Abstract
Rice is a daily staple for half of the world’s population. However, rice grains are poor in micronutrients such as Fe and Zn, the two most commonly deficient minerals in the human diet. In plants, Fe and Zn must be absorbed from the soil, distributed and stored, so that their concentrations are maintained at sufficient but non-toxic levels. The understanding of mechanisms of Fe and Zn homeostasis in plants has the potential to benefit agriculture, improving the use of micronutrients by plants, as well as to indicate approaches that aim at biofortification of the grains. ZIP transporters are commonly associated with Zn uptake, but there are few reports about their physiological relevance in planta. Here we describe a Tos17 loss-of-function line for the Zn plasma membrane transporter OsZIP7 (oszip7). We showed that the absence of functional OsZIP7 leads to deregulated Zn partitioning, increasing Zn accumulation in roots but decreasing in shoots and seeds. We also demonstrated that, upon Zn deficiency, oszip7 plants slightly increase their photosynthetic performance, suggesting that these plants might be primed for Zn deficiency which makes them more tolerant. On the other hand, we found that Zn excess is more deleterious to oszip7 plants compared to wild type, which may be linked to secondary effects in concentrations of other elements such as Fe. Our data suggest that OsZIP7 is important for Zn homeostasis under physiological Zn concentrations, and that Fe homeostasis might be affected due to loss of function of OsZIP7.
Keywords: Rice, ZIP transporter, Zinc, Iron, Tos17
Introduction
Zinc (Zn) is a key micronutrient to animals and plants, being the second most abundant transition metal in living organisms after iron (Fe). Many proteins use Zn as a structural cofactor, and it is estimated that nearly 10% of the proteome of eukaryotic organisms bind Zn (Andreini et al. 2006). While low Zn levels can be detrimental to cell metabolism, Zn excess can lead to oxidative stress, and therefore Zn homeostasis should maintain optimal concentrations. In plants, several Zn transporters and Zn binding proteins have been described to be involved in controlling Zn deficiency responses or Zn detoxification (Ricachenevsky et al. 2015).
Rice (Oryza sativa) is one the most important crops in the world, being a staple food for half of the world’s population. It is estimated that 19% of the calories consumed by humans are derived from rice grains (Elert 2014). However, rice grains are a poor source of minerals such as Zn and Fe, which are the most commonly lacking nutrients in human diet (Ricachenevsky et al. 2015; Sperotto et al. 2012). Rice also has the lowest concentration of both minerals and the narrowest genetic variability among cereals (Garcia-Oliveira et al. 2018). Considering its widespread consumption and low nutritional quality, rice biofortification has been proposed as one of the best solutions to deliver grains with increased levels of both Fe and Zn through the diet to humans. Biofortification consists in making plants accumulate higher concentrations of bioavailable nutrients in their edible parts, such as grains, leaves and roots (Garcia-Oliveira et al. 2018; Sperotto et al. 2012).
In order to do that, understanding the regulation of uptake, distribution and accumulation of nutrients is necessary. The ZIPs (Zinc-regulated/Iron-regulated Proteins) were the first Zn/Fe transporter family described in plants, with the cloning of AtIRT1 from Arabidopsis thaliana (Eide et al. 1996). Since then, several ZIPs were functionally characterized, and some were shown to transport Zn (Li et al. 2013; Milner et al. 2013; Tiong et al. 2015). Although most examples come from the model species A. thaliana, characterization of ZIP transporters in graminaceous plants have been performed, with available comprehensive surveys in maize (Li et al. 2013) and barley (Tiong et al. 2015). In rice, three ZIP transporters were functionally characterized: OsZIP4 (Ishimaru et al. 2005), OsZIP5 (Lee et al. 2010a) and OsZIP8 (Lee et al. 2010b), all implicated in Zn transport. However, their clear physiological roles are not yet understood.
Recently, the molecular characterization of OsZIP7 showed that heterologous expression of OsZIP7 in A. thaliana results in Zn accumulation in leaves and seeds. A. thaliana plants constitutively expressing OsZIP7 showed increased sensitivity to Zn excess. Authors demonstrated that OsZIP7 is a plasma membrane Zn-specific transporter (Ricachenevsky et al. 2018). OsZIP7 orthologs from barley (HvZIP7) and maize (ZmZIP7) were also characterized (Li et al. 2016; Tiong et al. 2014). While ZmZIP7 was heterologously expressed in A. thaliana (Li et al. 2016), HvZIP7 was over-expressed in barley. It was also shown that HvZIP7 is up regulated by Zn deficiency and down regulated by Zn excess, and authors proposed that HvZIP7 functions as a low-affinity Zn transporter (Tiong et al. 2014). Therefore, the orthologous group of OsZIP7 seems to perform low-affinity Zn transport in Poaceae species. However, the role of OsZIP7 in plant exposed to varying Zn concentrations is not yet clear.
In this study, we used a loss-of-function rice mutant oszip7 to better understand the role of this transporter in rice physiological responses when submitted to Zn deficiency and excess. We found that loss of OsZIP7 function results in deregulated Zn homeostasis. Interestingly, growth and photosynthetic parameters were generally increased under Zn deficiency, indicating that oszip7 plants are more acclimated to low Zn, while WT plants showed increased stress under excessive Zn. Our data support that OsZIP7 is involved in Zn homeostasis, and that loss of function mutation of this transporter may induce compensatory mechanisms that result in improved photosynthesis under low Zn conditions. Our data also supports that OsZIP7 might function as a low affinity Zn transporter, with a primary role under physiological Zn concentrations (i.e., not deficiency or excess).
