Abstract
Purpose
The purpose of this study was to investigate the molecular mechanism of LncRNA LOXL1-AS1 in non-small cell lung cancer (NSCLC).
Methods
Lung cancer cell lines (H1299, A549, H520 and H596) and human normal lung epithelial cell line (BEAS-2B) were used in this study. Gene expression was measured by qRT-PCR (quantitative real-time PCR). The bioinformatics databases (miRDB and TargetScan7) were used to predict target genes. Luciferase assay and pull-down assay were processed for verifying the binding sites. CCK8 assay was used for detecting proliferation, and transwell assay was undertaken for migration and invasion.
Results
LncRNA LOXL1-AS1 was higher expressed in lung cancer tissues and cells. Moreover, LOXL1-AS1 expression was upregulated in tumor tissues with advanced stages and metastasis. After knocking down LOXL1-AS1, proliferation, invasion and migration of H1299 and A549 cells were inhibited. Interestingly, miR-3128 was negatively regulated by LncRNA LOXL1-AS1, which inhibited the expression of RHOXF2. Rescue assay also confirmed that miR-3128 inhibitor and oeRHOXF2 could rescue the effect of down-regulated LOXL1-AS1 on proliferation, invasion and migration progression.
Conclusion
LOXL1-AS1 promotes the progression of NSCLC by regulating miR-3128/RHOXF2 axis, which might be a new potential target for the diagnosis and treatment of NSCLC.
Keywords: NSCLC, LOXL1-AS1, miR-3128, RHOXF2
Introduction
Lung cancer is one of the diseases threatening to the health and life of the population with the fastest growing morbidity and mortality rates. The clinical symptoms of lung cancer are closely related to tumor size, type, stage of development, location, occurrence of complications and metastasis.1 For early lung cancer, more sophisticated and less invasive surgical methods are increasingly pursued.2 Surgery is the preferred treatment for lung cancer.3 Radical surgery is still the only treatment that may cure lung cancer patients.4 In addition, chemotherapy and radiation therapy have certain clinical value as adjuvant treatment methods.5 The advent of immunotherapy has brought new hope to wild-type lung cancer patients.6 However, the 5-year survival rate is only 19.7%.7 To solve the many problems of lung cancer prevention and treatment, the key is to further promote and improve early diagnosis and treatment strategies of lung cancer. For advanced lung cancer, targeted therapy is deeply rooted. Molecular targeted therapy is a major breakthrough in the treatment of lung cancer in recent years, but few target drugs have effective for SCLC.8 There are many effective drugs for NSCLC, and mainly targets of drugs were epidermal growth factor receptor and vascular endothelial growth factor.9
Long noncoding RNAs (lncRNAs) are a kind of noncoding RNAs and characterized as over 200 nucleotides in length and the lack of protein-coding ability.10 MicroRNAs (miRNAs) are another kind of noncoding RNAs and have ~25 nucleotides in length. miRNAs could target the response element of specific mRNAs to regulate their expression. Of note, lncRNAs are acknowledged as the potential competing endogenous RNAs (ceRNAs) for miRNAs.10 In recent years, various lncRNAs and miRNAs were confirmed to be differentially expressed in various cancers, which played cell functions related to cancer. Yang et al processed bioinformatics analysis, and several lncRNAs, such as ENST00000366408, AK126698 and BX648420 were identified to be close with cisplatin resistance in lung cancer based on co-expression network analysis.10 Besides, lncRNA HOTAIR was verified to be higher expressed in lung cancer, which improved proliferation, survival, deterioration and drug resistance processes.11 In addition, overexpression of lncRNA-UCA1, lncRNA SNHG1 and PVT1 were also confirmed to be closely associated with progression of lung cancer.12–14 Interestingly, LncRNA LOXL1-AS1 has been researched in 74 human cancers, such as medulloblastoma and glioblastoma.15,16 However, the molecular mechanism of LncRNA LOXL1-AS1 in lung cancer has not been reported.
