Abstract
The aim of this study was to examine the feasibility of cognitive assessment from pre-surgery through two-year follow-up in a sample of pediatric brain tumor (BT) patients. We sought to investigate cognitive function over the course of diagnosis and treatment, and as a function of presenting problems, tumor location, treatment type, and tumor severity. Using a prospective, longitudinal design, standardized IQ measures were administered to pediatric BT patients (ages 6-16) prior to surgery (n=25), 6 months post-diagnosis (n=24), and 24 months post-diagnosis (n=23). Group differences emerged based on tumor severity and treatment type at multiple time points, including prior to surgical intervention; children with high grade tumors performed more poorly than children with low grade tumors, and children receiving surgery plus adjuvant therapy performed more poorly than children who received surgery only. When considered together, an analysis of covariance demonstrated that tumor grade significantly accounted for variability in cognitive functioning, while treatment type did not. Although there is overlap clinically between tumor severity and treatment received, results suggest that tumor severity is an important factor contributing to variability in cognitive functioning and should also be considered when monitoring risk for cognitive deficits in children diagnosed with BT.
Keywords: cancer, oncology, longitudinal assessment, medical predictors, risk factors
Brain and central nervous system (CNS) tumors are the most common solid tumor diagnosis of childhood (Porter, McCarthy, Freels, Kim, & Davis, 2010). The incidence rate is approximately 5.7 cases per 100,000 children annually and it is estimated that 4,770 new cases of childhood primary non-malignant and malignant CNS tumors will be diagnosed annually in the United States in coming years (Ostrom et al., 2016). Malignant brain tumors (BT) are the leading cause of cancer-related death in children, however, treatment advances have significantly improved survival rates with the overall 5-year survival rate reaching approximately 74% following diagnosis for children under age 20 (Ostrom et al., 2016). Despite increased survival, the aggressive nature of treatment may contribute to adverse late effects in multiple domains including chronic health, psychosocial, and neurocognitive problems (Desjardins et al., 2018; Ness & Gurney, 2007; Oeffinger et al., 2006; Panigrahy & Bluml, 2009).
In survivors of childhood BT, neurocognitive problems are well documented and one of the most prominent late effects. A meta-analysis of 39 studies published between 1992 and 2009 found large effects compared to test norms on multiple measures of cognitive functioning (Robinson et al., 2010). However, previous research has been limited by several methodological problems. Most importantly, most patients were assessed on average five years post-diagnosis and four years post-treatment and the majority of studies were cross-sectional, with cognitive function assessed at only one time-point (Robinson et al., 2010). The relative absence of prospective longitudinal studies prevents researchers from disentangling tumor related presenting problems (e.g., hydrocephalus) from disease characteristics (e.g., tumor type, location, severity) or treatment factors, including receiving adjuvant therapies (e.g., chemotherapy and radiation therapy). Focusing on late effects due to treatment processes in the absence of pre-treatment assessment, could mask possible direct effects that initial tumor characteristics may have on functioning.
Testing pre-diagnosis is rarely available and very difficult, if not impossible, to obtain for the majority of patients. Assessing children’s cognitive function after diagnosis, but prior to surgery, is important for establishing neurocognitive baselines and understanding what problems may exist pre-treatment (Meyers & Brown, 2006). Furthermore, relatively few studies of pediatric BT patients have followed children prospectively and have typically not obtained an adequate sample assessed prior to surgery (i.e., less than 10%) (Knight et al., 2014; Merchant, Conklin, Wu, Lustig, & Xiong, 2009; Spiegler, Bouffet, Greenberg, Rutka, & Mabbott, 2004; Stargatt, Rosenfeld, Maixner, & Ashley, 2007). Therefore, pre-surgical assessment could provide a valuable early index of cognitive functioning and is a high priority for research.
A wide range of characteristics and correlates of brain tumors may be important in predicting cognitive late effects. First, presenting problems, such as hydrocephalus, have documented negative effects on neurocognitive function and complicate understanding of a brain tumors’ impact on children’s neurocognitive functioning (Hardy, Bonner, Willard, Watral, & Gururangam, 2008; Merchant et al., 2004). Second, tumor location has demonstrated effects on cognitive outcomes in BT patients (Iuvone et al., 2011; Nathan et al., 2007; Willard, Conklin, Boop, Wu, & Merchant, 2013). Third, tumor severity can impact functioning; for example, in a prospective study of children with primary BT, those with high grade tumors scored lower on measures of academic achievement and processing speed compared to those with low grade tumors (Shortman et al., 2014). In addition, tumor grade and size may affect cognitive function as a result of tumor compression and infiltration that may be associated with white matter damage even before treatment (Law et al., 2011). Fourth, treatment intensity, particularly radiation, is related to deficits in neurocognitive function (Kahalley et al., 2013; Robinson, Fraley, Pearson, Kuttesch, & Compas, 2013; Wolfe, Madan-Swain, & Kana, 2012). Tumor type, location, and severity also inform treatment decisions; i.e., which children receive surgical resection alone versus adjuvant therapies such as chemotherapy, radiation, or both.