Materials and methods
Plant material and growth conditions
Experiments were conducted in hydroponic system, using seeds from Oryza sativa mutant oszip7 and wild type from the cultivar Nipponbare. The oszip7 mutant was obtained from the Rice Genome Resource Center bank website (www.rgrc.dna.affrc.go.jp; line ND7016). Seeds were surface sterilized and imbibed in distilled water in the dark at 25 °C for 24 h. Seeds were then transferred to Petri dishes for germination for seven days at 25 °C with a 16 h/8 h day/night photoperiod. Seven day old plants were transferred to vermiculite, grown for further seven days, and then transferred to plastic containers with 2 L nutrient solution, as described (Ricachenevsky et al. 2011). Plants were acclimated for seven days, and then treated with control nutrient solution (as described by Ricachenevsky et al. (2011), −Zn (no Zn added) and Zn excess (200 µM Zn) for 24 days. Nutrient solutions were changed every three days.
Superoxide dismutase activity
Samples were homogenized (0.5 g) in 3 mL of sodium phosphate buffer 0.05 M (pH 7,8) with 1 mM EDTA and 0.5% Triton X-100. Homogenates were centrifuged at 13,000g for 20 min at 4 °C. Supernatant was used in enzymatic activity and protein content determinations as described (Bradford 1976; Zhu et al. 2004). Superoxide dismutase (SOD) activity was evaluated according to the method described by Giannopolitis e Ries (Giannopolitis and Ries 1977).
Photosynthetic parameters
The Infra Red Gas Analyzer (IRGA) LI-COR model LI-6400 XT was used after 24 days of treatment in the middle third of the last completely expanded leaf, using a photosynthetic radiation of 1500 μmol m−2 s−1 and CO2 concentration of 400 μmol mol−1. Quantifications of CO2 liquid assimilation (A—μmol CO2 m−2 s−1), stomatal conductance (GS—mol H2O−1 m−2 s−1), transpiration rate (E—mmol H2O−1 m−2 s−1) and water use efficiency (WUE—mol CO2 mol H2O−1) were performed.
Chlorophyll fluorescence emission was measured after 24 days of treatment using a handheld modulated light fluorometer (Junior-Pam Chlorophyll Fluorometer Walz Mess-und-Regeltechnik, Germany). Measurements were performed in the morning (8:00–11:00 am) in the first completely expanded leaf (Souza et al. 2013), using three plants per treatment per genotype.
Elemental concentrations
Plants at the end of the experiment had shoots and roots collected (n = 4) and dried at 65 °C. Afterwards, samples were ground and digested with HNO3-HClO4 (Embrapa 1997) to determine concentrations of Cu, Zn, Fe e Mn using atomic absorption spectrometry (Perkin Elmer, Analyst 200, United States). For seed analyses, we used n = 3, each sample being a pool of 250 mg seeds of one plant.
Gene expression analyses by RT-qPCR
Samples were pools of three plants per genotype per treatment, and four biological replicates were used. RNA extraction was performed using TRIzol® according to the manufacturer’s instructions. RNA was quantified using Nanodrop®. cDNA synthesis was performed with M-MLV (Invitrogen- Life Technologies Corporation) reverse transcriptase. RT-qPCR reactions were conducted in a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and relative gene expression was quantified using the method described by Livak and Schmittgen (Livak and Schmittgen 2001) with modification described by Ricachenevsky et al. (2011). Primers used are listed in Table 1.
Table 1.
Gene-specific primers used in this work
| Gene name | Primer Forward (5′–3′) | Primer Reverse (5′–3′) |
|---|---|---|
| OsYSL15 | GGTGCGGGGATGATTTG | CCATACAAACTTGTCATGCTG |
| OsIRT1 | ACTGGTGCCCATTCTGC | GCGAGGATGGGGATGG |
| OsIRO2 | CGGATTTGGGAACAGGACA | GTTCCTGACGACTTTCTCCA |
| OsUBQ5 | ACCACTTCGACCGCCACTACT | ACGCCTAAGCCTGCTGGTT |
Statistical analyses
Means were compared using the Student’s t-test, and were considered significantly different when p < 0.05.
Results
OsZIP7 expression regulated by Zn and isolation of a Tos17 loss of function mutant
To gain insight into the possible OsZIP7 function in Zn homeostasis, we analyzed OsZIP7 gene expression in roots of plants grown under control conditions, Zn deficiency (no Zn added) and Zn excess (200 µM) for 24 days. We found that OsZIP7 is up regulated upon Zn deficiency and down regulated by Zn excess (Fig. 1A). To further understand the role of OsZIP7 in Zn homeostasis in rice, we isolated a homozygous mutant from the Tos17 insertion line ND7016. The Tos17 insertion in OsZIP7 is at the boundary of the second intron and third exon (Fig. 1B). Expression of OsZIP7 was not detectable in oszip7 homozygous lines in all conditions (Fig. 1A). Therefore, we conclude that the Tos17 oszip7 mutant is a knockout line.
Fig. 1.
