Abstract
Introduction
Peptic ulcer disease represents a worldwide health problem because of its high morbidity, mortality and economic loss. It is a very prevalent condition affecting around 10%–15% of the general population worldwide. Most of the available antiulcer drugs are costly and have an incidence of relapse, drug interactions and several side effects upon chronic usage. Hence, the use of herbal medicine may be safe, economical and effective in such cases when drugs are used for long periods. Ethnobotanical reports showed traditional claims on the use of Cordia africana seeds for the treatment of gastric ulcers. However, the safety and efficacy of these remedies are not well known. The aim of this study is, therefore, to evaluate the antiulcer activity and safety of a crude extract of C. africana seeds in animal models.
Methods
Shade-dried seeds of C. africana were extracted by 80% methanol and dried by the rotator evaporator and lyophilized. The crude extract was used to evaluate antiulcer activity in vivo with pylorus ligation method, on Wistar albino rats weighing 230–250g. Preliminary phytochemical screening was performed using a standard procedure. Acute toxicity study was carried out in Swiss albino mice before antiulcer activity tests.
Results
No sign of toxicity was observed upon the administration of 2000 mg/kg of the crude extract to mice. Single-dose administration of 400 and 600 mg/kg extract showed a significant reduction in the volume of secretion and acidity of the stomach (p <0.01). The doses 400 and 600 mg/kg have reduced the ulcer score by 83.58% and 88%.
Conclusion
The result of this study showed that the hydromethanolic crude extract of C. africana has strong antisecretory and ulcer protective activities against ulcers produced by pylorus ligation.
Keywords: antiulcer, Cordia africana, seeds, pylorus ligation, ulcer parameters, ulcer score, ulcer index, percent protection
Introduction
A peptic ulcer is defined as the disruption of the mucosal integrity of the stomach and/or duodenum due to imbalance between aggressive factors like Helicobacter pylori (H. pylori), nonsteroidal anti-inflammatory drugs, alcohol, fatty foods and smoking, acid-pepsin hypersecretion, stress, free radical generation (ROS & LPOs)1,2 bile, and reduced local blood flow are the major factors that disrupt this balance3–6 and protective factors like alkali mucus secretion, mucosal microcirculation, PGE PGI, NO,6,7 gastric mucosal glycoproteins HSPs6 and GSH.1
Number of modern drugs including Proton pump inhibitors, prostaglandin analogs, histamine receptor antagonists and cytoprotective agents are costly and have an incidence of relapse, drug interaction and several side effects like impotence, gynecomastia, galactorrhea and GI infections.8,9 Hence, herbal medicines are generally used in such cases when drugs are used for chronic periods and may also be safe, economical and effective as well as ease of availability.8,9 The extracts from medicinal plants and other natural products have become the widely accepted sources of therapeutic agents for the treatment of peptic ulcers.4,5,8
Peptic ulcer disease represents a worldwide health problem because of its high morbidity, mortality and economic loss. It is a very prevalent condition affecting around 10–15% of the general population worldwide.5,10
Cordia africana, a vernacular name, “Wanza” (Amharic), is a tree of the family Boraginaceae and genus Cordia. Ethnopharmacological studies have shown that different parts of C. africana have been traditionally used for different ailments. In Burkina Faso, it is used for its appetite suppressant (fruit) and antibacterial activity.11 In Ethiopia use of C. africana for liver disease (leaves), amoebiasis (root),12 trachoma stomach ache and diarrhea (root, root bark),12,13 gastric ulcer (seeds),14 Ascariasis, rabis eye disease wound dressing (stem bark)15 and acute febrile illness11 have been reported.
Different parts of C. africana (leaves, stem, park, and fruit) were screened to show their antioxidant activity via DPPH assay. The extract of leaves, stem, bark, and fruit with different solvents gave antioxidant activity of 74–91%. An in vitro antibacterial and antifungal evaluations of Cordia africana conducted in Sudan reported that the zone of inhibition of C. africana extracts was 18–20 and 20 −30 for bacteria and fungi, respectively.16 An in vivo Antidiarrheal activity screening of methanolic extract of the root bark of Cordia africana in Ethiopia reported that C. africana has antidiarrheal activity comparable to atropine.17 No in - vivo or in - vitro studies have been done on the seed of C. africana in Ethiopia.
