Abstract
Backgrounds
The relationship between cardiovascular outcomes and the Controlling Nutritional Status (CONUT) score in heart failure (HF) with preserved ejection fraction (HFpEF) patients is unknown. This study aimed to evaluate the relationship between the score and cardiovascular outcomes in HFpEF patients.
Methods and results
A total of 506 consecutive HFpEF patients were prospectively observed for up to 1500 days or until the occurrence of cardiovascular events. The mean age was 71.6 ± 9.4 years. Cardiovascular outcomes were compared between the CONUT score 0–1 group with a normal nutritional state (normal group), the CONUT score 2–4 group with a light degree of undernutrition (light group), and the CONUT score 5–8 group with a moderate degree of undernutrition (moderate group). In this study, there were no patients who scored 9–12, which was defined as a severe state of undernutrition. Overall, 238 cardiovascular events were observed during the follow-up period (median: 1159 days). Kaplan–Meier analysis showed that the moderate group was at higher risk of composite cardiovascular events than the normal group (P < 0.001) and the light group (P = 0.031). The analysis also showed that the light group was at higher risk of composite cardiovascular events than the normal group (P = 0.038). Multivariable Cox proportional hazards analysis with the significant factors from the univariate analysis showed that the CONUT score (hazard ratio: 1.12, 95% confidence interval: 1.03–1.21, P = 0.005) significantly predicted future cardiovascular events.
Conclusion
Nutritional screening using the CONUT score may be useful for predicting cardiovascular events in HFpEF patients.
Keywords: CONUT score, HFpEF, Clinical outcome
1. Introduction
For the purpose of treating heart failure (HF), it is important to (1) suppress the progression of cardiac dysfunction, (2) improve symptoms, exercise skills and quality of life (QOL), and (3) prevent readmission and improve the life prognosis [1]. In the progressive stage of HF, physical function and nutritional status change in parallel, and the nutritional status of patients with HF worsens toward the end of life. Serum albumin and cholesterol levels, commonly used as indicators of nutritional status, are predictors of prognosis independent of age and severity of HF [2], [3]. It has been reported that hypoalbuminemia progression after hospitalization is associated with a worse prognosis in patients with acute HF [4].
Serum albumin has a long half-life of approximately 20 days, is susceptible to invasion and is not suitable for individuals evaluation. For this reason, the Controlling Nutritional Status (CONUT) method was developed as a tool to evaluate nutritional status using three biomarkers: protein metabolism, immunocompetence, and lipid metabolism [5]. The serum albumin level reflects protein metabolism, the total lymphocyte count reflects immunity, and the total cholesterol level reflects lipid metabolism; these items are scored, and the nutritional status is comprehensively and pleiotropically evaluated according to the three biological indices. The CONUT index has been reported as a useful index for the early screening of malnutrition in patients with HF [5]. It has been reported that undernutrition in chronic HF patients could be evaluated with the CONUT index and was correlated with subsequent cardiovascular events [6], and a correlation was found between the CONUT score and mortality in patients with HF [7], [8].
Accumulating clinical studies have demonstrated that HF with reduced left ventricular (LV) ejection fraction (EF) (HFrEF) and HF with preserved LVEF (HFpEF) are separate pathological conditions because of differences in survival rates [9], [10] and effective drug therapies; thus, we have proposed that HFrEF and HFpEF patients should be managed differently [11].
In the present study, we sought to evaluate the relationship between the CONUT score and cardiovascular outcomes in patients with HFpEF.
2. Methods
This study was a prospective, single-center, observational study.
2.1. Ethics statement
All procedures were conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the institutional review board of Kumamoto University (approval number, Senshin 2225). This study is registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000036884). Opt-out materials are available at http://www.kumadai-junnai.com/home/wp-content/uploads/houkatsu.pdf.
2.2. Study design and patients
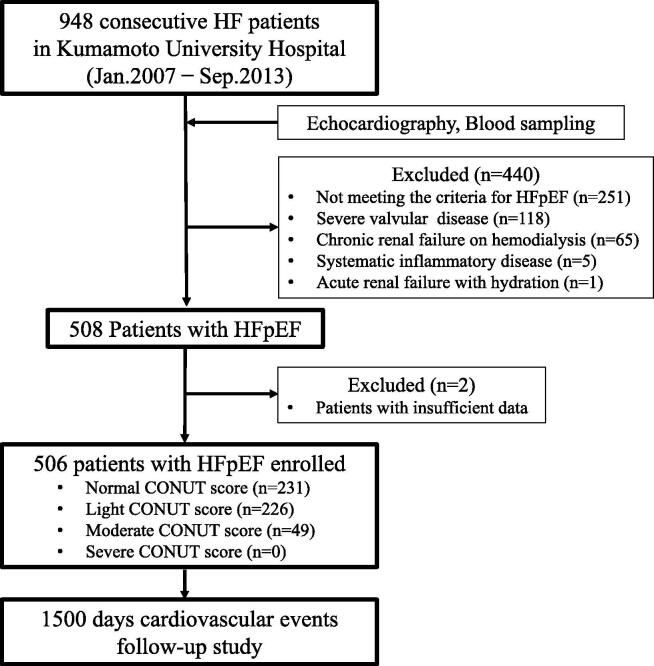
We prospectively investigated 948 consecutive patients with HF who were hospitalized in Kumamoto University Hospital between January 2007 and September 2013. We recorded each patient’s medical history and relevant clinical characteristics. We excluded 440 patients for the following reasons: severe valvular disease (n = 118), chronic renal failure requiring hemodialysis (n = 65), systemic inflammatory disease (n = 5), acute renal failure with dehydration (n = 1), and not meeting the diagnostic criteria for HFpEF as subsequently described (including HF with a reduced LVEF [HFrEF]; n = 251). Finally, 506 patients of the remaining 508 HFpEF patients, excluding those with insufficient data, were enrolled in this study. We subsequently calculated the CONUT score in these HFpEF patients, and the subjects were subdivided into normal- (0–1), light- (2–4), moderate- (5–8), and severe-score (9–12) groups according to the original concepts of CONUT score[5], with the occurrence of cardiovascular events followed for up to 1500 days. The study flow chart is shown in Fig. 1.
Fig. 1.
Flow chart showing the enrollment protocol. Abbreviations: HF, heart failure; HFpEF, HF with preserved left ventricular ejection fraction; CONUT, Controlling Nutritional Status.
