Abstract
Aldehydes, which are present within the air as well as food and beverage sources, are highly reactive molecules that can be cytotoxic, mutagenic, and carcinogenic. To prevent harm from reactive aldehyde exposure, the enzyme aldehyde dehydrogenase 2 (ALDH2) metabolizes reactive aldehydes to a less toxic form. However, the genetic variant of ALDH2, ALDH2*2, significantly reduces the ability to metabolize reactive aldehydes in humans. Therefore, frequent environmental aldehyde exposure, coupled with inefficient aldehyde metabolism, could potentially lead to an increased health risk for diseases such as cancer or cardiovascular disease.
Here, we discuss the environmental sources of reactive aldehydes and the potential health implications particularly for those with an ALDH2*2 genetic variant. We also suggest when considering the ALDH2*2 genetic variant the safety limits of reactive aldehyde exposure may have to be reevaluated. Moreover, the ALDH2*2 genetic variant can also be used as an example for how to implement precision medicine in the field of environmental health sciences.
Keywords: Reactive aldehyde, Aldehyde dehydrogenase 2, Cigarette, Alcohol, 4-HNE, ALDH2*2
1. Introduction
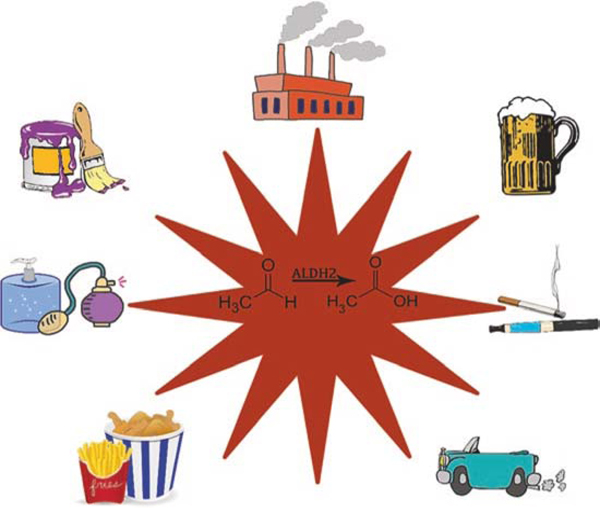
Aldehydes are highly reactive electrophiles abundant within our environment. Many sources of aldehydes exist and are present within the air we inhale in addition to the products we use and consume. Exposure to these aldehydes occurs outdoors and indoors, including within the workplace. Lifestyle choices such as tobacco cigarettes, e-cigarettes, and alcohol also expose people to aldehydes. Aldehydes are also present in foods, nonalcoholic beverages, cosmetics, and hand sanitizers (Fig. 1). Together, exposure to these aldehyde sources, coupled with genetic differences which reduce aldehyde metabolism, may influence the risk of developing diseases such as cancer and cardiovascular disease.
Fig. 1.
Exogenous sources of reactive aldehyde exposure. These include air pollution produced by industrial power plants and automobiles, alcoholic beverages, tobacco products including cigarettes and e-cigarettes, fried foods, cosmetics, and lacquers in paints. As pictured, the ALDH2 enzyme reduces an aldehyde to a less harmful acid. However, for those with an ALDH2*2 variant, the efficiency of this metabolism is reduced by >60%
Environmental aldehyde sources include those such as acrolein, acetaldehyde, and formaldehyde. Acrolein is considered a human carcinogen that irritates the upper respiratory tract when inhaled. Acetaldehyde and formaldehyde are also classified as Group 1 carcinogens by the International Agency for Research on Cancer (IARC) [ 1 ]. These toxic properties of aldehydes are due to their electrophilic nature, which can modify DNA and proteins, thus the label “reactive” aldehydes [2, 3]. For example, acrolein reacts with proteins specifically on histidine, cysteine, and lysine amino acids by Michael addition or Schiff base formation [4]. Acrolein can also block protein sulfhydryl group formation on lysine residues and result in impaired protein function [5]. Overall, these effects from exogenous sources of reactive aldehydes can be cytotoxic, mutagenic, and carcinogenic.
A critical enzyme responsible for reducing aldehydes to less reactive forms is aldehyde dehydrogenase 2 (ALDH2). ALDH2, primarily known for metabolizing acetaldehyde (produced as an intermediate in the metabolism of alcohol to acetic acid), is also important in the detoxification of other reactive aldehydes such as formaldehyde and acrolein [6, 7], However, individuals with an ALDH2 genetic variant have significantly reduced enzymatic activity to metabolize these aldehydes. In homozygotes for the ALDH2 variant (ALDH2*2/*2), the metabolism of aldehydes is severely limited (~96% activity loss), whereas in heterozygotes (ALDH2*l/*2) the enzymatic activity is reduced by 60–80% relative to those individuals that are without the variant (ALDH2*1/*1) [2, 8]. Throughout this rest of this chapter, we will denote the ALDH2 variant as ALDH2*2.
With exposure, the limited ability to metabolize reactive aldehydes for those with the ALDH2*2 variant can potentially pose an increased risk for developing diseases such as cancer or cardiovascular disease. However, some of these environmental exposures could be easily modifiable. Here we discuss the sources of reactive aldehydes within the environment and their effects on human health, particularly for those with a limited ability to metabolize reactive aldehydes.
2. Outdoor, Indoor, and Occupational Aldehyde Exposure
In this section, we will discuss the outdoor, indoor, and occupational exposure of reactive aldehydes and how these exposures may potentially impact people with an ALDH2*2 genetic variant. These exposures include air pollution from automobiles and industrial waste, indoor sources such as paint, and occupational sources such as working with aldehydes and surgical smoke.
2.1. Outdoor Exposures
2.1.1. Air Pollution
Air pollution is a significant concern for Asian countries but also a concern for the United States. According to the World Health Organization (WHO), air pollution is the greatest environmental risk to human health [9]. Sources of air pollution can be natural or anthropogenic and include biomass and fossil fuel combustion, automobile exhaust, and industrial power plant and wood burning fumes [10–12]. Typical city or urban air pollutant exposure consists of aldehydes, ketones, hydrocarbons, and particulate matter. The studies below detail how aldehydes contribute to air pollution.
At six locations in Japan including in urban cities (Sanda, Nishiwaki, Toyooka. and Sumoto), at roadside (Ashiya), and at an industrial site (Takasago), researchers measured 101 volatile organic compound (VOC) concentrations, including acetaldehyde and formaldehyde [13]. Relative to carcinogenic effect, hazardousness of these compounds was evaluated by calculating the factor called excess cancer incidence. For formaldehyde at all sites, the excess cancer incidence exceeded 10−5 per μg m−3. This is a level of concern for a carcinogenic effect since the United States Environmental Protection Agency quantitatively estimates that there is a carcinogenic risk for formaldehyde at or above 1.3 × 10−5 per μg m3 [13]. The authors concluded that elevated VOCs, such as formaldehyde, were partially attributed to higher traffic levels generating greater amounts of automobile exhaust in the designated areas [12, 13]. Overall, these studies emphasized the potential harm one exogenous source of pollution, automobile exhaust, can potentially have on changing the risk for carcinogenesis.