To further improve the diagnosis and treatment of NSCLC, the molecular mechanism of LncRNA LOXL1-AS1 in NSCLC was researched in this study. In addition, related miRNA and genes were also explored. This study might lay theoretical foundation for NSCLC research and provide a new potential target for the diagnosis and treatment of NSCLC.
Materials and Methods
Patients and Tissue Samples
Total 43 NSCLC patients (Stage I–II: 20 samples; Stage III–IV: 23 samples) and their corresponding adjacent normal tissues were collected from The First Affiliated Hospital of China Medical University. The study was approved by the ethics committee of The First Affiliated Hospital of China Medical University. Signed informed consent was obtained from each patient. The general information of patients was recorded in detail, and the medical records were well preserved. After surgery, the lung cancer tissue and the adjacent normal tissues were immediately placed in liquid nitrogen for freezing, and then stored in a −80°C refrigerator.
Cell Culture and Transfections
To confirm the upregulation of LOX1-AS1 in lung cancer cells, Lung cancer cell lines (H1299, A549, H520 and H596) and Human normal lung epithelial cell line (BEAS-2B) were purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The cells were inoculated into DMEM medium and placed in a 5% CO2 cell incubator at 37°C for subculture. LOXL1-AS1 negative control (si-NC), LOXL1-AS1 (si-LOXL1-AS1-1 and si-LOXL1-AS-2), miR-3128 mimics, miR-3128 inhibitor, negative control (miR-NC) and oeRHOXF2 were design and synthesis by Shanghai Gima Biotechnology Co., Ltd. These sequences were transfected into H1299 and A549 cells in accordance with the instructions of the liposome Lipofectamine 2000 instructions (Takara). Briefly, the RNA sequence and Lipofectamine 2000 was diluted separately in the medium. After incubation for 5 min at room temperature, they were mixed thoroughly and incubated for 20 min at room temperature to form a transfection complex. The above-mentioned transfection complexes were added to each well, and gently shake the cross to mix. After transfection for 6 h, the medium was changed and cultured for 48 h. The transfection efficiency was measured by qRT-PCR. After successful transfection, it was used for subsequent experiments.
RNA Isolation and qRT-PCR
A real-time fluorescent quantitative polymerase chain reaction (qRT-PCR) kit was purchased from Dalian Bao Biological Engineering Co., Ltd. According to the instructions of fast 200 (Shanghai Feijie Biotechnology Co., Ltd., China), total RNA was extracted from tissues and cells, respectively, and detected by ultraviolet spectrophotometer. Samples with OD260/OD280 between 1.9 and 2.1 were qualified. 500 ng of total RNA was taken out as a template, and a reverse transcription reaction system was prepared according to the instructions of the RT-PCR kit. Random 6 and Oligo-dT double primer methods were used to reverse transcribe lncRNA and mRNA in cells. After reverse transcription was completed, 2 μL of cDNA was used to prepare the qRT-PCR system, and the target gene was amplified according to the qRT-PCR instructions.
Target Gene Prediction
The bioinformatics databases using miRDB (MicroRNA Target Prediction Database; http://mirdb.org/index.html) and TargetScan7 were used to predict target genes for subsequent research.
CCK8 Assay
The cells in logarithmic growth phase were taken and divided into si-LOX1-AS1 group, si-LOX1-AS1 + inhibitor group, si-LOX1-AS1 + oeRHOXF2 group and control group, and 4 x 103 cells were inoculated into per well of 96-well plates. Three duplicate wells for each group were set up and placed them in a cell incubator. After culturing cells for 0, 24, 48, 72, and 96 h, 10 μL CCK8 was added to each well, and placed it in the incubator for 2 h. Microplate reader was used to measure the optical density (OD) at 450 nm. The experiment was repeated 3 times, and the average of the experimental results was taken as the final result.