To address these issues, the present study used a prospective, longitudinal design to examine neurocognitive function over the first two years after diagnosis of a pediatric BT. First, we report on the feasibility of carrying out a prospective study of this type during the highly stressful course of a child’s BT diagnosis and treatment. Second, we examined the relations between complications related to the tumor at initial presentation, tumor location, treatment type, and tumor severity on neurocognitive function. We hypothesized that children who presented with hydrocephalus (vs. without), had supratentorial or cortical tumors (vs. infratentorial or posterior fossa), underwent a combination of surgery and chemotherapy and/or radiation (vs. surgery alone), and had high grade (vs. low grade) tumors would have lower cognitive functioning.
Methods
Participants
Eligible participants for the current study included patients ages six to 16 years old identified as having a primary BT over the course of two years of recruitment through the Department of Pediatric Neurosurgery at a university-affiliated children’s hospital. Of the 31 eligible patients who were approached regarding the study, 25 agreed to participate (81% of those eligible). Reasons for patients not enrolling prior to surgery included insufficient time between referral and scheduled resection for cognitive testing to take place (n=4) and referral after surgery (n=2), as children often undergo surgery within days of diagnosis. Children in this age range were selected for three reasons: first, significant adverse effects on school achievement have been documented in school-age children with CNS tumors (Robinson et al., 2010). Second, it allowed for a homogeneous set cognitive measures to be used, as tests for younger children would introduce additional heterogeneity. Third, significant differences in neurocognitive functioning have been found among CNS tumor patients ranging from age five to 15 years, suggesting that this age range is sufficiently broad to capture age differences (Merchant et al., 2009). Exclusion criteria included the presence of a pre-existing neurodevelopmental disorder or disability (e.g., intellectual disability), pervasive developmental disorder (e.g., autism), or diagnosis of neurofibromatosis because illness and treatment course is significantly different from other children with CNS tumors.
Measures
Demographics and medical chart review.
Parents provided demographic information including the child’s age and gender. Patients’ charts were reviewed to collect medical diagnostic information including presence of hydrocephalus, tumor type, tumor location, tumor severity (World Health Organization [WHO] grade), and treatment type. Children were considered to have hydrocephalus if obstructive hydrocephalus was present on the initial MRI scan. Tumor location included the posterior fossa (n = 13), temporal lobes (n = 4), parietal lobes (n = 4), frontal lobes (n = 3), suprasellar region (n = 2), thalamus (n = 2), ventricles (n = 1), pineal gland (n = 1), and brainstem (n = 1). Adjuvant therapy consisted of chemotherapy (n = 2), radiation (n = 2), or both (n = 5). Medical variables were coded dichotomously to test group differences (Shortman et al., 2017). Hydrocephalus was rated as present or not present, tumor location was recorded as infratentorial or supratentorial, WHO grade was divided into low (I/II) versus high (III/IV), and treatment type was divided as surgery only versus surgery plus adjuvant treatment (Shortman et al., 2017). Individual participant data are presented in Supplementary Table 1 and summarized in Table 1.
Table 1.
Summary statistics for demographic, medical, and neurocognitive variables.