Materials
Chemicals and Drugs
The chemicals and drugs used in the study are; Ranitidine tablet (ACICLOC Cadila pharm, Ethiopia), conc.H2SO4 (Mettle-Toledo Ltd, Switzerland), Glacial acetic acid (Lobe chemi, India), aqueous 5% ferric chloride and 10% alcoholic ferric chloride (Lab Tech Chemicals, India), conc. HCL & 1% Aqueous hydrochloric acid (Nice chemicals Ltd India), 20% NaOH solution and 0.1 N NaOH solution (Central drugs house (p) Ltd India), phenolphthalein (Rankem India), Distilled water (Gondar hospital lab), Normal saline (Ethiopia pharmaceutical factory, Ethiopia), chloroform, Wagner reagent and Mayer reagent (Mettle-Toledo Ltd, Switzerland), dilute ammonia (Techno. Pharm. Chem., India), Disposable glove, catgut chromic reverse cutting with a needle (Swico Sweden), Centrifuge, Desiccators, Sensitive balance, separator funnel, beaker, Erlenmeyer flasks, measuring cylinder, cotton gauze, Watt man paper (EXACL, India), Oral gavages, scissors, centrifuge, Rotary evaporator and lyophilizer (Ningbo-Scientz-Biotechnology).
Plant Collection and Authentication
Fresh ripen C. africana fruits were collected from Tinsaye about 15 km North of Gondar town. The plant was identified and authenticated as C. africana by a botanist (Mr. Abiyu Eneyew) and a Voucher specimen was deposited (No.YE001) at the Herbarium, Department of Biology, College of Natural Computational Sciences, Gondar University, for future reference.
Experimental Animals
Wistar albino rats of mixed-sex weighing between 230 and 250 g.
Methods
Preparation of the Extract
The fruits of C. africana were processed to remove the mucilage from the seeds. Then, the seeds were shade dried and powdered using the electrical grinding mill. After grinding, 1.6 kg of fine powder (150gm in 500 mL of 80% methanol) was macerated in a conical flask with frequent steering for 3 days. After 3 days the mixture was filtered using a fine muslin cloth followed by paper filtration (Whatman No. 1). The residue was macerated for another 3 days. Methanol from the combined filtrates was removed under reduced pressure by rotary evaporator at 45 rpm and 40°C to obtain the crude extract. Then, the extract was further concentrated to dryness with a lyophilizer at 40°C. A total of 425 g (percentage yield of 26.56%) of the dried extract was obtained and stored in a desiccator with an airtight container.
Acute Oral Toxicity Test
Acute toxicity studies were conducted for the crude methanolic extract of the seeds of C. africana (single dose 2000 mg/kg) using the OECD guideline for the testing of chemicals. Five female Swiss albino mice weighing 25–35g were used. Each mouse received the extracts at a dose of 2000 mg/kg body weight.18 Signs of toxicity were continuously observed for the first 2 hours and every 2-hour for 6 hours. Finally, the number of survivors was noted after 24 hours. Then for any gross physical and behavioral changes further up to 72 h, and followed for 14 days for any mortality. Based on the results of the acute toxicity study, 1/10 of the limit dose was used as the starting dose.
Phytochemical Chemical Screening Test
The 80% methanolic crude extract of Cordia africana seeds were screened for the presence of secondary metabolites that may have correlations with the antiulcer activity of the extract. Hence, tests for alkaloids, saponins, cardiac glycosides, flavonoids, terpenoids, steroids, phenols, and tannins were performed using standard tests as stated below.19–22
Animal Grouping and Treatments
Albino rats weighing 230 to 250 g were randomly divided into 5 groups (n=6 in each group).
