Abstract
Background: The clinical and prognostic value of programmed death-ligand 1, PD-L1, in glioblastoma remains controversial. The present study aimed to identify the expression of PD-L1 for its prognostic value in glioblastoma.
Methods: A comprehensive literature search was performed using the PubMed and CNKI databases. The overall survival (OS) and disease-free survival (DFS) of GBM was analyzed based on Hazard ratios (HRs) and 95% confidence intervals (CIs). Furthermore, Odds ratios (ORs) and 95% CIs were summarized for clinicopathological parameters. The statistical analysis was using RevMan 5.3 software.
Results: The meta-analysis was performed by using total nine studies including 806 patients who had glioblastoma. The pooled results indicated that PD-L1 expression in tumor tissues was significantly related to a poor OS (HR = 1.63, 95%CI: 1.19–2.24, P = 0.003, random effects model) with heterogeneity (I2 = 51%). In subgroup analyses, PD-L1 positivity was significantly associated with a worse OS for patients of American and Asian regions, but not for those of European regions. Moreover, PD-L1 expression implied a trend toward the mutation status of the IDH1 gene [coding the Isocitrate Dehydrogenase (NADP(+))-1 protein] (HR = 9.92, 95%CI: 1.85–53.08, P = 0.007, fixed effects model). However, the prediction overall survival (OS) of the patients showed that PD-L1 expression was independent from other clinicopathological features, such as gender and age.
Conclusions: Our analyses indicated that high expression of PD-L1 in glioblastoma tumor tissues is associated with poor survival of patients, and PD-L1 may act as a prognostic predictor and an effective therapeutic target for glioblastoma.
Keywords: glioblastoma, PD-L1, prognostic, clinicopathological, meta-analysis
Introduction
Glioblastoma represents the most commonly seen primary malignant brain tumor in adults, characterized by high aggressive behavior and high recurrence rate (1). Multimodality therapies have been suggested and practiced according to NCCN Guidelines, including surgical resection, radiotherapy with alkylating agents such as temozolomide (TMZ) and adjuvant TMZ chemotherapy. However, the outcomes of the treatment are far from satisfactory with the 5-year overall survival being <10% (2–4).
Differently from many other tumors, molecularly targeted therapies for glioblastoma produced very limited advances in prolonging life expectancies of the patients, reasons at least partly attributable to the poor penetration of the Blood Brain Barrier (BBB) by therapeutic agents or by rapidly developing drug resistance (5, 6). In the recent years, it is increasingly recognized that the central nervous system (CNS) interacting actively with the systemic immune system have offered a new exciting theoretical basis and promising opportunities for brain tumor immunotherapy (7–10).
Tumor cells can display immune evasion to weaken antitumor immunity by activating the so-called immune Checkpoint molecules (ICs) (11). Programmed cell death ligand-1 (PD-L1), a “classic” IC molecule, has the effect on induction of T-cell-mediated immune tolerance in tumor local microenvironment, leading to tumor immune escape and tumor growth stimulation, by combination with programmed cell death-1 (PD-1) located on the surface of activated T cells (12). PD-L1 has been shown to be upregulated in various cancer cells and associated with unfavorable prognosis (13–18). Over the past decade, immunotherapies targeting PD-1/PD-L1 axis have made a series of remarkable breakthroughs in prognosis improvement of hard-to-treat solid tumors (including head and neck squamous cell carcinoma, non-small cell lung cancer, gastric cancer, urothelial cancer, cervical cancer, and melanoma) and have entered in the standard clinical practice (19–24). Recently, the expression of PD-L1 on glioma cells has been documented (25, 26). Researches have increasingly concerned over the prognostic evaluation of PD-L1 in patients with glioblastoma. However, whether PD-L1 expression correlates with prognosis in glioblastoma patients remains controversial. Therefore, we assessed the consistency and magnitude of the prognostic and clinical significance of PD-L1 in glioblastoma patients through a systematic review and meta-analysis.
Materials and Methods
Literature Search Strategy
The implementation of this systematic review and meta-analysis followed the guideline of PRISM, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We systematically reviewed the literature published in the PubMed and CNKI (China National Knowledge Infrastructure) databases (dated to July 2019). The following key words were adopted: (“glioblastoma” OR “GBM” OR “glioma” OR “brain tumor” OR “brain cancer” OR “cerebral tumor” OR “intracranial tumor”) AND (“CD 274” OR “PD-L1” OR “Programmed Cell Death 1 Ligand 1”) without restrictions on languages, regions and publication types.
Inclusion and Exclusion Criteria
The study adopted the following inclusion criteria: (1) All patients were diagnosed with glioblastoma by histological examination; (2) Hazard ratio (HR) and 95% confidence intervals (CIs) could be available; or the association between PD-L1 and overall survival (OS) or disease-free survival (DFS) with sufficient data were provided.
The study excluded the following: (1) conference abstracts, case reports, reviews, basic research, clinical trials; (2) studies missing available data.