2.3. Definition of HFpEF
HFpEF was clinically defined according to the European Society of Cardiology task force as follows:
-
1.
symptoms or signs of HF;
-
2.
normal or mildly reduced LVEF (LVEF > 50% and LV end-diastolic volume index < 97 mL/m2);
-
3.
evidence of abnormal LV relaxation, filling, diastolic distensibility, and diastolic stiffness.
We excluded HFpEF patients who had shown even a transient reduction in ejection fraction. Hence, HFpEF patients whose LVEF was <50% and was improved by optimal medical therapy were not included in the present study. In our study, we stratified patients by the E/e′ ratio, grouped by either a ≥15 ratio or >8 but <15 ratio, and by plasma B-type natriuretic peptide (BNP) levels, with a cut-off of 100 pg/mL. Physicians further confirmed that patients had HF by determining the New York Heart Association (NYHA) functional class [12], which was assessed by the standard questionnaire while the patient was in a stable condition after optimal therapy.
2.4. Calculation of the CONUT score
The CONUT score was calculated as described previously [5]. In brief, 3 parameters were used to calculate the score: (i) serum albumin level (g/dL); (ii) total cholesterol level (mg/dL); and (iii) total lymphocyte count (count/mL) (Supplemental Table 1). Thus, the CONUT score enables assessment of protein reserves, caloric depletion, and immune defenses in each patient.
2.5. Clinical parameters
The clinical parameters were described previously [11], [13], [14], [15], [16], [17]. In brief, the baseline demographic data, cardiovascular risk factors, and medications on discharge were documented. Hypertension was defined as a recorded blood pressure >140/90 mmHg or the use of any antihypertensive medications as described previously. Diabetes mellitus (DM) was defined as the presence of symptoms of diabetes and a random plasma glucose concentration ≥200 mg/dL, fasting plasma glucose concentration ≥126 mg/dL, or a 2-hr plasma glucose concentration ≥200 mg/dL according to a 75 g oral glucose tolerance test or the use of any medications for DM. Dyslipidemia was defined as low-density lipoprotein levels ≥140 mg/dL (≥3.63 mmol/L), high-density lipoprotein levels <40 mg/dL (1.04 mmol/L), triglycerides ≥150 mg/dL (≥1.7 mmol/L) or the use of medications for dyslipidemia.
2.6. Echocardiographic examinations
Echocardiography was performed while the patient was in a stable condition on admission by experienced cardiac sonographers who had no knowledge of the study data. Left ventricular ejection fraction (LVEF) was measured using a modified Simpson’s method. The LVEF and the ratio of early transmitral flow velocity to early diastolic mitral annular velocity (E/e′), which was assessed by tissue Doppler, were measured by echocardiography (Vivid 7®; GE-Vingmed Ultrasound, Horton, Norway; Aplio XG®; Toshiba, Tokyo, Japan) as previously reported [11], [14], [16].
2.7. Biomarker measurement
Blood samples were obtained in stable and fasting conditions in the early morning. The patient’s BNP levels were analyzed using a commercially available assay (Abbott Japan, Matsudo, Japan) in the hospital clinical laboratory on admission. The BNP levels were transformed into natural logarithmic levels (ln-BNP) to achieve a normal distribution. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese Society of Nephrology formula [18].
2.8. Follow-up and outcomes
Patients were followed up prospectively at our outpatient clinics or by the primary care physician every month until July 2017 or until the occurrence of a cardiovascular event, including the following: cardiovascular death, hospitalization for HF decompensation, nonfatal myocardial infarction (MI), unstable angina pectoris, coronary revascularization for a new diagnosis of angina or in-stent restenosis after percutaneous coronary intervention, and nonfatal ischemic stroke. Cardiovascular death was defined as death within 30 days of documented sudden death without apparent noncardiovascular causes, MI, death from HF, or death from stroke. Hospitalization for HF decompensation was defined if the patient was admitted for at least an overnight stay in the hospital because of HF with typical symptoms and had objective signs of worsening HF requiring intravenous drug administration. MI was diagnosed by an increase or decrease in cardiac biomarkers (plasma creatine kinase-MB or cardiac troponin) above the 99th percentile of the upper limit of the normal range together with evidence of myocardial ischemia and at least 1 of the following symptoms: electrocardiographic changes (new ST-T changes, left bundle branch block, or pathological Q wave) or imaging evidence of new viable myocardial loss, or a new regional wall motion abnormality [19]. Unstable angina pectoris was diagnosed according to new or accelerating symptoms of myocardial ischemia accompanied by new ischemic ST-T-wave changes. Ischemic stroke was diagnosed according to the documented focal neurological deficit with radiological evidence of brain infarction excluding intracranial hemorrhage. Cardiovascular events were ascertained by reviewing medical records and were confirmed by direct contact with the patients, their families, and physicians or by annual telephone interview with each patient. An Events Committee comprising at least 3 independent physicians reviewed all events to avoid intraobserver biases.
2.9. Statistical analysis
Continuous variables are expressed as the mean ± standard deviation for normally distributed variables according to the Shapiro–Wilk test. Variables with a non-normal distribution are expressed as the median value with the interquartile range. Categorical variables are presented as frequencies and percentages. Differences between groups were determined using Fisher’s exact test for categorical variables. Differences in continuous variables were analyzed by the unpaired t test or the Mann–Whitney U test, as appropriate. Missing data were excluded from the analyses. A Kaplan–Meier curve was used to determine the cumulative incidence of composite cardiovascular events, and the log-rank test was used to compare the incidence of composite cardiovascular event groups. The Cox proportional hazards model was used to estimate composite cardiovascular event hazard ratios (HRs) by univariable and multivariable analyses with forced inclusion modeling. HRs and 95% confidence intervals (CIs) are presented. Furthermore, the estimates of C-statistics in the Cox proportional hazards regression models were compared after the addition of higher CONUT scores to conventional factors identified in the subanalysis of the Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction (I-PRESERVE) trial (age, previous hospitalization for HF, diabetes mellitus, and ln-BNP; 4 prognostic factors [PF4]) [20]. We also assessed the incremental effects of adding a higher CONUT score to the PF4 to predict composite cardiovascular events using the net reclassification index (NRI). A P value <0.05 was considered statistically significant. The Harrell’s C-statistic, NRI and IDI were performed by R package of PredictABEL. The software Statistical Package for Social Sciences (SPSS) ver. 26.0 (IBM Japan, Tokyo, Japan) was used for other statistical analyses.