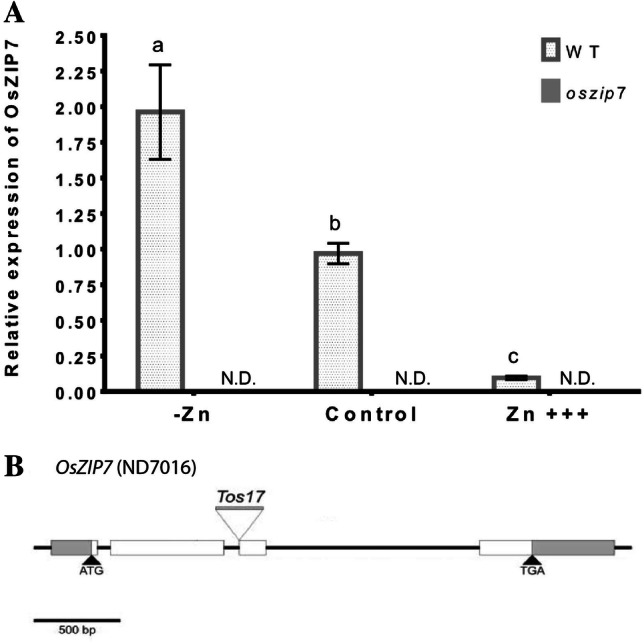
OsZIP7 gene expression under varying Zn concentrations and characterization of oszip7 line. A OsZIP7 expression under Zn deficiency (no Zn added), control conditions and Zn excess (200 µM). Different letters indicate statistically significant differences between means compared to control. Data is shown as mean ± SEM. B Gene model of OsZIP7 and insertion site of Tos17 in our mutant line. c OsZIP7 expression in the oszip7 mutant line
Biomass accumulation of WT and ozip7 under varying Zn conditions
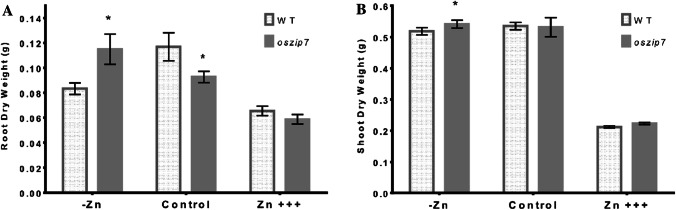
We evaluated how ozip7 and its respective WT accumulated biomass under control conditions, Zn excess and Zn deficiency. We observed that Zn excess clearly reduced biomass accumulation as shown by the lower shoot and root dry mass of both WT and oszip7 (Fig. 2A and B). Comparing WT to oszip7 plants, we found that roots of oszip7 have lower dry mass under control condition. Under Zn deficiency, oszip7 roots have higher dry mass compared to WT (Fig. 2A). In shoots, dry mass was similar in both WT and oszip7 in control and Zn excess conditions, while oszip7 plants showed slight increased biomass compared to WT under Zn deficiency (Fig. 2B).
Fig. 2.
Dry mass accumulation in WT and oszip7 plants under different Zn treatments. A Root dry mass. B Shoot dry mass. Asterisks indicate differences between WT and oszip7
Elemental quantification in roots and shoots of WT and ozip7 under varying Zn conditions
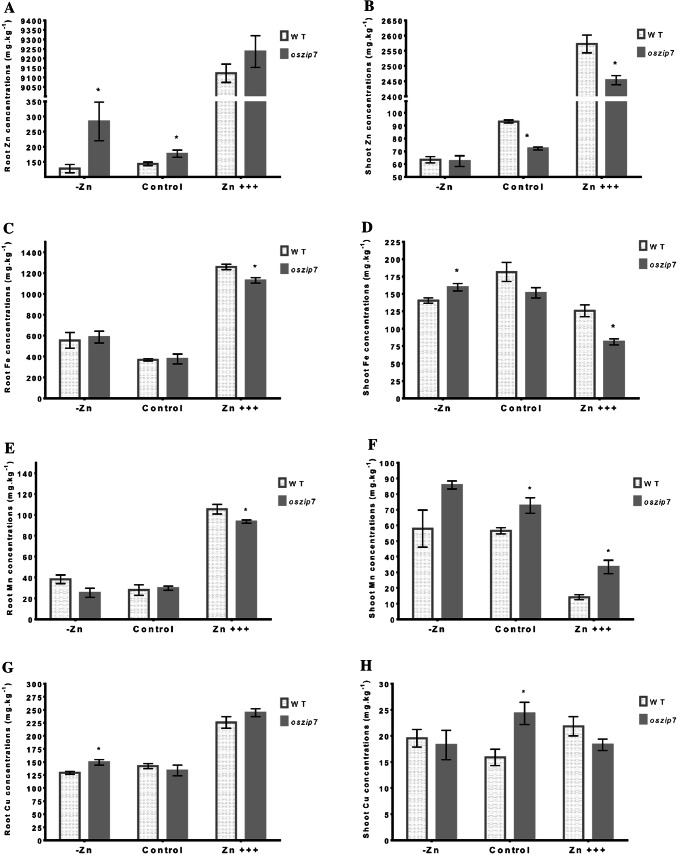
We evaluated the concentrations of micronutrients in WT and oszip7 roots and shoots. Since OsZIP7 is a Zn transporter (Ricachenevsky et al. 2018), we focused on Zn concentrations. We also quantified Fe, Mn and Cu in order to have a more complete picture of possible indirect effects of OsZIP7 loss-of-function in the homeostasis of these elements. As expected, Zn concentrations were much higher in both genotypes under Zn excess in roots and shoots. We found that Zn concentrations in roots of oszip7 plants were higher than in WT under both Zn deficiency and control conditions (Fig. 3A). In shoots, oszip7 Zn concentrations were lower compared to WT (Fig. 3B). Under Zn excess, oszip7 plants also showed lower Zn concentrations compared to WT. These results indicate that OsZIP7 loss-of-function decreases root to shoot translocation in rice.
Fig. 3.
Elemental accumulation in WT and oszip7 plants under different Zn treatments. A Root Zn concentration. B Shoot Zn concentration. C Root Fe concentration. D Shoot Fe concentration. E Root Mn concentration. F Shoot Mn concentration. G Root Cu concentration. H Shoot Cu concentration. Asterisks indicate differences between WT and oszip7
We also observed changes in concentrations of other metals. Under Zn excess, roots of OSZIP7 accumulated slightly less Fe than WT roots (Fig. 3C). Also under Zn excess, shoots of oszip7 accumulated higher levels of Fe, compared to WT (Fig. 3D). Under Zn deficiency, oszip7 mutant plants accumulated slightly higher Fe levels in shoots, whereas under control conditions there were no differences between the two genotypes (Fig. 3D). Mn concentrations were also altered in oszip7, compared to WT: roots of mutant plants accumulated less Mn under Zn excess (Fig. 3E), whereas shoots showed higher concentration under control conditions and Zn excess (Fig. 3F). Finally, we also found slightly increased Cu concentration in roots of oszip7 under Zn deficiency (Fig. 3G), and increased Cu concentration in shoots of oszip7 under control conditions (Fig. 3H), compared to WT. These results indicate that oszip7 loss-of-function changes metal accumulation in rice plants.
