Abstract
Objectives:
Older self-perceived age is associated with poor health and higher healthcare utilization in the geriatric population. We evaluated the associations of self-perceived age with geriatric assessment (GA) domain impairments in older adults with cancer
Methods:
This was a secondary analysis of baseline data from a GA cluster-randomized trial (URCC 13070; PI: Mohile). We included patients aged ≥70 with incurable stage III/IV solid tumor or lymphoma considering or receiving treatment and had ≥1 GA domain impairment other than polypharmacy. Multivariate analyses were used to evaluate the associations of age difference between chronological and self-perceived age (categorized into “feeling younger than chronological age” vs. “feeling the same or older than their chronological age”) with GA domain impairments.
Results:
We included 533 patients; mean age was 76.6 (SD 5.2). On multivariate analyses, compared to those who felt younger than their chronological age, those who felt the same or older were more likely to have impairments in physical performance [Adjusted Odds Ratio (AOR) 5.42, 95% Confidence Interval (CI) 1.69–17.40)], functional status (AOR 2.31, 95% CI 1.73–3.07), comorbidity (AOR 1.62, 95% CI 1.20–2.19), psychological health (AOR 2.62, 95% CI 1.85–3.73), and nutrition (AOR 1.65, 95% CI 1.20–2.28). They were also more likely to screen positively for polypharmacy (AOR 1.86, 95% CI 1.30–2.65).
Conclusions:
Older adults with cancer who felt the same or older than their chronological age were more likely to have GA domain impairments. Further studies are needed to better understand the relationships between self-perceived age, aging-related conditions, and outcomes in this population.
Keywords: Self-perceived age, geriatric assessment, physical performance, functional status, psychological health
Introduction
Self-perceived age is a measure of how an individual perceives their own aging process, and it is commonly assessed by asking patients “How old do you feel?” or “What age do you feel most of the time?”7,8 In the general older adult population, it is known that many perceive themselves to be younger (on average 5–10 years) than their chronological age.9 Younger self-perceived age is associated with better underlying health status, physical function, cognition, psychological health, and life satisfaction, as well as lower healthcare utilization.10–12 Based on a systematic review of 19 studies including a heterogeneous adult population with a mean age of 57–85 years, self-perceived age has a small but independent association on health, health behaviors, and longevity.13 To the authors’ knowledge, only one study evaluated self-perceived age in patients with cancer. In 292 patients receiving chemotherapy, 63% perceived themselves as younger, 15% as older, and 19% as the same age.14 There was no association of self-perceived age with symptoms and survival.14
Understanding how older adults with cancer perceive their own aging process has several implications. First, self-perceived age may be an indicator of poor health, disability, and vulnerability to adverse outcomes.12 Therefore, identifying this subset of patients early on may help with treatment decision-making and guide behavioral and supportive care interventions. Second, a study has shown that in older adults, lower perceived age was associated with higher uptake of a healthy aging program.15 Therefore, tailoring behavioral and supportive care interventions with self-perceived age in mind may prove useful in increasing uptake. Third, it is possible that self-perceived age may be modifiable, thereby mitigating negative outcomes such as functional decline.16 In fact, a “counterclockwise” intervention is currently being investigated to help older adults relive their younger selves.16 In another study, strengthening positive self-perceptions of aging among older individuals improved physical function.17
In this study of older adults with cancer, we evaluated the associations of self-perceived age with physical performance, functional status, comorbidity, psychological status, nutritional status, cognition, social support, and polypharmacy, as measured using a geriatric assessment (GA). We hypothesized that compared to patients who felt younger than their chronological age, patients who felt approximately the same or older than their chronological age were more likely to have impairment in these GA domains.
Methods
Study design, setting, and population
This is a secondary analysis of baseline data from a cluster-randomized controlled trial that evaluated the effect of a standardized GA and GA-guided recommendations on communication and satisfaction in older patients with cancer and their caregivers (University of Rochester Cancer Center (URCC) 13070; https://register.ClinicalTrials.gov/: NCT02107443; PI: Mohile). Detailed description of the primary study was previously reported.4,18–20 Briefly, patients aged ≥70 with incurable stage III/IV solid tumor or lymphoma who were considering any line of cancer treatment and had at least one impairment in GA domain other than polypharmacy were recruited between October 2014 and April 2017 from 31 community oncology practices. For the purpose of this study, we analyzed the data prior to their exposure to the study intervention.