Transwell Assay for Migration and Invasion
Total 100 μL of dissolved Matrigel (diluted 1:6 in serum-free culture medium) was added to the upper transwell chamber of a 24-well plate, shaked and placed in a CO2 incubator at 37°C for 6 hrs. After blotting the culture solution, 500 μL of serum-free culture medium was added to the lower chamber and stayed for half an hour to hydrate the basement membrane. Cell suspensions were prepared by serum-free medium after transfection for 24 h. Approximately, 100 μL of cell suspension (1 × 105 cells) was added to the upper chamber and 500 μL of complete culture medium was added to the lower chamber. After treatment overnight, the remaining cells in the upper chamber were wiped off, washed with PBS, and fixed with 4% paraformaldehyde for 30 min. After that, the cells were stained with 0.1% crystal violet for 20 min, and then washed with PBS. Total of 5 fields of view were randomly selected under the microscope to take pictures and count. The migration experiment procedure was similar to the invasion experiment but without Matrigel.
Luciferase Assay
The sequence of LOXL1-AS1 was inserted into the luciferase reporter gene PGL3 vector. The luciferase reporter plasmid and Renilla plasmid co-transfected cells were placed in an incubator and cultured for 48 hours. According to the instructions of the Promega dual-luciferase reporter gene detection kit, the cells were rinsed twice with PBS. 100 μL of PLB solution was added to each well, shake for 15 min until it was fully lysed. 20 μL of sample was taken from each well for testing on the machine. Besides, the regulation between LOXL1-AS1 and miR-3128 was also explored by luciferase assay as the above steps.
Western Blot Assay
Cell samples were added with RIPA cell lysate, and ice-bathed for 30 mins. The cell lysate was transferred to an EP tube and centrifuged at 12,000 g at 4°C for 20 mins. The supernatant was transferred to an EP tube, and the protein concentration was measured. After quantification, 10 × SDS-PAGE loading buffer was added and the solution was boiled for 10 min. GAPDH was used as the reference. SDS-PAGE was used. The protein bands were transferred to a PVDF membrane (0.22 μm) by wet electrophoresis, blocked with a blocking solution containing 5% skimmed milk powder for 2 h. Primary antibody (1:1000) was added and incubated at 4°C overnight. The membrane was washed for 3 times with TBST. HRP-labeled goat anti-rabbit IgG antibody (1:2000) was added and incubated for 2 hrs. After washing the membrane with TBST, the ECL chemiluminescence reagent was used for color-reaction.
Statistical Analysis
Data analysis was performed using SPSS21.0 statistical software. Results were expressed as mean ± standard deviation (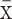 ± s). The t-test was used to compare the mean of the two groups of samples. Pearson correlation analysis was processed to calculate the expressed relationship between RHOXF2 and miR-3128. Significant difference was with the threshold of P <0.05.
± s). The t-test was used to compare the mean of the two groups of samples. Pearson correlation analysis was processed to calculate the expressed relationship between RHOXF2 and miR-3128. Significant difference was with the threshold of P <0.05.
