 |
| Fibrinogen, mg/dL | 152(112, 208) | 213 (165,276) | 307(251,405) | <0.001 |
| D-dimer, μg/mL | 6.2 (0.8, 7.4) | 0.8 (0.3, 5.1) | 0.8 (0.3, 2.1) | 0.038 |
| Base deficit | 6.0 (2.6, 12.8) | 1.6 (1.2, 5.3) | 3.8 (1.8, 9.2) | 0.001 |
| EXTEM CT, s | 101 (82, 160) | 59 (50, 69) | 52 (42, 63) | <0.001 |
| EXTEM α, degrees | 54 (50–61) | 72 (69, 75) | 79 (77, 81) | <0.001 |
| EXTEM MCF, mm | 45 (37, 47) | 63 (60–66) | 73 (72–75) | <0.001 |
| EXTEM ML, % | 16 (8, 100) | 14 (10, 18) | 12 (9, 17) | 0.031 |
| Crystalloid, first 24 h, mL | 3,725 (2,871, 6,466) | 3,046 (1,200, 5,200) | 2,900 (1,000, 5,975) | 0.214 |
| RBC, first 24 h, units | 1 (0, 15) | 0 (0, 1) | 0 (0, 0) | 0.015 |
| Plasma, first 24 h, units | 0 (0, 9) | 0 (0, 0) | 0 (0, 0) | 0.067 |
| Platelets, first 24 h, units | 0 (0, 2) | 0 (0, 0) | 0 (0, 0) | 0.062 |
| VTE prophylaxis | 11% | 49% | 38% | 0.045 |
| Outcomes | ||||
| Hospital LOS, d | 2(1,4) | 4 (2, 11) | 4 (1, 10) | 0.127 |
| ICU LOS, d | 1 (0, 3) | 1(0,4) | 1 (0, 2) | 0.505 |
| Ventilator-free days, first 28 d | 1 (0, 27) | 28 (25, 28) | 28 (27, 28) | <0.001 |
| ARDS | 56% | 12% | 6% | 0.003 |
| VTE | 0% | 6% | 4% | 0.810 |
| MOF | 11% | 7% | 4% | 0.514 |
| Mortality | 47% | 11% | 2% | <0.001 |
α, alpha angle; AIS, Abbreviated Injury Scale; ARDS, acute respiratory distress syndrome; BMI, body mass index; GCS, Glasgow Coma Scale; HYPER, hypercoagulable (MCF >71 mm); HYPO, hypocoagulable (MCF <49 mm); INR, international normalized ratio; ISS, Injury Severity Score; LOS, length of stay; MCF, maximum clot firmness; ML, maximum lysis; MOF, multiorgan failure; NORM, normocoagulable (MCF 49–71 mm); PTT, partial thromboplastin time; RBC, red blood cells.
Changes in Coagulability Over Hospital Course
We began by examining how the prevalence of hypocoagulability evolved over the first 5 days after injury (Fig. 1). Consistent with previous studies on TIC, a group of patients were HYPO early in their hospital course. This proportion peaked at 9% at 6 hours before declining significantly to 6% at 12 hours and to 2% at 24 hours, after which it remained less than 1%. Ninety-seven percent of patients were NORM by 24 hours. After this time, however, normocoagulability down-trended. We observed this change because 24 hours was an inflection point for hypercoagulability, which was 7% at arrival and escalated significantly to 24% at 72 hours, to 34% at 96 hours, and to 53% at 120 hours.
Figure 1.