Data Extraction and Quality Assessment
Two investigators independently reviewed potentially relevant studies in order to minimize bias. A third reviewer was brought in when there were disagreements. We extracted the following data from the included studies: authors, name of the journal, year of publication and ethnicity, number of enrolled patients, tumor histology, PD-L1 expression level, cut off value, detection area, detection methods, and follow-up.
If only survival curves were available, the data could be extracted from the Kaplan Meier curves. The quality of each retrieved article was assessed independently by two assessors according to the Newcastle-Ottawa Quality Assessment Scale (NOS). A total score of 0–9 was assigned to each included study, and studies with a NOS score≥5 were considered to be of high quality (27).
Statistical Analysis
The association between PD-L1 expression with OS and DFS of patients with glioblastoma was evaluated according to the HR and 95%CI. Statistical heterogeneity among studies was quantified with the Cochran's Q test and the I2 statistic. We used a random-effects model to pool the data when evidence suggested significant heterogeneity (I2>50% or P < 0.1), while a fixed-effects model was conducted otherwise. Subgroup analyses and sensitivity analyses were attempted to explain the origin of heterogeneities. The potential publication bias was estimated by the Begg's and Egger's tests with significance of P < 0.05. Review Manager Version 5.3 (Cochrane Collaboration, Oxford, UK) and STATA 15 were statistical packages used in the study.
Results
Characteristics of Included Studies
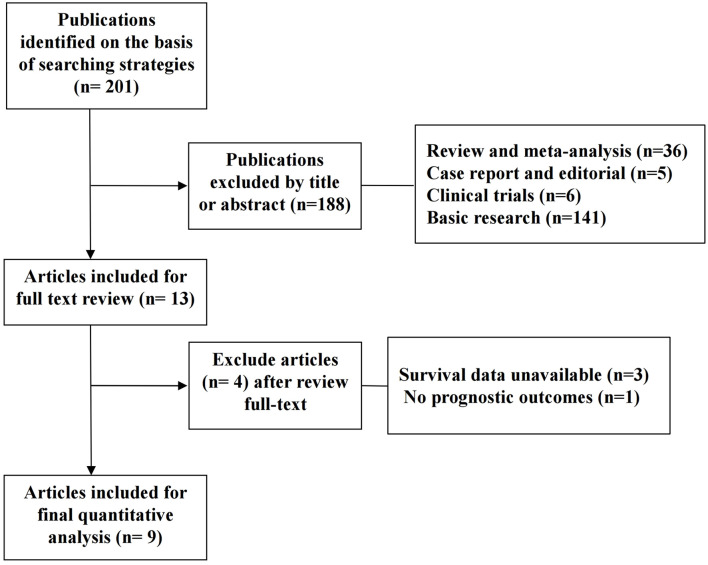
A total of 201 potentially relevant records were obtained according to the search strategy mentioned above. One hundred and eighty-eight studies were rejected after screening the titles and abstracts. Thirteen studies were included for further evaluation, of which 4 articles without eligible survival data were excluded. Finally, nine studies with 806 patients fulfilled the criteria and entered the meta-analysis. The selection flowchart and the baseline information of the studies are, respectively, displayed in Figure 1 and Table 1.
Figure 1.
Flow chart of the selection process of studies for inclusion in this meta-analysis.
Table 1.
General characteristics of included studies.
| References | Journal | Year | Country | No. of patients | Treatment | Methods | Cut-off point (high/low) | HR estimation | Quality assessment | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liu et al. (28) | The Journal of Neuroscience | 2013 | Denmark | 17 | Surgery | IFC | 10–100 positive cells | OS | 6 |
| 2 | Berghoff et al. (25) | Neuro-Oncology | 2015 | Austria | 135 | Surgery | IHC | 5% of positive cells | OS | 7 |
| 3 | Nduom et al. (29) | Neuro-Oncology | 2016 | USA | 94 | Surgery | IHC | 5% of positive cells | OS | 7 |
| 4 | Zeng et al. (30) | Oncotarget | 2016 | China | 229 | Surgery | IHC | 5% of positive cells | OS +DFS | 8 |
| 5 | Han et al. (31) | Journal of Pathology and Translational Medicine | 2017 | Korea | 54 | Surgery | IHC | 5% of positive cells | OS +DFS | 8 |
| 6 | Miyazaki et al. (32) | Journal of Neuro-Oncology | 2017 | Japan | 16 | Surgery | IHC | 50% of positive cells | OS +DFS | 7 |
| 7 | Lee et al. (33) | Journal of Neuro-Oncology | 2017 | Korea | 115 | Surgery | IHC | 5% of positive cells | OS | 7 |
| 8 | Pratt et al. (34) | Neurosurgery | 2018 | USA | 125 | Surgery | IHC | 5% of positive cells | OS | 8 |
| 9 | Hwang et al. (35) | Journal of Neuro-Oncology | 2018 | South Korea | 21 | Surgery | IHC | Score≥2 (infrequent small clusters of positive cells) | OS | 7 |
Association Between PD-L1 Expression and Prognostic Parameters
PD-L1 Expression and the Overall Survival (OS) of the Patients
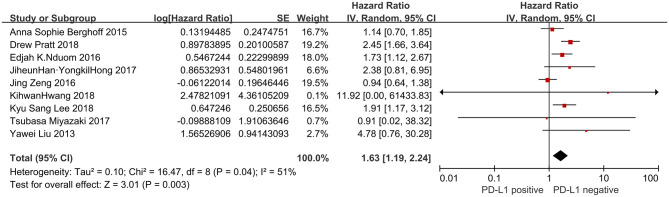
Nine studies presented OS data (n = 806). Significant heterogeneity existed amongst studies included in the analyses (I2 = 51%, P = 0.04). Pooled result by a random-effects model revealed a significantly inverse correlation between PD-L1 overexpression and OS of patients with glioblastoma (HR = 1.63, 95% CI: 1.19–2.24, P = 0.003) (Figure 2).