| T1 | T2 | T3 | |
|---|---|---|---|
| Sex (N(%)) | |||
| Male | 14(56%) | 17(71%) | 15(65%) |
| Female | 11(44%) | 7(29%) | 8(35%) |
| Hydrocephalus (N(%)) | |||
| Present | 13(52%) | 10(42%) | 10(43%) |
| Absent | 12(48%) | 14(58%) | 13(57%) |
| Location (N(%)) | |||
| Supratentorial | 12(48%) | 14(58%) | 12(52%) |
| Infratentorial | 13(52%) | 10(42%) | 11(48%) |
| Treatment (N(%)) | |||
| Surgery Only | N/A | 12(50%) | 11(48%) |
| Surgery + Adjuvant | N/A | 12(50%) | 12(52%) |
| Tumor Severity (N(%)) | |||
| Low Grade | 15(60%) | 15(62%) | 14(61%) |
| High Grade | 10(40%) | 9(38%) | 9(39%) |
| Child Age (M(SD)) | 10.44(2.96) | 11.08(3.26) | 10.83(3.07) |
| Cognitive Function (M(SD)) | 99.08(14.90) | 96.63(14.85) | 96.35(15.40) |
Note. T1 pre-surgery n=25; T2 6-month follow-up n=24, T3 24-month follow-up n=23. Cognitive function reflects standardized IQ scores with a population mean of 100 and a standard deviation of 15.
Cognitive functioning.
Due to the need for an abbreviated battery at pre-surgery (T1) because children often undergo surgery within days of diagnosis, the two-subtest version of the Wechsler Abbreviated Scales of Intelligence (WASI) was administered, including one verbal (Vocabulary) and one nonverbal (Matrix Reasoning) subtest. The association between these individual subtests and their overall broader indices are highly correlated (r’s = .87 to .93). At the first follow-up assessment at 6 months post-surgery (T2) and the second at 24 months post-surgery (T3), the standard battery of the Wechsler Intelligence Scale for Children (WISC-IV) was administered (Block Design, Similarities, Digit Span, Picture Concepts, Coding, Vocabulary, Letter-Number Sequencing, Matrix Reasoning, Comprehension, and Symbol Search subtests; Wechsler, 2003). To be comparable with the intelligence quotient that is computed on the WASI, the General Ability Index (GAI) was calculated for the WISC-IV and used in analyses, as this approximates FSIQ in children (Prifitera, Weiss, Rolfhus, & Saklofske, 2005).1 Henceforth, FSIQ scores computed from the WASI and the GAI from the WISC-IV are referred to as “cognitive functioning.”
Procedure
The study was approved by the Institutional Review Board (IRB #091096), and written informed consent and assent were signed by parents and children, respectively. Children and parents completed all questionnaires and children completed assessments. Measures were administered by graduate and postdoctoral-level research assistants and supervised by a clinical psychologist. For follow up assessments, families were contacted via phone calls, emails, and contacting families while in for clinic appointments to inform them of the upcoming assessments and schedule their study visit. Participants were compensated for their time.
Data Analyses
To test the main research questions, first a series of independent samples t-tests were conducted using data from all available participants at each of the three time points. Discreet time points were used in order to maximize the available sample size and increase power to detect meaningful group differences in cognitive functioning on key medical variables. Second, a supplementary analysis of covariance (ANCOVA) was conducted at each of the follow-up time points to examine whether the independent medical variables that were significant continued to have an effect on cognitive functioning when considered simultaneously. Tests of skewness and kurtosis on all variables yielded results all within acceptable ranges. We used Cohen’s guidelines to interpret effect sizes (Cohen, 1988).
Results
Feasibility
Twenty-nine (29) of 31 eligible patients were enrolled into the study (94%). Twenty-five of the patients enrolled completed a pre-surgical assessment (T1, n=25), tested on average 1.72 days (SD = 1.02) prior to surgery. Follow-up assessments occurred at 6-months (T2, n=24) and 24-months (T3, n=23) post-diagnosis. Every attempt was made to assess the children at each time point, however, a substantial proportion missed at least one time point due to a variety of reasons related to both the illness of the child, treatments, and logistical issues. At various time points, reasons included inability to reach the family (n=7 out of 31), family declined further assessment (n=1), family transferred care to another facility (n=1), child experienced disease relapse (n=1), and child death (n=2) (See Supplementary Table 1 for details of which time points were completed for each participant).2 Of those who participated over the course of the study, six children experienced disease relapse, while two had disease progression. Neurocognitive testing was completed at all three time points in 18 of 29 patients (62%), in at least two time points for an additional 7 patients (25 of 29 patients [86%]), and at only one time point for four participants. Chi-square and independent-samples t-tests were conducted to compare included versus excluded participants across demographic and medical variables. All comparisons were non-significant at each time point except gender, which was significant only at T1, with more males completing the T1 assessment.