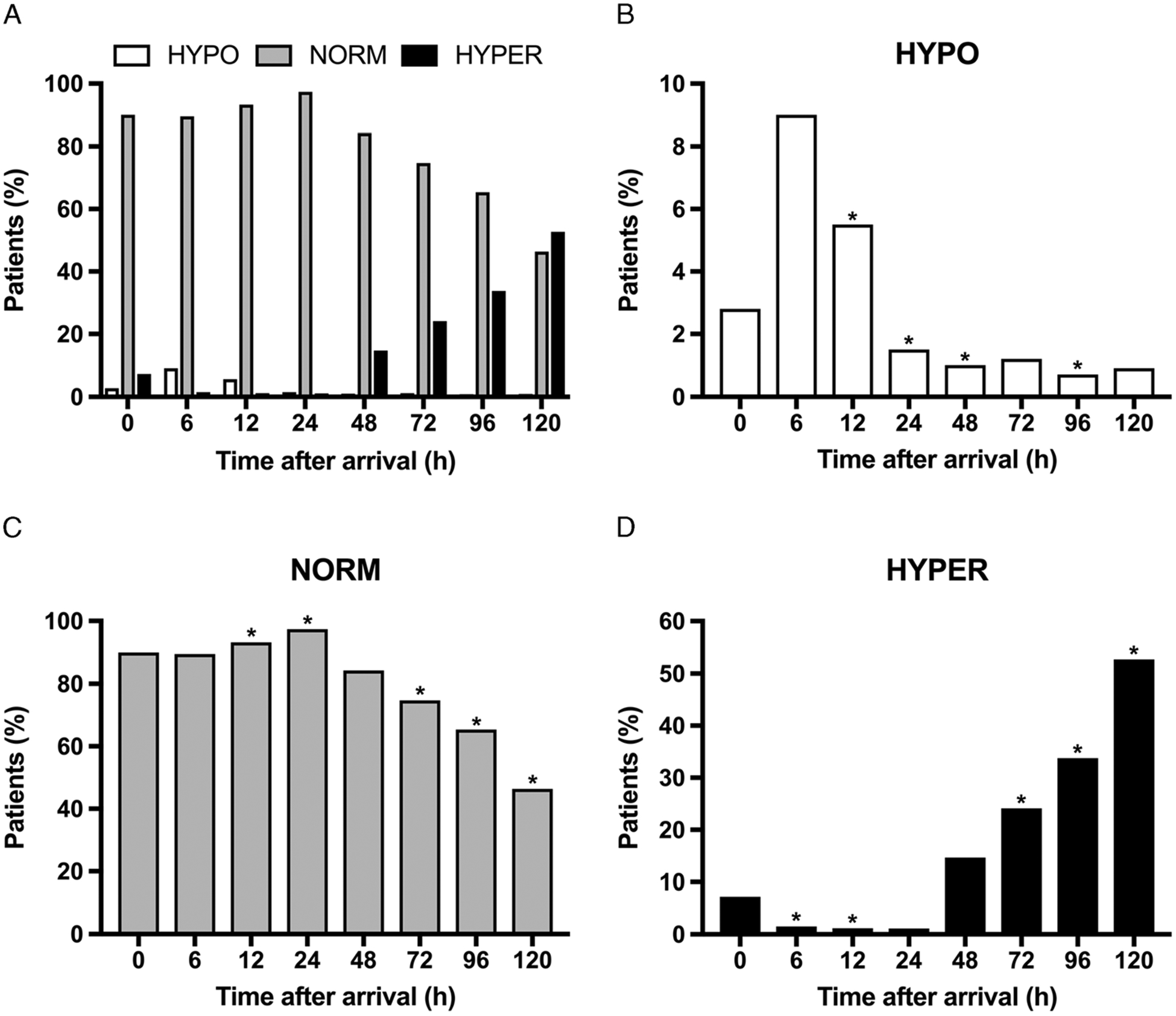
Changes in coagulability over hospital course. Proportions of HYPO, NORM, and HYPER patients at each time point are depicted collectively in (A) and individually in HYPO (B), NORM (C), and HYPER (D). *p < 0.05 when compared with previous time point; HYPER (MCF >71 mm); HYPO (MCF <49 mm); NORM (MCF, 49–71 mm).
As a corollary to overall coagulability, we analyzed the trends of each EXTEM parameter over the first 5 days for the entire population (Fig. 2). Median MCF began in the NORM range, increased gradually after 6 hours, and entered the HYPER range at 120 hours. While CT and α remained within normal limits throughout the study, α followed a similar course to MCF, approaching the threshold between normocoagulability and hypercoagulability after 48 hours.
Figure 2.