In Asia, ambient carbonyl compound concentrations were assessed in seven major cities (Beijing, Chengdu, Guangzhou, Shanghai, Wuhan, Xiamen, and Yantai) and two rural areas (Qinghai Lake, Qinghai, and Lhasa, Tibet) during both the summer and winter seasons. These average concentrations showed seasonal variability dependent on temperature. Among the nine sampling sites measured, the average concentrations of propionaldehyde, formaldehyde, and acetaldehyde were found to be 0.25, 5.07, and 1.91 ppb by volume in the summer and 0.17, 2.04, and 1.42 ppb by volume during the winter, respectively [14], This study also found the relatively high levels of acetaldehyde emissions were a direct result of increased use of ethanol-blended gasoline (e-gasoline) in vehicles during the summer season.
In Beijing, ambient air aldehyde contents were also measured, and the concentrations of formaldehyde, acetaldehyde, and acrolein were found to be 29.3 ±15.1 μg/ m3, 27.1 ± 15.7 μg/m3, and 2.3 ±1.0 μg/m3. These levels are considered at the high end of concentration ranges measured in cities around the world [15]. For example, in Savannah, Georgia, the relative levels of formaldehyde and acetaldehyde were 2.0 μg/m3 and 2.3 μg/m3, compared to Beijing concentrations of 29.3 ± 15.1 μg/m3 and 27.1 ± 15.7 μg/m3, ~14 times lower than that of Beijing [16].
Air pollution-mediated aldehyde exposure is highly concerning for those with an ALDH2*2 variant especially living in major East Asian cities. Air pollution continues to be a significant source of environmental reactive aldehyde exposure throughout East Asia and in particular China. Therefore, the overall risk for exposure for individuals with an ALDH2*2 variant, particularly with the increased migration to urban cities, is of potential concern. Below, we will discuss in more detail automobile exhaust and industrial waste which also contribute to outdoor levels of reactive aldehydes.
2.1.2. Automobiles
Automobile exhaust includes the reactive aldehydes formaldehyde, acetaldehyde, and acrolein [26]. These aldehydes are produced during the burning of fossil fuels and constitute ~1–2% of the volatile organic compounds produced from vehicle exhaust [13]. In one study, diesel engine exhaust gas emission was monitored using three types of diesel fuel (red diesel, biofuel, and gas oil). In all types of diesel fuel, the most abundant reactive aldehydes were formaldehyde, acetaldehyde, and acrolein that were all within a range of 1000–2000 ppb [17]. Biofuel diesel had the greatest levels of acetaldehyde when compared to red diesel and gas oil.
With their increasing popularity, reformulated automotive fuels are an additional source of aldehyde emission. Depending on whether ethanol or methanol is added to the automotive fuels, an increased amount of acetaldehyde or formaldehyde is emitted in automobile exhaust. In a study comparing ethanol-blended fuel exhaust with gasoline exhaust, ethanol-blended exhaust emits predominantly acetaldehyde (1.2–12 g/kWh), whereas gasoline exhaust emits predominantly formaldehyde (0.74–2.3 g/kWh) [18]. With increased ethanol blending, acetaldehyde emissions increased so that pure ethanol used as fuel had acetaldehyde emissions 35–44 times higher than gasoline. Ethanol also influenced formaldehyde emissions so that addition of 50% ethanol to gasoline resulted in a 30–50% increase in formaldehyde emissions [18].
The increased popularity of gasoline-electric, hybrid cars and electric cars facilitates a reduction in automobile emission of acetaldehyde and formaldehyde that may potentially assist in alleviating the elevated levels measured in major cities described above. For ALDH2*2 variant, vehicle selection could also potentially reduce direct reactive aldehyde exposure while driving an automobile.
2.1.3. Industrial Waste
During industrial manufacturing, aldehydes are released as a by-product into the atmosphere. Significant sources of reactive aldehydes produced during the manufacturing process include formaldehyde, butyraldehyde, acetaldehyde, and acrolein. Formaldehyde and butyraldehyde are used as precursors for resin and plasticizer (softener) production, respectively [19]. The reactivity and availability of butyraldehyde makes it a popular material in plasticizer production and other industrial materials [20], Formaldehyde is used extensively in commercial processes such as in the synthetic resin industry, due to its high chemical reactivity and thermal stability. In Hong Kong, an industrial workplace pilot study found formaldehyde as the most abundant carbonyl compound among all workplace air samples, accounting for 22.0–44.0% of total carbonyls measured on a molar basis. When formaldehyde levels were measured in a paint and wax manufacturing plant and a food-processing factory, formaldehyde levels exceeded the WHO air quality guideline of 81.8 ppb [20].
During the manufacturing processes, combustion emissions are of optimal importance. Combustion sources include power plant petrochemical, diesel-fueled engines, and polyethylene plastic, which release formaldehyde, acetaldehyde, and acrolein [21, 22], Manufacturing-based acrolein emissions also include volatilization from treated waters and contaminated waste streams, formation as a photooxidation product of various hydrocarbon pollutants, and use in petroleum operations [23–25].
Industrial waste carbonyl contents should be closely monitored particularly in East Asian countries where there is a high prevalence of people with the ALDH2*2 variant. Most standards in regard to what is considered a safe level of industrial product exposure are based upon the ability to efficiently metabolize by-products from industrial manufacturing such as aldehydes to less toxic forms. Therefore, reconsidering the standards for what are the safe levels of exposure for these aldehydes in the industrial workplace or from industrial waste may need to be considered for those who have an ALDH2*2 genetic variant.
2.2. Indoor Exposures
Within a building, aldehydes can infiltrate from external sources described above or can also be present in the air as a result of generation indoors. In six different New Jersey housing sites, levels of indoor formaldehyde, acetaldehyde, and seven other aldehydes were measured. The combined concentration of these nine aldehydes measured indoors was 63 ± 22 ppb compared to outdoor measured values of 19 ± 11 ppb. Of the nine aldehydes assessed, formaldehyde was the most abundant at 55 ppb with acetaldehyde the second most abundant at 3 ppb [26].
