Abstract
Malaria, an important parasitic disease worldwide, still has diagnostic challenges in the laboratory. Many studies have been conducted on the detection ability of haematology analysers for malaria. We evaluated the Sysmex XN-series analyser as a tool for detection of malaria by analysing the leukocyte cell population data (LCPD), scattergrams and associated Flow Cytometry Standard (FCS) data from both the WNR (white cell nucleated) and WDF (white cell differential) channels. 1281 clinically suspected cases of malaria were screened for malaria by peripheral blood smear examination and were run in the Sysmex XN-1000 for analysis of haematological parameter data, LCPD, all the scattergrams and FCS data. 1281 clinically suspected cases of malaria were screened for malaria by peripheral blood smear examination and were run in the Sysmex XN-1000 for analysis of haematological parameter data, LCPD, all the scattergrams and FCS data. 48 cases had malarial parasite on microscopy; of which, 41 cases were of Plasmodium vivax, 6 cases of Plasmodium falciparum and 1 case of mixed infection. 46 malaria-positive samples showed certain patterns of clusters in the scattergrams of both WDF and WNR channels. A case with only a few ring forms of P. vivax and another with very low parasite load having only gametocyte of P. falciparum didn’t show the distinctive cluster. The most distinctive clusters for all other cases were seen in WNR (SFL-SSC) and WNR (SSC-FSC) scattergrams. FCS data for the same were analysed to gate for those events. The gated events correlated (Spearman ρ = 0.77, p < 0.01) with the parasite load of the patients. By observing the scattergrams and different parameters in the Sysmex XN-series analyser, malaria can be detected from the analyser itself.
Keywords: Sysmex, Malaria, Autoanalyzer, FCS, Haematology
Introduction
Malaria is a highly prevalent febrile illness with dreaded complications. In routine clinical practice, all febrile cases undergo complete blood count. Automated haematology analysers are now widely used for this. Although peripheral smear microscopy is still the gold standard for diagnosis of malaria, the haematology analysers are documented for assisting in the identification of malaria [1].
Cell-Dyn (Abbott Diagnostic, Santa Clara, CA, USA) uses the depolarization potential of haemozoin pigment of malaria [2–4]. Numerous works have been done on Beckman Coulter (Miami, Fl, USA) haematology analysers [5–7]. Fourcade et al. [6] determined that the lymphocyte and monocyte standard deviation were the most accurate for malaria detection. Briggs et al. [5] formulated a discriminant factor (malaria factor) using the same. The Sysmex series of analysers (Sysmex Corp., Kobe, Japan) have been documented for the identification of malaria [8–11]. Amongst the Sysmex series of machines, research work has been done on the XE and XT haematology automated analysers regarding malaria, but limited literature is found regarding XN series [1, 10, 12–14].
To the best of our knowledge, the Flow Cytometry Standard (FCS) data exported from the XN-series has not been evaluated as a tool to diagnose malaria. We wished to evaluate the Sysmex XN-10 analyser module as a tool for detection of malaria by analysing the leukocyte cell population data (LCPD), the scattergrams and associated FCS data from both the WNR (white cell nucleated) and WDF (white cell differential) channels.
Materials and Methods
We analysed blood samples from 1281 clinically suspected cases of malaria who presented in our institute from June 2018 till November 2018.
Peripheral Smear Examination and Malarial Antigen Detection
Thick and thin smears were prepared from all 1281 samples, which were stained with Giemsa stain in accordance with the standard operating procedure (SOP) for malaria detection by World Health Organization (WHO) [15]. The smears were subjected to microscopy by two independent pathologists, unaware of the antigen detection status. Two-hundred oil immersion fields at a magnification of 1000× were examined before calling the sample negative for malaria. The evidence of the malarial parasites and the species identified were documented. Parasite load taking into the account both the asexual and sexual forms of the parasite was estimated.
All the samples were also subjected to MPDA assay with SD Bioline Malaria Ag Pf/Pan (Standard Diagnostics Inc., Suwon City, South Korea). The results were interpreted according to the manufacturer instructions.
