Abstract
Research suggests that physical activity may influence sleep, yet more research is needed before it can be considered a front line treatment for insomnia. Less is known about how this relationship is moderated by age. Using multilevel modelling, we examined self-reported physical activity and insomnia symptoms in 18,078 respondents from the U.S. nationally representative Health and Retirement Study (2004 through 2014). Mean baseline age was 64.7 years with 53.9% female. Individuals who reported more physical activity (B = −0.005, p < .001) had fewer insomnia symptoms. Over ten years, respondents reported fewer insomnia symptoms at times when they reported more physical activity than average (B = −0.003, p < .001). Age moderated this relationship (B = 0.0002, p< .01). Although modest, these findings concur with literature suggesting moderate benefits of physical activity for sleep in older adults. Future research should aim to further elucidate this relationship among adults at advanced ages.
Keywords: Physical activity, Insomnia symptoms, Multilevel models, Health and Retirement Study
Introduction
As life expectancy in the United States (U.S.) continues to increase, there is growing research and policy focus on ensuring that those extra years are good quality years with an emphasis, for example, on understanding factors associated with active life expectancy (Freedman & Spillman, 2016). Indeed, Rowe and Kahn’s seminal 1998 publication Successful Aging led to a generation of research focused on the causes and consequences of positive aging (Whitley, Popham, & Benzeval, 2016). One area that has received less attention in the context of an aging population, however, is the relationship between physical activity and insomnia. Insomnia—largely characterized by difficulty falling asleep and/or difficulty staying asleep—is prevalent in adulthood (Mander, Winer, & Walker, 2017). The National Sleep Foundation (National Sleep Foundation, 2013) reports that 44% of adults in the U.S. aged 55–84 years report at least a few nights of disturbed sleep each week; those with insomnia may experience related symptoms such as fatigue, difficulty concentrating, and mood changes. In addition, insomnia has been shown to be associated with shorter survival (Ancoli-Israel & Cooke, 2005). Therefore, treating insomnia may improve quality and even length of life (Bjornsdottir et al., 2015; Singh, Clements, & Fiatarone, 1997).
Given the risks associated with pharmacological treatments for insomnia symptoms, especially for older adults (Campanelli, 2012), physical activity has been advanced as a safe and effective alternative method of addressing these problems at older ages (Varrasse, Li, & Gooneratne, 2015). A major advantage of physical activity in this regard is that is has the potential to be widely disseminated since it does not require access to medical care (Kredlow, Capozzoli, Hearon, Calkins, & Otto, 2015). A variety of potential physiological mechanisms have been articulated to explain the connection (Buman & King, 2010). For example, physical activity may reduce arounsal, anxiety, and depression, which can improve sleep. Exercise also tends to increase bodily temperature, and the later drop can help induce sleep. Buman and King (2010) conclude that it is likely several mechanisms are at play simultaneously.
Studies investigating a possible connection between physical activity and sleep utilize a range of study designs and statistical methods, and therefore make unique contributions. Some of the strongest causal evidence for an association comes from intervention studies (Baron, Reid, & Zee, 2013; Buman, Hekler, Bliwise, & King, 2011a; Buman, Hekler, Bliwise, & King, 2011b; Buman & King, 2010; Hartescu, Morgan, & Stevinson, 2015; King, Oman, Brassington, Bliwise, & Haskell, 1997; Reid et al., 2010; Tang & Sanborn, 2014). Given the relatively small and restricted samples of these studies, however, they have limited generalizability to U.S. adults.
Relatively few studies have reported on national level data—and only two in the in the U.S.—to explore effects of physical activity on sleep. The National Sleep Foundation conducted a cross-sectional survey using a national sample and identified a relationship between self-reported activity and sleep quality (National Sleep Foundation, 2003). Older adults who exercised less than once per week were more likely to report experiencing a symptom of insomnia, such as difficulty falling asleep, compared to those who exercised three or more times per week (58% vs. 43%). Another study used data from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. Those who met recommended physical activity guidelines reported better sleep than those not meeting guidelines (Loprinzi & Cardinal, 2011). These studies were drawn from nationally representative samples yet feature cross-sectional designs. Researchers have long called for longitudinal epidemiologic studies that allow for the study of change over time (Youngstedt & Kline, 2006).
Recent studies have begun to use community samples with longitudinal designs to study the connection between exercise and sleep, leveraging their longitudinal data to study both within and between person effects (e.g., Dzierzewski et al., 2014; Holfeld & Ruthig, 2014). While cross-sectional studies can inform us on whether individuals who are more physically active have fewer insomnia symptoms than those who are less active (between persons effects), longitudinal studies featuring multilevel designs can also inform us on within-person effects, or whether or not individuals experience better sleep at a time when they are more physically active than is average for them. However, few studies have examined within-person trajectories of change over many years. More research is needed in larger samples featuring longitudinal designs with long-term follow-up.
Finally, age may serve to moderate the relationship between physical activity and sleep given that sleep tends to worsen, albeit modestly (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004) and physical activity tends to diminish (DiPietro, 2001; Milanović et al., 2013) with advancing age. A recent meta-analysis of studies on this topic indicated preliminary evidence of a moderating effect of age (Kredlow et al., 2015).
In summary, while physical activity may influence sleep, more research is needed before physical activity can be considered a “first-line treatment” for sleep problems (Varrasse et al., 2015). Therefore, using a multilevel modelling approach that takes advantage of multiple observations over a long follow-up period, we considered the association between physical activity and insomnia symptoms. We leverage the rich data resources of the Health and Retirement Study (HRS), a national probability sample of noninstitutionalized U.S. adults aged 51 and older who were interviewed up to four times over a 10 year period. While each of these features of study design and multilevel modelling statistical methods have been featured in the literature, this is the first study to combine all of them in one study. Our longitudinal model extends prior research by exploring how physical activity varies over a long period of follow-up (ten years) and whether individual variation is associated with changes in insomnia symptoms. Specifically, we hypothesized that over ten years, times when individuals are more active will be associated with fewer insomnia symptoms. Furthermore, given the possible importance of age in these associations, we hypothesized that physical activity has a stronger impact on insomnia symptoms in late middle age than at older ages.