Independent variable: Age difference
Patients self-reported their chronological and perceived age (“How old do you feel?”). Age difference was defined as the difference between chronological and perceived age.
Dependent variables
Dependent variables included eight individual GA domains: physical performance, functional status, comorbidity, psychological status, nutritional status, cognition, social support, and polypharmacy.4,18–21 Each domain was assessed using established tools and scored as impaired or not impaired using pre-established cut points (Supplemental Table 1).
Covariates
Covariates included demographics (age, gender, marital status, race, education, and annual household income) and cancer type.
Statistical analyses
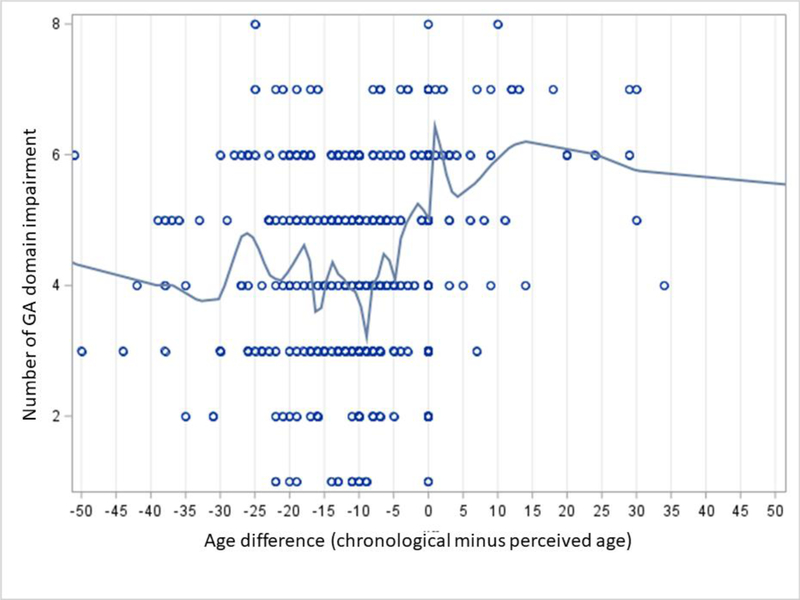
Descriptive analyses were used to summarize the demographics, cancer type, and distribution of age difference. We then dichotomize the age difference variable to enhance clinical usability. Prior studies have selected various cut-off (felt the same age or within one to two years of their chronological age).7,23 Therefore, we adopted an empirical approach, as there is no strong a priori theoretical guidance. We examined the distribution of age difference across the sample (in years) and visualized the relationship between age difference and the number of impaired GA domains using a scatter plot, and then fit a locally estimated scatterplot smoothing (LOESS) curve (PROC LOESS procedure in SAS). Based on the curve (Figure 1), we established a binary cut-off of 0 for age difference (“felt younger” vs. “felt older or felt exactly the same as chronological age).
Figure 1.
Relationship between age difference (defined as the difference between chronological and perceived age) and number of impairment in geriatric assessment domains
First, bivariate analyses were used to assess the associations between age difference and eight individual GA domains. Second, separate multivariate logistic regressions were used to assess these associations adjusting for demographic and cancer type. We used generalized estimating equation method to adjust for clustering at the practice level. All analyses were conducted using the SAS statistical software (Version 9.3, Cary, NC).
Results
The primary study included 541 patients.4 We included 533 patients because 8 patients had missing data on perceived age. Mean age was 76.6 (SD 5.2, range 70–96); 51% were male, 65% were married, 89% were white, and 26% had lung cancer (Supplemental Table 2). Mean number of impaired GA domains was 4.5 (SD 1.5). Percentage of impairment in each of the GA domains is shown in Table 1.
Table 1.