Photosynthetic performance of WT and oszip7 plants under varying Zn concentrations
We used IRGA to evaluate how photosynthetic performance might change in oszip7 under Zn deficiency, control condition and Zn excess. Stomatal conductance and transpiration rate were similar in all tested conditions comparing the two genotypes (data not shown). We found that CO2 assimilation and carboxylation instantaneous efficiency were increased in oszip7 under Zn deficiency compared to WT (Fig. 4A, B), indicating that oszip7 plants might be more acclimated to Zn deficiency than the WT. Water use efficiency was also increased in oszip7 plants under Zn deficiency compared to WT (Fig. 4C). Interestingly, the opposite was observed under Zn excess, with oszip7 plants showing lower water use efficiency than WT (Fig. 4C).
Fig. 4.
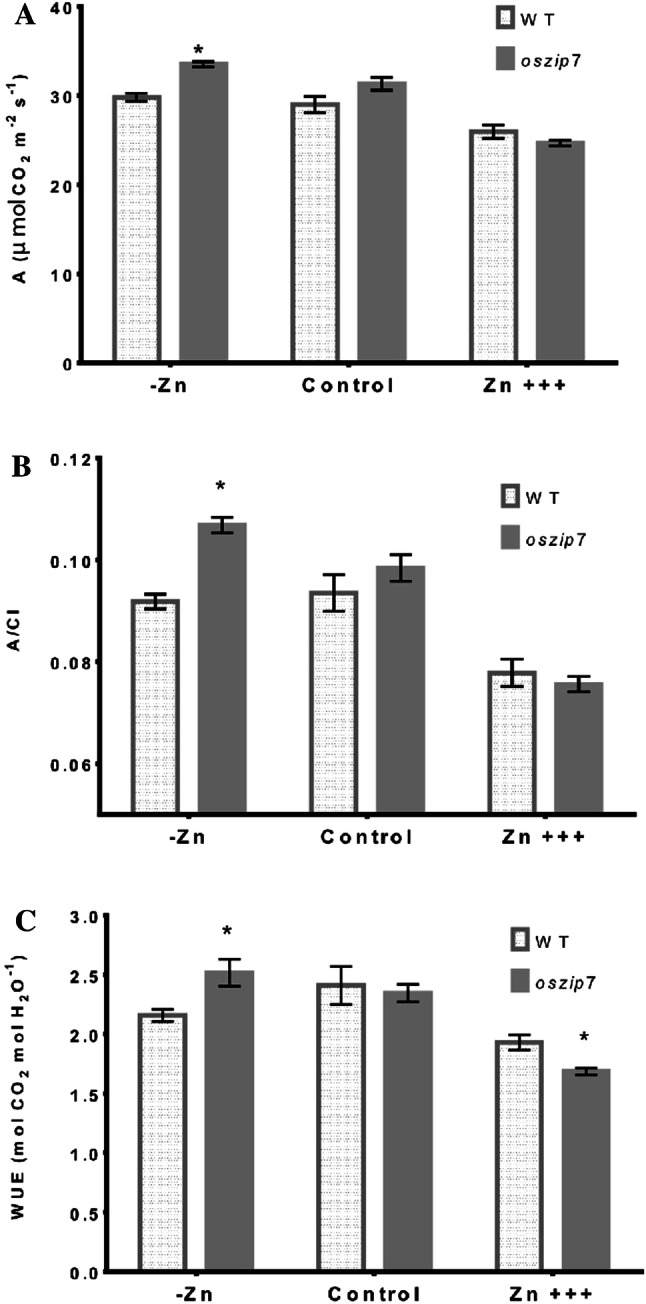
Photosynthetic performance of WT and oszip7 plants under different Zn treatments. A CO2 assimilation rate. B Carboxylation instantaneous efficiency. C Water use efficiency. Asterisks indicate differences between WT and oszip7
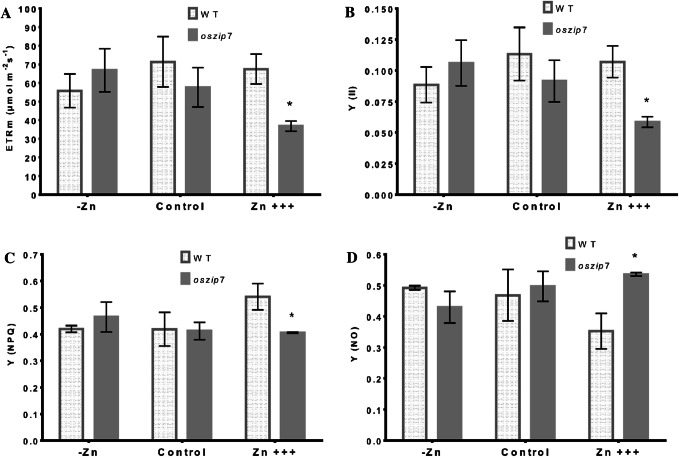
We also used chlorophyll fluorescence to access how the two genotypes responded to variations in Zn concentration, and found no differences comparing WT and oszip7 under Zn deficiency or control conditions. Under Zn excess, however, we observed decreased electron transfer rate (ETR) in oszip7 compared to WT (Fig. 5A), which suggests that oszip7 plants were more stressed upon high Zn concentrations. We also compared the quantum yield of photochemical energy conversion in PSII (Y (II); Fig. 5B); quantum yield of regulated non-photochemical energy dissipation losses (Y (NPQ); Fig. 5C); and quantum yield of non-regulated non-photochemical energy dissipation losses (Y (NO); Fig. 5D). Under Zn excess, oszip7 plants had lower Y (II) and Y (NPQ) as well as higher Y (NO) than WT plants (Fig. 5B–D. Therefore, it is likely that oszip7 has higher energy dissipation through non-regulated mechanisms, and reduced photochemical yield. These data suggest that OsZIP7 loss of function leads to increased sensitivity of the photosynthetic apparatus to Zn excess, and slight tolerance to low Zn conditions.