Results
LncRNA LOXL1-AS1 Highly Expressed in Lung Cancer Tissues and Cells
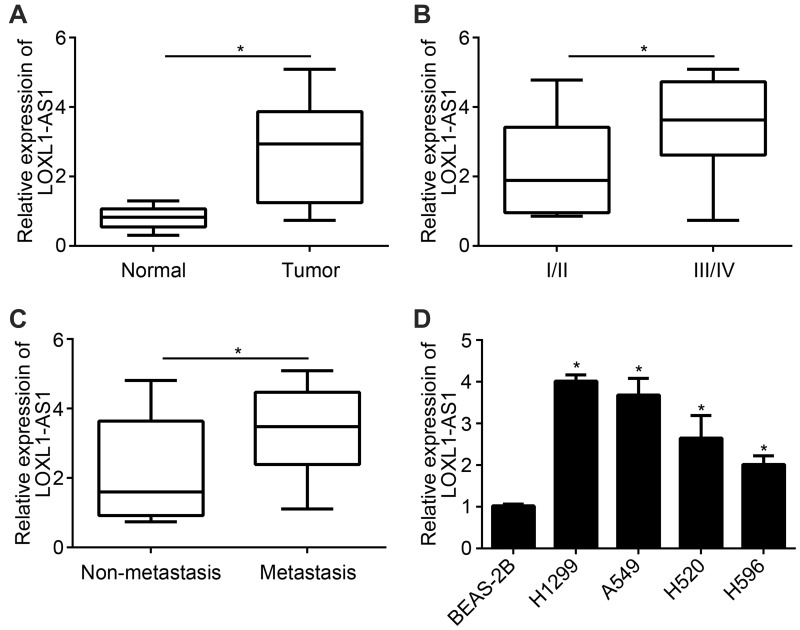
QRT-PCR assay was processed to detect the expression of LncRNA LOXL1-AS1 in lung cancer tissues and cells. As a result, LncRNA LOXL1-AS1 was higher expressed in lung cancer tissues than normal (Figure 1A). Moreover, patients with higher disease stage or metastasis were always combined with higher LncRNA LOXL1-AS1 expression (Figure 1B and C). The similar results were also obtained in cell lines. LncRNA LOXL1-AS1 was higher expressed in lung cancer cell lines (H1299, A549, H520 and H596) than that in BEAS-2B cells (Figure 1D). Moreover, LncRNA LOXL1-AS1 expressed highest in H1299 and A549 cell lines. Thereby, H1299 and A549 cell lines were used for the following experiments.
Figure 1.
LncRNA LOXL1-AS1 highly expressed in lung cancer tissues and cells (A) Analysis of LOXL1-AS1 expression in lung cancer and adjacent tumors. (B) The expression of LOXL1-AS1 in different stages of lung cancer. (C) The expression of LOXL1-AS1 in lymph node metastases and non-metastatic lung cancer tissues. (D) The expression of LOXL1-AS1 in lung cancer and normal cell lines. *P < 0.05.
Si-LOXL1-AS1 Inhibited Proliferation, Migration and Invasion of NSCLC Cells
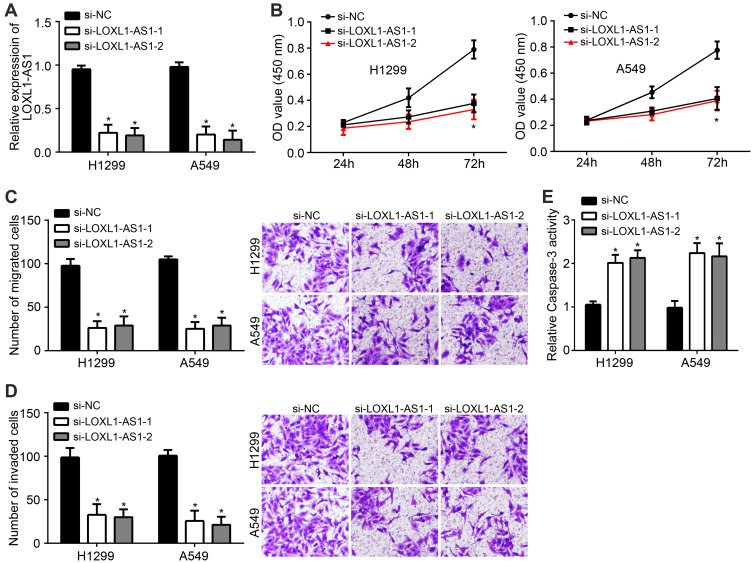
After transfected by si-LOXL1-AS1, the expression of lncRNA LOXL1-AS1 was significantly lower than normal control, indicating that the transfection was successful (Figure 2A). The results of CCK8 assay confirmed that si-LOXL1-AS1 inhibited proliferation of NSCLC cells and the inhibition was with significance after si-LOXL1-AS1 transfection for 72 h (Figure 2B). Moreover, migration and invasion of NSCLC cells were also inhibited by si-LOXL1-AS1 (Figure 2C and D). Interestingly, the expression of Caspase-3 was upregulated by si-LOXL1-AS1 (Figure 2E).