Cognitive Functioning at Discrete Time Points
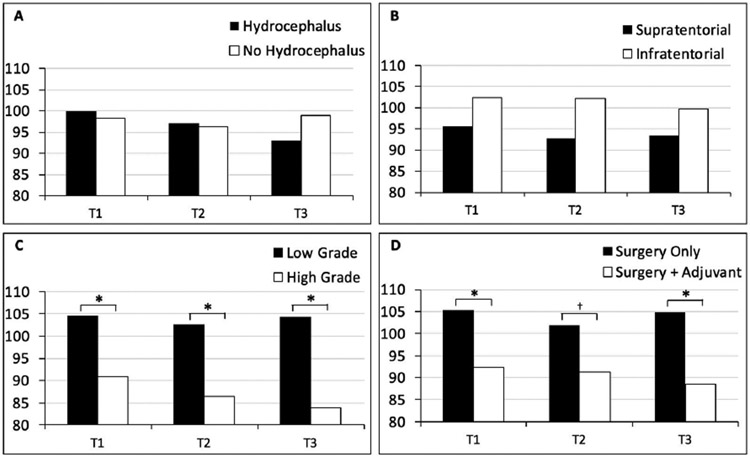
Demographics, medical characteristics, and cognitive functioning of the participants included across time points are summarized in Table 1. Cognitive functioning was not significantly related to participant age at study onset or age at diagnosis at any time point. With regard to presence of hydrocephalus, t-tests demonstrated no differences in cognitive function at any time point. In addition, there were no group differences in cognitive scores as a function of tumor location. Tumor severity had a statistically significant effect on cognitive functioning at pre-surgery (t=2.47, p=.02, d=1.01), 6 months (t=3.03, p=.01, d=1.28), and 24 months (t=4.06, p=.001, d=1.74), indicating that children with high grade tumors scored lower than children with low grade tumors at all three time points. For treatment type, there was a significant difference in cognitive functioning prior to surgery (i.e., at T1; t=2.38, p=.03, d=.95) and at 24-month follow-up (t=2.97, p=.01, d=1.24), with children receiving surgery plus adjuvant therapy scoring significantly lower than those who received surgery only at both time points. See Table 2 for comparisons of mean scores on cognitive functioning at each time point and Figure 1 for a graphical representation of these differences.
Table 2.
Subgroup comparisons of cognitive functioning at pre-surgery, 6 months and 24 months post-diagnosis.
| Cognitive Functioning | |||
|---|---|---|---|
| T1 M(SD) |
T2 M(SD) |
T3 M(SD) |
|
| Hydrocephalus | |||
| Present | 99.85(14.16) | 97.10(11.61) | 93.00(13.30) |
| Absent | 98.25(16.25) | 96.29(17.22) | 98.92(16.89) |
| t(df) | −.26(23) | −.13(22) | .91(21) |
| Tumor Location | |||
| Supratentorial | 95.50(11.71) | 92.71(13.73) | 93.25(13.86) |
| Infratentorial | 102.38(17.13) | 102.10(15.31) | 99.73(16.91) |
| t(df) | 1.16(23) | 1.58(22) | 1.01(21) |
| Treatment | |||
| Surgery Only | 105.31(14.44) | 102.00(10.18) | 104.91(9.51) |
| Surgery + Adjuvant | 923.3(12.70) | 91.25(17.16) | 88.50(15.85) |
| t(df) | 2.38(23)* | 1.87(22)† | 2.97(21)* |
| Tumor Severity | |||
| Low Grade | 104.53(13.59) | 102.73(13.30) | 104.36(12.92) |
| High Grade | 90.90(13.44) | 86.44(11.75) | 83.89(9.67) |
| t(df) | 2.47(23)* | 3.03(22)* | 4.06(21)* |
Note. T1 pre-surgery n=25; T2 6-month follow-up n=24, T3 24-month follow-up n=23.
p<.05
p<.10.
Figure 1. Cognitive functioning across medical variables.
Comparisons of participants’ mean cognitive functioning across time as a function of (A) presence of hydrocephalus, (B) tumor location, (C) tumor severity, and (D) treatment type.
*p < .05, †p < .10
Because significant effects were found for treatment type and tumor severity, both factors were considered together in ANCOVAs at 6- and 24-month follow-ups to determine if cognitive function varied as a function of tumor severity controlling for treatment type (treatment type was not analyzed at pre-surgery because no adjuvant therapies had been initiated at that time). Tumor severity remained statistically significant in accounting for variability in cognitive functioning at 6-month, F(1,23)=4.51, p=.02,3 and 24-month follow-ups, F(1,22)=7.96, p=.003, while cognitive function did not vary independently as a function of treatment type.