Analyser
The samples were processed in the Sysmex XN-1000, which incorporates the Sysmex XN-10 analyser module with a sampler. The analyser was run in accordance with the SOP and was checked daily with internal quality checks of low, normal and high levels. Calibration of the analyser was done once a year. All the clinically suspected samples were run within 4 h of collection in dipotassium ethylenediaminetetraacetic acid (K2-EDTA) tubes. It analyses cells based on impedance and flow-cytometric characteristics, using semiconductor laser and fluorescent dye. The cell scatters the light into FSC (Forward Scatter) which detects the cell size, SSC (Side Scatter) which detects nucleus and internal granularity and SFL (Side Fluorescence) which detects nucleic acid contents of the cell. Based on the combinations of FSC, SSC and SFL for both WNR and WDF channels, 6 different scattergrams are generated by the analyser. By use of a default gating system, WNR channel distinguishes total leucocytes, nucleated RBCs and basophils. WDF channel distinguishes different leucocyte populations except basophils. In the WNR channel, the RBCs and platelets are lysed using a strong lysing reagent, Lysercell-WNR which permeabilises the WBC, keeping the basophils intact. Then the fluorescent dye solution, the Fluorocell-WNR (polymethine) stains the nucleic acids. In the WDF channel, the RBCs and platelet are lysed using a milder lysing reagent, Lysercell-WDF which permeabilises the WBC and the Fluorocell-WDF then stains the nucleic acids.
The following haematological parameters were evaluated and compared between the malaria-positive and negative samples as confirmed by microscopy: haemoglobin, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), Red cell distribution width-standard deviation (RDW-SD), platelet, platelet-large cell ratio (P-LCR), plateletcrit (PCT), mean platelet volume (MPV), platelet distribution width (PDW), differential leukocyte count, white blood cell count in WNR channel (WBC-N), white blood cell count in WDF channel (WBC-D), neutrophil side scatter (NE-SSC), neutrophil side fluorescence (NE-SFL), neutrophil forward scatter (NE-FSC), lymphocyte side scatter (LY-X), lymphocyte fluorescence (LY-Y), lymphocyte forward scatter (LY-Z), monocyte side scatter (MO-X), monocyte fluorescence (MO-Y), monocyte forward scatter (MO-Z). The scattergrams were studied for any merging of neutrophil with eosinophils, irregularities of neutrophil events, etc. which were previously described [12].
FCS data of both WNR and WDF channels for each of the samples were exported and analysed by Flowing Software v.2.5.1 (Perttu Terho, Turku Centre for Biotechnology, Finland). The plots generated were analysed for any abnormal clustering of events. Further, gating and event counts were done.
Statistical Analysis
Statistical analysis was performed with SPSS v.23 (IBM Corp., USA). Receiver operating characteristic (ROC) curve was generated. The rates of sensitivity and specificity were calculated by 2 × 2 truth tables. Logistic regression was used to generate an equation for a predictive model for malaria from the observed parameters. p values less than 0.05 were considered significant. For group comparison, Welch’s t test was used as the group sizes were considerably large and they were having unequal variances. Hence, although there was considerable skewness in the data for some parameters, the use of robust Welch’s t-test in place of non-parametric tests like the Mann–Whitney U test is justified [16].
Results
A total of 1281 samples of clinically suspected cases of malaria were analysed by microscopy. Forty-eight cases were confirmed with malarial parasite. Out of which, 41 cases of Plasmodium vivax, 6 cases of Plasmodium falciparum and 1 case of mixed infection (P. vivax and P. falciparum). The morphology of the parasites ranged from early and late trophozoites, schizonts to gametocytes for the P. vivax. In the case of the P. falciparum, gametocytes and early trophozoites were detected. Both the mixed infections showed all the stages of P. vivax and trophozoites along with gametocytes of P. falciparum. MPDA interpretation corroborated with peripheral smear findings in all cases.
Out of 48 cases, 28 (58.3%) were males and 20 (41.7%) were females. Amongst the non-infected 1233 cases, 656 (53.2%) were males and 577 (46.8%) were females. The mean age of the malaria-positive group was 23.7 years (range 6 years-68 years) and that of the malaria-negative group was 37.8 years (range 2–81 years).
Amongst the haematological parameters in the malaria-positive group, mean haemoglobin was 116 ± 34.8 g/L while in the malaria-negative group, it was 123 ± 21.4 g/L. Mean RDW-SD was 51.27 (range 37.9–90.3) fL in case of the malaria-positive group and 49.17 (range 34.5–100.1) fL in the malaria-negative group.