3. Results
3.1. Clinical characteristics of enrolled patients with HFpEF
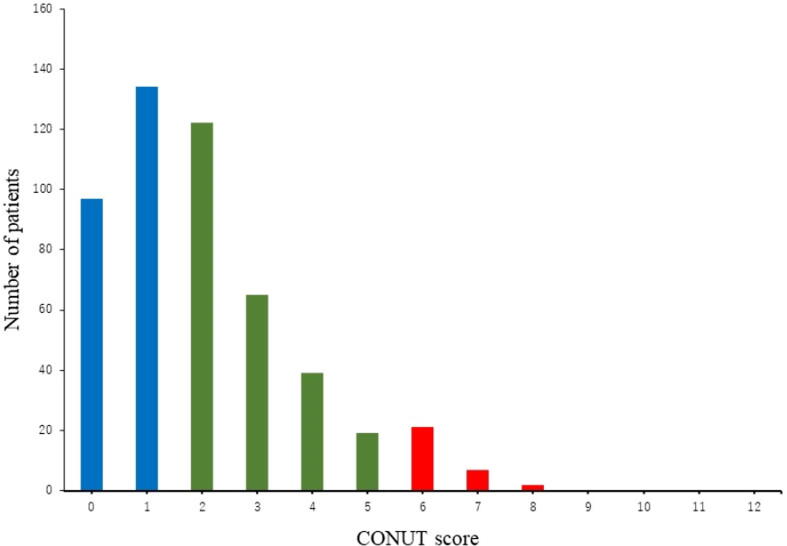
A total of 506 patients with HFpEF were enrolled in this study. The numbers of patients (percentage) with normal, light, moderate, and severe scores were 231 (45.6%), 226 (44.6%), 49 (9.4%), and 0 (0%), respectively. Fig. 2 shows the distribution of CONUT scores among HFpEF patients. The baseline characteristics of the HFpEF patients are shown in Table 1. Overall, the patients had a mean age of 71.6 ± 9.4 years, and 54.7% were male. The prevalence of ischemic heart disease (IHD) was significantly lower in both the light group and moderate group than in the normal group. Plasm BNP levels were significantly higher, and the prevalence of DM, hypertension and dyslipidemia and the use of beta-blockers were significantly lower in the light group than in the normal group. While the tricuspid regurgitation pressure gradient (TR-PG) and pulmonary artery systolic pressure (PAP) were significantly higher in the moderate group than in the normal group, NYHA class III or IV and transthoracic echocardiographic parameters, including LVEF, E/e′, stroke volume index (SVI), and left atrial diameter (LAD), were not significantly different between these groups.
Fig. 2.
Distribution of the CONUT score. Blue indicates a CONUT score of 0–1 point (normal degree of undernutrition). Green indicates a CONUT score of 2–5 points (light degree of undernutrition). Red indicates a CONUT score of 6–8 points (moderate degree of undernutrition). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Baseline characteristics of HFpEF patients according to group determined by CONUT (controlling nutritional status) scores.
| All HFpEF patients n = 506 |
Normal group n = 231 |
Light group n = 226 |
Moderate group n = 49 |
P value | |
|---|---|---|---|---|---|
| Age, years | 71.6 ± 9.4 | 71.7 ± 9.4 | 71.0 ± 9.5 | 74.1 ± 8.6 | 0.114 |
| Male, n (%) | 277 (54.7) | 133 (57.5) | 117 (51.7) | 27 (55.1) | 0.461 |
| BMI (kg/m2) | 24.1 ± 3.6 | 24.5 ± 3.5 | 23.8 ± 3.8 | 23.4 ± 2.9 | 0.061 |
| NYHA III or IV, n (%) | 86 (16.9) | 35 (15.1) | 39 (17.2) | 12 (24.4) | 0.363 |
| Diabetes mellitus, n (%) | 156 (30.8) | 92 (39.8) | 52 (23.0)** | 12 (24.4) | <0.001 |
| Hypertension, n (%) | 396 (78.2) | 198 (85.7) | 163 (72.1)** | 35 (71.4) | 0.001 |
| Dyslipidemia, n (%) | 393 (77.6) | 192 (83.1) | 163 (72.1)* | 38 (77.5) | 0.013 |
| IHD, n (%) | 266 (52.5) | 145 (62.7) | 100 (44.2)** | 21 (42.8)* | <0.001 |
| Atrial fibrillation, n (%) | 145 (28.6) | 54 (23.3) | 74 (32.7) | 17 (34.6) | 0.054 |
| SBP (mmHg) | 130.1 ± 21.2 | 129.6 ± 20.1 | 130.2 ± 22.4 | 132.7 ± 20.7 | 0.646 |
| DBP (mmHg) | 71.0 ± 13.1 | 70.2 ± 12.3 | 71.9 ± 13.4 | 70.3 ± 12.8 | 0.328 |
| Hemoglobin (g/dL) | 12.7 ± 1.8 | 12.8 ± 1.8 | 12.7 ± 1.9 | 12.5 ± 1.7 | 0.508 |
| hs-CRP (mg/L) | 0.44 ± 1.9 | 0.27 ± 0.8 | 0.39 ± 2.0 | 1.5 ± 3.9 | 0.073 |
| eGFR (mL/min/1.73 m2) | 62.2 ± 19.5 | 62.0 ± 18.6 | 63.6 ± 20.6 | 56.9 ± 17.4 | 0.095 |
| BNP (pg/mL) | 175.9 ± 291.2 | 120.3 ± 184.0 | 225.2 ± 374.1** | 209.3 ± 291.2 | <0.001 |
| LVEF (%) | 62.7 ± 5.8 | 63.1 ± 5.3 | 62.1 ± 6.0 | 63.1 ± 6.8 | 0.120 |
| SVI | 40.2 ± 9.9 | 40.9 ± 9.7 | 39.8 ± 9.5 | 39.3 ± 12.5 | 0.410 |
| LAD (mm) | 39.5 ± 7.0 | 39.5 ± 7.3 | 39.2 ± 6.9 | 40.8 ± 6.6 | 0.391 |
| E/e′ | 17.5 ± 5.0 | 17.0 ± 4.0 | 17.9 ± 5.7 | 18.3 ± 5.7 | 0.054 |
| TR-PG (mmHg) | 25.3 ± 8.0 | 24.6 ± 8.1 | 25.3 ± 7.4 | 28.6 ± 9.7 * | 0.017 |
| PAP (mmHg) | 31.6 ± 9.1 | 30.5 ± 9.2 | 31.9 ± 8.6 | 35.5 ± 10.2** | 0.006 |
| Diuretics, n (%) | 124 (24.5) | 48 (20.7) | 58 (25.6) | 18 (36.7) | 0.082 |
| ACE-I or ARB, n (%) | 317 (62.6) | 159 (68.8) | 124 (54.8)** | 34 (69.3) | 0.006 |
| CCB, n (%) | 294 (58.1) | 139 (60.1) | 122 (53.9) | 33 (67.3) | 0.154 |
| Beta-blocker, n (%) | 225 (44.4) | 121 (52.3) | 86 (38.1)** | 18 (36.7) | 0.006 |
| Statin, n (%) | 303 (59.8) | 145 (62.7) | 161 (71.2)** | 29 (59.1) | <0.001 |
| CONUT score | 4.3 ± 1.2 | 0.5 ± 0.4 | 2.6 ± 0.7** | 5.8 ± 0.8**,‡ | <0.001 |
Data are presented as the mean ± SD, median (interquartile range), or number (percentage).
