Watch a video presentation of this article
Watch an interview with the author
Answer questions and earn CME
Abbreviations
- ASL
argininosuccinate lyase
- ASS
argininosuccinate synthetase
- ATP
adenosine triphosphate
- CPSI
carbamyl phosphate synthetase 1
- HE
hepatic encephalopathy
- IEM
inborn error of metabolism
- NAG
N‐acetylglutamate
- NAGS
N‐acetylglutamate synthetase
- NH3
ammonia
- OTC
ornithine transcarbamylase
- PVT
portal vein thrombosis
- TPN
total parenteral nutrition
Introduction: Pathophysiology
Ammonia (NH3) is a known potent neurotoxin commonly implicated in the development of hepatic encephalopathy (HE), a clinical marker of decompensated cirrhosis that produces a spectrum of neurological or psychiatric abnormalities. Furthermore, the development of HE is a significant predictor of mortality in patients with cirrhosis.1
In a healthy individual with functioning hepatocytes, ammonia enters the portal circulation from the gastrointestinal tract and is subsequently converted to urea through the urea cycle (Fig. 1). Urea is then excreted physiologically via the colon or kidneys. However, in cases of severe hepatic dysfunction, or in the presence of blood shunting around the portal circulation, the burden of ammonia excretion falls on the kidneys, skeletal muscle, and brain. Under these circumstances, ammonia enters the systemic circulation, inhibits both excitatory and inhibitory postsynaptic potentials, and impairs neuronal function (Fig. 2).2, 3, 4
Figure 1.
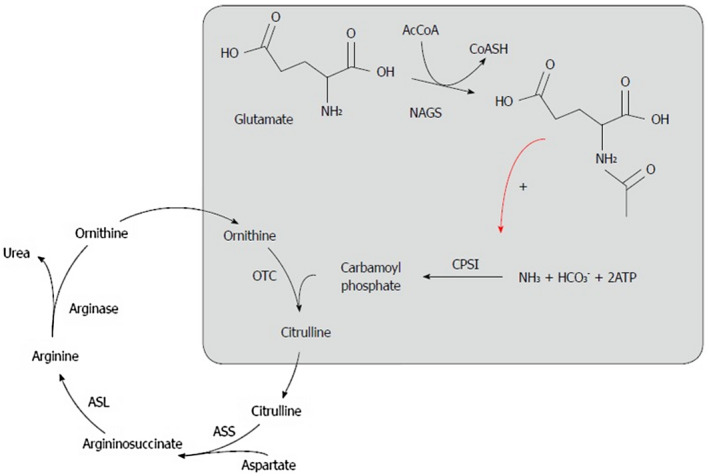
The urea cycle.19 Reproduced with permission from World Journal of Gastroenterology.19 Copyright 2015, Baishideng Publishing Group, in accordance with the Creative Commons Attribution Non Commercial (CC BY‐NC 4.0) license: http://creativecommons.org/licenses/by-nc/4.0/.
Figure 2.
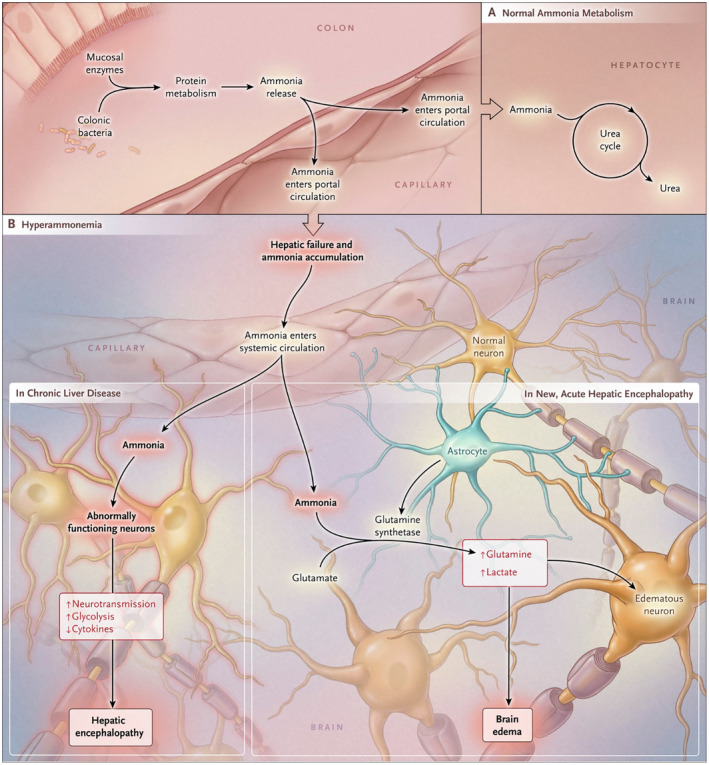
A proposed pathophysiological mechanism for ammonia causing encephalopathy. Reproduced with permission from New England Journal of Medicine.4 Copyright 2016, Massachusetts Medical Society.