Methods
Data
We analyzed data from the 2004 to 2014 waves of the HRS, a nationally representative longitudinal survey of adults over the age of 50 in the US. HRS also enrolls spouses or partners of any age. The study began in 1992 with a core interview administered every two years that includes information on health, employment, and financial status as well as family connections. In 1998, HRS instituted a steady-state design, enrolling a new birth cohort every six years. HRS features a complex sample design with oversampling of African American and Hispanic headed households. Sampling weights and design variables are provided, which adjust for differential selection and correct variance estimation for geographic stratification and clustering, respectively. Further details of the study design and content are available elsewhere (Sonnega et al., 2014). For most variables, we used the RAND data, a cleaned and ready-to-use version of much of the HRS data (Bugliari et al., 2016). All respondents have provided consent, and the study protocol has been approved by the University of Michigan Institutional Review Board (IRB).
Analytic Sample
While the insomnia symptom variables were available beginning in the HRS in 2002, we chose 2004 as our baseline in order to maximize our sample for longitudinal analysis since 2004 was the enrollment year for the Early Baby Boomer cohort, born 1948–53. HRS collected information on insomnia symptoms biennially until 2006 and then only in alternate waves thereafter. Thus, our analytic sample included all respondents impaneled in 2004 with potential follow-up interviews in 2006, 2010, and 2014. We began with 20,129 respondents who participated in the 2004 wave of the HRS. We selected 18,954 respondents who were over the age of 50. We further selected respondents with a positive value on the sampling weight (n=18,495). Finally, we excluded study respondents who were experiencing insomnia (defined as >=2.75 on the mean scale, corresponding to experiencing all four symptoms most of the time) at baseline. The final analytic sample was 18,078 respondents (~72,000 person-waves). Figure 1 portrays the sample selection. We conducted comparisons of excluded study respondents to those included on several basic demographics. Comparing the original sample to the final analytic sample, the analytic sample was older with a slightly smaller percentage of female respondents.
Figure 1.
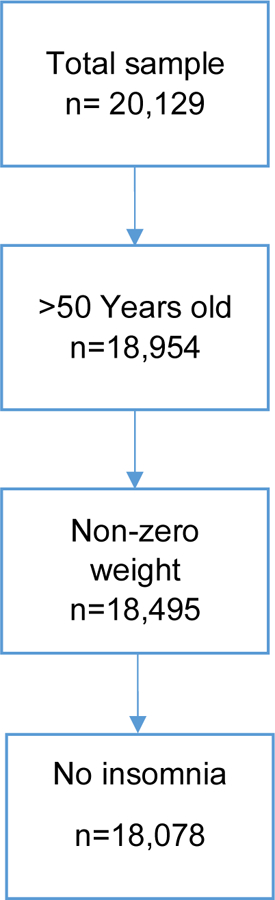
Flowchart of sample selection at baseline in 2004
Measures
Insomnia symptoms.
HRS includes a set of questions that have been used in research to indicate symptoms of insomnia (e.g., Leggett, Pepin, Sonnega, and Assari, 2016; Kaufman et al., 2013). Respondents were asked how often “do you have trouble falling asleep,” “do you have trouble with waking up during the night,” “do you have trouble with waking up too early and not being able to fall asleep again” and “do you feel really rested when you wake up in the morning?” Response categories were 1=“most of the time,” 2=“sometimes,” and 3=“rarely or never.” Note that that the questions ask respondents to recall these behaviors without reference to a particular time frame. We reverse-scored the first three items and created a mean scale for each wave that ranged from 1 to 3 with a higher number indicating worse insomnia.
Physical activity.
Respondents were asked three questions about their frequency of vigorous, moderate, and mild physical activity: “We would like to know the type and amount of physical activity involved in your daily life. How often do you take part in sports or activities that are vigorous, such as running or jogging, swimming, cycling, aerobics or gym workout, tennis, or digging with a spade or shovel?” “And how often do you take part in sports or activities that are moderately energetic such as, gardening, cleaning the car, walking at a moderate pace, dancing, floor or stretching exercises?” and “And how often do you take part in sports or activities that are mildly energetic, such as vacuuming, laundry, home repairs?” As with the questions about insomnia, these questions also do not reference to a particular time frame. The response categories are 1=”hardly ever or never,” 2=“one to three times a month,” 3=“once a week,” 4=“more than once a week,” and 4=“everyday.”
Given our interest in capturing the full range of physical activity reported in these items that might reflect the changing patterns of physical activity with age (from relatively more to less energy expended), we followed others (He & Baker, 2005; Wen, Li, & Su, 2014) in creating a scale that accounted for the relative energy expended across levels of intensity and frequency reported. This method approximates the metabolic equivalent of task (MET) within one scale. Thus, for each wave we created a summed scale for physical activity with activity points weighted such that each level of intensity was doubled across the frequencies as follows. Mild activity: 0=“hardly ever or never,” .5=“one to three times a month,” 1=“once a week,” 4=“more than once a week,” and 7=“everyday”. Moderate activity: 0=“hardly ever or never,” 1=“one to three times a month,” 2=“once a week,” 8=“more than once a week,” and 14=“everyday”. Vigorous activity: 0=“hardly ever or never,” 2=“one to three times a month,” 4=“once a week,” 16=“more than once a week,” and 28=“everyday”. We created a summed score for each wave and divided by three to ease interpretation of point estimates. The final physical activity variable ranged from 0 to 16.33.
Covariates.
We controlled for several covariates that may be associated with insomnia symptoms (Chen et al., 2015) and physical activity (DiPietro, 2001). Several covariates were taken from the respondent’s baseline interview including; gender coded as 1=female, 0=male; race coded as 1=white, 0=non-white; ethnicity coded as 1=Hispanic, 0=non-Hispanic; marital status coded as 1=married, 0=not married; and education coded as years of schooling (0–17). For all other covariates, we included wave-specific measures. Age in years was the respondents’ age in each wave. Chronic medical conditions was a count of up to 8 conditions: high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, psychiatric problems, and arthritis (range 0–8). A modified 8-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) was used for measurement of depressive symptoms. Respondents reported on the extent to which in the previous week, they felt: depressed, everything was an effort, sleep was restless, that he or she could not get going, lonely, that he or she enjoyed life, sad, and happy. To deal with differences in response categories over waves, RAND codes these dichotomously as yes or no. To minimize operational confounding of this covariate with the outcome, we removed the item about restless sleep. We reverse coded the positive items and summed the remaining 7 items for a count of recent depressive symptoms ranging from 0 to 7. Previous research using the 8- item version has indicated that a cut-off of 4 or more symptoms corresponds to a clinically significant level of depressive symptoms (Zivin et al., 2010). Body mass index (BMI) was calculated according to the standard formula (weight in kilograms by the square of height in meters) from self-reported height and weight at each wave. Wave specific indicators for any current consumption of alcohol (1=yes, 0=no) and current smoking (1=yes, 0=no) were included. To account for season when the interview was conducted, we created a dummy variable where interviews conducted from November 1 through March 31=1 and interviews conducted from April 1 through October 31=0.