Figure 2.
Forest plot of 9 studies evaluating the association between PD-L1 expression and OS in glioblastoma patients.
PD-L1 Expression and Association With the Diseases Free Survival (DFS)
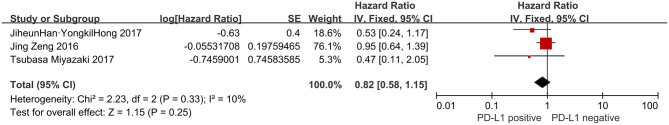
As shown in Figure 3, three studies (n = 299) focused on DFS and no heterogeneity was existed amongst the studies (I2= 10%, P = 0.33). However, pooled analysis by fixed model did not reveal any significant link between PD-L1 and DFS of patients (HR = 0.82, 95% CI: 0.58–1.15, P = 0.25).
Figure 3.
Forest plot of 3 studies evaluating the association between PD-L1 expression and DFS in glioblastoma patients.
PD-L1 Expression and Clinicopathological Characteristics
Age
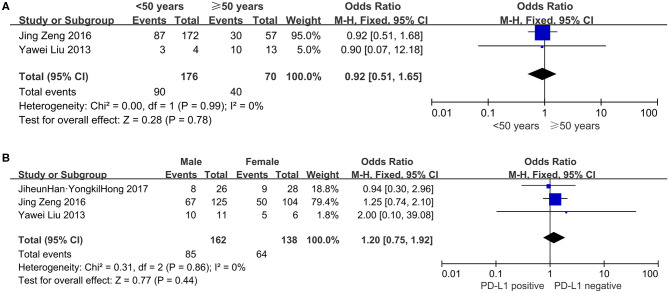
Two studies, consisting of 246 patients, assessed the correlation between age and PD-L1 expression. As shown in Figure 4A, 90 (51.14%) of 176 younger patients (whose age defined as younger than 50 yrs) showed PD-L1 expression, compared with 57.14% (40 of 70) of older patients (≥ 50 years of age) who had PD-L1 overexpression. PD-L1 expression did not correlate significantly with age (OR = 0.92, 95% CI: 0.51–1.65, P = 0.78).
Figure 4.
Forest plots for the association of PD-L1 expression with clinicopathological features in glioblastoma patients (A) age; (B) gender.
Gender
The dependability between PD-L1 expression and gender was assessed in three studies involving 300 patients. Eighty-five (52.47%) of 162 male patients and 64 (46.38%) of 138 female patients were PD-L1 overexpression. The results indicated that PD-L1 overexpression had no significant association with gender (OR = 1.20, 95% CI: 0.75–1.92, P = 0.44; Figure 4B).
Ethnicity
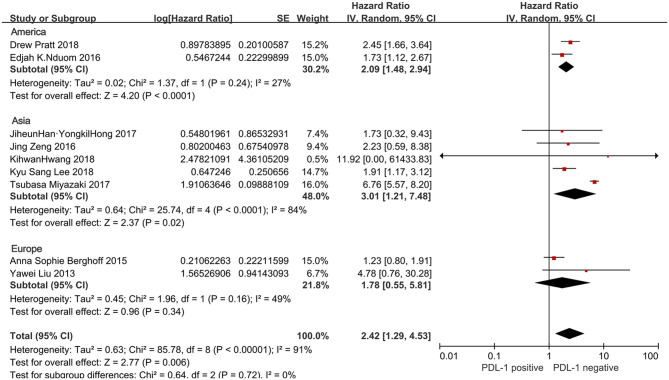
In ethnicity subgroup, the stratified analysis revealed PD-L1 positivity was linked to unfavorable OS in patients from the Asian regions (five studies with 435 cases: HR = 3.01, 95%CI:1.21–7.48, P = 0.02) and the American regions (two studies with 219 cases: HR = 2.09, 95% CI:1.48–2.94, P < 0.0001), but not in patients from the European studies (two studies with 152 cases: HR = 1.78, 95% CI:0.55–5.81, P = 0.34) (Figure 5).