Supplemental Analyses of Cognitive Functioning Over Time
The four medical variables were then considered to explore group, time, and group by time effects in separate repeated measures ANOVAs. Neither time effects nor group by time interactions were observed for any of the medical variables tested. Confirming previous analyses, treatment type yielded a significant group effect, F(1,16)=7.88, p=.01, with participants who received both surgery and adjuvant therapy having worse cognitive functioning than those who received surgery alone. Tumor severity also yielded a significant group effect, F(1,16)=6.71, p=.02,3 with children with high grade tumors performing worse than those with low grade tumors. As expected, patients with low grade tumors were more likely to be treated with surgery only than patients with high grade tumors, X2 =11.46, p=.001.
Discussion
Pediatric BT survivors experience significant neurocognitive consequences years into survivorship. There is a need for pre-surgical assessment to establish baseline functioning and to follow patients prospectively to disentangle how factors such as diagnostic information (e.g., tumor complications, tumor location, tumor severity) and treatment type affect the course of functioning. The current study reports on a sample of children with primary brain tumors who were tested prior to surgery and followed longitudinally and highlights the feasibility of continued cognitive assessment in this population and demonstrates important differences in cognitive abilities as a function of tumor severity and treatment type.
An aim of the current study was to establish the feasibility of enrolling and retaining pediatric BT patients in a two-year prospective study, starting at pre-surgery and through ongoing disease treatment. Our retention rates ranged from 74-81% over the course of the study, above those of prior longitudinal studies of similar nature in this population, which report retention rates ranging from 40-70% (Merchant et al., 2009; Stargatt et al., 2007). Several factors such as time constraints, the demands of multiple treatment appointments, fluctuating health status of the child, and treatment location change made it difficult to enroll and retain participants. Key features for enrolling and maintaining continued participation of children and their families include strong support from the neurosurgery team who made initial contact and referrals; extensive availability of the psychological team to administer assessments; and close proximity of the assessment location to the academic medical center where the majority of children received treatment. In addition, families were highly motivated to participate; anecdotally, many reported that their motivation stemmed from the desire to understand their child’s cognitive functioning and to help other families. Future research should explore ways to make cognitive testing more easily available to pediatric BT patients, including offering testing at home and integrating testing as part of regular medical appointments.
A second primary aim of this study was to determine if cognitive functioning differed among participants in the context of tumor and therapy-related characteristics, including presence of hydrocephalus, tumor location, treatment type, and tumor severity. Neither hydrocephalus nor tumor location was associated with significant differences in neurocognitive function. Previous studies that have found cognitive deficits associated with shunted hydrocephalus have had relatively small sample sizes with testing at only one time point over two years after treatment completion on average, and authors have called for the need for longitudinal studies of the effects of this complication (Hardy et al., 2008). While the present study did not assess additional shunt placements, the absence of an effect for hydrocephalus in the current study warrants further attention to determine if effects may be limited to patients requiring shunts who develop other post-surgical complications, or those tested farther post-treatment. Similarly, no significant differences were found for tumor location (supratentorial vs. infratentorial); future studies with larger samples and greater statistical power are needed to examine possible effects of tumor location.
Importantly, there were significant differences in functioning between children with low grade tumors compared to children with high grade tumors at pre-surgery, 6-, and 24-month follow-ups in the independent samples t-tests and repeated measures ANOVA. This is consistent with research showing differences in academic achievement scores and processing speed in children with high grade primary brain tumors compared to children with low grade tumors (Shortman et al., 2017). Further, these findings support the proposition that tumor grade and size may affect cognitive abilities as a result of tumor compression and infiltration that may be associated with white matter damage even before treatment (Law et al., 2011). Diffuse decreased white matter integrity has been shown to be related to cognitive deficits in children with BT and is suggested to occur as a result of treatment effects, yet these patterns may be present prior to treatment as well (Aleksonis et al., 2019). Future research is needed to examine specific tumor characteristics that may have the greatest effect on children’s cognitive function.