In newly installed and painted buildings, higher acetaldehyde levels were found indoors relative to outdoors, suggesting paint and/or lacquers are a significant acetaldehyde source which declines with building age [27]. Dry lacquers which contain aldehydes such as propanol, n-nonanal, and n-hexanal are also used to paint radiators [28], This is concerning because after a radiator is painted, the heal generated during radiator use causes dry lacquer combustion and aldehydes to be aerosolized into the air. Other lesser-known indoor aldehyde sources include wood burning fireplaces, gas stoves, gas heaters, and synthetic carpets, which can emit formaldehyde and acrolein [11, 26].
2.3. Occupational Exposure
Persons working in aldehyde production industries or as laboratory technicians, healthcare professionals, or funeral home employees may be exposed to higher levels of aldehydes, especially when working with formaldehyde, acrolein, and acetaldehyde [29, 30].
Occupational exposure to these reactive aldehydes may occur via inhalation of reactive aldehyde vapor or direct skin exposure. For people working directly with formaldehyde in an industrial setting, it was found that ~3.5% of workers were exposed to formaldehyde air concentrations greater than 3 ppm, well above the workplace formaldehyde exposure limit set by OSHA at ~0.75 ppm over an 8-hour workday. In this same study, less than 12% of workers were exposed to concentrations greater than I ppm; however, over 88% of workers were exposed to concentrations of 0.5 ppm or higher, nearing the OSHA limit [31].
For healthcare professionals, additional sources of reactive aldehyde exposure include electrocautery smoke and widely used hospital sanitizers and disinfectants. During surgery, electrocautery surgical smoke contains formaldehyde, acetone, benzene, and acrylamide [32]. Hospital disinfectants also contain reactive aldehyde sources including formaldehyde and ortho-phthalaldehyde (OPA) [33], Laboratory researchers can also be exposed to high levels of aldehydes. For example, in a cancer research institute study, formaldehyde exposure levels for laboratory workers ranged from 4.9 to 268.7 μg/m2 [29].
In humans, evidence suggests when using liver mitochondrial fractions that formaldehyde metabolism is ~3x slower for those with an ALDH2*2 variant compared to those that do not [34], This suggests that exposure to formaldehyde could perhaps be more toxic and potentially carcinogenic for those with an ALDH2*2 variant since formaldehyde cannot be metabolized as efficiently to the less reactive formic acid. Further, exposure of ALDH2 knockout mice to 500 ppm of inhalational acetaldehyde leads to higher erosion and degeneration of the respiratory epithelium (55.6% vs. 22.2%) and dorsal skin (77.8% vs. 0.0%) and hemorrhaging of the nasal cavity for the ALDH2 knockout mice when compared to the wild-type ALDH2 mice [35]. This suggests that workers with the ALDH2*2 variant are potentially more susceptible to harm from inhalational exposure to reactive aldehydes in the workplace. Therefore, standard limits in regard to workplace exposure may have to be re-examined with the ALDH2*2 genetic variant in mind.
Overall, these studies suggest that outdoor, indoor, and occupational sources of reactive aldehydes are prevalent and of potential concern. Elevated exposure to these aldehyde sources could pose an environmental risk for human health especially for those with an ALDH2*2 variant, and the thresholds considered safe for exposure in these environments need to be potentially re-examined.
3. Lifestyle Choices
Lifestyle choices contribute to an increased risk of aldehyde exposure including drinking alcohol, smoking tobacco products, consuming certain dietary sources, and using particular cosmetic products including perfumes. Here, we will discuss specific lifestyle choices that are sources of aldehyde exposure and their implication on human health, especially in the context of people with an ALDH2*2 variant who cannot catabolize aldehydes efficiently.
3.1. Alcohol
A well-known source of reactive aldehyde exposure is alcohol. Upon ingestion, alcohol is converted to acetaldehyde by the enzyme alcohol dehydrogenase (ADH). Acetaldehyde is then metabolized by ALDH2 to the less reactive acetic acid. Within the cell, ALDH2 has the highest enzymatic affinity for acetaldehyde [36, 37]. The biochemical effect of acetaldehyde after alcohol consumption, which includes DNA- and protein-induced adduct formation, has been detailed in recent reviews [38, 39].
Individuals who are heterozygotes for the ALDH2*2 variant result in an accumulation of acetaldehyde which is ~5-fold higher compared to the wild-type ALDH2 enzyme after alcohol consumption [40], This acetaldehyde accumulation produces the phenotype of facial flushing and elevated heart rate seen in those with an ALDH2*2 variant. Those homozygous for the ALDH2*2 variant have an aversion to alcohol consumption since the ability to metabolize acetaldehyde is <4% compared to the wild-type ALDH2 enzyme. Thus, the ALDH2*2 variant is considered protective from developing alcoholism, supported since the occurrence of alcoholism is reduced for those individuals with an ALDH2*2 variant [41].
Although the ALDH2*2 variant may protect from alcoholism, individuals who are heterozygotes for the ALDH2*2 variant, ~560 million people worldwide, still consume alcohol. For example, in Korea, where ~20% of the population carries an ALDH2*2 variant, alcohol consumption is one of the highest per capita in the world [42].
In particular, the reduced metabolism of acetaldehyde resulting from an ALDH2*2 variant after alcohol consumption is linked to a risk for developing esophageal cancer and cardiovascular disease. In regard to esophageal cancer, a seminal study by Yokoyama was the first to link an increased incidence of esophageal cancer caused by alcohol consumption to those with an ALDH2*2 genetic variant [43]. The odds of developing esophageal cancer are also dose-dependently related to the amount of alcohol consumed. Even a moderate consumption of alcohol (9–17.9 units/week) for those with an ALDH2*2 variant increases the odds ratio to 40 for esophageal cancer [43, 44], The importance of relaying this significant health risk to the public in addition to healthcare professionals is critical and is stressed in several studies [45, 46]. Further, the impact of the ALDH2 enzyme on aldehyde metabolism and alcohol-induced cardiomyopathy was recently reviewed [47].