The platelet count was significantly less in the malaria-positive group (Welch’s t-test, p < 0.05) ranging from 7 to 424 × 109/L with a mean of 116.04 × 109/L, while in the malaria-negative group, it ranged from 8 to 1173 × 109/L with a mean of 217.8 × 109/L. The platelet parameters like PDW, MPV, P-LCR, PCT were not calculated by the analyser in 10 out of the 48 malaria-positive cases (Fig. 1).
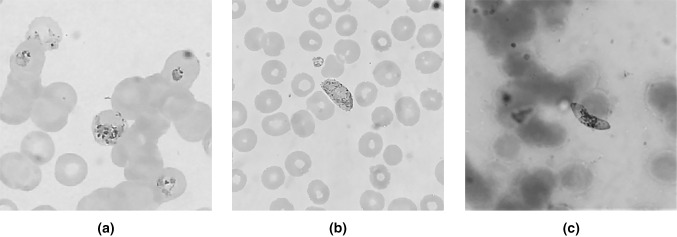
Fig. 1.
Photo-micrograph of a schizont and trophozoite of P. vivax, b gametocyte of P. vivax, c gametocyte of P. falciparum (Giemsa stain, × 1000)
The parameters found significantly higher in the malaria-positive group were MO-X, MO-Y, NE-SSC, LY-X, LY-Y; MO-Z was significantly lower (Welch’s t-test, p < 0.05) (Table 1).
Table 1.
Comparison between leukocyte cell population data (LCPD) of malaria-positive and malaria-negative group
| Parameters | Malaria-negative (n = 1233) | Malaria-positive (n = 48) | p value (Welch’s t-test) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| NE-SSC | 147.112 | 4.2801 | 150.092 | 4.8508 | <0.001 |
| NE-SFL | 51.967 | 3.2618 | 55.498 | 7.0967 | 0.001 |
| NE-FSC | 88.900 | 3.3084 | 73.215 | 28.0904 | <0.001 |
| LY-X | 77.752 | 2.5786 | 80.929 | 2.7424 | <0.001 |
| LY-Y | 70.589 | 4.0092 | 77.481 | 8.1997 | <0.001 |
| LY-Z | 58.988 | 1.4859 | 58.544 | 6.5567 | 0.638 |
| MO-X | 116.713 | 2.5079 | 120.475 | 5.4249 | <0.001 |
| MO-Y | 116.687 | 6.8289 | 122.708 | 20.5486 | 0.049 |
| MO-Z | 66.987 | 2.6773 | 64.283 | 6.5705 | 0.007 |
WBC-D and WBC-N were significantly different in the malaria positive group. This difference [(WBC-D) − (WBC-N)] is referred to as ΔWBC. WBC-D was higher (Welch’s t-test, p < 0.05) than the WBC-N. The difference ranged from −0.14 to 13.72 × 109/L with a mean of 1.84 × 109/L in the malaria-positive group. The ΔWBC in case of the malaria-negative group ranged from −0.62 to 0.54 × 109/L with a mean of −0.045 × 109/L. The ROC curve for ΔWBC showed (Fig. 2) the area under the curve (AUC) is 0.9364 with 95% confidence interval (CI) being 0.9009–0.9784 (p < 0.001). On taking the cut off of ΔWBC as 0.125 × 109/L, the sensitivity and specificity for detection of malaria came to be 85.42% (95% CI 72.24–93.93%) and 88.16% (95% CI 86.22–89.91%) respectively which gives the maximum Youden index. The WBC-D and WBC-N ratio can also be alternatively used for discrimination. Further, the ratio between the WBC-D over the WBC-N range from 0.998 to 14.3 with a mean of 1.3 in the malaria-positive group. This ratio in the malaria-negative group came in the range of 0.92 to 1.06 with a mean of 0.995. Spearman’s correlation analysis revealed the statistically significant (p < 0.01) strong positive correlation between ΔWBC and parasite load (Spearman ρ = 0.69) as well as WBC-D/WBC-N ratio and parasite load (Spearman ρ = 0.7).
Fig. 2.