HFpEF, heart failure with preserved ejection fraction; BMI, body mass index; NYHA, New York Heart Association; IHD, ischemic heart disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high sensitivity C reactive protein; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; SVI, stroke volume index; LAD, left atrium diameter; TR-PG, tricuspid regurgitation pressure gradient; PAP, pulmonary artery systolic pressure; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin Ⅱ receptor blocker; CCB, calcium channel blocker
P < 0.01 vs. light group.
P < 0.05.
P < 0.01 vs. normal group.
3.2. Cardiovascular events at follow-up
Overall, 238 cardiovascular events were recorded during the follow-up period (median: 1159 days). Table 2 shows the details of cardiovascular events during follow-up. We found significantly higher rates of composite cardiovascular events in the patients in the moderate group than in the patients in the normal (P = 0.005) and light groups (P < 0.001) and significantly higher rates of cardiovascular death in the patients in the moderate group than in the patients in the normal and light groups (P < 0.001). Moreover, the rate of hospitalization for HF decompensation was significantly higher in the moderate group than in the normal group (P = 0.003).
Table 2.
Cardiovascular events according to CONUT score.
| Total (n = 506) |
Normal group (n = 231) |
Light group (n = 226) |
Moderate group (n = 49) |
P value | |
|---|---|---|---|---|---|
| Total cardiovascular events, n (%) | 238 (47.0) | 95 (41.1) | 110 (48.6) | 32 (65.3)** | 0.005 |
| Cardiovascular death, n (%) | 31 (6.1) | 7 (3.0) | 10 (4.4) | 14 (28.5)**,‡ | <0.001 |
| Hospitalization for HF decompensation, n (%) | 110 (21.7) | 36 (15.5) | 62 (27.4)** | 12 (24.4) | 0.003 |
| Non-fatal myocardial infarction, n (%) | 6 (1.1) | 2 (0.8) | 2 (0.8) | 2 (4.0) | 0.544 |
| Unstable angina pectoris, n (%) | 15 (2.9) | 8 (3.4) | 5 (2.2) | 1 (2.0) | 0.811 |
| Coronary revascularization, n (%) | 61 (12.0) | 32 (13.8) | 27 (11.9) | 2 (4.0)* | 0.023 |
| Nonfatal ischemic stroke, n (%) | 15 (2.9) | 10 (4.3) | 4 (1.7) | 1 (2.0) | 0.278 |
P < 0.01 vs. light group.
P < 0.05.
P < 0.01 vs. normal group.
3.3. Kaplan–Meier curves
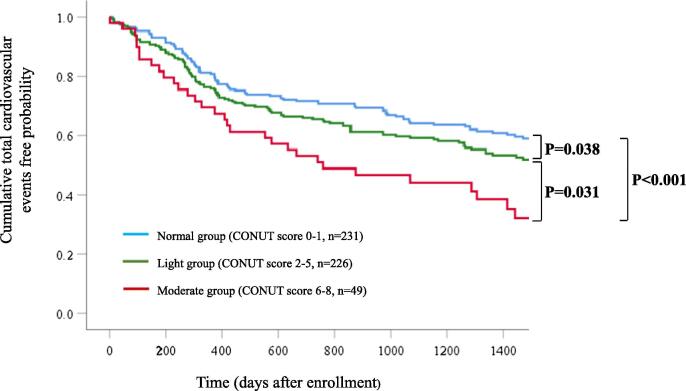
We performed a Kaplan–Meier analysis and observed that the moderate group was at higher risk of composite cardiovascular events than the normal group (P < 0.001; Fig. 3) and the light group (P = 0.031; Fig. 3). It also showed that the light group was at higher risk of composite cardiovascular events than the normal group (P = 0.038; Fig. 3).
Fig. 3.
Kaplan–Meier analyses after 1500 days of follow-up for cardiovascular events according to CONUT scores. The 0 time point on the x-axis indicates the discharge day of the qualifying cardiovascular events.
3.4. Cox proportional hazards analyses
Table 3 shows the results of univariate and multivariable Cox proportional hazards analyses for cardiovascular events. Univariate Cox proportional hazards analysis identified age (HR: 1.01, 95% CI: 1.00–1.03, P = 0.031), previous hospitalization for HF (HR: 1.62, 95% CI: 1.19–2.21, P = 0.002), NYHA III or IV (HR: 1.80, 95% CI: 1.33–2.43, P < 0.001), hemoglobin (HR: 0.87, 95% CI: 0.81–0.93, P < 0.001), ln-BNP (HR: 1.16, 95% CI: 1.05–1.28, P = 0.003), LAD (HR: 1.01, 95% CI: 1.00–1.03, P = 0.047), PAP (HR: 1.01, 95% CI: 1.00–1.03, P = 0.018), diuretic usage (HR: 1.48, 95% CI: 1.12–1.95, P = 0.005) and CONUT score (HR: 1.16, 95% CI: 1.08–1.24, P < 0.001) as significant factors associated with cardiovascular events. In a multivariate Cox proportional hazard analysis including PF4 by forced entry methods (model 1), previous hospitalization for HF (HR: 1.42, 95% CI: 1.02–1.99, P = 0.036) and CONUT score (HR: 1.14, 95% CI: 1.06–1.22, P < 0.001) were independently and significantly associated with cardiovascular events. Multivariable Cox proportional hazards analysis using the abovementioned 5 significant factors from the univariate analysis (model 2) identified hemoglobin (HR: 0.90, 95% CI: 0.83–0.99, P = 0.033) and CONUT score (HR: 1.12, 95% CI: 1.03–1.21, P = 0.005) as independent predictors of cardiovascular events in patients with HFpEF.