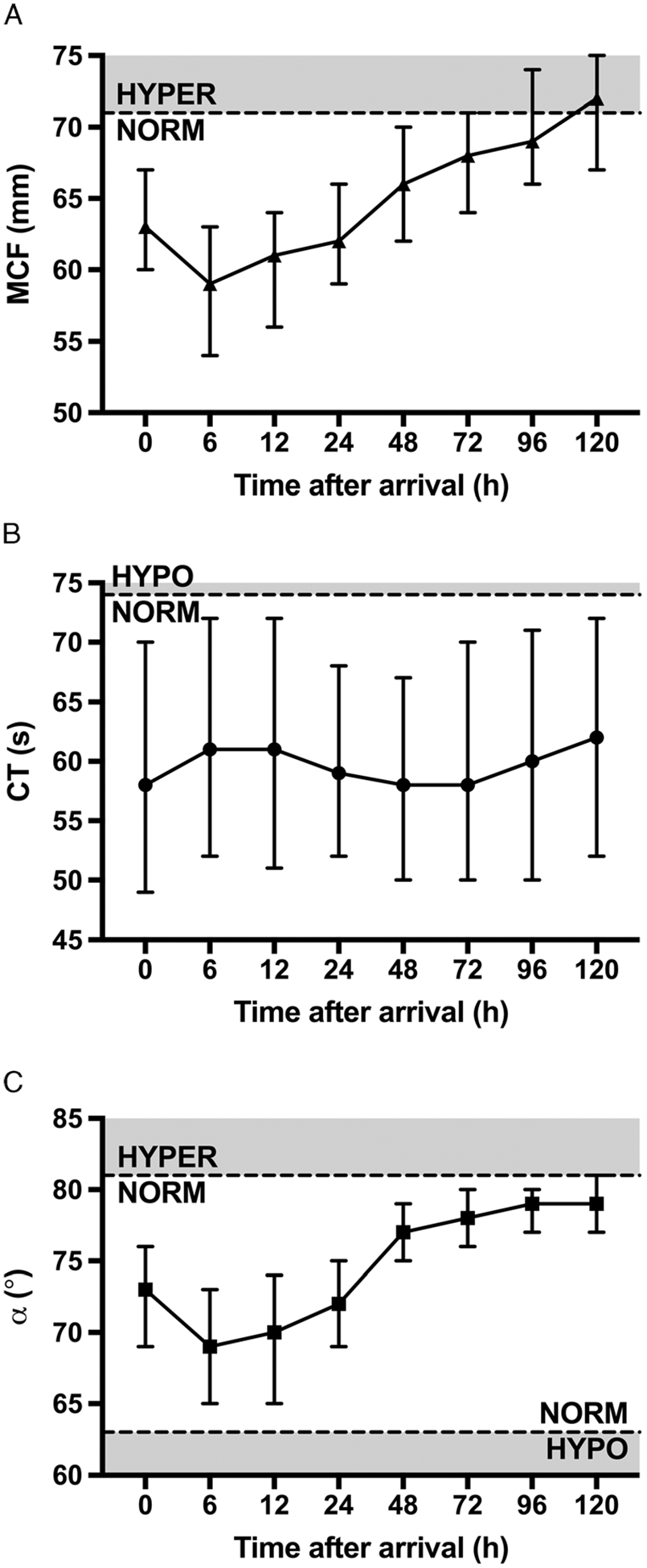
Trends in thromboelastometry parameters over first 5 days. (A) Maximum clot firmness (MCF; normal range, 49–71 mm). (B) Clotting time (CT; normal range, 42–74 seconds). (C) Alpha angle (α; normal range, 63–81 degrees).
To explore the relationship between injury pattern and coagulability, patients were categorized into three groups: no TBI, isolated TBI, and polytrauma (TBI and AIS score ≥ 3 in at least one body region other than head or face). At each time point, the individual proportions of HYPO and HYPER patients were not different among these three groups (all p > 0.02 for multiple comparisons).
Coagulability and Thromboembolic Events
Of the 71 patients with a symptomatic VTE, 36 (51%) developed a DVT, and 35 (49%) developed a PE. Venous thromboembolisms affected 25% of patients who were HYPER at 48 hours, 26% at 72 hours, 30% at 96 hours, and 28% at 120 hours; however, these rates were not significantly different from those of NORM patients at the same time points.
Timing of Chemoprophylaxis Initiation
Sixteen percent of inpatients at 24 hours had received their first dose of VTE chemoprophylaxis, 44% at 48 hours, 71% at 72 hours, 88% at 96 hours, and 99% at 120 hours. Because coagulopathy had largely resolved by 24 hours, we were interested in whether the decision to withhold anticoagulation was associated with the development of a symptomatic VTE (Fig. 3). Venous thromboembolism incidence was lower for inpatients who had received prophylaxis by 72 hours (16%) than for those who had not (27%, p < 0.05). This advantage persisted at 96 hours (16% vs 40%, p < 0.05) and 120 hours (19% vs 50%); however, at the latter time point, the number of patients who were still admitted and had not received prophylaxis was too small to detect statistical significance.
Figure 3.