Fig. 5.
Chlorophyll fluorescence parameters of WT and oszip7 plants under different Zn treatments. A Electron transport rate. B Quantum yield of photochemical energy conversion in PSII. C Quantum yield of regulated non-photochemical energy dissipation losses. D Quantum yield of non-regulated non-photochemical energy dissipation losses. Asterisks indicate differences between WT and oszip7
Superoxide dismutase activity of WT and oszip7 plants under varying Zn concentrations
Formation of reactive oxygen species (ROS) such as superoxide radical (O•−2) is often increased in chloroplasts of plants under stress, and superoxide dismutase activity can be necessary to counteract overproduction (Das and Roychoudhury 2014). In order to access if WT and oszip7 plants were activating stress responses to different degrees in leaves due to varying Zn concentrations, we quantified superoxide dismutase (SOD) activity in shoots. We found that SOD activity increased with increasing Zn concentrations, with plants under Zn deficiency showing the lowest values (Fig. 6). Comparing WT and oszip7, we found no difference under Zn deficiency or control conditions, whereas oszip7 showed higher SOD activity than WT under Zn excess (Fig. 6). Thus, these data suggest that oszip7 plants are undergoing increased superoxide formation, which might be as least partially counteracted by SOD.
Fig. 6.
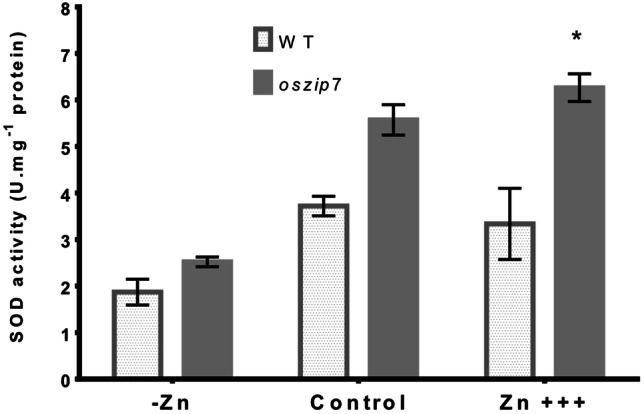
Superoxide dismutase activity in shoots of WT and oszip7 plants under different Zn treatments. Asterisks indicate differences between WT and oszip7
Gene expression of Fe homeostasis genes in WT and oszip7 plants under varying Zn concentrations
Since we found that oszip7 plants are more stressed under Zn excess, and that Fe concentrations are decreased in shoots of oszip7 compared to WT in these conditions, we tested how known Fe deficiency responsive genes were expressed in oszip7 and WT plants. OsYSL15, OsIRT1 and OsIRO2 expression was evaluated in roots of plants cultivated under Zn deficiency, control condition and Zn excess. Expression of the tested genes under Zn deficiency and control condition were not detected (data not shown). Under Zn excess, however, we found high expression of all genes. Interestingly, oszip7 plants showed higher expression of OsYSL15 and OsIRO2 than WT, whereas OsIRT1 expression was similar comparing both genotypes (Fig. 7). These data are in agreement with the observation that the Fe concentration in shoots is lower in oszip7 than in WT plants under Zn excess (Fig. 3B).
Fig. 7.
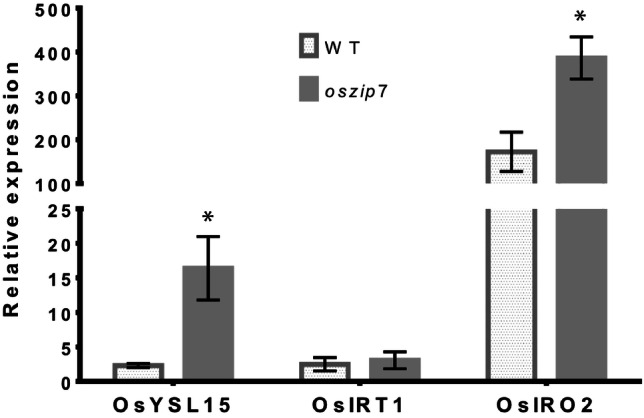
Gene expression of Fe deficiency up regulated genes in WT and oszip7 plants exposed to Zn excess. Asterisks indicate differences between WT and oszip7
Elemental accumulation in WT and oszip7 seed at maturity
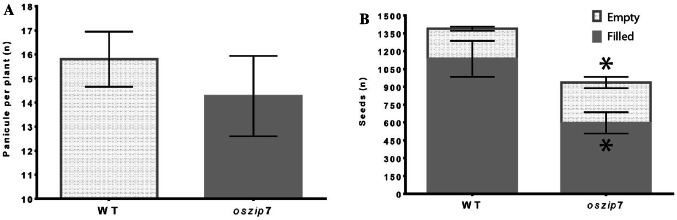
We cultivated WT and oszip7 plants until maturity in greenhouse conditions, and found that oszip7 plants showed decreased number of spikelets compared to WT, as well as markedly decreased number of filled seeds at maturity (Fig. 8A, B). We also analyzed elemental accumulation in mature seeds of WT and oszip7 plants. Interestingly, we found that concentration of Fe and Zn were decreased, while concentration of Mn was slightly increased (Fig. 9). Cu concentration, on the other hand, was unchanged. These results indicate that OsZIP7 loss-of-function and the consequent imbalance in Zn homeostasis reduce Zn and Fe in seeds.
Fig. 8.
Seed set comparison between WT and oszip7 plants. A Number of panicle per plant. B Number of seeds produced per plant. Seeds were further broke down in empty spikelets and filled seeds. Asterisks indicate differences between WT and oszip7
Fig. 9.