Table 3.
Cox proportional hazards regression analyses for cardiovascular outcome within 1500 days follow-up.
| Univariable Regression |
Multivariable Regression |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 (I-PRESERVE) |
Model 2 |
||||||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (years) | 1.01 | 1.00–1.03 | 0.031 | 1.01 | 0.99–1.02 | 0.075 | 1.00 | 0.99–1.02 | 0.433 |
| Male sex (yes) | 0.90 | 0.73–1.17 | 0.453 | ||||||
| BMI (kg/m2) | 0.96 | 0.93–1.00 | 0.091 | ||||||
| Previous hospitalization for HF (yes) | 1.62 | 1.19–2.21 | 0.002 | 1.42 | 1.02–1.99 | 0.036 | 1.47 | 0.97–2.24 | 0.068 |
| NYHA III or IV (yes) | 1.80 | 1.33–2.43 | <0.001 | 1.16 | 0.78–1.73 | 0.453 | |||
| Diabetes mellitus (yes) | 1.14 | 0.87–1.49 | 0.327 | 1.26 | 0.96–1.66 | 0.94 | |||
| Hypertension (yes) | 0.77 | 0.57–1.03 | 0.083 | ||||||
| Dyslipidemia (yes) | 0.99 | 0.72–1.36 | 0.977 | ||||||
| IHD (yes) | 1.04 | 0.80–1.34 | 0.740 | ||||||
| Atrial fibrillation (yes) | 1.18 | 0.89–1.59 | 0.237 | ||||||
| SBP (mm Hg) | 1.00 | 0.99–1.00 | 0.877 | ||||||
| DBP (mm Hg) | 0.99 | 0.98–1.00 | 0.126 | ||||||
| Hemoglobin (g/dL) | 0.87 | 0.81–0.93 | <0.001 | 0.90 | 0.83–0.99 | 0.033 | |||
| hs-CRP (mg/L) | 1.02 | 0.97–1.07 | 0.422 | ||||||
| eGFR (mL/min/1.73 m2) | 1.00 | 0.97–1.01 | 0.351 | ||||||
| ln-BNP | 1.16 | 1.05–1.28 | 0.003 | 1.08 | 0.96–1.20 | 0.160 | 1.01 | 0.88–1.16 | 0.823 |
| LVEF (%) | 0.99 | 0.97–1.01 | 0.552 | ||||||
| SVI (L/min) | 0.99 | 0.98–1.00 | 0.555 | ||||||
| LAD (mm) | 1.01 | 1.00–1.03 | 0.047 | 1.00 | 0.98–1.02 | 0.690 | |||
| E/e′ | 1.02 | 0.99–1.04 | 0.082 | ||||||
| TR-PG (mm Hg) | 1.01 | 0.99–1.03 | 0.124 | ||||||
| PAP (mm Hg) | 1.01 | 1.00–1.03 | 0.018 | 1.01 | 0.95–1.02 | 0.175 | |||
| Diuretics (yes) | 1.48 | 1.12–1.95 | 0.005 | 1.09 | 0.75–1.59 | 0.629 | |||
| ACE-I or ARB (yes) | 1.07 | 0.82–1.40 | 0.578 | ||||||
| CCB (yes) | 0.90 | 0.69–1.16 | 0.437 | ||||||
| Beta-blocker (yes) | 0.96 | 0.74–1.24 | 0.792 | ||||||
| Statin (yes) | 1.05 | 0.80–1.38 | 0.714 | ||||||
| CONUT score | 1.16 | 1.08–1.24 | <0.001 | 1.14 | 1.06–1.22 | <0.001 | 1.12 | 103–1.21 | 0.005 |
Model 1: age, previous hospitalization for HF, diabetes mellitus, ln-BNP and CONUT score.
Model 2: variables of statistical significance in the univariable analyses (P < 0.05).
Abbreviations as shown in this table. HR: hazard ratio CI: confidence interval ln-BNP: natural logarithmic transformed B-type natriuretic peptide level
3.5. Receiver operating characteristic (ROC) analysis for composite cardiovascular events and CONUT score
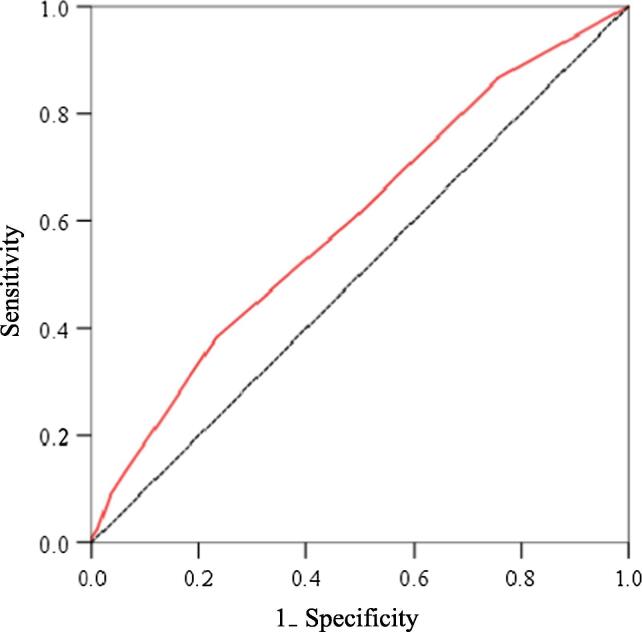
ROC curves were constructed to assess the ability of the CONUT score to predict composite cardiovascular events (Fig. 4). The area under the curve of the CONUT score for the detection of composite cardiovascular events was 0.599 (95% CI 0.550–0.648; P < 0.001). Using the cutoff value for the CONUT score (2.5), the sensitivity and specificity were 38.2% and 76.9%, respectively, for the detection of composite cardiovascular events.