Statistical Analyses
Descriptive statistics were generated using sampling weights and design variables to adjust for the complex sample design (Heeringa, West, & Berglund, 2017). We also described the intensity of physical activity within age groups. We conducted multilevel modelling (MLM) using PROC MIXED (Littell, Milliken, Stroup, & Wolfinger, 1996) in SAS version 9.4 (SAS Institute Inc., Cary, NC) to examine variability in physical activity and insomnia symptoms between respondents and within respondents across waves for up to four waves over up to 10 years. This approach has the advantage of utilizing all available waves of data (Raudenbush & Bryk, 2002). We first obtained the intraclass correlation coefficient for the insomnia and physical activity variable. We evaluated the effect of age as a moderator of the association between physical activity and insomnia symptoms by including an interaction between time-varying age and physical activity at both the between- and within-person levels. We evaluated three models. Model 1 examined the effect of just the control variables on insomnia symptoms. Model 2 added physical activity at both the between- and within-person levels. In Model 3, we added interaction terms for age and physical activity to test for moderation at both the between- and within-person levels. To further explore the region of significance for the interaction terms, we employed the Johnson-Neyman technique for probing interactions (Bauer & Curran, 2005) using an online calculator (http://www.quantpsy.org/interact/hlm2.htm). The time-varying covariates age, chronic medical conditions, depressive symptoms, BMI, current smoking, current drinking, season of interview and physical activity were included at the between-person level as person-specific means, which represent an individual’s average on that variable across the four waves. Gender, race, Hispanic ethnicity, marital status, and education were entered from the baseline interview. Within-person level predictors represented deviations in a given wave from an individual’s own mean across up to four waves such that a positive score indicates a wave value that is above the individual’s mean and a negative score indicates a wave value below the individual’s mean (Hoffman & Stawski, 2009). The equation presented below represents the final model.
Insomnia symptoms for the wth wave for the ith person were modeled as:
Level 1: Insomnia symptomswi = β0i + β1i (Agewi) + β2i (Chronic conditionswi) + β3i (Depressive symptomswi) + β4i (Body mass indexwi) + β5i (Smokingwi) + β6i (Drinkingwi) + β7i (Physical activitywi) + β8i (Age * Physical activitywi) + ewi
Level 2: β0i = γ00 + γ01(Genderi) + γ02 (Racei) + γ03 (Hispanic ethnicityi) + γ04 (Marital statusi) + γ05 (Educationi) + γ06 (Seasoni) + γ07 (Chronic conditionsi) + γ08 (Depressive symptomsi) + γ09 (Body mass indexi) + γ10 (Smokingi) + γ11 (Drinkingi)+ γ12 (Physical activityi) + γ13 (Age * Physical activityi) + u0i
| (Model 3) |
where the intercept (β0i) represents the mean level of insomnia symptoms for each individual (averaged across waves). The first slope (β1i) represents the effect of age at a given wave w (w = 1–4) on the respondent’s insomnia symptoms at wave w. Similarly, the second and third slope parameters (β2i, β3i) are within-person level controls for chronic condition and depressive symptoms. The fourth, fifth, and sixth slope parameters (β4i, β5i. β6i) represents the effect of body mass index, smoking, and drinking. The seventh slope represents the effect of physical activity. As described, these covariates reflect deviations from an individual’s own mean and were centered around the person-mean. The eighth slope parameter (β8i) tests whether the within-person association between physical activity and insomnia symptoms is moderated by age.
At Level 2, person-mean level of physical activity (γ12) is entered as a between-person covariate predicting the level of insomnia symptoms at the intercept (β0i)—likewise for season (γ06), chronic conditions (γ07), depressive symptoms (γ08), and body mass index (γ09), smoking(γ10), an drinking (γ11), Each of these covariates indicates an individuals’ average level of that characteristic across waves. Gender (γ01), race (γ02), Hispanic ethnicity (γ03), marital status (γ04), and education (γ05) were also entered as wave invariant, between-person controls. All models also adjust for a wave indicator variable (w = 1–4). γ10, γ20, γ30, and γ40 represent the average effects of within-person slopes from Level 1 for chronic conditions, depressive symptoms, body mass index, drinking, smoking, and physical activity, and the physical activity by age interaction, respectively. γ00 reflects the group mean level of individual insomnia symptom levels, and u0i reflects individual deviations from that mean.
We also conducted a set of sensitivity analyses including: restricting the initial age range to 51 to 70, conducting a stratified analyses by gender, excluding study respondents with elevated depressive symptoms (>=4 symptoms) from the analysis, and excluding study respondents with any chronic medical conditions. All analyses were conducted in 2019.
Results
We report summary statistics for all study variables across waves in Table 1. The average age of the sample at baseline in 2004 was 64.38 years. Respondents reported a mean insomnia symptom score of 1.60 to 1.66 (se = 0.01; range 1–3 for the mean scale) across waves, indicating that on average respondents were experiencing at least some insomnia symptoms rarely to sometime. Respondents reported a mean physical activity score of 4.00 to 4.45 (se = 0.04–0.05; range 0–16.33) across waves. These averages on the weighted score correspond roughly to weekly participation in moderate physical activity. The level of depressive symptoms was relatively low to moderate (1.28 to 1.43) and below what may be considered clinically significant (>=4 on the summary score).
Table 1.