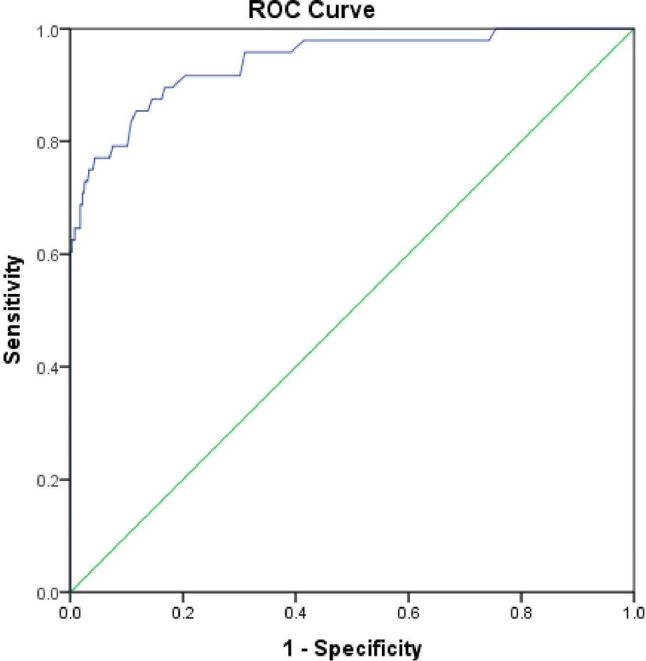
Receiver operating characteristic curve for ΔWBC [(WBC-D)-(WBC-N)]. The area under the curve (AUC) for ΔWBC is 0.9364 (95% CI 0.9009–0.9784)
By using logistic regression, we generated a predicted probability equation for malaria positivity. Predicted probability (PP) ≥ 0.5 will be considered positive for malaria. Platelet count (in 109/L), NE-SSC, MO-X, MO-Z and ΔWBC (in 109/L) was included in the predictive model.
Unlike malaria-negative group, the WDF scattergrams of the malaria-positive samples showed certain patterns. The patterns were double/multiple/abnormal or irregular shapes of the neutrophil area, merging of the neutrophil and eosinophil area, greying out of the neutrophil and eosinophil area, a left-ward shift of the eosinophil area, pseudo-eosinophilia (i.e. a difference of ≥ 5% of the analyser and manual eosinophil count).
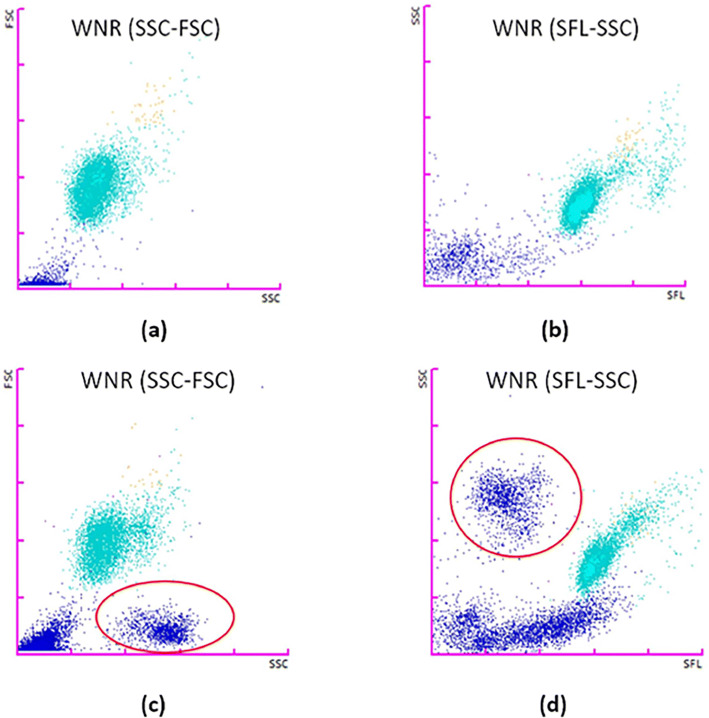
On analysing the WNR scattergrams of the total samples, we observed a typical clustering of events in the scattergrams of SSC-FSC and SFL-SSC in the malaria-positive samples (Fig. 3). Such typical plots were exclusively seen in all the malaria-positive samples except two cases. The two cases where the typical clustering was not observed were having very low parasitic count; one with only ring forms of P. vivax and another with only gametocytes of P. falciparum. The presence of such clustering shows sensitivity of 95.83% (95% CI 85.75–99.49%) and 100.00% (95% CI 99.70–100.00%) specificity.