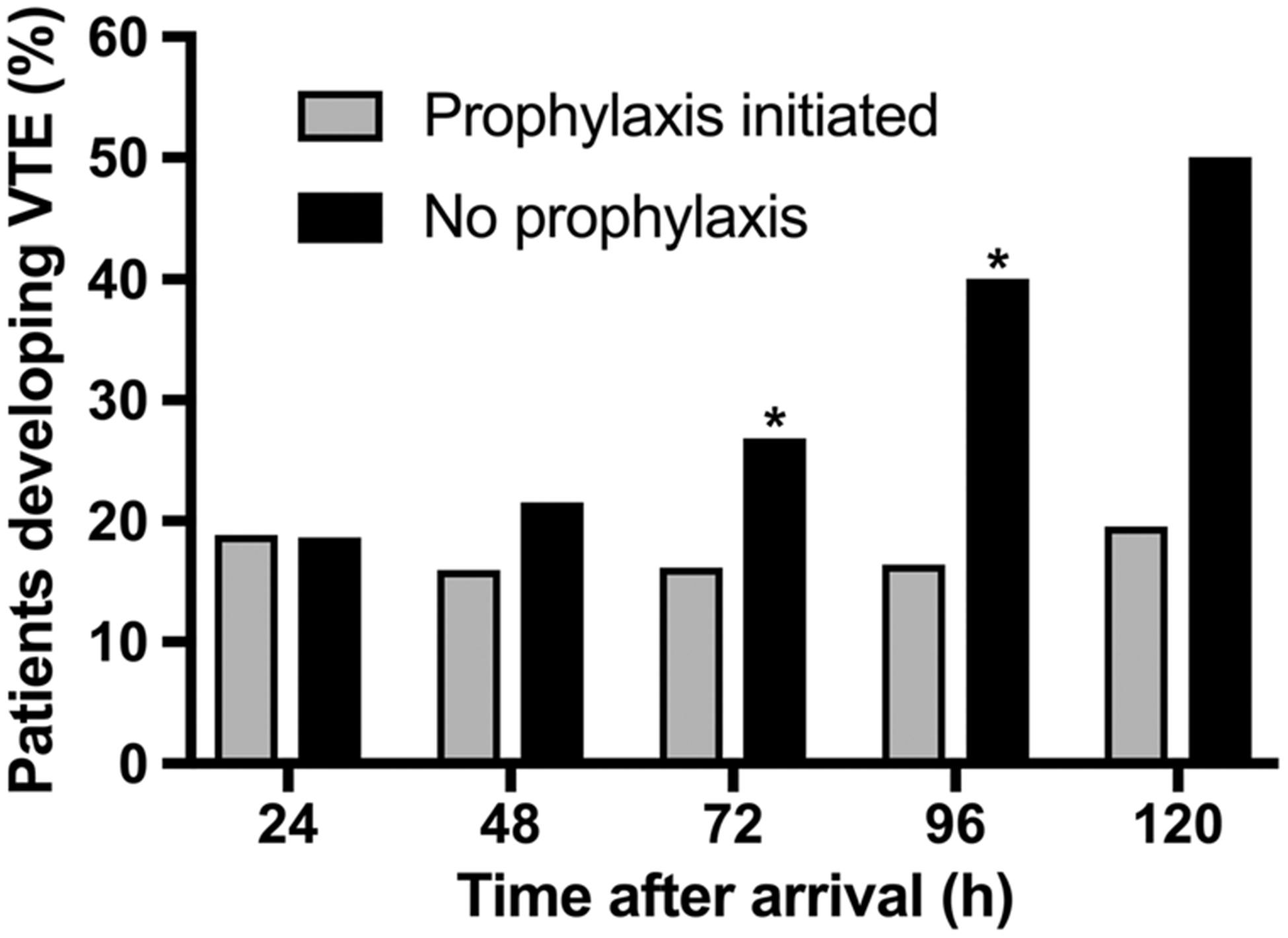
Venous thromboembolism development by timing of chemoprophylaxis initiation. At each time point, patients who had received their first dose of prophylaxis are compared with those who had not. *p < 0.05.
After establishing that failure to initiate prophylaxis by 72 hours is associated with VTE development, we compared patients who had and had not received their first dose of prophylaxis by that time (Table 3). Those who had not received prophylaxis experienced more blunt trauma (88% vs 60%) and were more severely injured (Injury Severity Score, 29 vs 21), especially with intracranial (head AIS score, 4 vs 0; TBI, 70% vs 27%; Glasgow Coma Scale score, 11 vs 14) and orthopedic (71% vs 48%) injuries (all p < 0.05). They had longer hospital (21 vs 15 days) and ICU (12 vs 6 days) stays and fewer ventilator-free days (22 vs 25, all p < 0.05). This cohort whose anticoagulation was withheld was not more coagulopathic at arrival (HYPO, 0% vs 1%) or 72 hours (2% vs 0%, all p > 0.05). After we controlled for age, sex, injury severity, and shock with logistic regression analysis, TBI and orthopedic injury remained independent predictors of delayed prophylaxis.