Fig. 4.
Receiver operating characteristic (ROC) curves of CONUT scores for the prediction of cardiovascular events.
3.6. C-statistic for regression models, continuous NRI and integrated discrimination improvement (IDI)
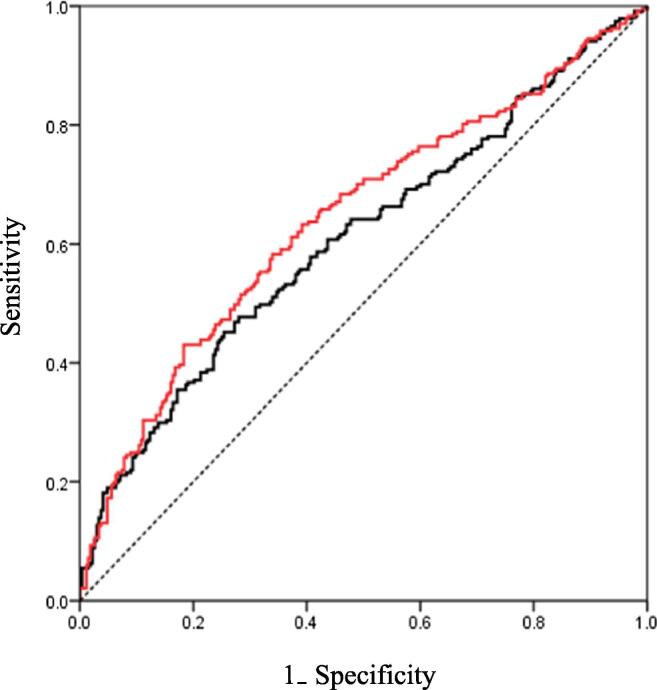
The C-statistic value for PF4 was 0.608 (95% CI: 0.559–0.658); after adding a CONUT score >2.5 as a factor, the value was 0.643 (95% CI: 0.594–0.691; P = 0.039). We reclassified the risk of cardiovascular events after adding a CONUT score >2.5 to PF4; the continuous NRI was 30.5% (P < 0.001), and the IDI was 2.2% (P < 0.001) (Table 4). The ROC curves for composite cardiovascular events are shown in Fig. 5.
Table 4.
C-statistics, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for the Cox hazard model to predict cardiovascular events in patients with HFpEF by the addition of CONUT score >2.5 to the PF4.
| C-statistic |
NRI |
IDI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | P value | Value | 95% CI | P value | Value | 95% CI | P value | |
| PF4 | 0.608 | 0.559–0.658 | |||||||
| PF4 + CONUT score >2.5 | 0.643 | 0.594–0.691 | 0.039 | 0.305 | 0.146–0.456 | <0.001 | 0.022 | 0.010–0.035 | <0.001 |
PF4 (4 prognostic factors): age + DM + previous hospitalization for HF + ln-BNP.
Abbreviations as shown in this table. CI, confidence interval.
Fig. 5.
ROC curves of PF4 and PF4 + CONUT score >2.5 for the prediction of cardiovascular events. The black curve indicates the PF4. The red curve indicates the PF4 + CONUT score >2.5. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussions
The main feature of this study is that the prognosis of HFpEF patients was classified by CONUT score, and the main findings of this study were as follows:
-
(i)
The Kaplan-Meier curve revealed that the higher the CONUT score, the higher the incidence of composite cardiovascular events.
-
(ii)
Multivariate Cox proportional hazards analysis revealed that the CONUT score was an independent and significant predictor of clinical outcome in HFpEF patients.
-
(iii)
The cutoff level of the CONUT score for composite cardiovascular events was 2.5.
-
(iv)
The NRI and IDI were significant when a CONUT score >2.5 was added to the PF4.
Obesity has been shown to be an independent risk factor for the future development of cardiovascular disease and a risk factor for the development of HF in the general population [21]. Therefore, conventional nutritional guidance for patients with HF has mainly focused on suppressing energy intake. However, Anker et al. reported that weight loss of 7.5% or more in HF patients followed for at least 6 months is a worse prognostic factor independent of HF prognosis factors such as age, NYHA functional classification, and LVEF and that preserved body weight is a better prognostic factor [22]. The concept of better prognosis when BMI is preserved was introduced. Similar large-scale multicenter trials revealed that low BMI was associated with poor prognosis, and some guidelines warn about underweight [23], [24]. Similar phenomena were reported in Japanese patients [25], [26], [27].
In Table 1, the reason that the moderate group had higher TRPG and PAP than the normal group suggests that malnutrition was more severe for more severe HF patients. The CONUT score should be interpreted with caution when medications for treating dyslipidemia due to IHD are used. The prevalence rates of dyslipidemia and IHD were significantly higher in the normal group than in the other groups. The rate of statin use was significantly higher in the light group than in the normal group.
Our findings indicate that a higher CONUT score significantly correlates with cardiovascular events in patients with HFpEF. After adjusting for various clinical parameters, a high CONUT score is still an independent predictor. Although the underlying mechanisms of cardiovascular events in the high CONUT group in HFpEF patients remain unknown, a large registry study of heart failure in Japan, JCARE-CARD [28], showed poor prognosis for low BMI patients [25]. The percentage of patients with HF with an LVEF of 50% or more in Japan was 50.6% in the CHART-1 study [29] and 68.7% in the subsequent CHART-2 study [30]. Thus, it would be reasonable to expect HFpEF patients to have poor prognoses.
In the present study, we mentioned the importance of nutritional management for HFpEF. There is a possibility for improving the prognosis of cardiovascular disease by positively intervening against malnutrition. Therefore, our present work provides data that indicate that the CONUT score not only provides important prognostic information regarding HFpEF patients but also that targeting the optimal CONUT score might be a promising therapeutic target for HFpEF. In recent years, it has been noted that both sarcopenia [31] and frail [32] are associated with a poor prognosis when they coexist with HF. Historically, low weight and malnutrition in HF patients have been described mainly by the depletion state called cachexia [33], which was attributed to changes in humoral factors. Hence, in the treatment of HF, diet therapy, especially nutrition therapy, is important together with exercise therapy.