Sample Characteristics of Key Variables across Waves and by Age (Physical Activity and Insomnia Symptoms)
| Wave 1 (2004) n=18,078 Mean or percent (se) |
Wave 2 (2006) n=16,182 Mean or percent (se) |
Wave 3 (2010) n=13,440 Mean or percent (se) |
Wave 4 (2014) n=10,952 Mean or percent (se) |
|
|---|---|---|---|---|
|
|
||||
| Age | 64.69 (0.17) | |||
| Range (51–107) | ||||
| Female (%) | 53.92 (0.31) | |||
| Non-White (%) | 14.30 (0.68) | |||
| Hispanic ethnicity (%) | 7.28 (0.88) | |||
| Married (%) | 66.78 (0.55) | |||
| Education | 12.75 (0.07) | |||
| Range (0–17 years) | ||||
| Chronic conditions | 1.71 (0.01) | 1.89 (0.02) | 2.23 (0.01) | 2.48 (0.02) |
| Range (0–8) | ||||
| Depressive symptoms | 1.39 (0.03) | 1.43 (0.03) | 1.29 (0.02) | 1.28 (0.03) |
| Range (0–7) | ||||
| Body Mass Index | 27.56 (0.07) | 27.93 (0.08) | 28.18 (0.07) | 28.20 (0.07) |
| Current Smoking (%) | 15.80 (0.01) | 14.02 (0.01) | 11.76 (0.00) | 9.22 (0.00) |
| Current drinking (%) | 79.23 (0.02) | 72.96 (0.02) | 74.43 (0.02) | 73.02 (0.02) |
| Weighted Physical | ||||
| Activity | ||||
| Total | 4.29 (0.05) | 4.45 (0.05) | 4.02 (0.04) | 4.00 (0.05) |
| 51–60 | 4.79 (0.07) | 5.00 (0.08) | 4.57 (0.07) | 4.52 (0.07) |
| 61–70 | 4.46 (0.07) | 4.58 (0.08) | 4.03 (0.07) | 3.89 (0.08) |
| 71–80 | 3.76 (0.07) | 3.86 (0.08) | 3.16 (0.08) | 2.86 (0.11) |
| 81–90 | 2.77 (0.10) | 2.71 (0.08) | 2.06 (0.10) | 1.87 (0.13) |
| 90+ | 1.73 (0.16) | 1.57 (0.23) | 1.05 (0.40) | 0.35 (0.30) |
| Range (0–16.33) | ||||
| Insomnia symptoms | ||||
| Total | 1.60 (0.01) | 1.62 (0.01) | 1.65 (0.01) | 1.66 (0.01) |
| 51–60 | 1.60 (0.01) | 1.62 (0.01) | 1.64 (0.01) | 1.65 (0.01) |
| 61–70 | 1.59 (0.01) | 1.60 (0.01) | 1.64 (0.01) | 1.67 (0.01) |
| 71–80 | 1.61 (0.01) | 1.63 (0.01) | 1.68 (0.01) | 1.68 (0.01) |
| 81–90 | 1.63 (0.01) | 1.65 (0.02) | 1.64 (0.02) | 1.64 (0.02) |
| 90+ | 1.64 (0.03) | 1.67 (0.04) | 1.63 (0.07) | 1.58 (0.11) |
| Range on mean scale (0–3) | ||||
Note: Percentages and means are weighted using sampling weights and standard errors corrected for the complex sample design as described in the text. Sample sizes reflect the unweighted n.
Source: Health and Retirement Study 2004–2014.
In Table 2, we show the mean level of physical activity by level of intensity using the original variables of mild, moderate, and vigorous activity by age groups. The mean value of 3.58 for 51–60 year olds suggests a high level (nearly every day) of mild activity but by age 81–90, this falls to 2.92 corresponding roughly to less than once a week. For vigorous activity, a mean of 2.26 for 51–60 year olds corresponds to several times a month. This declines to almost never at older ages.
Table 2.
Mean Level of Mild, Moderate, and Vigorous Physical Activity by Age Group (Baseline)
| Age group | Mild activity | Moderate activity | Vigorous activity |
|---|---|---|---|
| 51–60 | 3.58 (0.2) | 3.27 (0.02) | 2.26 (0.02) |
| 61–70 | 3.45 (0.2) | 3.19 (0.03) | 2.08 (0.02) |
| 71–80 | 3.26 (0.3) | 3.00 (0.03) | 1.80 (0.03) |
| 81–90 | 2.92 (0.3) | 2.62 (0.05) | 1.48 (0.04) |
| 90 and older | 2.13 (0.9) | 2.10 (0.09) | 1.23 (0.05) |
Note: Means are weighted using sampling weights. Corrected standard errors are in parentheses.
Source: Health and Retirement Study 2004–2014.
The intraclass correlation coefficient for insomnia symptoms indicated that 42% of the variance in insomnia symptoms was between-person (across respondents) and 54% was within-person (for the same person, across survey waves). Similar levels obtained for physical activity. These levels provided justification for sufficient variability at both levels for the MLM approach.
Results from the two-level multilevel model are displayed with coefficients and standard errors in Table 3. In Model 1, considering the within-person effects in the upper portion of the table, in a wave when an individual reported older age (γ10; Β = −0.002, p < .001), a greater number of chronic conditions (γ20; Β = 0.020, p < .001), a higher number of depressive symptoms (γ30; Β = 0.041, p < .001), higher BMI (γ40; Β = 0.002, p < .01), and current alcohol consumption (γ60; Β = 0.004, p < .05), their level of insomnia symptoms was higher. Considering between person effects revealed that individuals who were female (γ01; B = 0.085, p < .001), Black (γ02; B = 0.060, p < .001), non-Hispanic ethnicity (γ03; B = −0.051, p < .001), less educated (γ05; B = −0.006, p < .001) and with more chronic conditions (γ07; B = 0.048, p < .001) and a higher level of depressive symptoms (γ08; B = 0.107, p < .001) reported more insomnia symptoms.
Table 3.
Two-level Multilevel Model of Demographics, Health Characteristics, and Physical Activity on Insomnia Symptoms
| Insomnia Symptoms | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| B | (se) | B | (se) | B | (se) | |
|
|
||||||
| Fixed Effects | ||||||
| Intercept, γ00 | 1.587*** | (0.040) | 1.644*** | (0.042) | 1.69*** | (0.049) |
| Within-Person Covariates | ||||||
| Age, γ10 | −0.002*** | (0.001) | −0.003*** | (0.000) | −0.003*** | (0.001) |
| Chronic conditions, γ20 | 0.020*** | (0.003) | 0.018*** | (0.003) | 0.019*** | (0.003) |
| Depressive symptoms, γ30 | 0.041*** | (0.001) | 0.040*** | (0.001) | 0.040*** | (0.001) |
| Body mass index, γ40 | 0.002** | (0.001) | 0.002* | (0.001) | 0.002 | (0.001) |
| Smoking, γ50 | −0.006 | (0.011) | −0.005 | (0.011) | −0.006 | (0.013) |
| Drinking, γ60 | 0.004* | (0.002) | 0.005* | (0.002) | 0.004* | (0.002) |
| Physical activity, γ70 | -- | -- | −0.003*** | (0.001) | −0.016** | (0.005) |
| Age*Physical activity, γ80 | -- | -- | -- | -- | 0.0002** | (0.000) |
| Between-Person Covariates | ||||||
| Gender, γ01 | 0.085*** | (0.006) | 0.083*** | (0.006) | 0.083*** | (0.006) |
| Race, γ02 | 0.060*** | (0.008) | 0.062*** | (0.007) | 0.062*** | (0.008) |
| Hispanic ethnicity, γ03 | −0.051*** | (0.010) | −0.050*** | (0.011) | −0.050*** | (0.011) |
| Marital status, γ04 | 0.012 | (0.007) | 0.012 | (0.006) | 0.012 | (0.007) |
| Education, γ05 | −0.006*** | (0.001) | −0.006*** | (0.001) | −0.006*** | (0.001) |
| Season, γ06 | 0.001 | (0.001) | 0.001 | (0.000) | 0.001 | (0.000) |
| Chronic conditions, γ07 | 0.048*** | (0.002) | 0.046*** | (0.002) | 0.046*** | (0.002) |
| Depressive symptoms, γ08 | 0.107*** | (0.002) | 0.105*** | (0.002) | 0.105*** | (0.002) |
| Body mass index, γ09 | 0.001 | (0.001) | 0.000 | (0.001) | 0.000 | (0.001) |
| Smoking, γ10 | −0.024 | (0.010) | −0.030** | (0.010) | −0.031** | (0.010) |
| Drinking, γ11 | 0.005 | (0.003) | 0.006* | (0.002) | 0.006* | (0.003) |
| Physical activity, γ12 | -- | -- | −0.005*** | (0.001) | −0.017** | (0.006) |
| Age*Physical activity, γ13 | -- | -- | -- | -- | 0.0002 | (0.000) |
| Random Effects | ||||||
| Intercept, σ2u0 | 0.101*** | (0.002) | 0.101*** | (0.001) | 0.101*** | (0.002) |
| Residual, σ2e1 | 0.107*** | (0.001) | 0.107*** | (0.001) | 0.107*** | (0.001) |
| −2 Log Likelihood | 55051.1 | 55029.0 | 55052.7 | |||
Note. All models adjust for a wave indicator variable where w=1–4. Statistical significance indicated by
p<.05.