TABLE 3.
Comparison of Inpatients Who Did and Did Not Receive VTE Chemoprophylaxis by 72 Hours
| Prophylaxis Initiated (71%) | No Prophylaxis (29%) | p | |
|---|---|---|---|
| Blunt mechanism | 60% | 88% | <0.001 |
| ISS | 21(13, 29) | 29 (22, 35) | <0.001 |
| Head AIS score | 0 (0, 4) | 4 (3, 5) | <0.001 |
| TBI | 27% | 70% | <0.001 |
| Orthopedic injury | 48% | 71% | 0.001 |
| GCS score | 14(8, 15) | 11 (7, 14) | 0.001 |
| Vasopressors, day 3 | 27% | 49% | <0.001 |
| HYPO, arrival | 1% | 0% | 1.000 |
| HYPO, 72 h | 0% | 2% | 0.365 |
| Hospital LOS, d | 15(9, 26) | 21 (15, 33) | <0.001 |
| ICU LOS, d | 6 (3, 14) | 12 (7, 18) | <0.001 |
| Ventilator-free days, first 28 d | 25 (18, 27) | 22 (14, 25) | <0.001 |
AIS, Abbreviated Injury Scale; GCS, Glasgow Coma Scale; HYPO, hypocoagulable (MCF <49 mm); ISS, Injury Severity Score; LOS, length of stay.
DISCUSSION
The purpose of the current study was to characterize the changes in coagulation over the first 5 days after injury. While a subset of patients in this critically injured cohort experienced a brief period of hypocoagulability, 98% of patients were in a normal or hypercoagulable state by 24 hours, confirming our hypothesis. Hypercoagulability became increasingly more prevalent at subsequent time points, exceeding 50% among patients still in the ICU at 5 days, and these trends occurred independently of injury pattern. Although hypercoagulability on TEM did not correlate with thromboembolic events in this series, failure to initiate prophylactic anticoagulation by 72 hours, which was biased toward patients with intracranial injuries, was associated with VTE development.
Originally thought to result primarily from large-volume resuscitation, TIC is now understood as a complex endogenous response to tissue injury and hypoperfusion.2,3,12–14 This response disrupts the balance between clot formation and degradation that normally serves to stop hemorrhage while preventing microvascular thrombosis. As the balance is restored, patients can shift in a continuum between hypo- and hypercoagulable states over time. Despite much interest in deranged coagulation at isolated time points, only a few studies have accounted for the dynamic nature of coagulation status as patients progress through their hospital course, and they have focused on trends in hypercoagulability.6,20,21 Our longitudinal study is unique in this respect. We described the trend in hypocoagulability and determined that TIC rarely persists beyond 24 hours. The increasing prevalence of hypercoagulability after that time is consistent with MA6 but not reaction time (TEG equivalent of CT)20 data obtained at other institutions.
The lack of an independent association between hypercoagulability on TEM and eventual VTE diagnosis conforms with the mixed findings on this topic in the trauma literature. Kashuk et al.5 and Cotton et al.26 have observed that elevated G and MA values, respectively, on TEG correlate with thromboembolic events. However, Schreiber et al.20 and Van Haren et al.32 have reported that TEG values do not predict VTE occurrence. Several factors might explain our results. Growing recognition of PEs found incidentally or in the absence of DVT suggests that the time VTEs are identified might not correspond with the time they develop,33–35 and the use of protocolized VTE screening is varied among studies examining the clinical significance of hypercoagulability on viscoelastic assays. Since we did not screen, our true VTE incidence could have been higher. While we can conclude that blood becomes more prone to clotting as time from injury increases, we also appreciate that thrombosis is multifactorial and is influenced by the other components of Virchow’s triad, stasis and endothelial damage, in addition to biochemical clot strength.
Despite this issue, the increased risk of VTE after trauma remains well-established, along with the effectiveness of prophylactic anticoagulation in decreasing this risk.7,8,18,19 The decision to initiate or defer chemoprophylaxis must be individualized based on multiple considerations including the status of anatomic and coagulopathic bleeding and planned procedures. After certain injures like intracranial hemorrhages and blunt solid abdominal organ injuries, it is common to withhold anticoagulation empirically due to concerns for bleeding, but this practice is not evidence-based. Interrupting prophylaxis even by one dose is associated with DVT,36 and in the current study, we delineated the consequences of delaying initiation of prophylaxis by each day. Seventy-two hours was the point at which failure to initiate prophylaxis became associated with VTE development, and orthopedic injury and TBI were independent predictors of the decision not to anticoagulate. The latter finding is interesting because, although TBI is thought to promote a severe, prolonged coagulopathy that necessitates deferring prophylaxis, no differences in coagulability were shown among patients with no TBI, isolated TBI, and polytrauma at any time point in this study.