Negative Control (group I) animals were treated with vehicle (3% Tween 80). Test groups (II, III & IV) were treated with graded doses of 200, 400 and 600 mg/kg methanolic extract of C. africana seeds. Standard group (V) was treated with the standard antiulcer drug (ranitidine 50 mg/kg, p.o,).4 The 10-day repeated-dose study was carried out in 3 groups by using the most effective dose of the extract in the single-dose study; the other groups remain similar to the single-dose study.23
Ulcer Induction
The rats were placed in cages with elevated wide wire and fasted for 24 hours from food but water ad libitum before the experiment. Then
Group I animals were given the vehicle, group II, III &IV animals were given 200, 400 and 600 mg/kg methanolic extract of C. africana seeds, respectively. Group V animals were given ranitidine 50 mg/kg, p.o.4 After 60 minutes, the animals were anesthetized with ether, the abdomen was opened below the xiphoid process and pyloric end of the stomach was uplifted and ligated. The stomach was then returned and the abdomen was stitched by catgut. Care was taken not to damage the internal organs and blood supply to that area. 4 hours after surgery, rats were sacrificed with cervical dislocation and the abdomen was incised with care. Then, the gastric juice was collected for the estimation of free and total acids.2,3
Based on the results of the single-dose study, the most effective dose was 600 mg/kg and this dose was used for the 10 days repeated-dose study. For the 10 days repeated-dose study, Group I animals were given the vehicle, group II animals were given 600 mg/kg methanolic extract of C. africana seeds. Group III animals were given ranitidine 50 mg/kg, p.o. All groups of animals were given their respective treatments for 10 days and the pyloric ligation was done after the 10th day of treatment.23 After 60 minutes of the last dose, the animals were anesthetized with ether, the abdomen was opened below the xiphoid process and pyloric end of the stomach was uplifted and ligated. The stomach was then returned and the abdomen was stitched by catgut. Care was taken not to damage the internal organs and blood supply to that area. 4 hours after surgery, rats were sacrificed with cervical dislocation and the abdomen was incised with care. Then, the gastric juice was collected for the estimation of free and total acids.2,3
Determination of Gastric Secretion Parameters
Measurement of Acidity of Gastric Juice
After sacrificing the rats with cervical dislocation, the stomachs were removed and the contents of each stomach were squeezed into a test tube. The samples were centrifuged at 2000rpm for 10 minutes. 1mL of the aliquot was taken to a flask containing 10mL distilled water and analyzed for hydrogen ion concentration by colorimetric titration with 0.1 N NaOH solutions using phenolphthalein indicator. The acid content was calculated and expressed as mEq/l.2,24
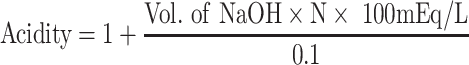 |
Determination of PH
After centrifugation, an aliquot of 1mL gastric juice was diluted with 1mL of distilled water and the pH of the solution was measured using PH meter.23
Quantification of Ulceration
After sacrificing the animals by cervical dislocation, stomachs were removed and open along the greater curvature. The macroscopic examination was carried out with a hand lens and the lesions were scored as follows:25
Normal stomach … … … … …. (0)
Red coloration … … … … … … (0.5)
Spot ulcer … … … … … … … …. (1)
Hemorrhagic streak … … … …. (1.5)
Ulcers … … … … … … … … …. (2)
Perforation … … … … … … … … (3)
The mean ulcer score for each animal was expressed as the ulcer index.
UI= UN+US+Upx10−1
UN, average of the number of ulcers per animal, US, average of severity scores, UP, percentage of animals with ulcer
The percentage of ulcer protection was determined as follows:
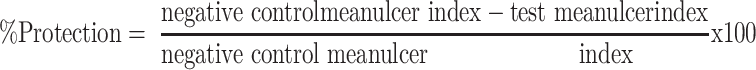 |
Statistical Analysis
The data analysis was done by SPSS version 20. The mean values of each parameter were analyzed by one way ANOVA followed by Tukey’s post hock multiple comparison test for significance of the difference of means.
Ethical Clearance
Throughout the experiments, all animals were handled with humanly caring according to the guideline for the housing of mice in scientific institutions26 and the ethical clearance with Reference no. of SOP4/70/09 was obtained from the Experimental Animals Ethics Committee, Department of Pharmacology, University of Gondar, Ethiopia.
Results
Phytochemical Screening
The preliminary phytochemical screening test of 80% methanolic crude extract of C. africana seeds revealed the presence of important secondary metabolites as indicated by Table 1.
Table 1.
Results of Phytochemical Screening of Cordia africana Seeds Extract
| Tests Were Done | Results |
|---|---|
| Tannins | + |
| Flavonoids | + |
| Glycosides | – |
| Alkaloids | – |
| Saponins | + |
| Phenols | + |
| Anthraquinones | – |
| Steroids | – |
Acute Oral Toxicity Test
No sign of toxicity or mortality was observed in mice after oral administration of the hydromethanolic seed extract of C. africana at a limit dose of 2000 mg/kg, signifying that the median lethal dose was quite above 2000mg/kg.