p< .01.
p< .001.
Source: Health and Retirement Study 2004–2014.
In Model 2, we tested within- and between-person associations between physical activity and insomnia symptoms in the presence of these control variables. For within person effects, respondents reported fewer insomnia symptoms at waves when they reported more physical activity (γ70; B = −0.003, p < .001) than their average levels. For between person effects, individuals who on average reported more physical activity had fewer insomnia symptoms (γ12; B = −0.005, p < .001). Note that, while statistically significant, the size of these effects is not large.
Lastly, Model 3 included interactions at both the within- and between-person levels to test whether age moderated the effect of physical activity on insomnia symptoms. The within-person level interaction was statistically significant, showing that age influenced the strength of the association between physical activity and insomnia symptoms (γ80; B = 0.0002, p < .01). The person-level interaction was not statistically significant (γ13; B = 0.0002, p=.06).
To provide a graphical display of the more statistically robust within-persons interaction (Figure 2), we demarcated late middle age as one standard deviation below the mean age at baseline (64 years) and older age as one standard deviation above the mean. The graphic representation of the interaction suggests that older adults derived less benefit to their sleep from physical activity than adults in late middle age. Results of the Johnson-Neyman analysis revealed that the moderating effect of insomnia symptoms lessened steadily with age across the entire age range analyzed.
Figure 2.
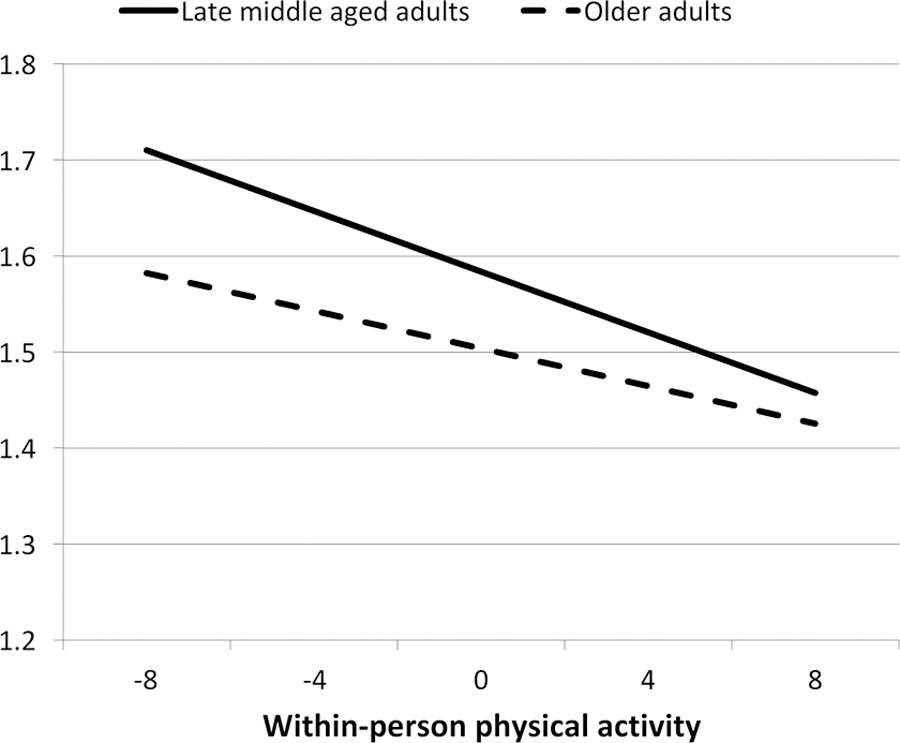
Age as a Moderator of the Physical Activity and Insomnia Symptoms Association
Note: Late middle age and older adults are indicated by 1 SD below and above mean age of 64 (baseline).
Discussion
In this nationally representative, longitudinal sample of older U.S. adults, we found that not only do individuals with higher levels of physical activity have fewer insomnia symptoms, but individuals who increase their level of physical activity have individual positive change in these symptoms. Age appears to moderate the latter relationship with stronger effects at in late middle age. This finding suggests that even over a long period of time, any individual level improvement in one’s level of physical activity may help to improve sleep, especially for adults in late middle age. These findings are generally in accord with a literature suggesting benefits of exercise for sleep (Baron et al., 2013; Buman et al., 2011a; Buman et al., 2011b; Buman & King, 2010; Dzierzewski et al., 2014; Hartescu et al., 2015; Holfeld & Ruthig, 2014; King et al., 1997; Kline, 2014; Kredlow et al., 2015; Loprinzi & Cardinal, 2011; National Sleep Foundation, 2013; Reid et al., 2010; Tang & Sanborn, 2014; Varrasse et al., 2015; Youngstedt & Kline, 2006).