3.2. Cigarettes and E-cigarettes
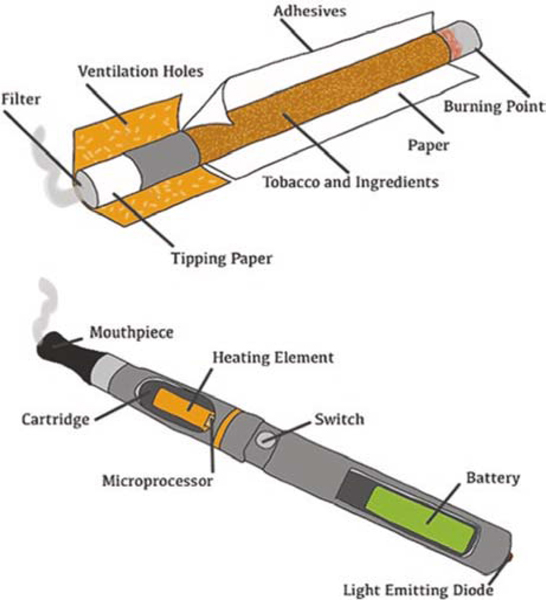
Smoking imposes a tremendous economic burden accounting for more than $170 billion each year in healthcare costs for adults in the United States and more than $422 billion each year worldwide [48, 49]. To date, smoking remains the leading cause of preventable death in the United States, accounting for one out of five deaths annually [50, 51]. The typical components of a tobacco cigarette are filters (designed to trap smoke), tipping paper (which wraps around the filter), tobacco fillers, cigarette paper (for holding the tobacco fillers), and adhesives. In contrast to cigarettes, the primary components of an e-cigarette are an e-liquid cartridge, an atomizer/heating element, a microprocessor, and a battery (Fig. 2). This is important to note since even though the structural compositions of cigarettes and e-cigarettes are quite different, both of these ways designed to deliver nicotine produce reactive aldehydes.
Fig. 2.
Schematic illustration describing cigarette and e-cigarette components. Although both are largely different in regard to appearance and the materials used for composition, both cigarette and e-cigarette aerosol contain reactive aldehydes
The smoke from a cigarette is composed of more than 4700 different chemical compounds. These include aldehydes such as formaldehyde, propionaldehyde, butyraldehyde, acetaldehyde, acrolein, and crotonaldehyde [52, 53]. When compared to non-smokers, a person smoking one cigarette produces a 3.5-fold increase in aldehyde levels in the saliva. After smoking ten cigarettes or more a day, a two-fold increase in these compounds was found in the saliva [54]. As these compounds when smoking are inhaled into the lungs, exhaled breath condensate of cigarette smokers also contains higher levels of endogenous aldehydes including malondialdehyde (57 ± 2 nmol/L), hexanal (64 ± 4 nmol/L), and heptanal (27 ± 4 nmol/L) compared to non-smokers (18 ± 6 nmol/L, 14 ± 4 nmol/L, and 19 ± 1 nmol/L, respectively) [54].
Epidemiological evidence also suggests that smoking is a key factor in developing cancer and cardiopulmonary disease [55]. Particularly reactive aldehydes in cigarette smoke, especially acetaldehyde and acrolein, are directly linked to a higher risk for developing lung, oral, and gastrointestinal cancer [38], Moreover, aldehyde levels in cigarette and e-cigarette smoke are responsible for 33% of deaths from cardiovascular diseases and 20% of deaths from ischemic heart disease [51]. Aldehydes derived from cigarette smoke affect heart contractile function and damage blood vessel structure and function by affecting vascular endothelial and epithelial cells [56].
Further in regard to the ALDH2*2 variant, a study consisting of 410 Japanese patients who underwent coronary angiography between 2010 and 2016 demonstrated that smokers had a significantly higher prevalence of coronary spastic angina (CSA) (63.1% vs. 48.9%) than non-smokers [57], Patients with the ALDH2*2 variant also had a higher risk for CSA when compared to non-smokers with a wild-type ALDH2 enzyme (58.5% vs. 40.8%), whereas the ALDH2*2 variant exaggerated the risk in smokers (82.5% vs. 56.6%) when compared to wild-type ALDH2.
Cigarette smoke exposure is also the primary risk factor for chronic obstructive pulmonary disease (COPD) with a general underlying hypothesis that reactive oxygen and reactive aldehydes contribute to the development of COPD [54, 58]. A recent study including 967 Japanese individuals revealed that the presence of an ALDH2*2 variant was not associated with the development of COPD but having an ALDH2*2 variant was associated with significantly lower lung functioning parameters when compared to individuals with wild-type ALDH2 [59]. Moreover, this study found that 16 weeks of cigarette smoke exposure increased the lung volume and resulted in mild emphysema for wild-type ALDH2 mice, whereas ALDH2*2 variant mice were resistant to emphysema development in response to cigarette smoke exposure. Although the link between cigarette smoking and COPD is clearly established, these data suggest how differences in the metabolism of reactive aldehydes may affect COPD requires further study.
E-cigarette aerosol, similar to cigarettes, also contains reactive aldehydes including acrolein, acetaldehyde, and formaldehyde [60–62], Moreover, a recent report suggests that when measuring reactive aldehyde content across 66 different e-cigarette liquids, acetaldehyde concentrations are generally ~2 fold higher than formaldehyde or acrolein [63]. However, the quantity of these three reactive aldehydes produced is variable and dependent upon the battery voltage, type of e-cigarette vaporizer, and amount and type of liquid within the e-cigarette cartridge. For example, changing the battery voltage from 3.7 to 4.8 V increases the combined reactive aldehyde production of acetaldehyde, formaldehyde, and acrolein by 3.5- fold. Additionally, a double-coil vaporizer when compared to a single-coil vaporizer will decrease resistance and increase the power. Therefore, higher-power e-cigarettes (9.1 W) can increase acetaldehyde levels ~65-fold, formaldehyde levels ~49-fold, and acrolein levels ~3-fold in the e-cigarette aerosol when compared to a lower- power e-cigarette (4.6 W) [60]. Clearly, e-cigarette research is a new and emerging field, and how e-cigarette aldehyde exposure in combination with genetic differences in reactive aldehyde metabolism may affect the risk of developing diseases such as cardiovascular disease or cancer is largely unknown.
3.3. Beverage and Food Sources
Aldehydes are abundantly found in several dietary sources. Besides alcoholic beverages, methanol and ethanol are natural constituents of fruit juice, which are enzymatically metabolized by the enzyme alcohol dehydrogenase (ADH) into formaldehyde (range from 3.7 to 60 ppm) and acetaldehyde (ranging from 0.0005 to 230 ppm), respectively [64, 65]. Aldehydes are natural components of fruits, vegetables, spices, and nuts. For example, peas contain traces of acetaldehyde, whereas cinnamon contains cinnamaldehyde. Almonds and chcrries contain benzaldehyde, whereas anisaldehyde and salicylaldehyde appear in anise and vanilla extracts [66], Moreover, fermentation is a widely known process used for millennia for preserving food and beverages. According to the WHO, fermented and processed foods including cheese, yogurt, meat, kefir, kimchi, and tofu also contain traceable amounts of formaldehyde (5.7–20 ppm) and acetaldehyde (0.2–0.6 ppm) [64].