By penetrating the blood‐brain barrier through passive diffusion or mediated transport, ammonia can produce cerebral edema by facilitating the production of glutamine, an osmolyte; this complication is known to be most severe in acute liver failure.4 Other factors, such as gastrointestinal bleeding, can enhance translocation of toxins from the portal to the systemic bloodstream. Cerebral edema, brain herniation, and seizures typically occur when acute hyperammonemia (i.e., in the setting of acute liver failure) leads to arterial ammonia levels greater than 200 μmol/L.5 As a result, arterial ammonia monitoring, which does not correlate with venous ammonia levels,5 is useful specifically in patients with acute liver failure.
Consulting hepatologists are commonly asked to interpret elevated serum venous ammonia levels. However, despite its implication as a neurotoxin, high serum ammonia levels alone add no diagnostic, staging, or prognostic value to patients with chronic liver disease who experience HE.6, 7 Alternately, in the case of otherwise unexplained encephalopathy in a patient without a known diagnosis of cirrhosis, an elevated serum ammonia level should prompt evaluation for causes of noncirrhotic hyperammonemic encephalopathy (Table 1).
Table 1.
Causes of Noncirrhotic Hyperammonemic Encephalopathy
| Decreased Ammonia Elimination | Increased Ammonia Production |
|---|---|
|
Increased muscle catabolism:
|
|
| Spontaneous portosystemic shunt* | TPN |
| PVT | Multiple myeloma |
|
Medications:
|
Infections:
|
|
Chemotherapeutic agents:
|
Often presents during childhood.
Noncirrhotic Hyperammonemic Encephalopathy: Decreased Ammonia Elimination
Metabolic Disorders Resulting From Inborn Errors of Metabolism
Inborn errors of metabolism (IEMs), such as organic acidurias or carnitine deficiency, typically present during childhood, but urea cycle disorders that can affect any enzyme in the urea cycle are notorious for presenting in adults.3, 8
Ornithine transcarbamylase (OTC) deficiency, characterized by an X‐linked recessive inheritance pattern, is the most common urea cycle disorder. The clinical presentation of OTC deficiency can vary from lethargy and disorientation to profound obtundation. The defect limits the liver’s ability to convert ammonia to urea, resulting in noncirrhotic hyperammonemia, and can remain asymptomatic until a precipitating event, such as infection, protein intake, or a medication, unmasks the disorder.2, 5
Portosystemic Shunts
Spontaneous portosystemic shunts are commonly seen in patients with cirrhosis and commonly lead to brittle HE. However, congenital shunts, which can be intrahepatic or extrahepatic and are almost always diagnosed in the neonatal period, are an obscure cause of adult‐onset, noncirrhotic hyperammonemic encephalopathy.