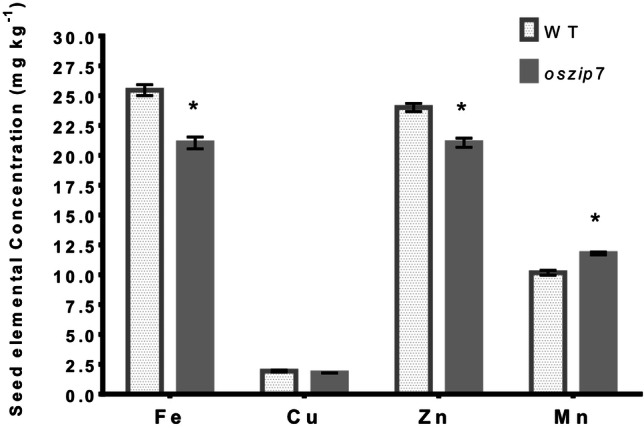
Elemental accumulation in seeds of WT and oszip7 plants. Asterisks indicate differences between WT and oszip7. Data is shown as mean ± SE
Discussion
Zn in an essential element to humans and animals, and understanding Zn homeostasis in rice is key for biofortification efforts in this important crop. Here we isolated a loss-of-function mutant for the Zn plasma membrane transporter OsZIP7 (Ricachenevsky et al. 2018), derived from the non-transgenic Tos17 insertional mutant collection. Under control conditions, lower shoot Zn concentration and higher root Zn concentration (Fig. 3A, B), supporting the hypothesis that OsZIP7 has a role in Zn root-to-shoot translocation. Our mutant line showed decreased Zn concentration in seeds (Fig. 9), which again indicates that OsZIP7 may have a role in Zn translocation. Since we found decreased seed set in oszip7 compared to WT (Fig. 8), the decrease in Zn concentration in seeds is not due to a lower number of seeds, which could therefore result in a concentration effect. Therefore, we showed that OsZIP7 has a role in Zn homeostasis in rice, likely being involved in Zn translocation to aerial tissues.
We exposed oszip7 and its respective WT to Zn deficiency, control condition and Zn excess. We found that in the absence of OsZIP7, rice plants show subtle, yet clear physiological alterations when external Zn concentrations are suboptimal. Small or even no clear changes are surprisingly common in mutants for genes of the ZIP family. In the model species Arabidopsis thaliana, the physiological roles of ZIP transporters are largely under characterized, since single mutants usually do not show phenotypic alterations, or show slight changes (Milner et al. 2013). Some transporters have been clearly demonstrated to respond to Zn deficiency, such as AtZIP4 (Assuncao et al. 2010; Sinclair et al. 2018), but not clear phenotype is associated with their loss-of-function. Considering that ZIP transporters are commonly large gene families in plant genomes (~ 15 members), a possible conclusion is that ZIPs are functionally overlapping, which suggests that higher-order mutants will be necessary to identify clear phenotypes and demonstrate physiological relevance (Ricachenevsky et al. 2015).
This hypothesis is further supported by work on ZIP genes in rice. OsZIP4 was shown to be responsive to Zn deficiency and to alter Zn homeostasis when over-expressed; OsZIP5, another Zn deficiency-responsive gene, showed decreased root-to-shoot Zn translocation when over-expressed (Lee et al. 2010a); and OsZIP8 over-expression also resulted in lower Zn translocation (Lee et al. 2010b). Only OsZIP5 loss-of-function mutants were described, and showed almost no changes compared to WT. Recently, OsZIP1 characterization showed a dual plasma membrane/ER localization. Interestingly, OsZIP1 seems to be important for metal excess tolerance, being required for Zn, Cu and Cd detoxification (Liu et al. 2019). Still, the precise mechanism by which OsZIP1 performs detoxification is unclear. Taken together, it is also likely that double/triple/quadruple mutants harboring mutations in phylogenetically related members would be necessary to demonstrate physiological roles for ZIP family genes in rice and other species. In this scenario, the phenotypic characterization performed in this work significantly contributes to the understanding of ZIP transporters in rice.
We found interesting changes in oszip7 plants exposed to Zn deficiency. Both shoots and roots of oszip7 accumulated more dry mass than WT under Zn deficiency (Fig. 2). Interestingly, it was previously shown that Zn deficiency leads to decreased root and shoot biomass accumulation in WT rice plants (Bandyopadhyay et al. 2017), similar to what we observed (Fig. 2). However, oszip7 plants have higher root dry weight compared to WT, slightly increased shoot dry weight, and do not show the general trend of decreased biomass under Zn deficiency, which indicates that OsZIP7 function has a role in acclimation to Zn deficiency. We also found that CO2 assimilation and water use efficiency were increased (Fig. 4). The increased carbon assimilated in oszip7 could be partitioned to root growth in order to overcome Zn deficiency. Corroborating the hypothesis that oszip7 plants might be less sensitive to Zn deficiency, we found that roots of oszip7 plants have higher Zn concentration than WT under Zn deficiency as well under control condition (Fig. 3). Shoot Zn concentrations, on the other hand, are similar between oszip7 and WT under Zn deficiency, but lower in oszip7 compared to WT under control condition (and Zn excess—see below; Fig. 3). Therefore, our data suggest that loss-of-function of OsZIP7 makes rice plants primed for Zn deficiency.