Effects of Cordia africana Seed Extract
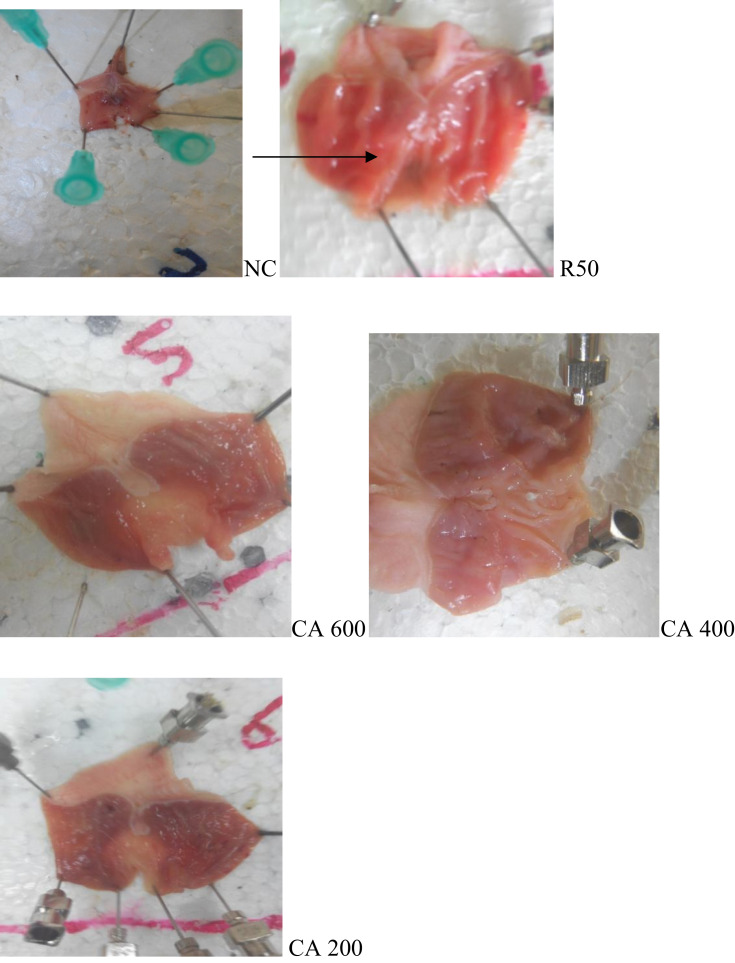
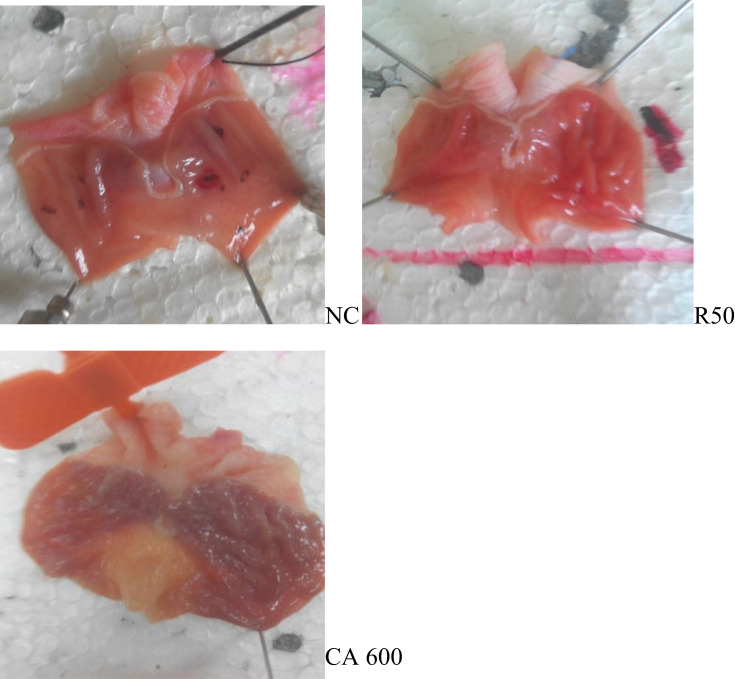
Antiulcer effects of the hydroalcoholic leaf extract of CA on gastric ulcers induced by pylorus ligation were assessed by macroscopic evaluation (Figures 1 and 2).