Fig. 3.
a WNR(SSC-FSC) and b WNR(SFL-SSC) scattergrams of a malaria-negative individual. c WNR(SSC-FSC) and d WNR(SFL-SSC) scattergrams of a malaria-positive patient with typical clusters circled in red (colour figure online)
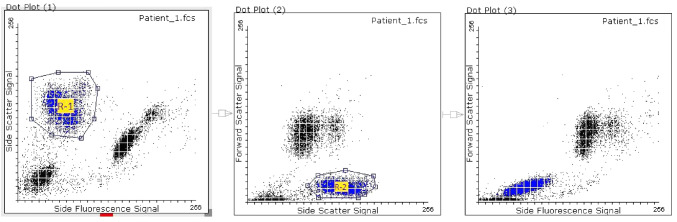
The FSC data of the WNR channel were analysed in the Flowing Software for the particular clusters as seen in our initial observation. The most well-separated clustering of events occurred in the SFL-SSC plot as shown in Fig. 4. A gate (R-1) was created in the SFL-SSC plot to separate the cluster. The gated events were found to match with a cluster in the SSC-FSC plot which was thereafter gated as another gate (R-2). The events which showed up in both the gates (i.e. R-1 and R-2) were considered to be the parasitised RBCs and the event count was done. These events again matched with the cluster in the SFL-FSC plot, although this cluster is not well separated from the RBC debris. It appears as a rightward shift of the RBC debris. The same set of gates was applied in FCS data of all the malaria-negative cases and event counts were obtained. The event count in the “malaria gate” of the malaria-positive samples ranged from 47 to 6167 with mean of 1184. The event count of the malaria-negative samples ranged from 0 to 5 with a mean of 0.9. The event count in the “malaria gate” showed a strong positive correlation with the parasite load (Spearman ρ = 0.77, p < 0.01).
Fig. 4.
Gating strategy applied in Flowing Software (v.2.5.1) on the FCS data of the WNR channel in a malaria-positive case. The events in the R-1 gated cluster in the WNR Side Fluorescence Signal-Side Scatter Signal scattergram was found to match with a cluster of events in the WNR Side Scatter Signal-Forward Scatter Signal scattergram, which was thereafter gated as R-2. These events again matched with a cluster of events in the WNR Side Fluorescence Signal-Forward Scatter Signal scattergram. The said clusters are shown in blue. These are the suspected parasitized RBCs
Discussion
Despite numerous works on detection of malaria by haematology analysers, limited literature is available regarding the Sysmex XN series of analysers. We report a difference in the WBC-D and WBC-N, i.e. ΔWBC ranging from −0.14 to 13.72 × 109/L with a mean of 1.84 × 109/L in the malaria-positive group which was significantly higher when compared to the malaria-negative group. Dumas et al. found a difference from −0.42 to 1.44 × 109/L in P. falciparum infection and from −0.26 to 7.63 × 109/L in other Plasmodium species [12]. Contrary to their findings, we found a strong correlation of ΔWBC with the parasite load in malaria positive cases (Spearman ρ = 0.69). According to our ROC curve analysis, on taking the cut off of ΔWBC as 0.125 × 109/L, we can detect malaria with a sensitivity and specificity of 85.42% (95% CI 72.24–93.93%) and 88.16% (95% CI 86.22–89.91%) respectively.
In our opinion, the difference in the physical properties of the lysing reagents is responsible for this effect. Lysercell-WNR (pH 2.95–3.05, 26–32 mOsm/kg H2O) lyses the parasitised RBCs more efficiently than Lysercell-WDF (pH 5.95–6.05, 98–108 mOsm/kg H2O) so that the lysed parasitised RBCs are not mistaken as WBCs in the WNR channel. The completely lysed parasitised RBCs in WNR channels shows very low FSC, but high SSC and SFL which separates it from the nucleated cells in the WNR scattergrams as seen in Fig. 3.
The findings from previous studies on Sysmex analysers showed WDF scattergram abnormalities like double-cluster in the neutrophil area, merging of the neutrophil and eosinophil plot area, greying of neutrophil eosinophil area and pseudo-eosinophilia which concurred with our findings [9, 10, 12].