Because time to resolution of TIC is most often less than 24 hours, this study provides additional support, but not complete justification, for earlier initiation of VTE chemoprophylaxis. One consideration we did not address is the safety of early anticoagulation in patients with injuries regarded high-risk for persistent or delayed bleeding, which has been the focus of previous work. In the setting of acute TBI, starting low–molecular-weight heparin (LMWH) or unfractionated heparin by 72 hours has retrospectively been shown not to increase radiographic progression of intracranial hemorrhage or the rate of subsequent neurosurgical interventions, and it decreased the risk of VTE compared with delayed anticoagulation.37,38 Like-wise, introducing LMWH 24 hours after intracranial hemorrhage had been deemed stable on imaging did not result in new or expanded lesions.39 Similar studies have demonstrated the efficacy and safety of early prophylaxis after spine fractures,40 blunt solid abdominal organ injuries,41,42 and major vascular injuries.43
We recognize several limitations of this study beyond those inherent in its prospective observational design. The TEM thresholds we used were based on reference ranges determined by the manufacturer using healthy patients,23 and while MCF greater than 71 mm has been associated with thromboembolic events in a surgical population,25 the clinical applicability of these thresholds has not been established in a trauma population. Patient attrition due to death and discharge from the ICU created a survivor bias and might have hindered our ability to detect statistical significance at later time points. Lastly, coagulation status at each time point was not controlled for the effects of prior resuscitation and blood transfusions.
Although viscoelastic assays have been shown to improve survival when used to guide resuscitation in trauma,15 their role in the management of the injured patient after correction of coagulopathy remains unclear and should be addressed in future work. Three studies have explored whether TEG could direct dosing of VTE chemoprophylaxis, but all were underpowered and failed to demonstrate a benefit over standard-dose LMWH.44–46 The most recent of these studies was a multicenter randomized controlled trial in which 185 patients were administered either 30 mg of enoxaparin twice daily or enoxaparin dose-adjusted to achieve a change in reaction time between standard and heparinase TEGs of 1–2 minutes,45 which had previously been associated with no DVT occurrence.47 Despite finding no difference in VTE rate between groups in this trial, the authors noted a lower overall VTE incidence than in their smaller pilot study (6.5% vs 14.9%),46 which they attributed partially to shorter time to prophylaxis initiation (1.0 vs 2.7 days). When this observation is considered in the context of the current study, we speculate that absence or resolution of coagulopathy on TEG or TEM might facilitate earlier prophylaxis initiation on an individual-patient basis.
Therefore, while we support continued investigation into the use of viscoelastic assays in guiding how to prophylax, future studies should also evaluate prospectively their efficacy and safety in directing when to prophylax. These same questions about using TEG and TEM to determine when and how to prophylax could also be asked for other conditions for which we anticoagulate trauma patients, like blunt cerebrovascular injuries. Finally, while the current study provides clinical insight into the dynamics of dysregulated coagulation after injury, further elucidating the biologic mechanisms behind the transition from hypo- to hypercoagulability remains the key to targeted treatment in these high-risk patients.
CONCLUSIONS
Based on the results of this study, we conclude that TIC largely resolves within 24 hours, after which hypercoagulability becomes increasingly more prevalent. These trends occur independently of injury pattern. Deferring initiation of prophylactic anticoagulation, which is often biased toward patients with intracranial and orthopedic injuries, is associated with VTE development. Further studies should examine the efficacy and safety of timing introduction of VTE chemoprophylaxis based on correction of coagulopathy.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (grants UM1HL120877 to MJC and K01ES026834 to RAC) and the United States Department of Defense (contract W911QY-15-C-0044 to MJC and RAC).
The authors thank Robert J. Torphy, MD, for his guidance with the statistical analyses in this study.