Figure 1.
Single-dose study pyloric ligation method.
Notes: As shown in CA600, the extract demonstrated a better antiulcer effect at the higher dose (CA600). As shown by the pictures; the crude extract decreases the number and severity of ulcer dose-dependently.
Abbreviations: NC, negative control; R50, ranitidine 50 mg/kg; CA600, Cordia africana 600mg/kg of extract; CA400, Cordia africana 400 mg/kg of extract; CA200, Cordia africana 200 mg/kg of extract.
Figure 2.
Ten days of pretreatment or repeated-dose study.
Notes: As shown by CA600n and R50 pretreatment with CA 600mg/kg for 10 days prevents ulceration comparably with ranitidine.
Abbreviations: NC, negative control; R50, ranitidine 50 mg/kg; CA600, Cordia africana 600mg/kg of extract.
Single-Dose Study
Untreated groups produce a gastric secretion of 5.92±0.63 but treatment with the ranitidine, 200mg/kg 400mg/kg and 600mg/kg crude extract reduces this secretion to 3.01+0.11 (p<0.001), 4.45+0.37, 4.05+ 0.29 (p<0.05) and 3.67+0.23 (p<0.01) respectively. The acidity of the stomach was 5.67 +3.07 for the negative control and significantly reduced by ranitidine (p<0.001), CA400 (p<0.05) and CA600 (p< 0.001) compared to the negative control. A significant difference was also observed between the lowest dose and ranitidine (p <0.01). Compared to the negative control, both ranitidine and CA 600 increase the stomach pH with a significance level of P <0.001 and P < 0.05, respectively. Ranitidine also significantly (P <0.01) increased the stomach pH compared to the lowest dose of the extract.
Regarding ulcer protection, ranitidine, the middle and highest dose of the crude extract significantly reduced the ulcer scores 91.04% (p <0.001), 83.58% (p < 0.01) and 88% (P <0.001) respectively compared to the negative controls. Ranitidine showed a significant reduction in ulcer number and ulcer index (P <0.05). But the effects of the crude extracts were not statistically significant for the two ulcer parameters (Table 2).
Table 2.
Effect of Cordia africana Single Dose on Pyloric Ligated Rats
| Test Group | The Volume of Gastric Juice | The Acidity of Gastric Juice | The PH of Gastric Juice | US | %Red. US | UN | UI | %Red. UI |
|---|---|---|---|---|---|---|---|---|
| NC | 5.92±0.63 | 51.67±3.07 | 3 ±0.25 | 5.58 ±1.51 | – | 4 ±1.24 | 15.92±1.04 | – |
| R 50 | 3.01±0.11c | 25.±3.07c | 5±0.36c | 0.5±0.34c | 91.04 | 0.83±0.54a | 4.36±2.76a | 72.6 |
| CA200 | 4.45±0.37 | 43.3±4.22y | 3.6±0.21x | 2.47±0.89 | 64.18 | 2.83±0.87 | 11.85±2.47 | 25.53 |
| CA400 | 4.05±0.29a | 36.67±5.57a | 4 ±0.22 | 0.91±0.3b | 83.58 | 1.5 ±0.43 | 10.32±2.09 | 35.18 |
| CA600 | 3.67±0.23 b | 29.17±2 c | 4.16±0.3a | 0.5±0.18 c | 88 | 1 ±0.37 | 8 ± 254 | 49.74 |
Notes: Values are expressed as mean ± SEM (n=6). aP<0.05, bP<0.01, cP<0.001 - compared to the negative control. xP<0.05, yP<0.01, - compared to the positive control.
Abbreviations: NC, negative control; R, ranitidine; CA, C. africana; SEM, standard error of the mean.