On analysing the LCPD, we found MO-X and MO-Y were increased in the malaria patients. MO-X signifies internal complexity, which depends on the vacuoles, granules and cytoplasmic inclusions of the monocytes, whereas higher MO-Y denotes activated monocytes, having a greater amount of cellular DNA and RNA. However, MO-Z, which indicates the size of monocyte after addition of diluting and lysing solution, was significantly less in the malaria-positive group. The reason for this could not be ascertained. The NE-SSC value was higher in the malaria-positive group. The parasitised RBCs, which remain unlysed or partially lysed, overlap with the ‘neutrophil gate’ in the WDF channel and this causes the factitious rise in the NE-SSC value as the internal complexity of the parasitised RBCs are greater than that of neutrophils.
Although rightward shift of RBC debris was mentioned as a scattergram abnormality in WBC/BASO plot of XE series (analogous to WNR channel of XN-series) in the previous literature, the SFL-FSC plot is not ideal for detection of malaria, especially when the parasite load is low, as it tends to overlap with the RBC debris [10, 11]. We suggest observation of WNR SFL-SSC and SSC-FSC scattergrams for the typical clusters as shown in Fig. 3. These clusters are well-separated, distinctive, easily demonstrable, and not previously described. ΔWBC, WBC-D/WBC-N ratio as well as the event count in the “malaria gate” strongly correlated with the parasite load. For practical purpose, we can get an approximate estimation of parasite load in the patient from the ΔWBC, WBC-D/WBC-N ratio and the density of the events in the region on the scattergrams as described.
But our study also shows the limitation of this method as a screening method for malarial parasite detection. We could not find typical event clusters in two malaria-positive cases where the parasite load was very low and only ring forms of P. vivax and gametocytes of P. falciparum were present. The parasites were too small with very little granularity or nucleic acid content to create high SSC or high SFL, which is necessary for its separation from RBC debris in the WNR scattergram.
Sysmex XN-30 analysers are now being introduced as a dedicated tool for diagnosis of malaria [17]. But the use of universal XN-10 series analysers as a screening tool can have much practical importance.
Conclusion
Malaria can be rapidly detected by automated Sysmex XN-series analyser by observing WBC-D, WBC-N, LCPD (particularly NE-SSC, MO-X, MO-Y, MO-Z), and all WDF and WNR scattergrams, especially SFL-SSC and SSC-FSC plots of WNR channel. Even in unsuspected cases, during routine screening in haematology analyser, a high suspicion of malaria can be instilled. Thus this method is unique compared to immunochromatographic methods as those are usually done only in suspected cases of malaria. It can be useful in laboratories with high sample volume for rapid screening. Although it can be used as an adjunct to peripheral smear examination or MPDA, it cannot be used as a replacement of these methods due to its limitations in the detection of certain forms of the parasite.
Acknowledgements
We wish to thank Dr. Irshad and Dr. Sudip Kumar Datta for their guidance and support. We would like to express our sincere gratitude to our technical staff, including Mr. Debasish Jash, Mr. Hariom Mishra, Mr. Narayan.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Aparna Ningombam, Abhirup Sarkar and Shreyam Acharya. The first draft of the manuscript was written by Abhirup Sarkar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No outside funding was taken for the study.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval was taken from the Institute Ethical Committee.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aparna Ningombam, Email: apning09@gmail.com.
Abhirup Sarkar, Email: abhirupsa@gmail.com.
Shreyam Acharya, Email: omkar.wbuhs@gmail.com.
Anita Chopra, Email: chopraanita2005@gmail.com.
Kundan Kumar, Email: drkundankumar2007@gmail.com.
Arulselvi Subramanian, Email: arulselvi.jpnatc@gmail.com.