There were significant group effects showing differences in functioning based on treatment type in the repeated measures ANOVA, as well as pre-surgery and at 24-month follow-up in t-tests. It is particularly noteworthy that this difference occurred pre-surgery, before any therapy (including surgery) was administered. While an abbreviated measure of cognitive function was used at the pre-surgical assessment, IQ estimated from abbreviated measures has been shown to underestimate FSIQ in children with BT, indicating that pre-surgical assessment differences may even underestimate deficits (Burgess et al., 2018). Findings at 24-month follow-up are in line with a large body of evidence demonstrating the deleterious effects of irradiation on neurocognitive functioning (Butler et al., 2013; Nathan et al., 2007; Robinson et al., 2013; Wolfe et al., 2012). This suggests that deficits that are typically attributed to adjuvant treatment may be related to characteristics of tumor severity as well. This is supported by our finding that the effect of tumor severity remained significant when covarying for adjuvant therapy, whereas the effects of adjuvant treatment were no longer significant when covarying for tumor severity. It should be highlighted that not all low grade tumors receive surgery alone and not all high grade tumors receive both forms of adjuvant therapy (i.e., chemotherapy and radiation).
When both treatment type and tumor grade were considered simultaneously as factors contributing to cognitive functioning, treatment type was no longer significant. This finding is particularly striking as much of the previous literature addressing differences in neurocognitive outcomes in patients with BT have attributed poor functioning to treatment with radiation therapy (Kahalley et al., 2013; Robinson et al., 2013). While we were underpowered to assess differences attributed to radiation alone, the current findings suggest that tumor severity is also an important factor in accounting for variability in cognitive functioning in pediatric BT patients at diagnosis, during treatment, and after its completion. The association between tumor grade and treatment suggests that the individual effects for these predictors in the ANCOVA should be interpreted with caution. However, the presence of an effect for tumor grade pre-surgery, prior to the initiation of any treatment, underscores the potential importance of examining tumor grade in future research.
Despite the important contributions of this study, it is not without limitations. First, only children ages six to 16 years were included. Second, although cognitive functioning was measured pre-surgically and provided an appropriate benchmark to compare to future functioning, pre-surgical assessment is not a measure of a true baseline. In addition, an abbreviated cognitive measure was used pre-surgically in order to accommodate families’ busy schedules at this time, yet this may underestimate actual deficits on cognitive domains not assessed (Burgess et al., 2018; Pulsifer et al., 2018). Obtaining a full comprehensive assessment pre-surgery is warranted and may be more feasible with testing children in the hospital. Both tumor size and velocity of growth may be important predictors of cognitive functioning, yet measurement prior to the presence of a tumor is extremely limited, and not feasible in this population, as children are mainly identified after becoming symptomatic. Third, although the present study is longitudinal in design, it did not follow children past two years following BT diagnosis. Some studies postulate that cognitive decline secondary to radiation therapy occurs three to four years after treatment, therefore, it is possible that our study does not capture all children who ultimately go on to experience cognitive problems (Butler et al., 2013; Nathan et al., 2007; Robinson et al., 2013). Fourth, we were limited to those who completed all three timepoints. A larger sample size would have allowed for more exploration of group, time, and group by time interactions, and greater statistical power to examine histologic tumor type, precise tumor grade, specific tumor location, specific adjuvant therapy received, and other complications or neurological impairments (e.g., neuroendocrine dysfunction, posterior fossa syndrome, need for anti-epileptics) in relation to children’s cognitive functioning. This should be a priority for research. Finally, while power to detect differences in cognitive functioning was increased by creating dichotomous groups for each medical variable considered, the finer details related to the tumor itself and treatment type were blurred.
The present study highlights the need for tracking of cognitive outcomes in children with BT, where multidisciplinary teams should aim to enroll and retain participants through close communication and collaboration with a child’s oncology team, since these providers often act as primary care providers for children even years into survivorship. Consideration should be given to incorporating neurocognitive assessment into multiple time points throughout a child’s treatment, so that it is given the same prioritization as adjuvant therapy or labs. Assessment is also important, as parent-administered questionnaires of child functioning often do not correspond with cognitive measures in children with neurological disorders (Coutinho et al., 2017). The present results demonstrate cognitive deficits in some patients, cognitive ability is closely tied to academic achievement (Wechsler, 2003). Adverse effects on school achievement have been documented in school-age children with CNS tumors, including deficits in reading, math, spelling, attention, verbal memory, and language (Robinson et al., 2010). Therefore, clinical psychologists should advocate for children to receive the proper academic accommodations to facilitate transitions back to school after treatment and continually monitor functioning for children who may continue to deal with cognitive deficits years after treatment for BT, including information for parents and teachers on cancer survivorship, tailoring accommodations for children’s specific needs (e.g., increased time on tests, language intervention) (Thompson et al., 2015).