Repeated-Dose Study
The dose of the seed extract that was noted as the most effective in the single-dose study produces a significant reduction in the volume of gastric juice and ulcer number was similar to that of the standard drug. It reduces the acidity of the stomach and ulcer severity score at a significant level of p <0.05 and p< 0.01, respectively, whereas that of the standard drug reduces both acidity of the stomach and ulcer severity score at a significant level of p <0.001. Even if it was not statistically significant, the dose of extract decreased the ulcer index. But ranitidine significantly (p <0.05) reduced the ulcer index compared to the negative control. The crude extract showed a better effect on the percent reduction of ulcers (75.61% -R50 Vs78.05%- CA600). The percentage reduction of ulcer index was shown to be 59.55% with ranitidine and 59.27% with CA600 (Table 3).
Table 3.
Effect of Cordia africana 10 Days Repeated Dose on Pyloric Ligated Rats
| Test Group | The Volume of Gastric Juice | The Acidity of Gastric Juice | The PH of Gastric Juice | US | %Red.US | UN | UI | %Red. UI |
|---|---|---|---|---|---|---|---|---|
| NC | 6.63±0.2 | 43.33 ±2.2 | 3.16±0.16 | 3.58±0.62 | 3.66±0.33 | 14.63±0.38 | – | |
| R 50 | 3.78±0.2c | 25.83±2c | 4.67±0.33b | 0.41±0.2c | 75.61 | 0.5±0.40c | 5.91±2.64a | 59.55 |
| CA600 | 4.58±0.2c | 33.33±2.2b | 4.33±0.21a | 0.33±0.17c | 78.05 | 0.67±0.47c | 5.96±2.67a | 59.27 |
Notes: Values are expressed as mean ± SEM; (n=6). aP< 0.05; bP < 0.01; cP < 0.001. All are compared to the negative control.
Abbreviations: US, ulcer score; UN, ulcer number; UI, ulcer index; NC, negative control; R 50, ranitidine 50mg/kg; CA, C. africana; SEM, standard error of mean.
Discussion
The extraction of CA seeds with 80% methanol produced a reddish-brown color crude extract with a 26.56% yield. This mucilaginous crude extract was used to evaluate the safety and antiulcer activity of C. africana in Swiss Albino mice and pyloric ligated rats, respectively. Cordia africana extract was safe at a limit dose of 2000 mg/kg in mice. It did not cause mortality or sign of toxicity in the test animals. This indicates that the median lethal dose of CA is quite above 2000 mg/kg.
Although the exact etiology of peptic ulcer is not known in most of the cases, it is generally accepted that it results from an imbalance between aggressive factors and the endogenous protective factors. To restore the balance, different therapeutic agents that reduce the aggressive factors and/or increase the defensive mechanism are used. Different plant extracts may also be used to restore the balance.25 Cordis Africana extract is one such herbal drug used in the present study primarily to evaluate the antiulcerogenic effect in pylorus ligation.
The most frequently prescribed antiulcer drugs are the proton pump inhibitors and the H2 receptor blockers. The mechanism of this group of drugs is the inhibition of acid secretion. Proton pump inhibitors irreversibly inhibit the H+/K+ ATPase in the gastric parietal cells and decrease gastric secretion. The H2 receptor blockers block H2 receptors and decrease histamine stimulated gastric secretion. The crude extract showed a significant reduction in gastric secretion and acidity of the stomach. It also showed a significant increase in pH. This action of the crude extract resembles the action of the above group of drugs. The antisecretory activity of this plant may be consistent with its flavonoid content. Flavonoids are known to decrease histamine secretion from mast cells by inhibition of histidine decarboxylase and inhibit H+/K+-ATPase.6,24,27,28 Treatment of animals with crude extract also significantly inhibited the formation of ulcers in the pylorus ligated rats. These activities of the extract may be attributed to the phytochemical constituents that have anti-inflammatory, antioxidant and mucosal secretion stimulants.
The phytochemical studies performed in the present study demonstrated that the seed of CA contains flavonoids, saponins, tannins, and phenols. The antiulcer activity of C. africana could be due to the presence of flavonoids tannins, saponin, and phenolic compounds. These secondary metabolites are effective as antioxidants, antineoplastic, antiulcer, anti-inflammatory, and immune-stimulating agents.