Figure 2.
Knockdown of lncRNA LOXL1-AS1 inhibited proliferation, migration and invasion of NSCLC cells and increased Caspase-3 activity. (A) LOXL1-AS1 is knocked down in H1299 and A549 cell lines. (B) CCK8 assay for proliferation. (C) Transwell assay for migration. (D) Transwell assay for invasion. (E) QRT-PCR detected the expression of Caspase after si-LOXL1-AS1 transfection. *P < 0.05.
LncRNA LOXL1-AS1 Targeted miR-3128
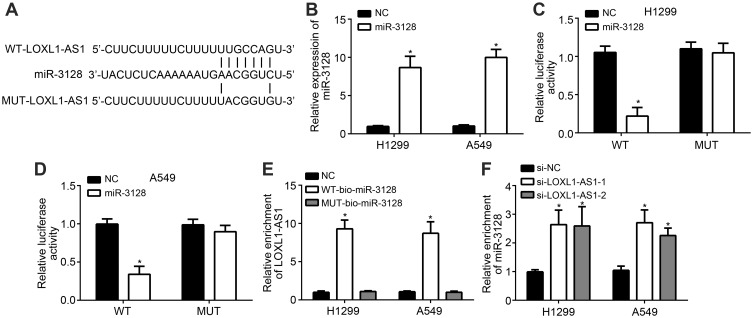
miRDB was used to predict the potential targets of LOXL1-AS1. Among all candidates, miR-3128 ranked the top. Their binding site is shown in Figure 3A. miR-3128 mimic was transfected into H1299 and A549 cell lines. Overexpressed miR-3128 confirmed the transfection was successful (Figure 3B). Then, the luciferase reporter assay was performed by transfection of WT- or Mut-LOXL1-AS1 luciferase reporter vector and miR-3128 mimics or negative controls. The results showed that relative luciferase activity was significantly lower in WT+miR-3128 group than that in MUT groups (Figure 3C and D). The association between LncRNA LOXL1-AS1 and miR-3128 in NSCLC cells was analyzed using the RNA pull-down experiment. The results showed that the binding activity of NSCLC cells LncRNA LOXL1-AS1 and miR-3128 was significantly stronger than that of the mutant and control groups, and the difference was significant (Figure 3E). Moreover, lncRNA LOXL1-AS1 knockdown promoted the expression level of miR-3128 (Figure 3F).
Figure 3.
LncRNA LOXL1-AS1 targeted miR-3128. (A) miRDB was used to predict that LOXL1-AS1 interacts with miR-3128. (B) Overexpressed miR-3128 confirmed the transfection was successful. (C and D) Luciferase assay. (E) The association between LncRNA LOXL1-AS1 and miR-3128 in NSCLC cells was analyzed using the RNA pull-down experiment. (F) lncRNA LOXL1-AS1 knockdown promoted the expression level of miR-3128. *P < 0.05.
miR-3128 Targeted RHOXF2
TargetScan7 online software was used to predict the target of miR-3128. Among all the candidates, RHOXF2 scored the highest. Their binding site is shown in Figure 4A. Relative luciferase activity was significantly lower in WT+miR-3128 group than that in control or MUT groups (Figure 4B and C). The results confirmed that miR-3128 targeted RHOXF2. miR-3128 overexpression inhibits RHOXF2 expression (Figure 4D). Correlation analysis showed that miR-3128 and RHOXF2 expression were negatively correlated in NSCLC tissues (Figure 4E).