Footnotes
This study was presented at the 48th annual meeting of the Western Trauma Association, February 25 to March 2, 2018, in Whistler, British Columbia, and was awarded third place in the Earl G. Young Resident Paper Competition.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Moore HB, Moore EE, Liras IN, Wade C, Huebner BR, Burlew CC, Pieracci FM, Sauaia A, Cotton BA. Targeting resuscitation to normalization of coagulating status: hyper and hypocoagulability after severe injury are both associated with increased mortality. Am J Surg. 2017;214(6):1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. [DOI] [PubMed] [Google Scholar]
- 3.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. [DOI] [PubMed] [Google Scholar]
- 4.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma—a unified approach. J Trauma. 1982;22(8):672–679. [DOI] [PubMed] [Google Scholar]
- 5.Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146(4):764–774. [DOI] [PubMed] [Google Scholar]
- 6.Chapman BC, Moore EE, Barnett C, Stovall RT, Biffl WL, Burlew CC, Bensard DD, Jurkovich GJ, Pieracci FM. Hypercoagulability following blunt solid abdominal organ injury: when to initiate anticoagulation. Am J Surg. 2013;206(6):917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24): 1601–1606. [DOI] [PubMed] [Google Scholar]
- 8.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3): 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874. [DOI] [PubMed] [Google Scholar]
- 10.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625–632. [DOI] [PubMed] [Google Scholar]
- 11.Cannon WB, Gray H. Factors affecting the coagulation time of blood. Am J Physiol. 1914;34(2):232–242. [Google Scholar]
- 12.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie SA, Kornblith LZ, Howard BM, Conroy AS, Kunitake RC, Nelson MF, Hendrickson CM, Calfee CS, Callcut RA, Cohen MJ. Characterization of distinct coagulopathic phenotypes in injury: pathway-specific drivers and implications for individualized treatment. J Trauma Acute Care Surg. 2017;82(6): 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menezes AA, Vilardi RF, Arkin AP, Cohen MJ. Targeted clinical control of trauma patient coagulation through a thrombin dynamics model. Sci Transl Med. 2017;9(371):1–11. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MJ. Translational approaches to coagulopathy after trauma: towards targeted treatment. PLoS Med. 2017;14(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, Hamilton PA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–707. [DOI] [PubMed] [Google Scholar]
- 19.Cothren CC, Smith WR, Moore EE, Morgan SJ. Utility of once-daily dose of low-molecular-weight heparin to prevent venous thromboembolism in multisystem trauma patients. World J Surg. 2007;31(1):98–104. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58(3):475–481. [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornblith LZ, Howard B, Kunitake R, Redick B, Nelson M, Cohen MJ, Callcut R. Obesity and clotting: body mass index independently contributes to hypercoagulability after injury. J Trauma Acute Care Surg. 2015; 78(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16(4):301–310. [DOI] [PubMed] [Google Scholar]
- 24.Van Haren RM, Valle EJ, Thorson CM, Guarch GA, Jouria JM, Andrews DM, Sleeman D, Levi JU, Livingstone AS, Proctor KG. Long-term coagulation changes after resection of thoracoabdominal malignancies. J Am Coll Surg. 2014;218(4):846–854. [DOI] [PubMed] [Google Scholar]
- 25.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18(5):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, Wade CE, Kozar RA, Holcomb JB. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72(6):1470–1477. [DOI] [PubMed] [Google Scholar]
- 27.Pommerening MJ, Rahbar E, Minei K, Holcomb JB, Wade CE, Schreiber MA, Cohen MJ, Underwood SJ, Nelson M, Cotton BA. Splenectomy is associated with hypercoagulable thrombelastography values and increased risk of thromboembolism. Surgery. 2015;158(3):618–626. [DOI] [PubMed] [Google Scholar]
- 28.Liras IN, Rahbar E, Harting MT, Holcomb JB, Cotton BA. When children become adults and adults become most hypercoagulable after trauma: an assessment of admission hypercoagulability by rapid thrombelastography and venous thromboembolic risk. J Trauma Acute Care Surg. 2016;80(5):778–782. [DOI] [PubMed] [Google Scholar]