In light of differences in cognitive function by tumor grade, future studies should examine the effect of tumor characteristics on cognitive functioning, including tumor volume and tumor microenvironment, as it may affect surrounding tissues. It is important to continue to identify factors that impact cognitive functioning, so that we can better understand the mechanisms that underlie deficits in this population, as well as identify protective factors that may buffer against cognitive decline. Finally, interventions focusing on cognitive remediation should be developed and implemented so that we can reverse or slow changes in brain structure and function in children diagnosed with a primary BT, as well as the necessary supports in school systems to assist with academic outcomes.
Children diagnosed with a BT can experience significant cognitive effects years after treatment, yet prospective longitudinal studies are sparse. Our results demonstrate the feasibility of pre-surgical assessment to establish baseline functioning, and longitudinal assessment over the course of treatment and its completion to disentangle how diagnostic factors (e.g., tumor complications, tumor location, tumor severity) and treatment type affect cognition. Despite testing children during this difficult time filled with appointments and treatments, overall, our protocol was feasible and showed differences in cognitive ability as early as pre-surgery. Primary BT survivors should be followed longitudinally, receiving continued monitoring and assessment, and tumor severity and treatment type in particular should be taken into consideration as these children progress through treatment and survivorship.
Supplementary Material
Acknowledgements.
This research was supported by a gift from an anonymous donor and in part by a grant from the National Cancer Institute (R21 CA175840).
Footnotes
Conflict of interest. The authors have no conflicts of interest to disclose.
Data availability statement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Of note, FSIQ, including working memory and processing speed subtests, was significantly lower than the calculated GAI, which does not include working memory and processing speed subtests, at T2, t = 4.50, p < .001, and at T3, t = 3.60, p = .002, each by approximately four IQ points. Therefore, all analyses were conducted with both GAI and FSIQ calculated from the standard 10 subtests administered from the WISC-IV at T2 and T3 in order to ensure that cognitive function across time points was comparable, and also account for potential effects altered by the inclusion of working memory and processing speed subtests which were only assessed at T2 and T3. Of note, all results but one remained significant when using FSIQ vs. GAI and is noted.
All analyses with run both with and without the two participants who had died over the course of the study. One participant had completed only a T1 assessment and the other had only completed a T2 assessment, as noted in Supplementary Table 1. No results changes as a result of their exclusion, therefore all results include these data where applicable.
This is the one result that became non-significant when using FSIQ, F(1,23) = 2.55, p = .10, instead of GAI.
References
- Aleksonis HA, Wier R, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF, … & Hoskinson KR (2019). Associations among diffusion tensor imaging and neurocognitive function in survivors of pediatric brain tumor: A pilot study. Applied Neuropsychology: Child, 1–12. [DOI] [PubMed] [Google Scholar]
- Burgess L, Pulsifer MB, Grieco JA, Weinstein ER, Gallotto S, Weyman E, … & Yock TI (2018). Estimated IQ systematically underestimates neurocognitive sequelae in irradiated pediatric brain tumor survivors. International Journal of Radiation Oncology* Biology* Physics, 101(3), 541–549. [DOI] [PubMed] [Google Scholar]
- Butler RW, Fairclough DL, Katz ER, Kazak AE, Noll RB, Thompson RD, & Sahler OJ (2013). Intellectual functioning and multi-dimensional attentional processes in long-term survivors of a central nervous system related pediatric malignancy. Life Sciences, 93, 611–616. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Coutinho V, Câmara-Costa H, Kemlin I, Billette de Villemeur T, Rodriguez D, & Dellatolas G (2017). The discrepancy between performance-based measures and questionnaires when assessing clinical outcomes and quality of life in pediatric patients with neurological disorders. Applied Neuropsychology: Child, 6(4), 255–261. [DOI] [PubMed] [Google Scholar]
- Desjardins L, Solomon A, Janzen L, Bartels U, Schulte F, Chung J, … & Barrera M (2018). Executive functions and social skills in pediatric brain tumor survivors. Applied Neuropsychology: Child, 1–9. [DOI] [PubMed] [Google Scholar]
- Hardy KK, Bonner MJ, Willard VW, Watral MA, & Gururangan S (2008). Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psycho-Oncology, 17, 1157–1161. [DOI] [PubMed] [Google Scholar]