Flavonoids are free radical scavengers and have the potential cytoprotective and antiulcerogenic effect. The antiulcerogenic activity of these compounds is since these compounds increase mucus, bicarbonate and prostaglandin secretion and counteract the deleterious effects of reactive oxidants in the gastrointestinal lumen.8,29,30 Saponins may activate mucous membrane protective factors, and tannins render the outermost layer of the mucosa less permeable, for instance, to chemical irritation.28,30
As evidenced by the phytochemical contents, CA seed extract may have anti-inflammatory, antisecretary and free radical scavenging activities. It is expected that the gastroprotective effect exerted by this plant could be credited to its aforementioned activities. Also, the extract may have direct physical protection to the mucosa from irritants like alcohol and NSAIDs as it is highly mucilaginous.
Conclusion
Based on the results of the present study we can conclude that the crude methanolic seed extract of Cordia africana is safe at doses as high as 2g/kg, and have significant ulcer protection effects against pylorus ligation induced ulcers. The free radical scavenging secondary metabolites (flavonoids and phenolic compounds) and the mucosal stimulating metabolites like saponins may contribute to the ulcer protective activity of the extract. The plant’s crude extract has shown significant antisecretory and acid-neutralizing activities in pyloric ligated rats, this effect may contribute to the antiulcerogenic activity of the plant.
Acknowledgments
First, I would like to express my sincerest gratitude and appreciation to Dr. Wubayehu Khaliw for his major and detailed comments on the finalization of this work. Secondly, I would like to thank Mr. Kefyalew A. for his valued comments. My sincere gratitude also goes to the laboratory technicians Ato Asemachew and Ato Gashaw for their cooperation. My appreciation is also extended to the animal attendant of the Pharmacology Department; W/ro Banchi for her constant care of the experimental animals. Finally, I would like to express my thanks to the laboratory animals which sacrificed their lives for the continuation of science.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dursun H, Bilici M, Albayrak F, et al. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009;9(1):36. doi: 10.1186/1471-230X-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onasanwo SA, Singh N, Olaleye SB, Mishra V, Palit G. Anti-ulcer & antioxidant activities of Hedrantherabarteri {(Hook F.) Pichon} with possible involvement of H+, K+ ATPase inhibitory activity. Indian J Med Res. 2010;132(4):442. [PubMed] [Google Scholar]
- 3.Siddique RA. Prevalence of peptic ulcer disease among the patients with abdominal pain attending the department of medicine in Dhaka Medical College Hospital, Bangladesh. IOSR J Dent Med Sci. 2014;13(1):05–20. [Google Scholar]
- 4.Srivani S, Sahoo SP, Ramchandran S, Dhanraju MD. Antiulcer and antisecretory activity of trychosantheslobata. Int J Pharm Chem Res. 2013;2(4):1798–1802. [Google Scholar]
- 5.Priya S, Nethaji S. Anti-ulcer activity of ethanolic extract of diospyrosVirginiana in rats. World J Pharm Res. 2015;4(6):1403–1408. [Google Scholar]
- 6.Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35(5):523–534. doi: 10.1590/s0100-879x2002000500003 [DOI] [PubMed] [Google Scholar]
- 7.Mohite M, Pramod HJ, Yadav AV, Raje VN, Wadkar GH. Evaluation of the antiulcer activity of aqueous extract of Borassusflabellifer (linn.) fruits. J Pharm Res. 2012;5(7):3782–3786. [Google Scholar]
- 8.Dashputre NL, Naikwade NS. Evaluation of the anti-ulcer activity of methanolic extract of Abutilon Indicum Linn leaves in experimental rats. Int J Pharm Sci Drug Res. 2011;3(2):97–100. [Google Scholar]
- 9.Priyanka V. Some of the medicinal plants with anti-ulcer activity – a review. J Pharm Sci Res. 2015;7(9):772–775. [Google Scholar]
- 10.Lauret M, Pérez I, Rodrigo L. Peptic ulcer disease. Austin J Gastroenterol. 2015;2(5):1055–1063. [Google Scholar]
- 11.Mesfin K, Tekle G, Tesfay T. Ethnobotanical study of traditional medicinal plants used by indigenous people of Gemad District, Northern Ethiopia. J Med Plants Stud. 2013;1(4):32–37. [Google Scholar]