Fats are also a significant source of aldehydes, and when cooked, over 20 different aldehydes are produced [66], Aldehydes are formed when frying food or heating oils to cook food. These aldehydes are mainly generated from the thermal oxidation of the polyunsaturated triacylglycerols [67]. Cooking oil heated at a temperature of 180 °C produces high amounts of aerosolized acrolein (canola oil 53.5 ± 3.9 mg/h and safflower oil 57.3 ± 6.7 mg/h) which are typically inhaled while standing over cooking food [68]. When soybean oil is used to cook deep-fried potatoes, 4-hydroxynonenal (4-HNE) is a major polar lipophilic compound in the thermally oxidized frying oil [69], Additional studies further validate that deep-frying food, especially at high temperatures and for prolonged periods of time, generate reactive aldehydes [70, 71]. For example, in Taiwan restaurant exhaust streams, 18 carbonyl species were measured. Formaldehyde, acetaldehyde, acetone, and butyraldehyde contributed 55.01–94.52% of total carbonyls in the dining areas for the restaurants measured [72].
3.4. Cosmetics
Cosmetic products including perfumes, deodorants, skin care products, and nail polish removers are common in daily lifestyle. According to the American Cancer Society, professional hair treatment products such as keratin smoothening products contain formaldehyde or formaldehyde-releasing agents which can raise indoor formaldehyde concentrations to a hazardous level (range 0.33–1.88 ppm) in hair salons [73], Various other aldehydes found in perfumes and deodorants are the most common cause of hypersensitivity to fragrances and allergic reactions [74], For example, cinnamaldehyde, a well-known chemical irritant and sensitizing agent, is a major component of the “fragrance mix” and commonly found in deodorants [75]. In 1991, when the EPA tested 31 fragrance products, among the 20 most commonly found toxic chemicals were propylene glycol, acetone, benzaldehyde, ethanol, and benzyl alcohol.
Aldehydes are also present in hand sanitizers where a hand sanitizer may contain 60–90% ethanol. During topical application of ethanol and aldehydes, the skin is the most susceptible organ to ethanol- and aldehyde-induced damage. Both ethanol and aldehydes are highly diffusible through the lipophilic layer of the outer skin and can covalently modify the epidermis to form an immunogenic antigen. The antigen-carrying cell can migrate to the lymph nodes, where the antigen is recognized by specific T cells that can proliferate and disseminate throughout the body via the blood, causing an inflammatory skin response [76]. Although there is a lack of evidence linking topical ethanol application to skin cancer, several studies confirm that when taken orally, ethanol increases the risk for skin cancer and other types of cancer [77]. Lastly, considering the susceptible nature of ALDH2*2 variant to ethanol and subsequently generated acetaldehyde-induced health disorders, it is advisable to avoid usage of ethanol-based cosmetic products.
Taken together, these studies suggest alcohol intake, use of tobacco products, fried foods, and cosmetic products are sources of reactive aldehydes. Reactive aldehyde exposure associated with these lifestyle choices increases the chances of developing cancer and cardiovascular diseases [51] specifically for those with an ALDH2*2 variant [78].
4. Impact on Humans with Rcduccd Rcactive Aldehyde Metabolism
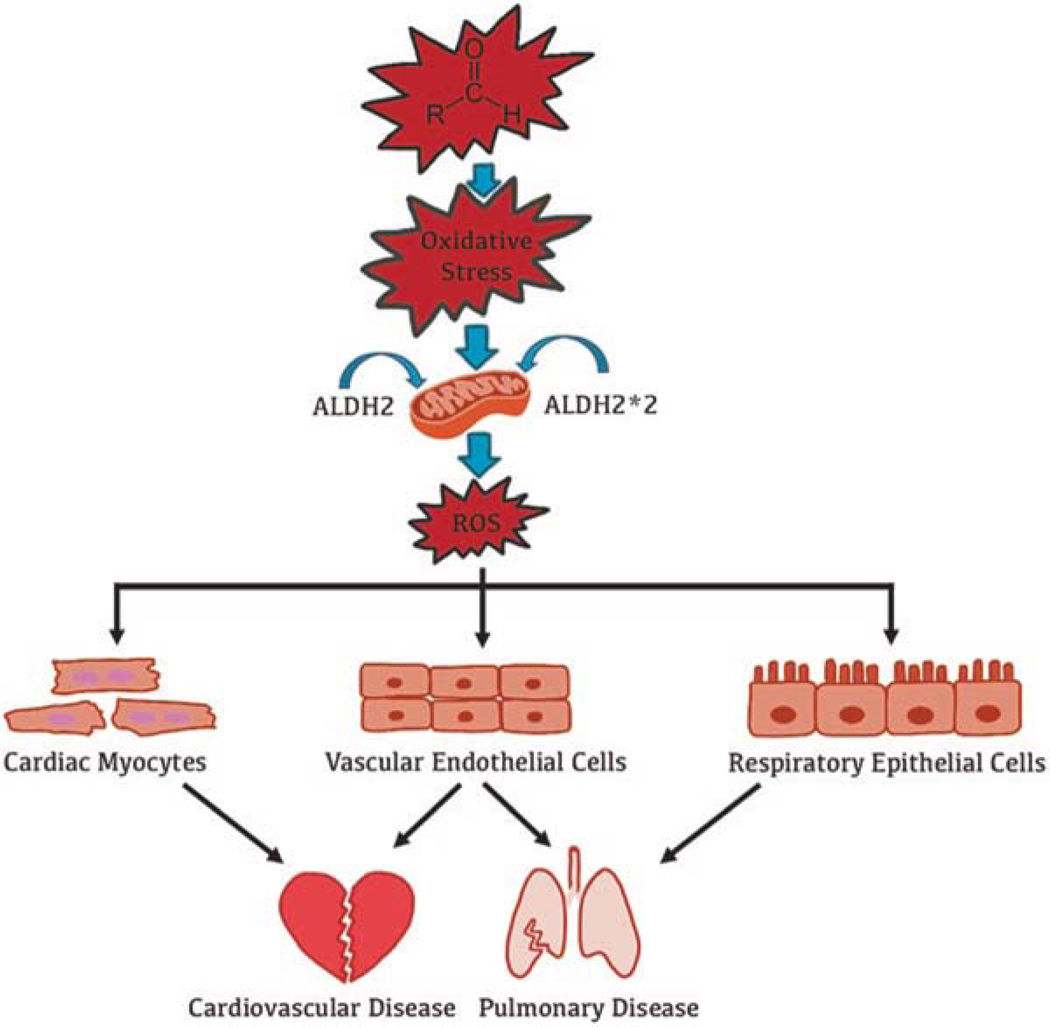
Reactive aldehyde exposure damages the electron transport chain, leading to an overproduction of mitochondrial rcactive oxygen spccics (Fig. 3). This in turn affects cellular properties contributing to various diseases [79]. The health implications of reactive aldehyde exposure from these environmental sources listed above are even more concerning for those ~560 million people worldwide who cannot efficiently metabolize these reactive aldehydes due to ALDH2*2 genetic variant. Therefore, people with an ALDH2*2 variant are vulnerable to reactive aldehyde exposure-induced oxidative stress and associated pathophysiological conditions.