To the best of our knowledge, this study is the first to investigate the association of the novel CONUT score with future cardiovascular events in HFpEF patients. Each component of the CONUT score is simple, and the calculation is not only easy in clinical practice but is also well validated and has a low cost, which indicates that the score can be widely applied. If this score further predicts subsequent cardiovascular events in HFpEF patients, it can also represent a useful indicator for general clinicians, as well as cardiologists in clinical practice. Although the CONUT score is strongly expected to have clinical value, large-scale clinical studies are required to confirm its value. Therefore, additional detailed, prospective, multicenter studies are warranted to verify this precise usefulness.
5. Study limitations
The present study has some limitations. First, it was a single-center study with a relatively small population. Therefore, a larger multiracial and multicenter study is required. Second, there were small numbers with only 49 with a “moderate” CONUT score and none with a severe score. Third, it is unclear which factors contribute—and the extent of their contribution—to the worse HF prognosis and malnutrition. Thus, further pathophysiological and molecular physiological studies, including animal experiments, are warranted. Additional detailed, large-scale clinical studies may be required to verify our results. Finally, the study population discussed in this study had the relatively non-obese nature which is less typical than what is seen in the West where the obese phenotype of HFpEF is more prevalent. This might decrease the generalizability of the results of the Western world.
6. Conclusion
Despite the limitations mentioned above, the results of the present study demonstrate the following: the CONUT score may be useful for predicting cardiovascular events in HFpEF patients. The CONUT score provides important prognostic information regarding HFpEF patients, and the optimal CONUT score might be a promising therapeutic target for HFpEF.
Acknowledgments
Acknowledgments
We thank all paramedical staff and clinical secretaries for their kind support during this work.
Funding sources
This study was supported in part by Grants-in-Aid for Scientific Research (#18K07720) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Declaration of Competing Interest
Dr. Sakamoto has received significant research grant support from Daiichi Sankyo Co., Ltd. Dr Kaikita has received significant research grant support from Bayer Yakuhin, Ltd.; Daiichi Sankyo Co., Ltd.; Novartis Pharma A.G.; and SBI Pharma Co., Ltd. and has received Honoraria from Bayer Yakuhin, Ltd. and Daiichi Sankyo Co., Ltd. Dr. Tsujita has received Honoraria from Amgen Astellas BioPharm K.K.; Bayer Yakuhin, Ltd.; Daiichi Sankyo Co. Ltd.; MSD K.K.; and Sanofi K.K. and has received grants from AstraZeneca K.K.; Astellas Pharma Inc.; Bayer Yakuhin, Ltd.; Boehringer Ingelheim Japan; Boston Scientific Japan K.K.; Chugai Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; Eisai Co., Ltd.; Kowa Pharmaceutical Co. Ltd.; Mitsubishi Tanabe Pharma; MSD K.K.; Pfizer Japan, Inc.; Sanofi K.K.; Shionogi & Co., Ltd.; and Takeda Pharmaceutical Co., Ltd. The remaining authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100563.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., Authors/Task Force M., Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.Rauchhaus M., Clark A.L., Doehner W., Davos C., Bolger A., Sharma R., Coats A.J., Anker S.D. The relationship between cholesterol and survival in patients with chronic heart failure. J. Am. Coll. Cardiol. 2003;42(11):1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Uthamalingam S., Kandala J., Daley M., Patvardhan E., Capodilupo R., Moore S.A., Januzzi J.L., Jr. Serum albumin and mortality in acutely decompensated heart failure. Am. Heart J. 2010;160(6):1149–1155. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama H., Koyama S., Kuragaichi T., Shiba M., Fujiwara H., Takatsu Y., Sato Y. Prognostic value of rising serum albumin during hospitalization in patients with acute heart failure. Am. J. Cardiol. 2016;117(8):1305–1309. doi: 10.1016/j.amjcard.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Ignacio de Ulibarri J., Gonzalez-Madrono A., de Villar N.G., Gonzalez P., Gonzalez B., Mancha A., Rodriguez F., Fernandez G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 6.Fukushima K., Ueno Y., Kawagishi N., Kondo Y., Inoue J., Kakazu E., Ninomiya M., Wakui Y., Saito N., Satomi S., Shimosegawa T. The nutritional index ‘CONUT’ is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J. Exp. Med. 2011;224(3):215–219. doi: 10.1620/tjem.224.215. [DOI] [PubMed] [Google Scholar]
- 7.Narumi T., Arimoto T., Funayama A., Kadowaki S., Otaki Y., Nishiyama S., Takahashi H., Shishido T., Miyashita T., Miyamoto T., Watanabe T., Kubota I. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013;62(5):307–313. doi: 10.1016/j.jjcc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Nochioka K., Sakata Y., Takahashi J., Miyata S., Miura M., Takada T., Fukumoto Y., Shiba N., Shimokawa H. Investigators C-.Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: a report from the CHART-2 Study. Circ. J. 2013;77(9):2318–2326. doi: 10.1253/circj.cj-13-0127. [DOI] [PubMed] [Google Scholar]
- 9.Somaratne J.B., Berry C., McMurray J.J., Poppe K.K., Doughty R.N., Whalley G.A. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur. J. Heart Fail. 2009;11(9):855–862. doi: 10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
- 10.Meta-analysis Global Group in Chronic Heart F. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur. Heart J. 2012;33(14):1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara T., Yamamoto E., Sueta D., Fujisue K., Usuku H., Oike F., Takae M., Arima Y., Araki S., Takashio S., Nakamura T., Suzuki S., Sakamoto K., Soejima H., Kawano H., Kaikita K., Tsujita K. Clinical significance of serum magnesium levels in patients with heart failure with preserved ejection fraction. Medicine (Baltimore) 2019;98(38):e17069. doi: 10.1097/MD.0000000000017069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NYHAC Committee, NYH Association . Brown Medical Division; Little: 1979. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. [Google Scholar]