- 12.Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-Awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525. doi: 10.1016/j.jep.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 13.Mengesha GG. Ethnobotanical survey of medicinal plants used in treating human and livestock health problems in ManduraWoreda of BenishangulGumuz, Ethiopia. Adv Med Plant Res. 2016;4(1):11–26. [Google Scholar]
- 14.Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in DebreLibanosWereda, Central Ethiopia. Afr J Plant Sci. 2014;8(7):366–379. doi: 10.5897/AJPS2013.1041 [DOI] [Google Scholar]
- 15.Geyid A, Abebe D, Debella A, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005;97(3):421–427. doi: 10.1016/j.jep.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 16.Alhadi EA, Khalid HS, Alhassan MS, Gader HA, Kabbashi AS, FadulAlla EF. Comparison of antimicrobial activity and thin layer chromatography profile of ethyl acetate fraction of leaves and stem of Cordia Africana (Boraginaceae). Int J Multidiscip Res Dev. 2015;2(11):665–669. [Google Scholar]
- 17.Asrie AB, Abdelwuhab M, Shewamene Z, Gelayee DA, Adinew GM, Birru EM. Antidiarrheal activity of methanolic extract of the root bark of CordiaAfricana. J Exp Pharmacol. 2016;8:53–59. doi: 10.2147/JEP.S116155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OECD. Guidelines for the testing of chemicals; Acute Oral Toxicity: up-and-down procedures. OECD Publ, No 425, Adopted Decem. Available from: http://www.oecdbookshop.org. Accessed May25, 2020.
- 19.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaceuticasciencia. 2011;1(1):98–106. [Google Scholar]
- 20.Suman Kumar R, Venkateshwar C, Samuel G, Rao SG. Phytochemical screening of some compounds from plant leaf extracts of HolopteleaIntegrifolia (Planch.) and CelastrusEmarginata (Grah.) used by Gondu tribes at Adilabad district, Andhra Pradesh, India. Int J Eng Sci Invention. 2013;2(8):65–70. [Google Scholar]
- 21.Jaradat N, Hussein F, Al Ali A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra AlataDecne. J Mater Environ Sci. 2015;6(6):1771–1778. [Google Scholar]
- 22.Zohra SF, Meriem B, Samira S, Muneer MA. Phytochemical screening and identification of some compounds from mallow. J Nat Prod Plant Resour. 2012;2(4):512–516. [Google Scholar]
- 23.Mohod SM, Bodhankar SL. Evaluation of the antiulcer activity of methanolic extract of leaves of Madhucaindica JF Gmel in rats. Pharmacologyonline. 2011;3:203–213. [Google Scholar]
- 24.Abebaw M, Mishra B, Gelayee DA. Evaluation of the anti-ulcer activity of the leaf extract of Osyris quadripartiteDecne. (Santalaceae) in rats. J Exp Pharmacol. 2017;9:1–11. doi: 10.2147/JEP.S125383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju D, Ilango K, Chitra V, Ashish K. Evaluation of anti-ulcer activity of methanolic extract of Terminaliachebula fruits in experimental rats. J Pharm Sci Res. 2009;1(3):101–107. [Google Scholar]
- 26.Fawcett A, Rose M. Guidelines for the housing of mice in scientific institutions. Animal Welfare Unit, NSW Department of Primary Industries, West Pennant Hills. Anim Res Rev Panel. 2012;1:1–43. [Google Scholar]
- 27.Pyloric pu. Screening for the anti-ulcer activity of convolvulus pluricaulis using the pyloric ligation method in Wistar rats. Int J Pharm Sci Res. 2015;6(1):89–99. [Google Scholar]
- 28.Borrelli F, Izzo AA. The plant kingdom as a source of anti‐ulcer remedies. Phytother Res. 2000;14(8):581–591. [DOI] [PubMed] [Google Scholar]
- 29.Inas ZA, Hala AK, Gehan HH. Gastroprotective effect of Cordiamyxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 2011;8(3):433–445. [Google Scholar]
- 30.Alrdahe SS, Abdulla MA, Razak SA, Kadir FA, Hassandarvish P. Gastroprotective activity of SwieteniaMahagoni seed extract on ethanol-induced gastric mucosal injury in rats. World Acad Sci Eng Technol. 2010;21(43):883–887. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- OECD. Guidelines for the testing of chemicals; Acute Oral Toxicity: up-and-down procedures. OECD Publ, No 425, Adopted Decem. Available from: http://www.oecdbookshop.org. Accessed May25, 2020.