Fig. 3.
Schematic representation of how reactive aldehyde exposure harms the cardiopulmonary system. ALDH2 metabolizes toxic reactive aldehydes into a less toxic form. For those with an ALDH2*2 variant, exposure to exogenous reactive aldehydes increases intracellular oxidative stress and impairs mitochondrial function due to an inefficient metabolism of reactive aldehydes. This is followed by an increase in mitochondrial reactive oxygen species (ROS) production. ROS directly affects the structure and function of cardiac myocytes, vascular endothelial cells, and pulmonary epithelial cells, resulting in cellular dysfunction that can ultimately lead to cardiopulmonary disease
Extensive research over the past several years has recognized the ALDH2*2 variant as a major contributor to several human diseases including cardiovascular disorders, stroke, and cancer [2, 38, 80], For example, a recent meta-analysis of nine case-control studies concluded that the ALDH2*2 variant is associated with an increased risk for developing coronary heart diseases, including myocardial infarction [81]. Therefore, those with an ALDH2*2 variant exposed to exogenous reactive aldehyde sources as discussed above are more likely to develop medical complications as a result of limited reactive aldehyde metabolism.
Since ALDH2*2 individuals are more susceptible to reactive aldehyde-associated health complications, it is important that information regarding the health risks associated with reactive aldehyde exposure be disseminated widely to the public. Many exogenous reactive aldehyde sources, such as alcohol consumption or cigarette use, are avoidable or modifiable. In situations where exposure cannot be avoided, safety precautions can be taken to limit or minimize exposure.
5. Conclusion
Over the past several years, extensive research has advanced the understanding of the relationship between exogenous reactive aldehyde sources and the harmful effects of their exposure. Whether outdoors, indoors, or at the place of occupational employment, the potential for reactive aldehyde exposure and the resultant negative health consequences are particularly important to consider for those with an ALDH2*2 variant.
At the forefront of protection from environmental sources of reactive aldehydes is public awareness. Unless knowledge is properly disseminated, individuals will not be cognizant of the risks associated with reactive aldehyde exposure or capable of taking the necessary steps to minimize exposure. Precautionary measures and lifestyle modifications to reduce reactive aldehyde exposure should be a focus of public health to ultimately reduce the potential risk for developing cancer or cardiovascular disease.
There are also several key questions that remain unanswered. More research is needed to understand how susceptible those with an ALDH2*2 variant are to reactive aldehydes from environmental sources. In addition, it is also important to further understand how much the relative risk for cancer or cardiovascular disease is increased with reactive aldehyde exposure for those with an ALDH2*2 genetic vaniant. Basic and clinical studies are also required to determine how reactive aldehydes produced with the workplace environment, including hospitals, may potentially and more preferentially affect those with an ALDH2*2 variant. New therapeutic strategies should also be encouraged to prevent, minimize, and treat health-related disorders arising as a result of reactive aldehyde exposure. Together, considering the genetics of reactive aldehyde metabolism with respect to environmental sources of aldehyde exposure can lead to developing a basis for a precision medicine platform in the field of environmental health sciences.
Acknowledgments
PSR and SM received career development awards from TRDRP (27FT-0037) and NICHD K99 (HD093858), respectively. All additional work was supported by a TRDRP High Impact Pilot Award (26IP-0038), and NHLBI (HL144388) to ERG.
References
- 1.O’Brien PJ, Siraki AG, Shangari N (2005) Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol 35(7):609–662 [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D (2014) Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev 94(1): 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda T, Yabushita H, Kanaly RA, Shibutani S, Yokoyama A (2006) Increased DNA damage in ALDH2-deficient alcoholics. Chem Res Toxicol 19(10): 1374–1378 [DOI] [PubMed] [Google Scholar]
- 4.Cai J, Bhatnagar A, Pierce WM Jr (2009) Protein modification by acrolein: formation and stability of cysteine adducts. Chem Res Toxicol 22(4):708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens JF, Maier CS (2008) Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52(l):7–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD (2010) Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol 17(2): 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambelli VO, Gross ER, Chen CH, Gutierrez VP, Cury Y, Mochly-Rosen D (2014) Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Sei Transl Med 6(251):251rall8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida A I Iuang IY, Ikawa M (1984) Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sei U S A 81(1 ):258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (2018) Air pollution, http://www.who.int/airpollution/en/ [Google Scholar]
- 10.Schauer JJ. Kleeman Ml. Cass GR, Simoneit BR (2001) Measurement of emissions from air pollution sources. 3. CI-C29 organic compounds from fireplace combustion of wood. Environ Sei Technol 35(9):1716–1728 [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Smith KR (1999) Emissions of carbonyl compounds from various cookstoves in China. Environ Sei Technol 33( I4):2311–2320 [Google Scholar]
- 12.Lipari F Dasch JM, Scruggs WF (1984) Aldehyde emissions from wood-burning fireplaces. Environ Sei Technol 18(5):326–330 [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Nakagoshi A. Tsurukawa M, Matsumura C, Eiho J, Nakano T (2012) Environmental risk assessment and concentration trend of atmospheric volatile organic compounds in Hyogo Prefecture. Jpn Environ Sei Pollut Res Int 19(1):201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho KF, Ho SSH, Huang RJ, Dai WT, Cao JJ, Tian L et al. (2015) Spatiotemporal distribution of carbonyl compounds in China. Environ Pollut 197:316–324 [DOI] [PubMed] [Google Scholar]