Bivariate and multivariate analyses evaluating the associations of self-perceived age with impairment in individual geriatric assessment domains
| N (%) | Self-perceived age | Bivariate analysesa | Multivariate analysesb | |||
|---|---|---|---|---|---|---|
| All patients (N=533) | Felt younger than chronological age (N=308) | Felt the same or older than chronological age (N=225) | OR (95% CI) | AOR (95% CI) | ||
| Physical performance | Impaired | 499 (93.6) | 279 (90.6) | 220 (97.8) | 4.57 (1.60–13.06) | 5.42 (1.69–17.40) |
| Functional status | Impaired | 316 (59.3) | 160 (52.0) | 156 (69.3) | 2.09 (1.61–2.71) | 2.31 (1.73–3.07) |
| Comorbidity | Impaired | 341 (64.0) | 184 (59.7) | 157 (69.8) | 1.56 (1.15–2.11) | 1.62 (1.20–2.19) |
| Psychological health | Impaired | 134 (25.1) | 54 (17.5) | 80 (35.6) | 2.60 (1.88–3.57) | 2.62 (1.85–3.73) |
| Nutritional status | Impaired | 320 (60.0) | 171 (55.5) | 149 (66.2) | 1.57 (1.19–2.07) | 1.65 (1.20–2.28) |
| Cognition | Impaired | 178 (33.4) | 100 (32.5) | 78 (34.7) | 1.10 (0.84–1.44) | 1.07 (0.83–1.39) |
| Instrumental social support | Impaired | 153 (28.7) | 86 (27.9) | 67 (29.8) | 1.09 (0.63–1.89) | 1.31 (0.67–2.56) |
| Polypharmacy | Screen positive | 447 (83.9) | 249 (80.8) | 198 (88.0) | 1.74 (1.18–2.56) | 1.86 (1.30–2.65) |
Adjusted for clustering at practice site
Adjusted for chronological age, gender, marital status, race, education, income, cancer type, and clustering at practice site Abbreviations: AOR, Adjusted Odds Ratio; CI, Confidence Interval; OR, Odds Ratio
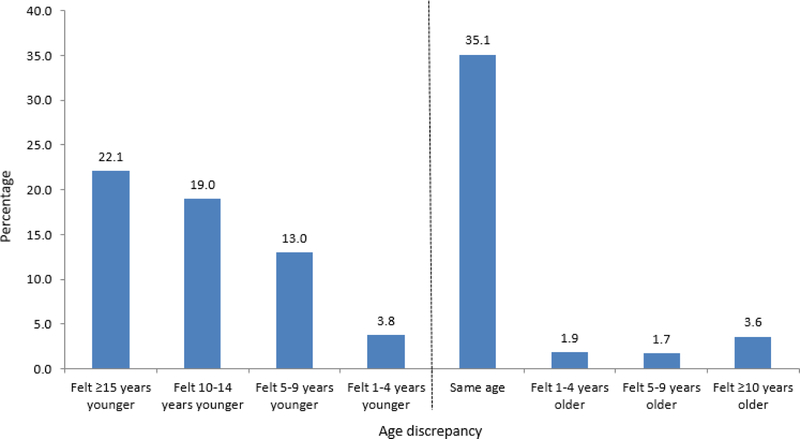
Mean perceived age was 69.3 (SD 14.1, range 7–175); over half of the patients felt younger than their chronological age (308/533; 58%). The distribution of age difference is shown in Figure 2. The relationship between age difference and the number of impaired GA domains is shown in Figure 1.
Figure 2.
Distribution of age difference (defined as the difference between chronological and perceived age)
On multivariate analyses, compared to those who felt younger than their chronological age, those who felt the same or older than their chronological age were more likely to be impaired in physical performance [Adjusted Odds Ratio (AOR) 5.42, 95% Confidence Interval (CI) 1.69–17.40), functional status (AOR 2.31, 95% CI 1.73–3.67), comorbidity (AOR 1.62, 95% CI 1.20–2.19), psychological health (AOR 2.62, 95% CI 1.85–3.73), and nutritional status (AOR 1.65, 95% CI 1.20–2.28). They were also more likely to screen positively for polypharmacy (AOR 1.86, 95% CI 1.30–2.65). Age difference was not significantly associated with impairment in cognition or instrumental social support.
Discussion
Consistent with studies in the general older adult population,7,8,23,24 we showed that older adults with cancer who reported feeling the same or older than their chronological age were more likely to experience poor health as captured by the GA. Specifically, they were more likely to have impairments in various GA domains including physical performance, functional status, comorbidity, psychological health, nutritional status, and polypharmacy. To our knowledge, the associations of self-perceived age and GA domains have not been shown previously in older adults with cancer.