Although we found statistically significant associations at both the within- and between-person levels between physical activity and insomnia symptoms, the relative size of the effects were modest. This result supports the broad finding that, to the extent that exercise affects sleep, the sizes of the effect are generally found to be moderate. For example, a review by Yang, Ho, Chen, and Chien (2012) found moderate benefits to sleep quality of participation in exercise training programs in middle-aged to older adults. Similarly, a recent meta-analysis found small effects of acute exercise on sleep but moderate to large effects of regular exercise on some indicators of sleep and small effects on other indicators (Kredlow et al., 2014).
Fewer studies have employed a MLM approach to study the relationship between physical activity and sleep. Although the exact insomnia measures differ and the follow-up periods are dramatically different, our results are comparable to the findings of Dzierzewski et al. (2014). These authors collected 18 weekly personal assessments of exercise and sleep quality using a daily diary method from 79 sedentary older adults living in the community. Multilevel modeling identified a modest positive between-person effect of exercise and wake time after sleep onset and a small within-person association between exercise and a general sleep quality rating (Dzierzewski et al., 2014).
In this sample of adults over age 50, we found that the positive impact of physical activity on insomnia symptoms was stronger for adults in late middle age compared to adults on average 20 years older, growing steadily weaker with increasing age. To our knowledge, only one other study has found a moderating effect of age with a similar conclusion that the benefit of exercise for sleep lessened with advancing age (Kredlow et al., 2015). These researchers found that for at least one variable studied, namely sleep onset latency, physical activity had stronger effects on sleep for younger compared to older adults. Clearly more research is needed to understand the relationship between physical activity and insomnia symptoms across later life. As demonstrated in Table 1, levels of physical activity, using our weighted measure and using the original items (Table 2), declined precipitously at older ages. It may be that some threshold level of physical activity is necessary to achieve benefits for sleep. That is, older people may not generally experience enough physical exertion to affect their sleep. In addition, it is interesting to speculate on potential age differences in putative mechanisms for the effect of physical activity on sleep. For instance, without a certain level of physical activity, the body temperature mechanism may be less effective. Or as older adults sleep patterns change in relation to circadian rhythms, this potential mechanism may differ as well. It is premature, however, to conclude that physical activity does not help individuals at advance older ages to experience fewer insomnia symptoms.
Sensitivity Analyses
We conducted a series of sensitivity analyses to check the robustness of our results. Given the finding that women experience higher rates of insomnia than men, and that this difference is particularly marked at older ages (Zhang & Wing, 2006), we conducted analyses that stratified by gender. Perhaps surprisingly, the models for men and women shared very similar coefficients and level of statistical significance (of the main effects and interactions) to each other and to the combined sample. Furthermore, given research showing differences in insomnia symptoms for those with psychiatric and medical comorbities (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004), we also conducted our analyses excluding study respondents with chronic medical conditions and excluding those respondents with elevated depressive symptoms. The findings were robust to exclusion of those with elevated depressive symptoms. However, in the sample excluding those with any chronic medical condition, which reduced the sample size considerably, the main effects of both between and within person effect of exercise on sleep were reduced (p<01 for both). The interaction of age and within person physical activity was also reduced (p<.05).
As in any long-term longitudinal cohort study, especially of older adults, mortality selection is a concern. One implication of this is that the sample becomes “healthier” over time. Zajacova and Burgard (2013) found substantial changes in sample composition over nine waves of the HRS and noted that, especially for research results with the much older adults, findings should be interpreted with caution. There are no simple ways to account for mortality selection; however, we conducted sensitivity analyses restricting the initial age range to 51 to 70 to reduce the force of mortality selection. Interestingly, the results remained the same.
Strengths and Limitations
A strength of this study is the data source, which allows us to generalize our findings to the U.S. population over age 50. The large sample size and longer-term follow-up allowed us to detect even small effects of physical activity on sleep both with and between persons over a long period of time. That is, on average over 10 years, making even small changes in one’s activity level relative to one’s norm can benefit sleep. Despite their modest size, these findings lend support to the potential value of physical activity for sleep as even small improvements in sleep for individuals can translate into large effects on population health.
Because the HRS is a large epidemiological study of middle aged and older US adults with a very wide range of content, to date, it contains only limited self-reported measurement of both physical activity and insomnia symptoms. These measures can be biased. For example, because of social desirability bias, self-reports of physical activity are often overreported owing potentially to an “exercise identity” in some respondents (Brenner & DeLamater, 2014). Some research suggests, however, that self-reported physical activity, at least, provides reasonably valid information (Silsbury, Goldsmith, & Rushton, 2015). Nonetheless, given the expectancy that people may have about the sleep benefits of physical activity, it may be that those who reported more physical activity were also more likely to report better sleep. Although the HRS is an observational longitudinal study in which respondents should not be aware of research hypotheses, this source of bias is a concern. In addition, neither the physical activity nor the insomnia questions are anchored to any time reference. Rather they ask respondents to report what is usual for them. This could lead the respondent to encompass a relatively long period of time in thinking about what is normal for them rather than current time. The advantage of the analysis we employed, however, is that it is well-suited to characterizing what is average for individuals over time.
Regarding the measurement of sleep, common age-related changes in sleep such as a decrease in sleeping hours or increased in night waking were not measured in the HRS. The sleep measures in HRS, which are more reflective of insomnia symptoms, may in fact not be worse for the older compared to middle aged adults. Also, possibly past age 50, these symptoms are more stable. Regarding HRS’s measures of physical activity, potentially they do not well capture the kinds of activity much older individuals may engage. DiPietro (2001) notes the problem of assessing the physical activity habits of older individuals, which tend to be less intense and quite variable. We created a weighted measure using all three items in hopes of allowing the measures of “mild activity” to count, but this may not substitute for more accurate measurement of both aerobic and anaerobic activity. Indeed, table 2 showed the age breakdown by each level of intensity using the original physical activity variables for mild, moderate, and vigorous activity. This confirmed that at older ages, there is almost no participation in vigorous activity. Indeed, even mild activity is reported on average as only occurring only one to three times a month. Further verification of our findings using objective measurement of both sleep and physical activity is needed. Relatedly, we were also not able to characterize sedentarinesss. Out findings actually highlight the importance of capturing this in the survey context (for example, time spent sitting), as this may be especially relevant at advanced older ages.
Finally, this study addressed the direction of effect going from physical activity to sleep, but research suggests that the association is likely bidirectional (Baron, Reid & Zee, 2014; Holfeld & Ruthig, 2014; Kline, 2014). It is possible that improvements in insomnia symptoms could also lead to increased physical activity, and future research should explore the potentially complex interplay of both health behaviors in relation to potential moderators.