Figure 5.
Forest plot for the association of PD-L1 expression with clinicopathological feature of region in glioblastoma patients.
IDH1 [Isocitrate Dehydrogenase (NADP(+))-1 Coding Gene] Status
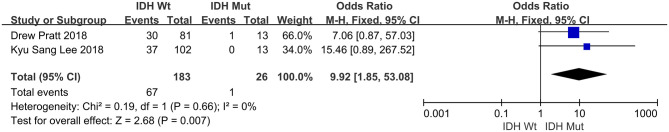
Two separate studies, encompassing 209 patients in total, evaluated a possible connection between IDH1 mutation (namely IDH1-wild type vs. with IDH1 mutation) and PD-L1. Of the 183 tumors which displayed IDH1-wild type, 67 (36.61%) were PD-L1 positive. Of the 26 tumors which had IDH1-mutantion, one (3.85%) was PD-L1 positive expression. The pooled OR indicated that PD-L1 positivity was closely related to IDH1 status (OR = 9.92, 95% CI: 1.85–53.08, P = 0.007) (Figure 6).
Figure 6.
Forest plot for the association of PD-L1 expression with clinicopathological feature of IDH1 status in glioblastoma patients.
In a subgroup analysis using a random effects model, heterogeneity was revealed in relation to PD-L1 and ethnicity of the patients (P < 0.00001, I2 = 91%).
Sensitivity Analysis and Publication Bias
Finally, we found no significant publication bias in the nine studies entered the current analysis, by, respectively, applying the Begg's test and the Egger's test (P = 0.917 and P = 0.527, respectively) (Figure 7).
Figure 7.
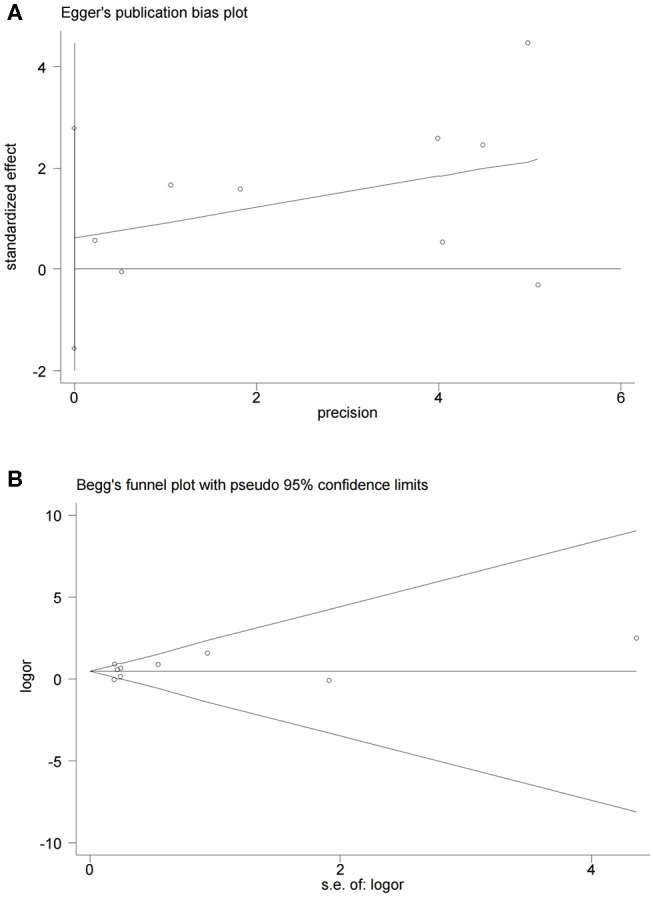
Begg's and Egger's funnel plot with 95% CI for OS publication bias in the included nine studies.
Discussion
PD-L1 is a coinhibitory ligand expressed in many types of tumor cells. It has been indicated that the binding of PD-L1 to its receptor PD-1 induces T cell dysfunction and apoptosis which plays a crucial role in tumor immune evasion. Gliomas have been recognized as an immunosuppressive tumor. The current understanding to glioma-mediated immunosuppression have generated increasing interest in the correlations between PD-L1 expression and survival for gliomas, particularly glioblastomas. However, the published results about glioblastomas remain controversial. In 2013 Liu et al. first reported that the expression of PD-L1 in seventeen patients with glioblastoma is a possible indicator for poor clinical outcome (28). Using level 3 Illumina RNASeq, Nduom et al. also found PD-L1 overexpression was indicative for shorter survival time in 149 patients with glioblastoma from (data from the Cancer Genome Atlas (TCGA) dataset) (29). Various other studies showed similar results (31, 33, 34). However, in a retrospective study of 117 newly diagnosed glioblastomas as well as a TCGA database analysis comprising 446 glioblastoma patients, researchersdid not find a significant connection between PD-L1 and the OS (25). The similar views were also taken in several other analyses (30, 32). To clarify a reasonable evidence-based conclusion, a meta-analysis including 9 studies with a total of 806 patients was performed. The present meta-analysis showed that PD-L1 positive expression was significantly associated with poor OS (HR = 1.63, 95% CI: 1.19–2.24, P = 0.003) in glioblastoma patients after surgery; however, there were insufficient evidence to suggest that PD-L1 was related to DFS (HR = 0.82, 95% CI: 0.58–1.15, P = 0.25). These results suggested that PD-L1 positive expression might be a negative prognostic factor in glioblastomas.