Figure 4.
miR-3128 targeted RHOXF2. (A) TargetScan7 online software was used to predict A. RHOXF2 interaction with miR-3128. (B and C) Luciferase assay. (D) miR-3128 inhibited RHOXF2 expression. (E) Correlation analysis verified the relationship between miR-3128 and RHOXF2 expression in NSCLC tissues. *P < 0.05.
miR-3128 Inhibitor and oeRHOXF2 Rescued the Effect of Si-LOXL1-AS1
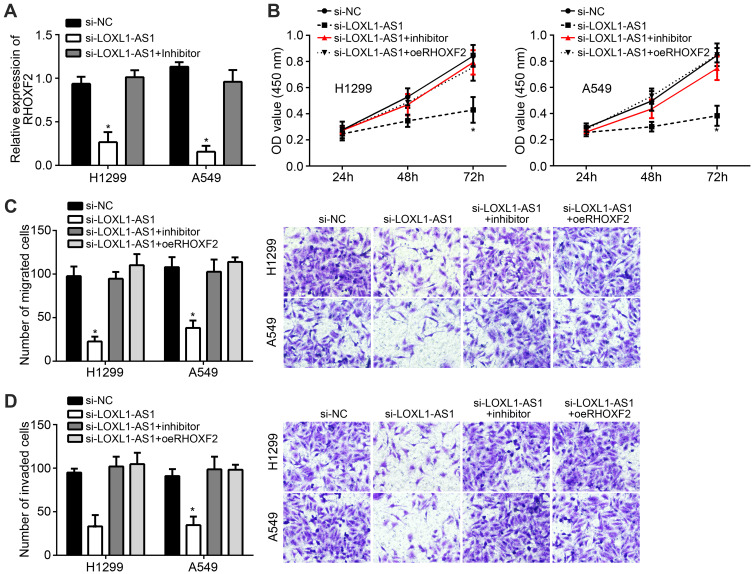
Si-LOXL1-AS1 inhibited the expression of RHOF2, while oeRHOXF2 upregulated the level (Figure 5A). Si-LOXL1-AS1 inhibited proliferation, invasion and migration of H1299 and A549 cells, while the effects were rescued after miR-3128 inhibitor or oeRHOXF2 simultaneously transfected (Figure 5B–D).
Figure 5.
Rescue assay. (A) LOXL1-AS1 regulated RHOXF2 expression. (B) CCK8 experiment for proliferation. (C and D) Transwell assay for migration and invasion. *P < 0.05.
Discussion
In order to verify the molecular mechanism of LncRNA LOXL1-AS1 in NSCLC, the expression of LncRNA LOXL1-AS1 was detected, and effects on proliferation, invasion and migration were researched. As a result, LncRNA LOXL1-AS1 was higher expressed in NSCLC tissues and cells. Moreover, its expression was elevated in tumor tissues with advanced stages and metastasis. After knocking-down, the expression of LncRNA LOXL1-AS1, proliferation, invasion and migration of H1299 and A549 cells were inhibited, and the expression of Caspase-3 was upregulated. Interestingly, miR-3128 was negatively regulated by LncRNA LOXL1-AS1, which inhibited the expression of RHOXF2. Rescue assay also confirmed that miR-3128 inhibitor and oeRHOXF2 could rescue the effect of down-regulated LOXL1-AS1 on proliferation, invasion and migration progression.
LncRNA is a single-stranded noncoding RNA with a length of 200bp to 100kb, which can regulate important biological processes such as cell division, growth, differentiation and apoptosis. LncRNA LOXL1-AS1 was an important factor, which effected the development of various cancers.16 In glioblastoma cancer, it was higher expressed, and knockdown of LncRNA LOXL1-AS1 inhibited the migration process by participating in NF-κB pathway.15–17 Besides, LncRNA LOXL1-AS1 was also confirmed to promote PI3K/AKT pathway, and further induce metastasis and proliferation of medulloblastoma disease.15 However, how LOXL1-AS1 affects NF-κB and PI3K/AKT pathways remains unclear. Chen et al referred that LncRNA LOXL1-AS1 could improve metastasis and proliferation in osteosarcoma cancer and predict poor prognosis.17 It worth noting that chemotherapy for NSLCL could induce the activity of NF-κB and inhibiting PI3K/AKT pathway improved chemotherapy-induced apoptosis.18 In addition, PI3K/AKT pathway was also confirmed to be closely related with migration, invasion, cell cycle and apoptosis of lung cancer.19,20 In this study, si-LOXL1-AS1 was confirmed to improve the expression of Caspase-3. Caspase-3 was a kind of cysteine protease, which was a critical factor for the apoptosis of lung cancer.21 Koomagi and Volm detected the relationship between Caspase-3 level and clinical outcome of NSCLC, and confirmed that patients with higher Caspase-3 expression were always with longer survival time.22 In lung cancer mouse models, Caspase-3 could also inhibit autophagy progress.23 Nevertheless, the mechanism through which LOXL1-AS1 regulates Caspase-3 activity requires to be defined in the future. Thereby, LncRNA LOXL1-AS1 was an important factor for proliferation, invasion and migration of NSCLC. Of note, we found that LOXL1-AS1 overexpression had no effect on proliferation or migration of the normal lung cell line BEAS-2B (data not shown).