Increasing Zn uptake in roots when root to shoot Zn translocation is defective (including transcriptional changes in roots) was already described in rice plants. Plants over-expressing OsHMA3, a Zn/Cd vacuolar transporter expressed in roots, up regulated expression of ZIP transporters (OsZIP4, OsZIP5, OsZIP8, OsZIP9 and OsZIP10) (Sasaki et al. 2014). Similar results were found with rice natural variation: when comparing rice genotypes harboring loss of function alleles or functional OsHMA3 alleles, plants with the later showed increased expression of OsZIP4, OsZIP5, OsZIP8 and OsZIP10 compared to the former (Cai et al. 2019). These data indicate that, when OsHMA3 functionality is higher, Zn sinking into root vacuoles is increased, resulting in lower Zn root to shoot translocation and consequently inducing a shoot derived Zn deficiency signal which up regulates Zn uptake genes (Cai et al. 2019; Sasaki et al. 2014). The nature of such signal is not known, but indirect evidence for its existence in Zn deficiency response in Arabidopsis was recently shown (Sinclair et al. 2018). Loss of function of OsZIP7 might lead to a similar response due to decreased Zn translocation. In agreement with that, OsZIP5 over-expression leads to Zn accumulation in roots and decreased Zn translocation to shoots, decreased plant height and lower tiller numbers (Lee et al. 2010a), resembling the phenotypes described for our oszip7 line. Therefore, oszip7 plants could be primed for Zn deficiency response under control condition due to lower Zn concentration in shoots, which would lead to an exacerbated Zn deficiency response under low Zn condition. This is in agreement with the proposed role for the barley ortholog HvZIP7, the closest member of OsZIP7 characterized (Tiong et al. 2014). Thus, our data suggest that loss of OsZIP7 function results in increased expression of other ZIP transporters and consequent changes in Zn uptake. These genes would be part of the “priming” of oszip7 plants. Upon Zn deficiency, these plants would perform better due to higher Zn uptake capacity. This hypothesis, however, remains to be tested.
Upon Zn excess, we found clear changes in oszip7 plants compared to WT. First, it was clear that Zn excess stressed plants of both genotypes, decreasing most photosynthetic parameters (Figs. 4, 5). Comparing oszip7 to WT, we found that water use efficiency was lower (Fig. 4). Chlorophyll fluorescence data also indicate that oszip7 plants were more affected by excessive Zn, as electron transfer rate and quantum yield of photochemical energy conversion in PSII were reduced compared to WT (Fig. 5). Interestingly, we found that Zn concentration in shoots of oszip7 was lower, suggesting that Zn excessive uptake is not involved in decreased photosynthesis (Fig. 3). We also observed that Fe concentration was markedly decreased in shoots of oszip7 plants, and in roots to a lesser degree. This indicates that oszip7 plants might be under an exacerbated Fe deficiency response, despite the normal Fe concentration in the growth media. Under Zn deficiency, Fe concentrations are higher in shoots of oszip7. Although OsZIP7 is not involved in Fe transport (Ricachenevsky et al. 2018; Tan et al. 2019), Fe and Zn homeostasis are known to interact with each other (Shanmugam et al. 2011). This is further corroborated by expression of OsYSL15 and OsIRO2, a transporter (Lee et al. 2009) and a transcription factor (Ogo et al. 2011) that are responsive to low Fe condition and to Zn excess (Ricachenevsky et al. 2011). Our data showed that both genes are induced under Zn excess compared to control conditions, indicating that Zn excess indeed leads to a secondary Fe deficiency response; and that oszip7 induces these responses to a higher degree than WT (Fig. 7). Thus, this is evidence that OsZIP7 function might be important in the crosstalk between Zn and Fe homeostasis.
We observed that oszip7 showed increased superoxide dismutase activity under Zn excess compared to WT (Fig. 6). Superoxide dismutase is involved in detoxifying superoxide, which can be generated by deregulated photosynthesis electron transport (Das and Roychoudhury 2014). This corroborates our observation that oszip7 plants show defects in photosynthesis, which may be leading to increased superoxide production. Another possibility is that lower Fe concentration are causing defects in Fe–S cluster formation, which are key for electron transport, and thus causing reactive oxygen species formation. Previous work has shown that Fe–S clusters are targets to abiotic stress with heavy metals as well as Fe deficiency (Liang et al. 2014). Thus, Zn excess could be leading to similar phenotypes, which would be exacerbated in oszip7 plants. Moreover, Mn accumulation in shoots of oszip7 could also lead to increased reactive oxygen species formation and stress (Fig. 3).
We also showed that oszip7 plants, despite having lower seed set compared to WT plants (Fig. 8), have decreased Zn concentration in seeds (Fig. 9). Considering our data, it is possible to suggest that OsZIP7 is involved in Zn translocation from roots to shoots, and to seeds to developing seeds. In our work, Fe concentrations were also found to be decreased in oszip7 mutant compared to WT (Fig. 9). These observations should be considered together with the finding that lower seed set could result in increased Zn and Fe concentrations if an unspecific, concentrating effect was in place, which would result in the opposite phenotype (Sperotto et al. 2013). Our results indicate that OsZIP7 is an interesting target for biofortification.
Conclusion
Our work demonstrated an important role of OsZIP7 in Zn homeostasis, showing its function under varying Zn concentration, and how its loss-of-function might affect photosynthesis, the ionome and homeostatic mechanisms of other metals, including Fe, which support its function as a low affinity transporter. We conclude that OsZIP7 loss-of-function leads to increased tolerance to low Zn conditions, but increased sensitivity to Zn excess in rice, possibly due to compensatory mechanisms. We also showed that OsZIP7 is involved in controlling Zn and Fe concentrations in seeds.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul). Authors also declare no competing interests, and that materials used in this work are available upon request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rafael Gonçalves Gindri and Bruno Bachiega Navarro have contributed equally.