To further explore the potential sources of heterogeneity in the relationship between PD-L1 and overall survival in glioblastoma, we utilized subgroup analyses. The results confirmed that the significance of PD-L1 in OS was not affected by gender and age, collectively suggesting that this relationship is independent of these factors in tumor type. The influence of PD-L1 on the OS of multiethnic patients was also explored. Patients were classified as from Asia, America and Europe. Different combined HRs and P-values for OS were shown in different ethnic groups: PD-L1 overexpression was significantly associated with poor OS for patients from Asia and America, while no significant association for the survival of patients from Europe survival, which suggested that racial differences may be a potential origin of heterogeneity in glioblastoma. This “ethnic biasedness” of PD-L1 has been observed in several clinical studies for patients with certain other types of solid tumors. KEYNOTE-181, a phase 3 trial of Pembrolizumab (P) vs. chemotherapy (paclitaxel, docetaxel or irinotecan) in patients with esophageal squamous cell carcinoma, showed that P was superior to chemotherapy for OS in patients with PD-L1 positive expression (CPS≥10) in the global cohort, especially in the Chinese subgroup (36). Similarly, in the KEYNOTE-062 (a study of Pembrolizumab vs. chemotherapy in patients who had advanced gastric or gastro-esophageal junction cancer), P didn't bring significant survival benefits as the first-line treatment for PD-L1-positive (CPS≥1) population in the full global cohort (37). However, in further ethnicity subgroup analysis, P showed lower risk of death in Asians with PD-L1 positive expression (CPS≥1) when compared to chemotherapy, but not in Europeans, Americans and Australians. The findings in stratified analyses revealed that PD-L1 holding a prognostic role in different ethnic groups may have potential implications inimmunotherapy and prognostication for stratify patients. It is possible that the immunogenetics might to some extent differ in different races (38). So the subgroup analysis stratified by ethnicity may be necessary for accurately assessing the potential prognostic value of PD-L1 and the efficacy of relevant immunotherapy drugs in further clinical studies for glioblastoma.
According to the updated 2016 World Health Organization (WHO) classification of diffuse gliomas, Isocitrate dehydrogenase (IDH) has been considered as one of the important molecular biomarkers that has diagnostic, prognostic and predictive application (39–42). Studies have reported that glioblastomas which are hotspot mutation in IDH1 (an isoform of IDH) generally have a significantly better prognosis compared with IDH1-wildtype glioblastomas (43–45). Moreover, the expression of PD-L1 in diffuse gliomas might be directly influenced by IDH1 mutational status (33, 46, 47). Therefore, the relation between PD-L1 expression and IDH1 status was further investigated in the stratified analysis. We found that IDH1-wildtype status in glioblastoma was PD-L1 expression positive. The result confirmed recent findings of a PD-L1/IDH1-wildtype association. The hypothesis about its mechanism of this connection was that IDH1 mutation can result in the PD-L1 promoter hypermethylation that downregulates the expression of PD-L1 (48, 49). So the PD-L1 immune checkpoint inhibitors might not be advisable because of the low PD-L1 expression in patients with IDH1-mutant glioblastomas.
Recent research has showed that tumor cells in gliomas can regulate PD-L1 expression via two major mechanisms to mediate immune evasion, “adaptive resistance” mechanism and “innate resistance” mechanism (50). The former is for extrinsic induction of PD-L1. IFN-γ, a proinflammatory cytokine primarily produced by tumor infiltrating lymphocytes (TILs), can induce PD-L1 upregulation via NF-κB /PKD2 signal pathway (51–53). The latter is proved to mediate intrinsic induction of PD-L1. When there is a lack of TILs, PD-L1 induction in glioma cells might depend on multiple oncogenic signaling pathways (such as JAK/STAT 3, PI3K/Akt/mTOR/S6K1 and EGFR/MAPK pathway) or oncogene mutations (such as ALK, EGFR and PTEN) (11, 54–60).