Self-perceived age is known to be associated with poor health, generally represented by poor physical performance, functional status, psychological health, and nutritional status, as well as polypharmacy in the general geriatric population.13 In our sample, self-perceived age was not associated with social support or cognition. Impairment in social support may not reflect a patient’s health status, but rather a protective element against adverse life events and having social support helps to increase resilience. We are unclear why self-perceived age was not associated with cognition. A prior study has shown that negative perceptions of aging were associated with decline of cognitive function.25 It should be noted that we recruited patients who were considering or receiving cancer treatment. Those with severe cognitive impairment, who may not be considered for treatment by their oncologist, would therefore be underrepresented in our study. It is possible that self-perceived age was associated with severe but not mild cognitive impairment. It is also possible that patients with cognitive impairment may not adjust their perceptions of their own age. Therefore, the association between self-perceived age and cognition among older patients with cancer needs to be further explored.
Our study is consistent with a recently published study in older adults with cancer.26 Among 101 patients with cancer (mean age=71.8 years), negative self-perception of aging (measured using the Attitudes to Aging Questionnaire) was associated with deleterious effects on physical and mental health, supporting the use of self-perceived age as a marker of vulnerability. On the other hand, in a separate study of 292 patients with cancer not limited to older adults, self-perceived age was not associated with symptoms or survival.14 Therefore, the associations of self-perceived age and survival in the context of cancer need to be further elucidated. It is worth mentioning that self-perceived age correlates with self-perceived health status27 and the latter is incorporated in the validated Geriatric 8 (G8) screening tool.28 G8 has been shown to be associated with morbidity and mortality in older adults with cancer,29,30 and is a widely accepted screening tool for identifying patients who may benefit from GA.28 In addition, older adults are susceptible to cancer- and treatment-related adverse events, which may be mitigated by behavioral and supportive care interventions.31 Understanding how self-perceived age plays a role in the uptake of these interventions is an intriguing area of investigation. Interventions focusing on modifying perceptions of aging and their effects on cancer- and treatment-related adverse events should also be explored.
Strengths of our study include a relatively large sample of older adults with cancer from multiple community practices and minimal missing data. Nonetheless, we only included patients who were considering or receiving cancer treatment. Therefore, those who were too frail to be considered for treatment by their oncologists were excluded. Future work should evaluate all older patients with cancer. In addition, the majority of our sample was white and future research should include a more racially diverse group of patients. Finally, give the cross-sectional nature of our study, we were unable to determine causality.
In summary, older patients who felt the same or older than their chronological age were more likely to have impairments in several GA domains including physical performance, functional status, psychological health, nutritional status, and polypharmacy. Further studies are needed to better understand the relationships between self-perceived age and outcomes in older adults with cancer.
Supplementary Material
Acknowledgement
The work was funded through a Patient-Centered Outcomes Research Institute (PCORI) Program contract (4634; Mohile), UG1 CA189961, and R01 CA177592 (Mohile), and K24 AG056589 from the National Institute of Aging (Mohile). Dr. Loh is supported by the Wilmot Research Fellowship Award and National Cancer Institute (K99 CA237744). This work was made possible by the generous donors to the Wilmot Cancer Institute (WCI) geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation: The abstract was presented at 2017 International Society of Geriatric Oncology Annual Meeting.
Disclosures and Conflict of Interest Statements:
Dr. Loh has served as a consultant to Pfizer and Seattle Genetics. All other authors have declared no conflicts of interest.