- 13.Nishihara T., Tokitsu T., Sueta D., Takae M., Oike F., Fujisue K., Usuku H., Takashio S., Hanatani S., Kanazawa H., Arima Y., Sakamoto K., Izumiya Y., Yamabe H., Kaikita K., Yamamoto E., Tsujita K. Serum potassium and cardiovascular events in heart failure with preserved left ventricular ejection fraction patients. Am. J. Hypertens. 2018;31(10):1098–1105. doi: 10.1093/ajh/hpy101. [DOI] [PubMed] [Google Scholar]
- 14.Tabata N., Sueta D., Yamamoto E., Takashio S., Arima Y., Araki S., Yamanaga K., Ishii M., Sakamoto K., Kanazawa H., Fujisue K., Hanatani S., Soejima H., Hokimoto S., Izumiya Y., Kojima S., Yamabe H., Kaikita K. Tsujita K, investigators Ks. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur. Heart J. Qual. Care Clin. Outcomes. 2018;4(4):290–300. doi: 10.1093/ehjqcco/qcx047. [DOI] [PubMed] [Google Scholar]
- 15.Fujisue K., Tokitsu T., Yamamoto E., Sueta D., Takae M., Nishihara T., Oike F., Usuku H., Ito M., Motozato K., Kanazawa H., Araki S., Arima Y., Takashio S., Izumiya Y., Suzuki S., Sakamoto K., Kaikita K., Tsujita K. Prognostic significance of polyvascular disease in heart failure with preserved left ventricular ejection fraction. Medicine (Baltimore) 2019;98(28):e15959. doi: 10.1097/MD.0000000000015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sueta D., Yamamoto E., Nishihara T., Tokitsu T., Fujisue K., Oike F., Takae M., Usuku H., Takashio S., Arima Y., Suzuki S., Nakamura T., Ito M., Kanazawa H., Sakamoto K., Kaikita K., Tsujita K. H2FPEF score as a prognostic value in HFpEF patients. Am. J. Hypertens. 2019;32(11):1082–1090. doi: 10.1093/ajh/hpz108. [DOI] [PubMed] [Google Scholar]
- 17.Tabata N., Sueta D., Yamamoto E., Takashio S., Arima Y., Araki S., Yamanaga K., Ishii M., Sakamoto K., Kanazawa H., Fujisue K., Hanatani S., Soejima H., Hokimoto S., Izumiya Y., Kojima S., Yamabe H., Kaikita K., Matsui K., Tsujita K. A retrospective study of arterial stiffness and subsequent clinical outcomes in cancer patients undergoing percutaneous coronary intervention. J. Hypertens. 2019;37(4):754–764. doi: 10.1097/HJH.0000000000001949. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial I Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 20.Anand I.S., Rector T.S., Cleland J.G., Kuskowski M., McKelvie R.S., Persson H., McMurray J.J., Zile M.R., Komajda M., Massie B.M., Carson P.E. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ. Heart Fail. 2011;4(5):569–577. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 21.Kenchaiah S., Evans J.C., Levy D., Wilson P.W., Benjamin E.J., Larson M.G., Kannel W.B., Vasan R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 22.Anker S.D., Ponikowski P., Varney S., Chua T.P., Clark A.L., Webb-Peploe K.M., Harrington D., Kox W.J., Poole-Wilson P.A., Coats A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 23.Writing Committee M., Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., Johnson M.R., Kasper E.K., Levy W.C., Masoudi F.A., McBride P.E., McMurray J.J., Mitchell J.E., Peterson P.N., Riegel B., Sam F., Stevenson L.W., Tang W.H., Tsai E.J., Wilkoff B.L., American College of Cardiology Foundation/American Heart Association Task Force on Practice G 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., ESCSD Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 25.Hamaguchi S., Tsuchihashi-Makaya M., Kinugawa S., Goto D., Yokota T., Goto K., Yamada S., Yokoshiki H., Takeshita A., Tsutsui H., Investigators J.-C. Body mass index is an independent predictor of long-term outcomes in patients hospitalized with heart failure in Japan. Circ. J. 2010;74(12):2605–2611. doi: 10.1253/circj.cj-10-0599. [DOI] [PubMed] [Google Scholar]
- 26.Takiguchi M., Yoshihisa A., Miura S., Shimizu T., Nakamura Y., Yamauchi H., Iwaya S., Owada T., Miyata M., Abe S., Sato T., Suzuki S., Suzuki H., Saitoh S., Takeishi Y. Impact of body mass index on mortality in heart failure patients. Eur. J. Clin. Invest. 2014;44(12):1197–1205. doi: 10.1111/eci.12354. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita M., Shirakabe A., Hata N., Shinada T., Kobayashi N., Tomita K., Tsurumi M., Okazaki H., Yamamoto Y., Asai K., Shimizu W. Association between the body mass index and the clinical findings in patients with acute heart failure: evaluation of the obesity paradox in patients with severely decompensated acute heart failure. Heart Vessels. 2017;32(5):600–608. doi: 10.1007/s00380-016-0908-9. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsui H., Tsuchihashi-Makaya M., Kinugawa S., Goto D., Takeshita A., Investigators J.-C. Clinical characteristics and outcome of hospitalized patients with heart failure in Japan. Circ. J. 2006;70(12):1617–1623. doi: 10.1253/circj.70.1617. [DOI] [PubMed] [Google Scholar]
- 29.Shiba N., Watanabe J., Shinozaki T., Koseki Y., Sakuma M., Kagaya Y., Shirato K., Investigators C. Analysis of chronic heart failure registry in the Tohoku district: third year follow-up. Circ. J. 2004;68(5):427–434. doi: 10.1253/circj.68.427. [DOI] [PubMed] [Google Scholar]
- 30.Shiba N., Nochioka K., Miura M., Kohno H., Shimokawa H. Investigators C-. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan–first report from the CHART-2 study. Circ. J. 2011;75(4):823–833. doi: 10.1253/circj.cj-11-0135. [DOI] [PubMed] [Google Scholar]
- 31.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., Chou M.Y., Chen L.Y., Hsu P.S., Krairit O., Lee J.S., Lee W.J., Lee Y., Liang C.K., Limpawattana P., Lin C.S., Peng L.N., Satake S., Suzuki T., Won C.W., Wu C.H., Wu S.N., Zhang T., Zeng P., Akishita M., Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A., Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.Evans W.J., Morley J.E., Argiles J., Bales C., Baracos V., Guttridge D., Jatoi A., Kalantar-Zadeh K., Lochs H., Mantovani G., Marks D., Mitch W.E., Muscaritoli M., Najand A., Ponikowski P., Rossi Fanelli F., Schambelan M., Schols A., Schuster M., Thomas D., Wolfe R., Anker S.D. Cachexia: a new definition. Clin. Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.