It has been indicated that PD-L1 may be a valuable therapeutic target in cancer immunotherapy (9, 61). Recently, promising preclinical data in murine models of glioma have provided support for PD-L1 checkpoint inhibitors implementation in GBM patients (62–64). However, early clinical trials on the effectiveness of PD-L1 blockade agents are still limited and elusive. A combination between Anti-PD-L1 mAb durvalumab and bevacizumab is now being tested in a phase 2 open label, non-randomized clinical trial for GBM (cohort B, NCT02336165) (65). Interim results of durvalumab monotherapy revealed low SAE (severe adverse events) rate of 10% and efficacy with OS-6 of 42% and PFS-6 (progression-free survival) of 20%. Trials for other cohorts (cohort A: newly diagnosed MGMT-promoter unmethylated GBM, cohort C: refractory recurrent GBM) are still ongoing. Atezolizumab, a humanized antibody to PD-L1 that has been approved for second-line treatment for patients with advanced or metastatic NSCLC (non-small cell lung cancer) and urothelial cancer, is also being studied in a phase 1a clinical trial for multiple solid tumors, including GBM (PCD4989g; NCT01375842) (66). Results showed that Atezolizumab was safe and well-tolerated in patients with GBM. Glioblastoma was considered a type of “immunologically cold tumor” due to the relative lack of tumor infiltrating T cells in the tumor micro-environment (TME) and high selectivity of BBB (67–69). The “cold” phenotype of GBM may limit immunotherapy efficacy. Combinatorial treatment approaches targeting immune-suppression or BBB permeability may help shift the “cold” microenvironment and enhance response to immune checkpoint blockade in GBM, including radiation therapy, chemotherapy, other immunotherapies (e.g., Chimeric antigen receptor T-cells, oncolytic virus), and bevacizumab (70, 71).
So far as we are aware, the present study is the first meta-analysis to systematically estimate the correlation between PD-L1 and clinical outcomes and clinicopathological factors in glioblastoma. While some limitations need attention. Firstly, different analysis strategy of IHC and inconsistent cut-off values of PD-L1 expression may lead to heterogeneity between studies. Thus, a standardized approach for protein expression should be set up to improve consistency and veracity in the measurement of PD-L1 for future studies. Secondly, some subgroups, such as the IDH1 status group, had small sample sizes. Thirdly, the investigation about the correlations between PD-L1 and clinical features, including as tumor size, tumor site and surgical approach are not performed due to the deficiency of related original information.
In conclusion, our meta-analysis shows that high PD-L1 expression is strongly correlated with unfavorable prognosis for GBM patients. PD-L1 may present itself as a valuable target for immunotherapy in clinical practice. Additional high-quality, larger-scale prospective studies are needed to provide validate the potential value of PD-L1 for the prognosis and treatment of GBM patients in the future.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
CH and GC designed this study, searched the literature, screened identified studies and extracted data, and wrote the manuscript. Disagreements were resolved by discussion with WJ and WL. HZhao, FC, YL, SL, HZhang, YZ, and JC assisted in the literature search and screening. WJ and WL assisted in the designing of the study and the data analyses, and supervised the study. All authors have read and approved the final version of this manuscript.
Conflict of Interest
GC was employed by the company Beijing Qinglian Biotech, Co., Ltd., Beijing, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. (2015) 152:63–82. 10.1016/j.pharmthera.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Komotar RJ, Otten ML, Moise G, Connolly ESJr. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma-a critical review. Clin Med Oncol. (2008) 2:421–2. 10.4137/CMO.S390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Affronti ML, Heery CR, Herndon JE, II, Rich JN, Reardon DA, Desjardins A, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. (2009) 115:3501–11. 10.1002/cncr.24398 [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumours diagnosed in the United States in 2008-2012. Neuro Oncol. (2015) 17 (Suppl. 4):iv1–62. 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumours: microvessel populations with distinctive structural and functional properties. Microvasc Res. (1999) 58:312–28. 10.1006/mvre.1999.2188 [DOI] [PubMed] [Google Scholar]
- 6.Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals. (2013) 6:1475–506. 10.3390/ph6121475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. (2011) 17:1603–15. 10.1158/1078-0432.CCR-10-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumours: no longer a matter of privilege. Clin Cancer Res. (2014) 20:5620–9. 10.1158/1078-0432.CCR-14-0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber ST, Hashimoto Y, Weathers SP, Xiu J, Gatalica Z, Verhaak RG, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. (2016) 18:1357–66. 10.1093/neuonc/now132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. (2019) 25:477–86. 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. (2015) 27:450–61. 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA. (2013) 110:E2480–9. 10.1073/pnas.1305394110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. (2006) 66:3381–5. 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. (2007) 56:1173–82. 10.1007/s00262-006-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Yuan SX, Chen C, Mao YX, Xu G, Wang XY. [Expression of B7-H1 protein in human pancreatic carcinoma tissues and its clinical significance]. Ai Zheng. (2009) 28:1328–32. 10.5732/cjc.009.10245 [DOI] [PubMed] [Google Scholar]