- 15.Altemose B, Gong J, Zhu T, Hu M, Zhang L, Cheng H et al. (2015) Aldehydes in relation to air pollution sources: a case study around the Beijing Olympics. Atmos Environ (1994) 109:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macintosh DL, Zimmer-Dauphinee SA, Manning RO, Williams PL (2000) Aldehyde concentrations in ambient air of coastal Georgia, U.S.A. Environ Monit Assess 63(3):409–429 [Google Scholar]
- 17.Smith D, Spanel P, Dabill D, Cocker J, Rajan B (2004) On-line analysis of diesel engine exhaust gases by selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom 18(23):2830–2838 [DOI] [PubMed] [Google Scholar]
- 18.Magnusson R, Nilsson C, Andersson B (2002) Emissions of aldehydes and ketones from a two-stroke engine using ethanol and ethanol-blended gasoline as fuel. Environ Sei Technol 36(8):1656–1664 [DOI] [PubMed] [Google Scholar]
- 19.Franz AW, Kronemayer H, Pfeiffer D, Pilz RD, Reuss G, Disteldorf W, Gamer AO, Hilt A (2016) Formaldehyde In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag: GmbH & Co. KGaA [Google Scholar]
- 20.Ho SS, Ip HS, Ho KF, Ng LP, Chan CS, Dai WT et al. (2013) Hazardous airborne carbonyls emissions in industrial workplaces in China. J Air Waste Manag Assoc 63(7):864–877 [DOI] [PubMed] [Google Scholar]
- 21.Hodgkin JH, Galbraith MN, Chong YK (1982) Combustion products from burning polyethylene. J Macromol Sei Part A Chem 17(l):35–44 [Google Scholar]
- 22.Jonsson A, Persson KA, Grigoriadis V (1985) Measurements of some low molecular-weight oxygenated, aromatic, and chlorinated hydrocarbons in ambient air and in vehicle emissions. Environ Int ll(2):383–392 [Google Scholar]
- 23.Eisler R (1994) Acrolein hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Dept, of the Interior, National Biological Survey, Washington, DC: Report. Laurel, MD: 1994 Report 28; Biological Report 23 [Google Scholar]
- 24.Ghilarducci DP, Tjeerdema RS (1995) Fate and effects of acrolein. Rev Environ Contam Toxicol 144:95–146 [DOI] [PubMed] [Google Scholar]
- 25.Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ (2008) Acrolein environmental levels and potential for human exposure. Toxicol Ind Health 24(8):543–564 [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Lioy PJ, He Q (1994) Characteristics of aldehydes: concentrations, sources, and exposures for indoor and outdoor residential microenvironments. Environ Sei Technol 28(1):146–152 [DOI] [PubMed] [Google Scholar]
- 27.Crump DR, Gardiner D (1989) Sources and concentrations of aldehydes and ketones in indoor environments in the UK. Environ Int 15(l):455–462 [Google Scholar]
- 28.Ullrich D, Nagel R, Seifert B (1982) The effect of lacquer coatings on indoor air quality using as example radiator lacquers. Schriftenr Ver Wasser Boden Lufthyg 53:283–298 [PubMed] [Google Scholar]
- 29.Pala M, Ugolini D, Ceppi M, Rizzo F, Maiorana L, Bolognesi C et al. (2008) Occupational exposure to formaldehyde and biological monitoring of Research Institute workers. Cancer Detect Prev 32(2): 121–126 [DOI] [PubMed] [Google Scholar]
- 30.Scarselli A, Corfiati M, Di Marzio D, Iavicoli S (2017) National estimates of exposure to formaldehyde in Italian workplaces. Ann Work Expo Health 61(1):33–43 [DOI] [PubMed] [Google Scholar]
- 31.Siegel DM, Frankos VH, Schneiderman MA (1983) Formaldehyde risk assessment for occupationally exposed workers. RTP 3(4):355–371 [DOI] [PubMed] [Google Scholar]
- 32.Krones CJ, Conze J, Hoelzl F, Stumpf M, Klinge U, Möller M et al. (2007) Chemical composition of surgical smoke produced by electrocautery, harmonic scalpel and argon beaming - a short study. Eur Surg 39(2):118–121 [Google Scholar]
- 33.Mwanga HH, Baatjies R, Jeebhay MF (2018) 646 exposure to aldehydes among health care workers in a large tertiary hospital in Cape Town, South Africa. Occup Environ Med 75(Suppl 2):A337 [Google Scholar]
- 34.Wang RS, Nakajima T, Kawamoto T, Honma T (2002) Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metab Dispos 30(l):69–73 [DOI] [PubMed] [Google Scholar]
- 35.Oyama T, Isse T, Ogawa M, Muto M, Uchiyama I, Kawamoto T (2007) Susceptibility to inhalation toxicity of acetaldehyde in Aldh2 knockout mice. Front Biosci 12:1927–1934 [DOI] [PubMed] [Google Scholar]
- 36.Parrilla R, Okawa K, Lindros KO, Zimmerman UJ, Kobayashi K, Williamson JR (1974) Functional compartmentation of acetaldehyde oxidation in rat liver. J Biol Chem 249(15):4926–4933 [PubMed] [Google Scholar]
- 37.Eriksson CJ, Marselos M, Koivula T (1975) Role of cytosolic rat liver aldehyde dehydrogenase in the oxidation of acetaldehyde during ethanol metabolism in vivo. Biochem J 152(3):709–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross ER, Zambelli VO, Small BA, Ferreira JC, Chen CH, Mochly-Rosen D (2015) A personalized medicine approach for Asian Americans with the aldehyde dehydrogenase 2*2 variant. Annu Rev Pharmacol Toxicol 55:107–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HeymannHM Gardner AM, Gross ER (2018) Aldehyde-induced DNA and protein adducts as biomarker tools for alcohol use disorder. Trends Mol Med 24(2): 144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y, Nakamura C, Mizukami T et al. (2008) Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydro-genase-2 genotype. Alcohol Clin Exp Res 32(9): 1607–1614 [DOI] [PubMed] [Google Scholar]
- 41.Harada S, Agarwal DP, Goedde HW (1985) Aldehyde dehydrogenase polymorphism and alcohol metabolism in alcoholics. Alcohol (Fayetteville, NY) 2(3):391–392 [DOI] [PubMed] [Google Scholar]
- 42.Monzavi SM, Afshari R, Rehman N (2015) Alcohol related disorders in Asia Pacific region: prevalence, health consequences and impacts on the nations. Asia Pac J Med Toxicol 4(1): 1–8 [Google Scholar]
- 43.Yokoyama A, Omori T (2005) Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Alcohol (Fayetteville, NY) 35(3):175–185 [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama A, Omori T, Yokoyama T (2010) Alcohol and aldehyde dehydrogenase polymorphisms and a new strategy for prevention and screening for cancer in the upper aerodigestive tract in East Asians. Keio J Med 59(4): 115–130 [DOI] [PubMed] [Google Scholar]
- 45.Brooks PJ, Enoch M A, Goldman D, Li TK, Yokoyama A (2009) The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med 6(3):e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAllister SL, Sun K. Gross F R (2016) Developing precision medicine for people of East Asian descent. J Biomed Sci 23:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Ren J (2011) ALDH2 in alcoholic heart diseases: molecular mechanism and clinical implications. PharmacolTher 132(1):86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Bishop EE, Kennedy SM, Simpson SA, Pcchacek TF (2015) Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med 48(3):326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodchild M, Nargis N, Tursan d’Espaignel E (2018) Global economic cost of smoking-attributable diseases. Tob Control 27:58–64. https://doi.Org/10.l136/tobaccocontrol-2016-053305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Federal Trade Commission (2017) United States Federal Trade Commission Cigarette Report for 2015. Published online www.fte.gov [Google Scholar]