- 29.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 30.Robles AJ, Kornblith LZ, Hendrickson CM, Howard BM, Conroy AS, Moazed F, Calfee CS, Cohen MJ, Callcut RA. Health care utilization and the cost of posttraumatic acute respiratory distress syndrome care. J Trauma Acute Care Surg. 2018;85(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996; 40(4):501–512. [DOI] [PubMed] [Google Scholar]
- 32.Van Haren RM, Valle EJ, Thorson CM, Jouria JM, Busko AM, Guarch GA, Namias N, Livingstone AS, Proctor KG. Hypercoagulability and other risk factors in trauma intensive care unit patients with venous thromboembolism. J Trauma Acute Care Surg. 2014;76(2):443–449. [DOI] [PubMed] [Google Scholar]
- 33.Spencer Netto F, Tien H, Ng J, Ortega S, Scarpelini S, Rizoli SB, Geerts W. Pulmonary emboli after blunt trauma: timing, clinical characteristics and natural history. Injury. 2012;43(9):1502–1506. [DOI] [PubMed] [Google Scholar]
- 34.Van Gent JM, Zander AL, Olson EJ, Shackford SR, Dunne CE, Sise CB, Badiee J, Schechter MS, Sise MJ. Pulmonary embolism without deep venous thrombosis: de novo or missed deep venous thrombosis? J Trauma Acute Care Surg. 2014;76(5):1270–1274. [DOI] [PubMed] [Google Scholar]
- 35.Coleman JJ, Zarzaur BL, Katona CW, Plummer ZJ, Johnson LS, Fecher A, O’Rear JM, Feliciano DV, Rozycki GS. Factors associated with pulmonary embolism within 72 hours of admission after trauma: a multicenter study. J Am Coll Surg. 2015;220(4):731–736. [DOI] [PubMed] [Google Scholar]
- 36.Louis SG, Sato M, Geraci T, Anderson R, Cho SD, Van PY, Barton JS, Riha GM, Underwood S, Differding J, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149(4):365–370. [DOI] [PubMed] [Google Scholar]
- 37.Koehler DM, Shipman J, Davidson MA, Guillamondegui O. Is early venous thromboembolism prophylaxis safe in trauma patients with intracranial hemorrhage. J Trauma. 2011;70(2):324–329. [DOI] [PubMed] [Google Scholar]
- 38.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, Neal M, Pirouzmand F, Nathens AB. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: a propensity-matched cohort study. J Am Coll Surg. 2016;223(4):621–631. [DOI] [PubMed] [Google Scholar]
- 39.Saadeh Y, Gohil K, Bill C, Smith C, Morrison C, Mosher B, Schneider P, Stevens P, Kepros JP. Chemical venous thromboembolic prophylaxis is safe and effective for patients with traumatic brain injury when started 24 hours after the absence of hemorrhage progression on head CT. J Trauma Acute Care Surg. 2012;73(2):426–430. [DOI] [PubMed] [Google Scholar]
- 40.Sharpe JP, Gobbell WC, Carter AM, Pahlkotter MK, Muhlbauer MS, Camillo FX, Fabian TC, Croce MA, Magnotti LJ. Impact of venous thromboembolism chemoprophylaxis on postoperative hemorrhage following operative stabilization of spine fractures. J Trauma Acute Care Surg. 2017;83(6):1108–1113. [DOI] [PubMed] [Google Scholar]
- 41.Joseph B, Pandit V, Harrison C, Lubin D, Kulvatunyou N, Zangbar B, Tang A, O’Keeffe T, Green DJ, Gries L, et al. Early thromboembolic prophylaxis in patients with blunt solid abdominal organ injuries undergoing nonoperative management: is it safe? Am J Surg. 2015;209(1):194–198. [DOI] [PubMed] [Google Scholar]
- 42.Kwok AM, Davis JW, Dirks RC, Wolfe MM, Kaups KL. Time is now: venous thromboembolism prophylaxis in blunt splenic injury. Am J Surg. 2016;212(6):1231–1236. [DOI] [PubMed] [Google Scholar]
- 43.Frank B, Maher Z, Hazelton JP, Resnick S, Dauer E, Goldenberg A, Lubitz AL, Smith BP, Saillant NN, Reilly PM, et al. Venous thromboembolism after major venous injuries: competing priorities. J Trauma Acute Care Surg. 2017;83(6):1095–1101. [DOI] [PubMed] [Google Scholar]
- 44.Harr JN, Moore EE, Chin TL, Ghasabyan A, Gonzalez E, Wohlauer MV, Banerjee A, Silliman CC, Sauaia A. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74(3):756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis SG, Van PY, Riha GM, Barton JS, Kunio NR, Underwood SJ, Differding JA, Rick E, Ginzburg E, Schreiber MA. Thromboelastogram-guided enoxaparin dosing does not confer protection from deep venous thrombosis: a randomized controlled pilot trial. J Trauma Acute Care Surg. 2014;76(4):937–942. [DOI] [PubMed] [Google Scholar]
- 46.Connelly CR, Van PY, Hart KD, Louis SG, Fair KA, Erickson AS, Rick EA, Simeon EC, Bulger EM, Arbabi S, et al. Thrombelastography-based dosing of enoxaparin for thromboprophylaxis in trauma and surgical patients: a randomized clinical trial. JAMA Surg. 2016;151(10):–e162069. [DOI] [PubMed] [Google Scholar]
- 47.Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66(6): 1509–1517. [DOI] [PubMed] [Google Scholar]