References
- 1.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. [DOI] [PubMed] [Google Scholar]
- 2.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohile SG, Epstein RM, Hurria A, et al. Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohile SG, Magnuson A, Pandya C, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw. 2018;16(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What Every Oncologist Should Know About Geriatric Assessment for Older Patients With Cancer: Young International Society of Geriatric Oncology Position Paper. J Oncol Pract. 2018;14(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rippon I, Steptoe A. Feeling old vs being old: associations between self-perceived age and mortality. JAMA Intern Med. 2015;175(2):307–309. [DOI] [PubMed] [Google Scholar]
- 8.Gendron TL, Inker J, Welleford A. “How Old Do You Feel?” The Difficulties and Ethics of Operationalizing Subjective Age. Gerontologist. 2018;58(4):618–624. [DOI] [PubMed] [Google Scholar]
- 9.Tréguer J-P. 50+ marketing: Marketing, communicating and selling to the over 50s generations. Palgrave Macmillan; 2002. [Google Scholar]
- 10.Stephan Y, Caudroit J, Chalabaev A. Subjective health and memory self-efficacy as mediators in the relation between subjective age and life satisfaction among older adults. Aging & mental health. 2011;15(4):428–436. [DOI] [PubMed] [Google Scholar]
- 11.Sun JK, Kim ES, Smith J. Positive Self-Perceptions of Aging and Lower Rate of Overnight Hospitalization in the US Population Over Age 50. Psychosom Med. 2017;79(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser C, Spagnoli J, Santos-Eggimann B. Self-perception of aging and vulnerability to adverse outcomes at the age of 65–70 years. J Gerontol B Psychol Sci Soc Sci. 2011;66(6):675–680. [DOI] [PubMed] [Google Scholar]
- 13.Westerhof GJ, Miche M, Brothers AF, et al. The influence of subjective aging on health and longevity: a meta-analysis of longitudinal data. Psychology and aging. 2014;29(4):793–802. [DOI] [PubMed] [Google Scholar]
- 14.Lim MY, Stephens EK, Novotny P, et al. Self-perceptions of age among 292 chemotherapy-treated cancer patients: Exploring associations with symptoms and survival. Journal of geriatric oncology. 2013;4(3):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza-Nunez VM, Sarmiento-Salmoran E, Marin-Cortes R, Martinez-Maldonado ML, Ruiz-Ramos M. Influence of the Self-Perception of Old Age on the Effect of a Healthy Aging Program. Journal of clinical medicine. 2018;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagnini F, Cavalera C, Volpato E, et al. Ageing as a mindset: a study protocol to rejuvenate older adults with a counterclockwise psychological intervention. BMJ open. 2019;9(7):e030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy BR, Pilver C, Chung PH, Slade MD. Subliminal strengthening: improving older individuals’ physical function over time with an implicit-age-stereotype intervention. Psychol Sci. 2014;25(12):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehoe LA, Xu H, Duberstein P, et al. Quality of Life of Caregivers of Older Patients with Advanced Cancer. J Am Geriatr Soc. 2019;67(5):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer. 2019;125(14):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh KP, Mohile SG, Lund JL, et al. Beliefs About Advanced Cancer Curability in Older Patients, Their Caregivers, and Oncologists. Oncologist. 2019;24(6):e292–e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. J Am Geriatr Soc. 1985;33(9):607–615. [DOI] [PubMed] [Google Scholar]
- 23.Uotinen V, Rantanen T, Suutama T. Perceived age as a predictor of old age mortality: a 13-year prospective study. Age Ageing. 2005;34(4):368–372. [DOI] [PubMed] [Google Scholar]
- 24.Christensen K, Thinggaard M, McGue M, et al. Perceived age as clinically useful biomarker of ageing: cohort study. BMJ. 2009;339:b5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson DA, King-Kallimanis BL, Kenny RA. Negative perceptions of aging predict longitudinal decline in cognitive function. Psychol Aging. 2016;31(1):71–81. [DOI] [PubMed] [Google Scholar]
- 26.Schroyen S, Missotten P, Jerusalem G, Van den Akker M, Buntinx F, Adam S. Association between self-perception of aging, view of cancer and health of older patients in oncology: a one-year longitudinal study. BMC Cancer. 2017;17(1):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephan Y, Demulier V, Terracciano A. Personality, self-rated health, and subjective age in a lifespan sample: the moderating role of chronological age. Psychology and aging. 2012;27(4):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. [DOI] [PubMed] [Google Scholar]
- 29.Agemi Y, Shimokawa T, Sasaki J, et al. Prospective evaluation of the G8 screening tool for prognostication of survival in elderly patients with lung cancer: A single-institution study. PLOS ONE. 2019;14(1):e0210499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middelburg JG, Mast ME, de Kroon M, et al. Timed Get Up and Go Test and Geriatric 8 Scores and the Association With (Chemo-)Radiation Therapy Noncompliance and Acute Toxicity in Elderly Cancer Patients. Int J Radiat Oncol Biol Phys. 2017;98(4):843–849. [DOI] [PubMed] [Google Scholar]
- 31.Mustian KM, Alfano CM, Heckler C, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017;3(7):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.