It must be mentioned that miR-3128 was the target of LncRNA LOXL1-AS1, both miRDB prediction and targeted experiments confirmed it in this study. Few studies reported the miRNA. In 2011, Persson et al firstly have undertaken generation sequencing expression analysis of breast cancer and screened miR-3128 as differentially expressed miRNA to identify this disease.24 Moreover, special expression of miR-3128 was also verified as a new biomarker for melanoma progression based on gene library sequencing.25 Interestingly, there was a negative correlation between miR-3128 and RHOXF2 expression in NSCLC tissues in this study. In a previous study, RHOXF2 has been confirmed to be higher expressed in various cancer cell lines, such as lung cancer, colon cancer and leukemia cell lines.26 In breast cancer patients, it was considered as a recognized biomarker at the transcript level.27 Compared with normal tissue, the expression of RHOXF2 in breast cancer was upregulated by 3.31 fold in breast cancerous tissues.28 Importantly, knocking-down of LncRNA LOXL1-AS1 could inhibit proliferation, invasion and migration of NSCLC, while miR-3128 inhibitor and oeRHOXF2 could rescue the effects in this study. Thereby, LOXL1-AS1 regulated miR-3128/RHOXF2 expression, and then improved the development of NSCLC. However, there are some limitations in this study. Due to experimental conditions, the molecular mechanism of LOXL1-AS1 has not been verified in animal models.
Conclusion
In conclusion, we found that LOXL1-AS1 was upregulated in NSCLC tissues and correlated with tumor progression. LOXL1-AS1 promoted the progression of NSCLC by regulating miR-3128/RHOXF2 axis. Our findings suggested LOXL1-AS1 might be a new potential target for the diagnosis and treatment of NSCLC.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fábián K, Gyulai M, Furák J, et al. Significance of primary tumor location and histology for brain metastasis development and peritumoral brain edema in lung cancer. Oncology. 2016;91(5):237–242. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood JT, Brock MV. Lung cancer: new surgical approaches. Respirology. 2007;12(3):326–332. doi: 10.1111/j.1440-1843.2007.01083.x [DOI] [PubMed] [Google Scholar]
- 3.Majem M, Juan O, Insa AI, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019;21(1):3–17. doi: 10.1007/s12094-018-1978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodera Y, Fujiwara M, Koike M, Hibi K, Nakao A. Current surgical management of gastric carcinoma: global standards and Japanese modifications. Cancer Rev Asia-Pac. 2004;02(1):39–50. doi: 10.1142/S0219836304000366 [DOI] [Google Scholar]
- 5.Van Rens MT, Schramel FM, Elbers JR, Lammers JW. The clinical value of lung imaging fluorescence endoscopy for detecting synchronous lung cancer. Lung Cancer. 2001;32(1):13–18. [DOI] [PubMed] [Google Scholar]
- 6.Sgambato A, Casaluce F, Gridelli C. The role of checkpoint inhibitors immunotherapy in advanced non-small cell lung cancer in the elderly. Expert Opin Biol Ther. 2017;17(5):565–571. doi: 10.1080/14712598.2017.1294157 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine. 2019;98(3):e13788. doi: 10.1097/MD.0000000000013788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzel JA, Fanucchi MP, Li Z. Recent advances of novel targeted therapy in non-small cell lung cancer. J Hematol Oncol. 2009;2(1):2. doi: 10.1186/1756-8722-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi A, Maione P, Claudia SP, Ambrosio R, Falanga M, Gridelli C. Vascular endothelial growth factor receptor as target for advanced non-small cell lung cancer therapy. Curr Drug Targets. 