References
- 1.Campuzano-Zuluaga G, Hänscheid T, Grobusch MP. Automated haematology analysis to diagnose malaria. Malar J BioMed Central. 2010;9:346. doi: 10.1186/1475-2875-9-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wever PC, Henskens YMC, Kager PA, Dankert J, van Gool T. Detection of imported malaria with the Cell-Dyn 4000 hematology analyzer. J Clin Microbiol. 2002;40:4729–4731. doi: 10.1128/JCM.40.12.4729-4731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh IB, Kim HJ, Kim JY, Lee SW, An SSA, Kim WJ, et al. Evaluation of the Abbott Cell-Dyn 4000 hematology analyzer for detection and therapeutic monitoring of Plasmodium vivax in the Republic of Korea. Trop Med Int Health. 2003;8:1074–1081. doi: 10.1046/j.1360-2276.2003.01135.x. [DOI] [PubMed] [Google Scholar]
- 4.Scott CS, Zyl D, Ho E, Meyersfeld D, Ruivo L, Mendelow BV, et al. Automated detection of malaria-associated intraleucocytic haemozoin by Cell-Dyn CD4000 depolarization analysis. Clin Lab Haematol. 2003;25:77–86. doi: 10.1046/j.1365-2257.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 5.Briggs C, Simon-Lopez R, Da Costa A, Freeman L, Aucamp I, Taylor I, et al. Development of an automated malaria discriminant factor using VCS technology. Am J Clin Pathol. 2006;126:691–698. doi: 10.1309/0PL3C674M39D6GEN. [DOI] [PubMed] [Google Scholar]
- 6.Fourcade C, Casbus MJC, Belaouni H, Gonzalez JJD, Garcia PJJ, Pepio MAE. Automated detection of malaria by means of the haematology analyser CoulterR GEN.STM. Clin Lab Haematol. 2004;26:367–372. doi: 10.1111/j.1365-2257.2004.00648. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava M, Sharma P, Sukhachev D. Discriminant value of volume, conductivity and scatter properties of leucocytes (VCS technology) for rapid and reliable diagnosis of malaria and dengue fever. Blood. 2011;118(21):4731. doi: 10.1182/blood.V118.21.4731.4731. [DOI] [Google Scholar]
- 8.Park TS, Lee K-A, Kim Y-A, Song J, Choi JR, Yoo J-H, et al. Automated detection of malaria-associated pseudoeosinophilia and abnormal WBC scattergram by the sysmex XE-2100 hematology analyzer: a clinical study with 1,801 patients and real-time quantitative PCR analysis in vivax malaria-endemic area. Am J Trop Med Hyg. 2010;82:412–414. doi: 10.4269/ajtmh.2010.09-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campuzano-Zuluaga G, Alvarez-Sánchez G, Escobar-Gallo GE, Valencia-Zuluaga LM, Ríos-Orrego AM, Pabón-Vidal A, et al. Design of malaria diagnostic criteria for the Sysmex XE-2100 hematology analyzer. Am J Trop Med Hyg. 2010;82:402–411. doi: 10.4269/ajtmh.2010.09-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohapatra S, Samantaray J, Arulselvi S, Panda J, Munot K, Saxena R. Automated detection of malaria with haematology analyzer sysmex xe-2100. Indian J Med Sci. 2011;65:26. doi: 10.4103/0019-5359.103163. [DOI] [PubMed] [Google Scholar]
- 11.Jain M, Gupta S, Jain J, Grover RK. Usefulness of automated cell counter in detection of malaria in a cancer set up-Our experience. Indian J Pathol Microbiol. 2012;55:467–473. doi: 10.4103/0377-4929.107782. [DOI] [PubMed] [Google Scholar]
- 12.Dumas C, Bienvenu A-L, Girard S, Picot S, Debize G, Durand B. Automated plasmodium detection by the sysmex XN hematology analyzer. J Clin Pathol. 2018;71:594–599. doi: 10.1136/jclinpath-2017-204878. [DOI] [PubMed] [Google Scholar]
- 13.Kb S, Naik P. Usefulness of automated hematology analyzer Sysmex XN 1000 in detection of Malaria. Indian J Pathol Oncol. 2016;3:658–661. doi: 10.5958/2394-6792.2016.00122.8. [DOI] [Google Scholar]
- 14.Alfaro-Arroyo L, Blanco-Chaves L, Rodriguez-Díaz A. Diagnosis and follow-up of a Plasmodium vivax malaria case through the Sysmex XT-1800i ® hematology analyzer. Int J Mol Biol. 2019;4:80–83. [Google Scholar]
- 15.WHO. Giemsa Staining of Malaria Blood Films. MM-SOP-07A
- 16.Ratcliffe J. The effect on the t distribution of non-normality in the sampled population. J R Stat Soc. 1968;17:42–48. [Google Scholar]
- 17.Tougan T, Suzuki Y, Itagaki S, Izuka M, Toya Y, Uchihashi K, et al. An automated haematology analyzer XN-30 distinguishes developmental stages of falciparum malaria parasite cultured in vitro. Malar J BioMed Central. 2018;17:59. doi: 10.1186/s12936-018-2208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]