Portal vein thrombosis (PVT) leading to a portosystemic shunt can lead to noncirrhotic hyperammonemia (shunting ammonia into the systemic circulation), although encephalopathy under these circumstances is usually mild given the patients’ preserved liver function.2, 8
Medications
Certain medications can disrupt the urea cycle. Valproic acid in particular is known to induce noncirrhotic hyperammonemic encephalopathy.9 Valproate increases propionic acid levels, which inhibit the rate‐limiting enzyme of the urea cycle, carbamyl phosphate synthetase. The reported prevalence rate of valproic acid–induced hyperammonemia is 35% to 45%, but patients are usually asymptomatic.8
Hyperammonemia can also result from administration of glycine (used during transurethral resection of the prostate) and has been described, via less clear mechanisms, with the use of carbamazepine, ribavirin, and sulfadiazine.5
Noncirrhotic Hyperammonemic Encephalopathy: Increased Ammonia Production
Increased Muscle Catabolism
Seizures, starvation, and trauma have all been associated with hyperammonemia.2, 8 These conditions can result in noncirrhotic hyperammonemic encephalopathy in patients (such as those with urea cycle disorders) whose livers cannot handle an increased nitrogen load.
Notably, a 2008 retrospective review of 17 cases of transient hyperammonemia found that hyperammonemia may be related to generalized seizures, and although the mechanisms require further investigation, the phenomenon may be related to persistent muscle contractions during a seizure.10 Compared with fluctuating ammonia levels in HE, patients with seizures demonstrate rapid normalization of ammonia levels.11
Total Parenteral Nutrition
The high protein load in total parenteral nutrition (TPN) can unmask previously unrecognized urea cycle disorders. The phenomenon has also been described in adults given TPN containing only essential amino acids. In these patients, the absence of ornithine (a substrate in the urea cycle) impairs ammonia detoxification.12
Malignant Myeloma
Increased amino acid metabolism by malignant cells produces excess ammonia, and plasma cell infiltration of the liver can produce portosystemic shunting, thereby bypassing the liver’s ability to metabolize ammonia.2, 3, 13
Infections Caused by Urease‐Producing Bacteria (e.g., Proteus mirabilis, Escherichia coli, Klebsiella)
Although infections caused by urease‐producing bacteria occur more commonly in children with congenital urinary tract abnormalities, they can occur in adult conditions predisposing to high urinary residuals, such as urinary retention or neurogenic bladder.2, 14 These infections produce an alkaline environment, which increases the proportion of NH3 compared with ammonium (NH4 +). The venous drainage of the bladder flows directly to the systemic circulation, bypassing the liver’s attempt to detoxify.8
Chemotherapy Regimens for Leukemia and Bone Marrow Transplantation
The exact mechanisms by which these hematological/oncological interventions produce hyperammonemia are likely multifactorial (increased protein catabolism, parenteral nutrition, gastrointestinal hemorrhage, sepsis, transient acquired enzyme reductions affecting urea synthesis, drug effects from chemotherapy agents). The precise mechanism remains elusive.7
Chemotherapeutic agents that have been implicated in noncirrhotic hyperammonemia include cytarabine, vincristine, amsacrine, etoposide, l‐asparaginase, cyclophosphamide, and 5‐fluorouracil.15
Conclusion
Assessing ammonia levels in a patient with known cirrhosis is unlikely to change clinical management and is therefore not recommend.16 Testing for elevated ammonia levels is appropriate in the absence of chronic liver disease to evaluate otherwise unexplained altered mental status,17 and if discovered, hyperammonemia, particularly in intensive care unit patients, should prompt urgent monitoring for complications such as brain edema and intracerebral hypertension. Hyperammonemia is associated with high morbidity and mortality in the critically ill population.5, 18 In the case of noncirrhotic hyperammonemic encephalopathy, the differential diagnosis should focus on mechanisms leading to either increased ammonia production or decreased ammonia elimination.
In particular, after ruling out acute liver failure, as well as underlying chronic liver disease, the consultant should perform a careful medication/nutrition review (including TPN), an assessment for seizure activity, abdominal imaging to evaluate for a portosystemic shunt or PVT, and a comprehensive evaluation for infection. If the etiology of hyperammonemia remains obscure, consideration of an occult urea cycle disorder, evaluating acid‐base status and consideration of quantitative plasma and urine amino acids, is a reasonable next step (Table 2).
Table 2.