Implications and Future Directions
Addressing directions for future research in this area, the findings of this study suggest that individuals at older ages may need special consideration in future studies. In particular, better measurement of the physical activities this age group is likely to engage such as light weight-bearing activities or normal daily movement that could be measured by an accelerometer might shed more light on the relationship between physical activity and sleep at older ages. In addition, more detailed characterization of sleep-related changes at older ages may improve the specification of this relationship.
Following the trend for large surveys of aging to begin integrating biological measurement, a major step forward for research on physical activity and sleep would be the inclusion of more objective measurement in the HRS, with accelerometers showing promise as measures of both sleep and physical activity (Hjorth et al., 2012). This would address concerns about bias inherent in self-reports and the lack of measurement of sedentariness. More objective measurement would add much needed depth to our understanding of the mechanisms that potentially link physical activity with sleep as we age. In addition, combined with the broad scope of psychological, social, and behavioral factors associated with health, objective measurement of physical activity and sleep would greatly increase the value of the HRS data.
In terms of implications for practice, our findings suggest that especially for individuals in late middle age, initiating a moderate intensity endurance physical activity program—such as walking for 30 minutes at least several times a week—is not likely to harm sleep and most likely to benefit sleep. Given the long-term nature of our findings, patients with insomnia symptoms could be encouraged to evaluate the effectiveness of exercise on sleep over time rather than just day-to-day. An important consideration for practitioners encouraging individuals to increase their physical activity to benefit sleep and for policy makers attempting to address this issue at the community level is potential physical barriers. The built environment has been shown to impact the amount of physical activity undertaken (Clarke & Gallagher, 2013; Grafova, Freedman, Kumar, & Rogowski, 2008). Walking, which is a common recommendation to patients, may be a relatively easy physical activity to take up, but it depends on being able to walk and on living in a walkable community. On the other hand, for some older adults walkability may not be as important as structured, supervised opportunities for physical activity.
Conclusion
The present study found a modest positive effect of engaging in physical activity on insomnia symptoms in a nationally representative sample of U.S. adults over age 50 followed longitudinally over ten years. Not only do individuals with higher levels of physical activity have fewer insomnia symptoms, but individuals who increase their level of physical activity have individual positive change in these symptoms. Age appears to moderate this relationship with stronger effects at late middle age relative to advance older age. Making even small changes over a long period of time relative to one’s own norm is helpful to getting better sleep, especially for adults in late middle age. These findings support a growing body of evidence for the moderate benefits of physical activity on sleep. The finding that age moderates the association bears further investigation with objective measurement of both physical activity and sleep a promising direction for future research in longitudinal surveys of older adults.
Acknowledgments
Funding
No funding sources to report.
Footnotes
Conflicts
No conflicts of interest to report.
Contributor Information
Amanda Sonnega, Institute for Social Research, University of Michigan, Ann Arbor, Michigan
Amanda Leggett, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan
Renee Pepin, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire
Shervin Assari, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan; Center for Ethnicity, Culture, and Health, School of Public Health, University of Michigan, Ann Arbor, Michigan
References
- Ancoli-Israel S, & Cooke JR (2005). Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. Journal of the American Geriatrics Society, 53(7), S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x [DOI] [PubMed] [Google Scholar]
- Baron KG, Reid KJ, & Zee PC (2013). Exercise to improve sleep in insomnia: Exploration of the bidirectional effects. Journal of Clinical Sleep Medicine, 9(8), 819. doi: 10.5664/jcsm.2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, & Curran PJ (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40(3), 373–400. doi: 10.1207/s15327906mbr4003_5 [DOI] [PubMed] [Google Scholar]
- Bjornsdottir E, Keenan BT, Eysteinsdottir B, Arnardottir ES, Janson C, Gislason T, … Benediktsdottir B (2015). Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. Journal of Sleep Research, 24(3), 328–338. doi: 10.1111/jsr.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner PS & DeLamater JD (2014). Social desirability bias in self-reports of physical activity: Is an exercise identity the culprit? Social Indicators Research, 117(2), 489–504. doi: s11205-013-0359-y [Google Scholar]
- Bugliari D, Campbell N, Chan C, Hayden O, Hurd M, Main R (2016). RAND HRS Data Documentation, Version N. Retrieved from University of Michigan Institute for Social Research Health and Retirement Study website: http://HRSonline.isr.umich.edu/modules/meta/rand/randHRSn/randHRSN.pdf
- Buman MP, Hekler EB, Bliwise DL, & King AC (2011a). Exercise effects on night-to-night fluctuations in self-rated sleep among older adults with sleep complaints. Journal of Sleep Research, 20(1pt1), 28–37. doi: 10.1111/j.1365-2869.2010.00866.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, Hekler EB, Bliwise DL, & King AC (2011b). Moderators and mediators of exercise-induced objective sleep improvements in midlife and older adults with sleep complaints. Health Psychology, 30(5), 579–587. doi: 10.1037/a0024293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP& King AC (2010). Exercise as a treatment to enhance sleep. American Journal of Lifestyle Medicine, 4(6), 500–514. doi: 10.1177/1559827610375532 [DOI] [Google Scholar]
- Campanelli CM (2012). American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults: the American Geriatrics Society 2012 Beers Criteria Update Expert Panel. Journal of the American Geriatrics Society, 60(4), 616. doi: 10.1111/j.1532-5415.2012.03923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Waite L, Kurina LM, Thisted RA, McClintock M, & Lauderdale DS (2015). Insomnia symptoms and actigraph-estimated sleep characteristics in a nationally representative sample of older adults Journals of Gerontology: Biological Sciences and Medical Sciences, 70(2), 185–192.doi: 10.1093/gerona/glu144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, & Gallagher NA (2013). Optimizing mobility in later life: The role of the urban built environment for older adults aging in place. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 90(6), 997–1009. doi: 10.1007/s11524-013-9800-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro L (2001). Physical activity in aging: Changes in patterns and their relationship to health and function. Journals of Gerontology: Biological Sciences and Medical Sciences, 56A, 13–22. 10.1093/gerona/56.suppl_2.13 [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Buman MP, Giacobbi PR, Roberts BL, Aiken-Morgan AT, Marsiske M, & McCrae CS (2014). Exercise and sleep in community-dwelling older adults: Evidence for a reciprocal relationship. Journal of Sleep Research, 23(1), 61–68. doi: 10.1111/jsr.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, & Spillman BC (2016). Active life expectancy in the older US population, 1982–2011: Differences between blacks and whites persisted. Health Affairs, 35(8), 1351–1358. doi: 10.1377/hlthaff.2015.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafova IB, Freedman VA, Kumar R, & Rogowski J (2008). Neighborhoods and obesity in later life. American Journal of Public Health, 98(11), 2065–2071. doi: 10.2105/ajph.2007.127712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartescu I, Morgan K, & Stevinson CD (2015). Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. Journal of Sleep Research, 24(5), 526–534. doi: 10.1111/jsr.12297 [DOI] [PubMed] [Google Scholar]