- 16.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. (2011) 28:682–8. 10.1007/s12032-010-9515-2 [DOI] [PubMed] [Google Scholar]
- 17.Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. (2015) 9:901–9. 10.2147/DDDT.S75152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enkhbat T, Nishi M, Takasu C, Yoshikawa K, Jun H, Tokunaga T, et al. Programmed cell death ligand 1 expression is an independent prognostic factor in colorectal cancer. Anticancer Res. (2018) 38:3367–73. 10.21873/anticanres.12603 [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. (2006) 108:19–24. 10.1016/j.acthis.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumour PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. (2014) 20:2773–82. 10.1158/1078-0432.CCR-13-2702 [DOI] [PubMed] [Google Scholar]
- 21.Faghfuri E, Faramarzi MA, Nikfar S, Abdollahi M. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther. (2015) 15:981–93. 10.1586/14737140.2015.1074862 [DOI] [PubMed] [Google Scholar]
- 22.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 23.Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumour cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumour-infiltrating lymphocytes. Oncoimmunology. (2017) 6:e1312240. 10.1080/2162402X.2017.1312240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotte A, D'orazi G, Bhandaru M. Nobel committee honors tumour immunologists. J Exp Clin Cancer Res. (2018) 37:262. 10.1186/s13046-018-0937-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, et al. Programmed death ligand 1 expression and tumour-infiltrating lymphocytes in glioblastoma. Neuro Oncol. (2015) 17:1064–75. 10.1093/neuonc/nou307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiland DH, Haaker G, Delev D, Mercas B, Masalha W, Heynckes S, et al. Comprehensive analysis of PD-L1 expression in glioblastoma multiforme. Oncotarget. (2017) 8:42214–25. 10.18632/oncotarget.15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Carlsson R, Ambjorn M, Hasan M, Badn W, Darabi A, et al. PD-L1 expression by neurons nearby tumours indicates better prognosis in glioblastoma patients. J Neurosci. (2013) 33:14231–45. 10.1523/JNEUROSCI.5812-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. (2016) 18:195–205. 10.1093/neuonc/nov172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng J, Zhang XK, Chen HD, Zhong ZH, Wu QL, Lin SX. Expression of programmed cell death-ligand 1 and its correlation with clinical outcomes in gliomas. Oncotarget. (2016) 7:8944–55. 10.18632/oncotarget.6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Hong Y, Lee YS. PD-L1 expression and combined status of PD-L1/PD-1-positive tumour infiltrating mononuclear cell density predict prognosis in glioblastoma patients. J Pathol Transl Med. (2017) 51:40–8. 10.4132/jptm.2016.08.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki T, Ishikawa E, Matsuda M, Akutsu H, Osuka S, Sakamoto N, et al. Assessment of PD-1 positive cells on initial and secondary resected tumour specimens of newly diagnosed glioblastoma and its implications on patient outcome. J Neurooncol. (2017) 133:277–85. 10.1007/s11060-017-2451-7 [DOI] [PubMed] [Google Scholar]
- 33.Lee KS, Lee K, Yun S, Moon S, Park Y, Han JH, et al. Prognostic relevance of programmed cell death ligand 1 expression in glioblastoma. J Neurooncol. (2018) 136:453–61. 10.1007/s11060-017-2675-6 [DOI] [PubMed] [Google Scholar]
- 34.Pratt D, Dominah G, Lobel G, Obungu A, Lynes J, Sanchez V, et al. Programmed death ligand 1 is a negative prognostic marker in recurrent isocitrate dehydrogenase-wildtype glioblastoma. Neurosurgery. (2019) 85:280–9. 10.1093/neuros/nyy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang K, Koh EJ, Choi EJ, Kang TH, Han JH, Choe G, et al. PD-1/PD-L1 and immune-related gene expression pattern in pediatric malignant brain tumours: clinical correlation with survival data in Korean population. J Neurooncol. (2018) 139:281–91. 10.1007/s11060-018-2886-5 [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Luo S, Qin S, Cheng Y, Li Z, Fan Y, et al. Pembrolizumab vs chemotherapy in patients with advanced/metastatic adenocarcinoma (ac) or squamous cell carcinoma (SCC) of the esophagus as second-line therapy: analysis of the chinese subgroup in KEYNOTE-181. Ann Oncol. (2019) 30:mdz247–086. 10.1093/annonc/mdz247.086 [DOI] [Google Scholar]
- 37.Tabernero J, Van Cutsem E, Bang YJ, Fuchs CS, Wyrwicz L, Lee KW, et al. Pembrolizumab with or without chemotherapy versus chemotherapy in advanced G/GEJ adenocarcinoma: the phase 3, KEYNOTE-062 study. J ClinOncol. (2019) 37:LBA4007 10.1200/JCO.2019.37.18_suppl.LBA4007 [DOI] [Google Scholar]
- 38.Zhang XC, Wang J, Shao GG, Wang Q, Qu X, Wang B, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. (2019) 10:1772. 10.1038/s41467-019-09762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, Van Den Bent MJ. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol. (2011) 12:83–91. 10.1016/S1470-2045(10)70053-X [DOI] [PubMed] [Google Scholar]