References
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5(11):3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Assuncao AG, Herrero E, Lin YF, Huettel B, Talukdar S, Smaczniak C, Immink RG, van Eldik M, Fiers M, Schat H, Aarts MG. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci USA. 2010;107(22):10296–10301. doi: 10.1073/pnas.1004788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay T, Mehra P, Hairat S, Giri J. Morpho-physiological and transcriptome profiling reveal novel zinc deficiency-responsive genes in rice. Funct Integr Genomics. 2017;17(5):565–581. doi: 10.1007/s10142-017-0556-x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai H, Huang S, Che J, Yamaji N, Ma JF. A tonoplast-localized OsHMA3 plays an important role in maintaining Zn homeostasis in rice. J Exp Bot. 2019 doi: 10.1093/jxb/erz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93(11):5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elert E. Rice by the numbers: a good grain. Nature. 2014;514(7524):S50–S51. doi: 10.1038/514S50a. [DOI] [PubMed] [Google Scholar]
- Garcia-Oliveira AL, Chander S, Ortiz R, Menkir A, Gedil M. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front Plant Sci. 2018;9:937. doi: 10.3389/fpls.2018.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977;59(2):315–318. doi: 10.1104/pp.59.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot. 2005;56(422):3207–3214. doi: 10.1093/jxb/eri317. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Suzuki M, Bashir K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J Exp Bot. 2007;58(11):2909–2915. doi: 10.1093/jxb/erm147. [DOI] [PubMed] [Google Scholar]
- Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009;150(2):786–800. doi: 10.1104/pp.109.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong HJ, Kim SA, Lee J, Guerinot ML, An G. OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol Biol. 2010;73(4–5):507–517. doi: 10.1007/s11103-010-9637-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim SA, Lee J, Guerinot ML, An G. Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol Cells. 2010;29(6):551–558. doi: 10.1007/s10059-010-0069-0. [DOI] [PubMed] [Google Scholar]
- Li S, Zhou X, Huang Y, Zhu L, Zhang S, Zhao Y, Guo J, Chen J, Chen R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol. 2013;13:114. doi: 10.1186/1471-2229-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhou X, Zhao Y, Li H, Liu Y, Zhu L, Guo J, Huang Y, Yang W, Fan Y, Chen J, Chen R. Constitutive expression of the ZmZIP7 in Arabidopsis alters metal homeostasis and increases Fe and Zn content. Plant Physiol Biochem. 2016;106:1–10. doi: 10.1016/j.plaphy.2016.04.044. [DOI] [PubMed] [Google Scholar]
- Liang X, Qin L, Liu P, Wang M, Ye H. Genes for iron-sulphur cluster assembly are targets of abiotic stress in rice, Oryza sativa. Plant Cell Environ. 2014;37(3):780–794. doi: 10.1111/pce.12198. [DOI] [PubMed] [Google Scholar]
- Liu XS, Feng SJ, Zhang BQ, Wang MQ, Cao HW, Rono JK, Chen X, Yang ZM. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019;19(1):283. doi: 10.1186/s12870-019-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Milner MJ, Seamon J, Craft E, Kochian LV. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot. 2013;64(1):369–381. doi: 10.1093/jxb/ers315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo Y, Itai RN, Kobayashi T, Aung MS, Nakanishi H, Nishizawa NK. OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol Biol. 2011;75(6):593–605. doi: 10.1007/s11103-011-9752-6. [DOI] [PubMed] [Google Scholar]
- Ricachenevsky FK, Sperotto RA, Menguer PK, Sperb ER, Lopes KL, Fett JP. ZINC-INDUCED FACILITATOR-LIKE family in plants: lineage-specific expansion in monocotyledons and conserved genomic and expression features among rice (Oryza sativa) paralogs. BMC Plant Biol. 2011;11:20. doi: 10.1186/1471-2229-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricachenevsky FK, Menguer PK, Sperotto RA, Fett JP. Got to hide your Zn away: molecular control of Zn accumulation and biotechnological applications. Plant Sci. 2015;236:1–17. doi: 10.1016/j.plantsci.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Ricachenevsky FK, Punshon T, Lee S, Oliveira BHN, Trenz TS, Maraschin FDS, Hindt MN, Danku J, Salt DE, Fett JP, Guerinot ML. Elemental profiling of rice FOX lines leads to characterization of a new Zn plasma membrane transporter, OsZIP7. Front Plant Sci. 2018;9:865. doi: 10.3389/fpls.2018.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Ma JF. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot. 2014;65(20):6013–6021. doi: 10.1093/jxb/eru340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Lo JC, Wu CL, Wang SL, Lai CC, Connolly EL, Huang JL, Yeh KC. Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana–the role in zinc tolerance. New Phytol. 2011;190(1):125–137. doi: 10.1111/j.1469-8137.2010.03606.x. [DOI] [PubMed] [Google Scholar]
- Sinclair SA, Senger T, Talke IN, Cobbett CS, Haydon MJ, Kramer U. Systemic upregulation of MTP2- and HMA2-mediated Zn partitioning to the shoot supplements local zn deficiency responses. Plant Cell. 2018;30(10):2463–2479. doi: 10.1105/tpc.18.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza TCPC, Evarito MC, Paulo EPA, Mauro AM. The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. Acta Physiol Plant. 2013;35:515–527. doi: 10.1007/s11738-012-1093-9. [DOI] [Google Scholar]
- Sperotto RA, Ricachenevsky FK, Waldow Vde A, Fett JP. Iron biofortification in rice: it's a long way to the top. Plant Sci. 2012;190:24–39. doi: 10.1016/j.plantsci.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Tan L, Zhu Y, Fan T, Peng C, Wang J, Sun L, Chen C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem Biophys Res Commun. 2019;512(1):112–118. doi: 10.1016/j.bbrc.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Tiong J, McDonald GK, Genc Y, Pedas P, Hayes JE, Toubia J, Langridge P, Huang CY. HvZIP7 mediates zinc accumulation in barley (Hordeum vulgare) at moderately high zinc supply. New Phytol. 2014;201(1):131–143. doi: 10.1111/nph.12468. [DOI] [PubMed] [Google Scholar]
- Tiong J, McDonald G, Genc Y, Shirley N, Langridge P, Huang CY. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare) New Phytol. 2015;207(4):1097–1109. doi: 10.1111/nph.13413. [DOI] [PubMed] [Google Scholar]
- Zhu ZWG, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.) Plant Sci. 2004;167:527–533. doi: 10.1016/j.plantsci.2004.04.020. [DOI] [Google Scholar]