- He XZ, & Baker DW (2005). Differences in leisure-time, household, and work-related physical activity by race, ethnicity, and education. Journal of General Internal Medicine, 20(3), 259–266. doi: 10.1111/j.1525-1497.2005.40198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa SG, West BT, & Berglund PA (2017). Applied survey data analysis (Second ed.). Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- Hjorth MF, Chaput JP, Damsgaard CT, Dalskov SM, Michaelsen KF, Tetens I, & Sjodin A (2012). Measure of sleep and physical activity by a single accelerometer: Can a waist-worn Actigraph adequately measure sleep in children? Sleep and Biological Rhythms, 10(4), 328–335. doi: 10.1111/j.1479-8425.2012.00578.x [DOI] [Google Scholar]
- Hoffman L, & Stawski RS (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6(2–3), 97–120. doi: 10.1080/1542700902911189 [DOI] [Google Scholar]
- Holfeld B, & Ruthig JC (2014). A longitudinal examination of sleep quality and physical activity in older adults. Journal of Applied Gerontology, 33(7), 791–807. doi: 10.1177/0733464812455097 [DOI] [PubMed] [Google Scholar]
- Kaufmann CN, Canham SL, Mojtabai R, et al. (2013). Insomnia and health services utilization in middle-aged and older adults: results from the Health and Retirement Study. Journals of Gerontology A Biological Sciences and Medical Science. 68,1512–1517. 10.1093/gerona/glt050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Oman RF, Brassington GS, Bliwise DL, & Haskell WL (1997). Moderate-intensity exercise and self-rated quality of sleep in older adults: a randomized controlled trial. Journal of the American Medical Association, 277(1), 32–37. doi: 10.1001/jama.1997.03540250040029 [DOI] [PubMed] [Google Scholar]
- Kline CE (2014). The bidirectional relationship between exercise and sleep: Implications for exercise adherence and sleep improvement. American Journal of Lifestyle Medicine, 8(6), 375–379. doi: 10.1177/1559827614544437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, & Otto MW (2015). The effects of physical activity on sleep: A meta-analytic review. Journal of Behavioral Medicine, 38(3), 427–449. doi: 10.1007/s10865-015-9617-6 [DOI] [PubMed] [Google Scholar]
- Leggett A, Pepin R Sonnega A, Assari S (2016). Predictors of new onset sleep medication and treatment utilization among older adults in the United States. Journals of Gerontology A Biological Sciences and Medical Science,71(7), 954–960. doi: 10.1093/gerona/glv227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, & Wolfinger R (1996). SAS systems for mixed models. Cary, NC: SAS Institute, Inc. [Google Scholar]
- Loprinzi PD, & Cardinal BJ (2011). Association between objectively-measured physical activity and sleep, NHANES 2005–2006. Mental Health and Physical Activity, 4(2), 65–69. doi: 10.1016/j.mhpa.2011.08.001 [DOI] [Google Scholar]
- Mander BA, Winer JR, & Walker MP (2017). Sleep and human aging. Neuron, 94(1), 19–36. doi: 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanović Z, Pantelić S, Trajković N, Sporiš G, Kostić R, & James N (2013). Age-related decrease in physical activity and functional fitness among elderly men and women. Clinical Interventions in Aging, 8, 549–556. doi: 10.2147/cia.s44112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. (2003). 2003 Sleep in America Poll. National Sleep Foundation. doi: 10.1016/j.sleh.2015.04.002 [DOI] [Google Scholar]
- National Sleep Foundation. (2013). Aging and sleeping. National Sleep Foundation. Retireved from https://sleepfoundation.org/sleep-topics/aging-and-sleep [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep, 27(7), 1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical linear models: Applications and data analysis methods (Vol. 1): Sage. [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, & Zee PC (2010). Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Medicine, 11(9), 934–940. doi: 10.1016/j.sleep.2010.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silsbury Z, Goldsmith R, & Rushton A (2015). Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations. BMJ Open, 5(9), e008430. doi: 10.1136/bmjopen-2015-008430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Clements KM, & Fiatarone MA (1997). A randomized controlled trial of the effect of exercise on sleep. Sleep, 20(2), 95–101. [DOI] [PubMed] [Google Scholar]
- Sonnega A et al. (2014). Cohort profile: the Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–85. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NK, & Sanborn AN (2014). Better quality sleep promotes daytime physical activity in patients with chronic pain? A multilevel analysis of the within-person relationship. PLoS One, 9(3), e92158. doi: 10.1371/journal.pone.0092158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrasse M, Li J, & Gooneratne N (2015). Exercise and sleep in community-dwelling older adults. Current Sleep Medicine Reports, 1(4), 232–240. doi: 10.1007/s40675-015-0028-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Li LF, & Su DJ (2014). Physical activity and mortality among middle-aged and older adults in the United States. Journal of Physical Activity & Health, 11(2), 303–312. doi: 10.1123/jpah.2011-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley E, Popham F, & Benzeval M (2016). Comparison of the Rowe-Kahn Model of Successful Aging with self-rated health and life satisfaction: The West of Scotland Twenty-07 Prospective Cohort Study. Gerontologist, 56(6), 1082–1092. doi: 10.1093/geront/gnv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Ho K, Chen H & Chien M (2012). Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy, 58, 157–163. doi: 10.1016/S1836-9553(12)70106-6 [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, & Kline CE (2006). Epidemiology of exercise and sleep. Sleep and Biological Rhythms, 4(3), 215–221. doi: 10.1111/j.1479-8425.2006.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, & Burgard SA (2013). Healthier, wealthier, and wiser: A demonstration of compositional changes in aging cohorts due to selective mortality. Population Research and Policy Review, 32(3), 311–324. doi: 10.1007/s11113-013-9273-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B & Wing YK (2006). Sex differences in insomnia: a meta-analysis. Sleep, 29 (1), 85–93. doi: 10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- Zivin K, Llewellyn DJ, Lang IA, Vijan S, Kabeto MU, Miller EM, Langa KM (2010). Depression among older adults in the United States and England. 18(11), 1036–44. doi: 10.1097/JGP.0b013e3181dba6d2 [DOI] [PMC free article] [PubMed] [Google Scholar]