- 40.Van Lith SA, Molenaar R, Van Noorden CJ, Leenders WP. Tumour cells in search for glutamate: an alternative explanation for increased invasiveness of IDH1 mutant gliomas. Neuro Oncol. (2014) 16:1669–70. 10.1093/neuonc/nou152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. (2017) 14:284–97. 10.1007/s13311-017-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. (2013) 19:764–72. 10.1158/1078-0432.CCR-12-3002 [DOI] [PubMed] [Google Scholar]
- 44.Kaminska B, Czapski B, Guzik R, Krol SK, Gielniewski B. Consequences of IDH1/2 mutations in gliomas and an assessment of inhibitors targeting mutated IDH proteins. Molecules. (2019) 24:968. 10.3390/molecules24050968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korshunov A, Casalini B, Chavez L, Hielscher T, Sill M, Ryzhova M, et al. Integrated molecular characterization of IDH-mutant glioblastomas. Neuropathol Appl Neurobiol. (2019) 45:108–18. 10.1111/nan.12523 [DOI] [PubMed] [Google Scholar]
- 46.Moxley KM, Wang L, Welm AL, Bieniasz M. Short-form ron is a novel determinant of ovarian cancer initiation and progression. Genes Cancer. (2016) 7:169–81. 10.18632/genesandcancer.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berghoff AS, Kiesel B, Widhalm G, Wilhelm D, Rajky O, Kurscheid S, et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol. (2017) 19:1460–8. 10.1093/neuonc/nox054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu L, Long Y, Yang C, Jin L, Tao H, Ge H, et al. The IDH1 mutation-induced oncometabolite, 2-hydroxyglutarate, may affect DNA methylation and expression of PD-L1 in gliomas. Front Mol Neurosci. (2018) 11:82. 10.3389/fnmol.2018.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rover LK, Gevensleben H, Dietrich J, Bootz F, Landsberg J, Goltz D, et al. PD-1 (PDCD1) Promoter methylation is a prognostic factor in patients with diffuse lower-grade gliomas harboring isocitrate dehydrogenase (IDH) mutations. EBioMedicine. (2018) 28:97–104. 10.1016/j.ebiom.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. (2017) 10:81. 10.1186/s13045-017-0455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. (2012) 217:385–93. 10.1016/j.imbio.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 52.Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS ONE. (2015) 10:e0123410. 10.1371/journal.pone.0123410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian J, Wang C, Wang B, Yang J, Wang Y, Luo F, et al. The IFN-γ/PD-L1 axis between T cells and tumour microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflammation. (2018) 15:290. 10.1186/s12974-018-1330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumour suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. (2007) 13:84–8. 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 55.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci USA. (2008) 105:20852–7. 10.1073/pnas.0810958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crane C, Panner A, Pieper RO, Arbiser J, Parsa AT. Honokiol-mediated inhibition of PI3K/mTOR pathway: a potential strategy to overcome immunoresistance in glioma, breast, and prostate carcinoma without impacting T cell function. J Immunother. (2009) 32:585–92. 10.1097/CJI.0b013e3181a8efe6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. (2010) 116:3268–77. 10.1182/blood-2010-05-282780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumours. Cancer Discov. (2013) 3:1355–63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lastwika KJ, Wilson W, III, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. (2016) 76:227–38. 10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- 60.Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. (2019) 25:462–9. 10.1038/s41591-019-0349-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. (2015) 125:3384–91. 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. (2013) 86:343–9. 10.1016/j.ijrobp.2012.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumours. Clin Cancer Res. (2014) 20:5290–301. 10.1158/1078-0432.CCR-14-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, Qu YM, et al. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS ONE. (2015) 10:e0134715. 10.1371/journal.pone.0134715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reardon DA, Kaley TJ, Dietrich J, Clarke JL, Dunn GP, Lim MMH, Cloughesy TF, et al. Phase 2 study to evaluate safety and efficacy of MEDI4736 (durvalumab [DUR]) in glioblastoma (GBM) patients: an update. J Clin Oncol. (2017) 35:2042 10.1200/JCO.2017.35.15_suppl.2042 [DOI] [Google Scholar]
- 66.Lukas RV, Rodon J, Becker K, Wong ET, Shih K, Touat M, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. (2018) 140:317–28. 10.1007/s11060-018-2955-9 [DOI] [PubMed] [Google Scholar]
- 67.Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. (2003) 33:2998–3006. 10.1002/eji.200323611 [DOI] [PubMed] [Google Scholar]
- 68.Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. (2013) 33:13–21. 10.1038/jcbfm.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colli LM, Machiela MJ, Myers TA, Jessop L, Yu K, Chanock SJ. Burden of nonsynonymous mutations among TCGA cancers and candidate immune checkpoint inhibitor responses. Cancer Res. (2016) 76:3767–72. 10.1158/0008-5472.CAN-16-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. (2017) 9:aaa0984. 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta K, Burns TC. Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front Oncol. (2018) 8:503. 10.3389/fonc.2018.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.