- Iuvone L, Peruzzi L, Colosimo C, Tamburrini G, Caldarelli M, Di Rocco C, … Ricardi R (2011). Pretreatment neuropsychological deficits in children with brain tumors. Neuro-Oncology, 13, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahalley LS, Conklin HM, Tyc VL, Hydson MM, Wilson SJ, Wu S, … Hinds PS (2013). Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psycho-Oncology, 22(9), 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SJ, Conklin HM, Palmer SL, Schreiber JE, Armstrong CL, Wallace D, … Gajjar A (2014). Working memory abilities among children treated for medulloblastoma: Parent report and child performance. Journal of Pediatric Psychology, 39(5), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law N, Bouffet E, Laughlin S, Laperrier N, Briere ME, Strother D, … Mabbott D (2011). Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: Impact on working memory. Neuroimage, 56(4), 2238–2248. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Lee H, Zhu J, Xiong X, Wheeler G, Phipps S, … Sanford RA (2004). The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. Journal of Neurosurgery: Pediatrics, 101(2), 159–168. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Conklin HM, Wu S, Lustig RH, & Xiong X (2009). Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. Journal of Clinical Oncology, 27, 3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA & Brown PD (2006). Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. Journal of Clinical Oncology, 24, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Nathan PC, Patel SK, Dilley K, Goldsby R, Harvey J, Jacobsen C, …Armstrong FD (2007). Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: A report from the Children’s Oncology Group. Archives of Pediatrics & Adolescent Medicine, 161(8), 798–806. [DOI] [PubMed] [Google Scholar]
- Ness KK & Gurney JG (2007). Adverse late effects of childhood cancer and its treatment on health and performance. Annu Rev. Public Health, 28, 279–302. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, & Barnholtz-Sloan JS (2016). CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncology, 18 (supp 5)I, v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, … Robison LL (2006). Chronic health conditions in adult survivors of childhood cancer. New England Journal Medicine, 355, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Panigrahy A & Bluml S (2009). Neuroimaging of pediatric brain tumors: From basic to advanced magnetic resonance imaging (MRI). Journal of Child Neurology, 24, 1343–1365. [DOI] [PubMed] [Google Scholar]
- Porter KR, McCarthy BJ, Freels S, Kim Y, & Davis FG (2010). Prevalance estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-Oncology, 12, 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prifitera A, Weiss LG, Rolfhus E, & Saklofske DH (2005). The WISC-IV in the clinical assessment context. In WISC-IV clinical use and interpretation (pp. 3–32). Academic Press. [Google Scholar]
- Pulsifer MB, Duncanson H, Grieco J, Evans C, Tseretopoulos ID, MacDonald S, … & Yock TI (2018). Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. International Journal of Radiation Oncology* Biology* Physics, 102(2), 391–398. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Kuttesch JF, Champion JE, Andreotti CF, Hipp DW, Bettis A, … Compas BE (2010). A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatric Blood & Cancer, 55, 525–531. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Fraley CE, Pearson MM, Kuttesch JF, & Compas BE (2013). Neurocognitive late effects of pediatric brain tumors of the posterior fossa: A quantitative analysis. Journal of International Neuropsychology Soc, 19, 44–53. [DOI] [PubMed] [Google Scholar]
- Shortman RI, Lowis SP, Penn A, McCarter RJ, Hunt LP, Brown CC, …Sharples PM (2014). Cognitive function in children with brain tumors in the first year after diagnosis compared to healthy matched controls. Pediatric Blood & Cancer, 61, 464–472. [DOI] [PubMed] [Google Scholar]
- Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, & Mabbott DJ (2004). Change in neurocognitive functioning after treatment with cranial radiation in childhood. Journal of Clinical Oncology, 22, 706–713. [DOI] [PubMed] [Google Scholar]
- Stargatt R, Rosenfeld JV, Maixner W, & Ashley D (2007). Multiple factors contribute to neuropsychological outcome in children with posterior fossa tumors. Developmental Neuropsychology, 32, 729–748. [DOI] [PubMed] [Google Scholar]
- Thompson AL, Christiansen HL, Elam M, Hoag J, Irwin MK, Pao M, … & Kelly KP (2015). Academic continuity and school reentry support as a standard of care in pediatric oncology. Pediatric Blood & Cancer, 62(S5), S805–S817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment, Inc.; 1999. [Google Scholar]
- Wechsler D WISC-IV: Wechsler Intelligence Scale for Children-4th edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Willard VW, Conklin HM, Boop FA, Wu S, & Merchant TE (2013). Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. International Journal of Radiation Oncology* Biology* Physics, 88(4), 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KR, Madan-Swain A, & Kana RK (2012). Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Developmental Neuropsychology, 37(2), 153–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.