2010;11(7):865–874. doi: 10.2174/138945010791320791 [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Li H, Hou S, et al. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8(5):e65309. doi: 10.1371/journal.pone.0065309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7(1):90. doi: 10.1186/s13045-014-0090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HM, Lu JH, Chen WY, Gu AQ. Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int J Clin Exp Med. 2015;8(7):11824–11830. [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Liu S, Wang H, Zhang Z, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8(11):5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 14.Yun C, Fuming Z, Chunkai Z, Liang G, Tongde T, Huaimin L. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(11):17785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao R, Zhang R, Zhang C, Liang Y, Tang W. LncRNA LOXL1-AS1 promotes the proliferation and metastasis of medulloblastoma by activating the PI3K/AKT pathway. Anal Cell Pathol. 2018;2018:1–11. doi: 10.1155/2018/9275685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Li L, Yin L. Silencing LncRNA LOXL1-AS1 attenuates mesenchymal characteristics of glioblastoma via NF-κB pathway. Biochem Biophys Res Commun. 2018;500(2):518–524. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Li W, Guo A. LOXL1-AS1 predicts poor prognosis and promotes cell proliferation, migration and invasion in osteosarcoma. Biosci Rep. 2019;39(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DR, Broad RM, Madrid LV, Baldwin AS, Mayo MW. Inhibition of NF-κB sensitizes non–small cell lung cancer cells to chemotherapy-induced apoptosis. Ann Thorac Surg. 2000;70(3):930–936. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Lai JM, Chou TY, et al. VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One. 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Zhang Y, Qu D, Jiang T, Li S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 2011;30(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata T, Tanaka F, Yamada T, Yanagihara K, Wada H. Clinical significance of caspase-3 expression in pathologic-stage I, nonsmall-cell lung cancer. Int J Cancer. 2001;96 Suppl(S1):54–60. doi: 10.1002/ijc.10347 [DOI] [PubMed] [Google Scholar]
- 22.Koomägi R, Volm M. Relationship between the expression of caspase-3 and the clinical outcome of patients with non-small cell lung cancer. Anticancer Res. 2000;20(1B):493–496. [PubMed] [Google Scholar]
- 23.Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha YI, Lu B. Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer. Autophagy. 2008;4(5):659–668. doi: 10.4161/auto.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson H, Kvist A, Rego N, et al. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res. 2011;71(1):78–86. doi: 10.1158/0008-5472.CAN-10-1869 [DOI] [PubMed] [Google Scholar]
- 25.Stark MS, Tyagi S, Nancarrow DJ, et al. Characterization of the melanoma miRNAome by deep sequencing. PLoS One. 2010;5(3):e9685. doi: 10.1371/journal.pone.0009685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura T. Identification of RHOXF2 (PEPP2) as a cancer-promoting gene by expression cloning. Int J Oncol. 2012;40(1):93–98. [DOI] [PubMed] [Google Scholar]
- 27.Davis JB. Palliation of metastatic breast cancer. Nebr Med J. 1958;43(11):498. [PubMed] [Google Scholar]
- 28.Kazemi-Oula G, Ghafouri-Fard S, Mobasheri MB, Geranpayeh L, Modarressi MH. Upregulation of RHOXF2 and ODF4 expression in breast cancer tissues. Cell J. 2015;17(3):471–477. doi: 10.22074/cellj.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]