- 51.CDC (2010) How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Centers for Disease Control and Prevention; (US), Atlanta: [PubMed] [Google Scholar]
- 52.Eschner MS, Selmani I, Groger TM, Zimmermann R (2011) Online comprehensive two-dimensional characterization of puff-by-puff resolved cigarette smoke by hyphenation of fast gas chromatography to single-photon ionization time-of-flight mass spectrometry: quantification of hazardous volatile organic compounds. Anal Chem 83(17):6619–6627 [DOI] [PubMed] [Google Scholar]
- 53.Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A et al. (2007) Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol 37(5):617–623 [DOI] [PubMed] [Google Scholar]
- 54.Corradi M, Rubinstein I, Andreoli R, Manini P, Caglieri A, Poli D et al. (2003) Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167(10):1380–1386 [DOI] [PubMed] [Google Scholar]
- 55.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT et al. (2004) Tobacco and cancer: recent epidemiological evidence. JNCI 96(2):99–106 [DOI] [PubMed] [Google Scholar]
- 56.DelloStritto DJ, Sinharoy P, Connell PJ, Fahmy JN, Cappelli HC, Thodeti CK et al. (2016) 4-Hydroxynonenal dependent alteration of TRPV1-mediated coronary microvascular signaling. Free Radic Biol Med 101:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizuno Y, Hokimoto S, Harada E, Kinoshita K, Yoshimura M, Yasue H (2017) Variant aldehyde dehydrogenase 2 (ALDH2*2) in East Asians interactively exacerbates tobacco smoking risk for coronary spasm—possible role of reactive aldehydes. Circ J 81(1):96–102 [DOI] [PubMed] [Google Scholar]
- 58.Kirkham PA, Barnes PJ (2013) Oxidative stress in COPD. Chest 144(l):266–273 [DOI] [PubMed] [Google Scholar]
- 59.Kuroda A, Hegab AE, Jingtao G, Yamashita S, Hizawa N, Sakamoto T et al. (2017) Effects of the common polymorphism in the human aldehyde dehydrogenase 2 (ALDH2) gene on the lung. Respir Res 18(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ et al. (2017) Aldehyde detection in electronic cigarette aerosols. ACS Omega 2(3): 1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinharoy P, McAllister S, Gross ER (2018) E-cigarette vapor elevates heart rate in mice with limited reactive aldehyde metabolism. FASEB J 32(1 suppl):848.16–16 [Google Scholar]
- 62.Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J et al. (2017) A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS One 12(l):e0169811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fagan P, Pokhrel P, Herzog TA, Moolchan ET, Cassel KD, Franke AA et al. (2017) Sugar and aldehyde content in flavored electronic cigarette liquids. Nicotine Tob Res 20(8):985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ (1991) Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res 259(3–4):363–385 [DOI] [PubMed] [Google Scholar]
- 65.Kavet R, Nauss KM (1990) The toxicity of inhaled methanol vapors. Crit Rev Toxicol 21(l):21–50 [DOI] [PubMed] [Google Scholar]
- 66.Guillen-Sans R, Guzman-Chozas M (1995) Aldehydes in food and its relation with the TBA test for rancidity. Lipid/Fett 97(7–8):285–286 [Google Scholar]
- 67.Ramirez MR, Estevez M, Morcuende D, Cava R (2004) Effect of the type of frying culinary fat on volatile compounds isolated in fried pork loin chops by using SPME-GC-MS. J Agric Food Chem 52(25):7637–7643 [DOI] [PubMed] [Google Scholar]
- 68.Katragadda HR, Fullana A, Sidhu S, Carbonell-Barrachina ÄA (2010) Emissions of volatile aldehydes from heated cooking oils. Food Chem 120(l):59–65 [Google Scholar]
- 69.Seppanen CM, Csallany AS (2004) Incorporation of the toxic aldehyde 4-hydroxy-2-trans-nonenal into food fried in thermally oxidized soybean oil. J Am Oil Chem Soc 81(12): 1137–1141 [Google Scholar]
- 70.Guilldn MD, Uriarte PS (2012) Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: presence of toxic oxygenated a,ß unsaturated aldehydes. Food Chem 131(3):915–926 [Google Scholar]
- 71.Katsuta I, Shimizu M, Yamaguchi T, Nakajima Y (2008) Emission of volatile aldehydes from DAG-rich and TAG-rich oils with different degrees of unsaturation during deep-frying. J Am Oil Chem Soc 85(6):513–519 [Google Scholar]
- 72.Cheng J-H, Lee Y-S, Chen K-S (2016) Carbonyl compounds in dining areas, kitchens and exhaust streams in restaurants with varying cooking methods in Kaohsiung, Taiwan. J Environ Sei 41:218–226 [DOI] [PubMed] [Google Scholar]
- 73.Golden R, Valentini M (2014) Formaldehyde and methylene glycol equivalence: critical assessment of chemical and toxicological aspects. Regul Toxicol Pharmacol 69(2):178–186 [DOI] [PubMed] [Google Scholar]
- 74.Patlewicz GY, Wright ZM, Basketter DA, Pease CK, Lepoittevin JP, Amau EG (2002) Structure-activity relationships for selected fragrance allergens. Contact Dermatitis 47(4):219–226 [DOI] [PubMed] [Google Scholar]
- 75.Smith CK, Moore CA, Elahi EN, Smart AT, Hotchkiss SA (2000) Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol Appl Pharmacol 168(3):189–199 [DOI] [PubMed] [Google Scholar]
- 76.Johansen JD (2003) Fragrance contact allergy: a clinical review. Am J Clin Dermatol 4(ll):789–798 [DOI] [PubMed] [Google Scholar]
- 77.Yen H, Dhana A, Okhovat J- P, Qureshi A, Keum N, Cho E (2017) Alcohol intake and risk of nonmelanoma skin cancer: a systematic review and dose-response meta-analysis. Br J Dermatol 177(3):696–707 [DOI] [PubMed] [Google Scholar]
- 78.Chen C- H, Sun L, Mochly-Rosen D (2010) Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res 88(l):51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manzo-Avalos S, Saavedra-Molina A (2010) Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health 7(12):4281–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S (2008) Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28(24):6239–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q, Zhou S, Wang L, Lei M, Wang Y, Miao C et al. (2013) ALDH2 rs671 polymorphism and coronary heart disease risk among Asian populations: a meta-analysis and meta-regression. DNA Cell Biol 32(7):393–399 [DOI] [PubMed] [Google Scholar]