Recommended Evaluation for an Adult With an Elevated Serum Ammonia Level
|
Potential conflict of interest: Nothing to report.
References
- 1. Stewart CA, Malinchoc M, Kim WR, et al. Hepatic encephalopathy as a predictor of survival in patients with end‐stage liver disease. Liver Transp 2007;13:1366‐1371. [DOI] [PubMed] [Google Scholar]
- 2. Upadhyay R, Bleck TP, Busl KM. Hyperammonemia: What urea‐lly need to know: Case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Rep Med 2016;2016:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cichoż‐Lach H, Michalak A. Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J Gastroenterol 2013;19:26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wijdicks EFM. Hepatic encephalopathy. N Engl J Med 2016;375:1660‐1670. [DOI] [PubMed] [Google Scholar]
- 5. Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest 2007;132:1368‐1378. [DOI] [PubMed] [Google Scholar]
- 6. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by AASLD and EASL. Alexandria, VA: American Association for the Study of Liver Diseases; 2014. Available at: https://www.aasld.org/sites/default/files/2019-06/141022_AASLD_Guideline_Encephalopathy_4UFd_2015.pdf. Accessed September 25, 2019. [Google Scholar]
- 7. Mallet M, Weiss N, Thabut D, et al. Why and when to measure ammonemia in cirrhosis? Clin Res Hepatol Gastroenterol 2018;42:505‐511. [DOI] [PubMed] [Google Scholar]
- 8. Laish I, Ben Ari Z. Noncirrhotic hyperammonaemic encephalopathy. Liver Int 2011;31:1259‐1270. [DOI] [PubMed] [Google Scholar]
- 9. Wadzinski J, Franks R, Roane D, et al. Valproate‐associated hyperammonemic encephalopathy. J Am Board Fam Med 2007;20:499‐502. [DOI] [PubMed] [Google Scholar]
- 10. Yanagawa Y, Nishi K, Sakamoto T. Hyperammonemia is associated with generalized convulsion. Intern Med 2008;47:21‐23. [DOI] [PubMed] [Google Scholar]
- 11. Hung TY, Chen CC, Wang TL, et al. Transient hyperammonemia in seizures: A prospective study. Epilepsia 2011;52:2043‐2049. [DOI] [PubMed] [Google Scholar]
- 12. Grazer RE, Sutton JM, Friedstrom S, et al. Hyperammonemic encephalopathy due to essential amino acid hyperalimentation. Arch Int Med 1984;144:2278‐2279. [PubMed] [Google Scholar]
- 13. Kwan L, Wang C, Levitt L. Hyperammonemic encephalopathy in multiple myeloma. N Engl J Med 2002;346:1674‐1675. [DOI] [PubMed] [Google Scholar]
- 14. De Jonghe B, Janier V, Abderrahim N, et al. Urinary tract infection and coma. Lancet 2002;360:996. [DOI] [PubMed] [Google Scholar]
- 15. Odigwe CC, Khatiwada B, Holbrook C, et al. Noncirrhotic hyperammonemia causing relapsing altered mental status. Proc (Bayl Univ Med Cent) 2015;28:472‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumral D, Qayyum R, Roseff S, et al. Adherence to recommended inpatient hepatic encephalopathy workup. J Hosp Med 2019;14:157‐160. [DOI] [PubMed] [Google Scholar]
- 17. Elgouhari HM, O’Shea R. What is the utility in measuring serum ammonia level in patients with altered mental status? Cleve Clin J Med 2009;76:252‐254. [DOI] [PubMed] [Google Scholar]
- 18. Prado FA, Delfino VDA. Letter to the Editor: Hyperammonemia in ICU patients: A frequent finding associated with high mortality. J Hepatol 2015;62:1216‐1218. [DOI] [PubMed] [Google Scholar]
- 19. Foschi FG, Morelli MC, Savini S, et al. Urea cycle disorders: A case report of a successful treatment with liver transplant and a literature review. World J Gastroenterol 2015;21:4063‐4068. [DOI] [PMC free article] [PubMed] [Google Scholar]


